Abstract
Infection by Plasmodium, the causative agent of malaria, is associated with hemolysis and therefore with release of hemoglobin from RBC. Under inflammatory conditions, cell-free hemoglobin can be oxidized, releasing its heme prosthetic groups and producing deleterious free heme. Here we demonstrate that survival of a Plasmodium-infected host relies strictly on its ability to prevent the cytotoxic effects of free heme via the expression of the heme-catabolyzing enzyme heme oxygenase-1 (HO-1; encoded by the Hmox1 gene). When infected with Plasmodium chabaudi chabaudi (Pcc), wild-type (Hmox1+/+) BALB/c mice resolved infection and restored homeostasis thereafter (0% lethality). In contrast, HO-1 deficient (Hmox1−/−) BALB/c mice developed a lethal form of hepatic failure (100% lethality), similar to the one occurring in Pcc-infected DBA/2 mice (75% lethality). Expression of HO-1 suppresses the pro-oxidant effects of free heme, preventing it from sensitizing hepatocytes to undergo TNF-mediated programmed cell death by apoptosis. This cytoprotective effect, which inhibits the development of hepatic failure in Pcc-infected mice without interfering with pathogen burden, is mimicked by pharmacological antioxidants such as N-acetylcysteine (NAC). When administered therapeutically, i.e., after Pcc infection, NAC suppressed the development of hepatic failure in Pcc-infected DBA/2 mice (0% lethality), without interfering with pathogen burden. In conclusion, we describe a mechanism of host defense against Plasmodium infection, based on tissue cytoprotection against free heme and limiting disease severity irrespectively of parasite burden.
Keywords: heme, heme-oxygenase-1, cell death, infection
Malaria, the disease caused by Plasmodium infection, remains one of the main causes of morbidity/mortality worldwide (1). Epidemiologically however, less than 1–2% of infected individuals succumb to severe forms of malaria (1). This suggests that Plasmodium has co-evolved with its human host to reach an evolutionary “tradeoff” in which infection “rarely” compromises host viability. This tradeoff is thought to rely almost exclusively on the ability of the host's immune system to control parasite burden (2), a defense strategy referred to as resistance to infection (3, 4). However, there is an additional host defense strategy that operates during Plasmodium infection and that limits disease severity irrespectively of parasite burden, i.e., tolerance to infection (3, 4). The mechanisms underlying host tolerance to Plasmodium infection remain poorly understood.
Plasmodium replication inside RBC leads to hemolysis and therefore to the production of cell-free hemoglobin (5, 6). When exposed to reactive oxygen species (ROS), cell-free hemoglobin is oxidized and releases its heme prosthetic groups (6). Circulating free heme is deleterious (6, 7), an effect countered by several host defense strategies (5), including the up-regulation of the heme catabolyzing enzyme heme oxygenase-1 (HO-1, encoded by the Hmox1 gene) (6).
HO-1 is a stress-responsive enzyme that converts the protoporphyrin IX ring of heme into biliverdin, releasing iron (Fe) and producing carbon monoxide (CO) (8). The heme/HO-1 system controls the pathogenesis of experimental cerebral malaria in mice (5, 6), a neuroinflammatory syndrome that resembles cerebral malaria in children infected by Plasmodium falciparum (9). We provide hereby a mechanistic basis for a broader involvement of the heme/HO-1 system in the pathogenesis of cerebral as well as noncerebral forms of severe malaria, such as those associated with hemolytic anemia and hepatic damage leading eventually to multi-organ failure. Moreover we demonstrate that the cytoprotective effect of HO-1 against free heme affords host tolerance against Plasmodium infection.
Results
Expression of HO-1 Sustains the Survival of Plasmodium chabaudi chabaudi (Pcc)-Infected Mice.
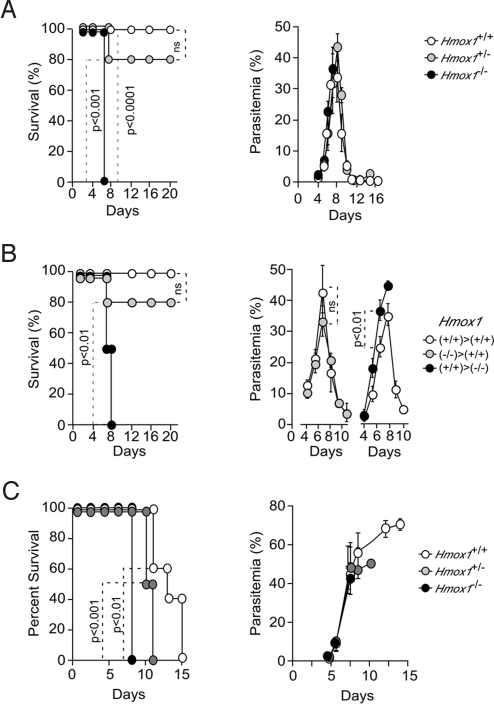
Contrary to wild-type (Hmox1+/+) BALB/c mice that survived Pcc infection (0% mortality) (Fig. 1A), Pcc infection was lethal in HO-1 deficient (Hmox1−/−) BALB/c mice (100% mortality) (Fig. 1A). Higher virulence of Pcc infection in Hmox1−/− mice was not attributable to higher parasitemia (Fig. 1A), suggesting that the protective effect of HO-1 acts irrespectively of pathogen burden, a host defense strategy referred to as tolerance to infection (3, 4).
Fig. 1.
Expression of HO-1 is required to sustain the survival of Pcc-infected mice. (A) Survival and parasitemia of Pcc-infected Hmox1+/+ (n = 8), Hmox1+/− (n = 10), and Hmox1−/− (n = 8) BALB/c mice. (B) Survival and parasitemia of Hmox1+/+ → Hmox1+/+ (n = 10), Hmox1−/− → Hmox1+/+ (n = 5), and Hmox1+/+ → Hmox1−/− (n = 4) bone marrow chimeric mice. (C) Survival and parasitemia of Pcc-infected Hmox1+/+ (n = 5), Hmox1+/− (n = 6), and Hmox1−/− (n = 6) SCID BALB/c mice. Parasitemias are shown as mean ± SD.
We asked whether the protective effect of HO-1 could be dissociated from the host immune response against Pcc infection. We used syngenic bone marrow transplants from Hmox1+/+ or Hmox1−/− donors into lethally irradiated Hmox1+/+ or Hmox1−/− recipients to generate chimeric mice in which Hmox1 was deleted specifically in nonhematopoietic (Hmox1+/+ → Hmox1−/−) vs. hematopoietic (Hmox1−/− → Hmox1+/+) tissues. The assumption being that host immunity against Pcc infection is confined to the hematopoietic compartment. Mortality associated with Pcc infection increased from 0% in control chimeric mice (Hmox1+/+ → Hmox1+/+) to 100% in chimeric mice in which the Hmox1 locus was deleted in nonhematopoietic tissues (Hmox1+/+ → Hmox1−/−) (Fig. 1B). This effect was associated with a slight, but significant increase in parasitemia (Fig. 1B). Chimeric mice in which the Hmox1 locus was deleted in hematopoietic tissues (Hmox1−/− → Hmox1+/+) were resistant to Pcc infection, i.e., 20% lethality (Fig. 1B). There was no modulation of parasitemia in these chimeric mice (Fig. 1B), suggesting that expression of HO-1 outside the host's adaptive immune system affords tolerance to Pcc infection.
We tested whether HO-1 would afford protection to Pcc infection in the absence of host adaptive immunity. SCID BALB/c mice, lacking T and B cells, did not clear Pcc, succumbing 11–15 days postinfection (100% mortality), from hyperparasitemia, i.e., >60% infected RBC (Fig. 1C). Onset of mortality in Hmox1−/− SCID BALB/c mice occurred 8 days postinfection, which was significantly earlier as compared to Hmox1+/+ SCID BALB/c mice (Fig. 1C). Earlier onset of mortality in Hmox1−/− SCID BALB/c mice did not correlate with modulation of parasitemia (Fig. 1C), suggesting that HO-1 can exert protective effects against Pcc infection in the absence of the host adaptive immunity.
Expression of HO-1 Suppresses Liver Failure in Pcc-Infected BALB/c Mice.
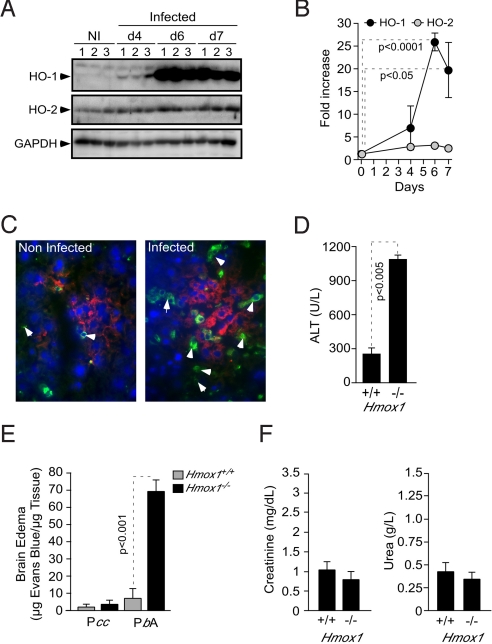
Pcc infection was associated with induction of HO-1 protein expression in the liver of BALB/c mice, i.e., average 27-fold induction at day 6 postinfection vs. naive mice, as detected by western blot (Fig. 2 A and B). Expression of HO-2, the constitutive HO isoform, was not significantly increased in response to Pcc infection (Fig. 2 A and B).
Fig. 2.
Induction of HO-1 expression in hepatocytes prevents the development of liver failure in Pcc-infected mice. (A) HO-1, HO-2, and GAPDH proteins were detected by western blot in the livers of noninfected (NI) BALB/c mice or 4, 6, 7 days post-Pcc infection. Numbers (1–3) represent individual mice. (B) Liver HO-1 and HO-2 protein expression normalized to GAPDH, 6 days post-Pcc infection, as detected by western blot (n = 3 mice/group). (C) HO-1 (green), F4/80 (red), and DNA (blue) staining in the liver of noninfected or Pcc-infected BALB/c mice, 6 days postinfection. Arrows indicate HO-1 staining in hepatocytes. (D) Mean plasma ALT concentration ± SD, 6 days post-Pcc infection (n = 4 mice/genotype). (E) Brain edema was measured by Evans blue accumulation, 7 days post-Pcc or P. berghei ANKA (PbA) infection in BALB/c mice. (F) Plasma creatinine and urea concentration 6 days post-Pcc infection in BALB/c mice. Results are mean ± SD (n = 3 per group).
Expression of HO-1 was restricted to hepatocytes with little or no HO-1 expression in resident monocyte/macrophages (F4/80+), as assessed by immunocytochemistry (Fig. 2C). Because HO-1 is protective against oxidative injury (10), we reasoned that mortality associated with Pcc infection in Hmox1−/− mice (Fig. 1) might be due to unfettered oxidative stress, presumably leading to hepatocyte cytotoxicity. This hypothesis was confirmed by the observation that Hmox1−/− mice developed hepatic failure in response to Pcc infection, i.e., average 4-fold increase in alanine aminotransferase (ALT) plasma concentration vs. Pcc-infected Hmox1+/+ mice (Fig. 2D). Pcc infection in Hmox1−/− mice did not lead to other forms of severe malaria, including cerebral malaria. This is revealed by the lack of blood brain barrier disruption and brain edema (Fig. 2E). These two hallmarks of experimental cerebral malaria were readily observed in Plasmodium berghei ANKA-infected Hmox1−/− BALB/c mice (Fig. 2E) (11). Moreover, Pcc infection did not lead to kidney failure in Hmox1−/− BALB/c mice, as revealed by creatinine and urea plasma levels, two serological markers of kidney dysfunction (Fig. 2F).
HO-1 Suppresses Liver Failure in Pcc-Infected DBA/2 Mice.
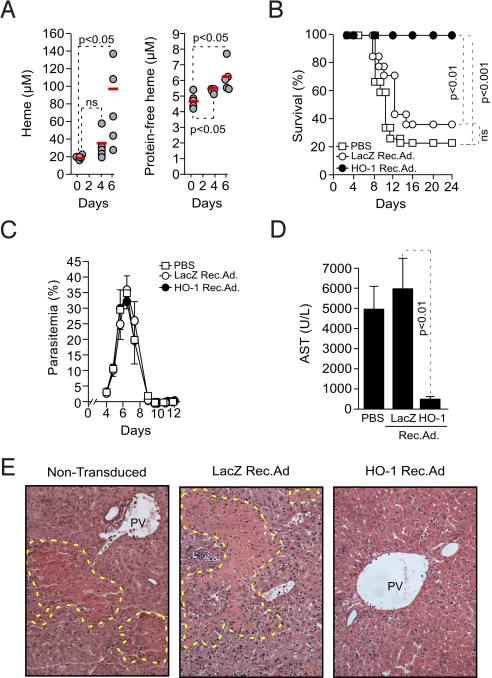
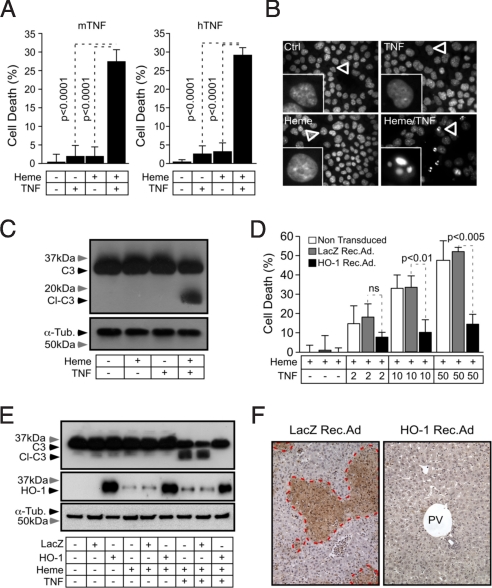
We have previously shown that Pcc-infected DBA/2 mice succumb to a lethal form of severe malaria associated with severe anemia and irreversible hepatic failure, without apparent injury to other vital organs, including brain, kidneys, heart, and lungs (12). Hepatic failure in Pcc-infected DBA/2 mice was associated with the accumulation in plasma of protein-bound and nonprotein-bound heme (i.e., free heme), i.e., 5–8 μM at day 6 postinfection (Fig. 3A). Lethality was reduced to 0% in mice transduced with a recombinant adenovirus (Rec.Ad.) expressing HO-1 in the liver (Fig. S1). In control mice receiving vehicle (PBS) or transduced with a LacZ Rec.Ad., lethality was 75% and 62.5%, respectively (Fig. 3B). The protective effect of HO-1 was not due to modulation of parasitemia (Fig. 3C), suggesting that expression of HO-1 in the liver affords tolerance to Plasmodium infection.
Fig. 3.
Expression of HO-1 in the liver suppresses liver failure in Pcc-infected mice. (A) Total and protein-free heme in plasma of Pcc-infected DBA/2 mice. Each circle represents an individual mouse. (B) Survival and (C) mean parasitemia ± SD in Pcc-infected DBA/2 mice receiving vehicle (PBS) (n = 12), transduced with HO-1 (n = 12) or LacZ (n = 8) Rec.Ad. (D) Mean plasma AST concentration ± SD, 8 days post-Pcc infection (n = 4 mice/group). (E) Hematoxylin & eosin-stained livers from DBA/2 mice, 7 days post-Pcc infection (200×; representative of eight mice). PV, portal vein. Yellow dotted line surrounds necrosis/apoptosis area.
Survival of Pcc-infected DBA/2 mice transduced with the HO-1 Rec.Ad. was due to suppression of hepatic failure as revealed by an average 10-fold reduction of aspartate aminotransferase (AST) plasma concentration (Fig. 3D) and liver portal and centrilobular vein necrosis (Fig. 3E), as compared to nontransduced or LacZ Rec.Ad.-transduced controls. This suggests that expression of HO-1 in the liver prevents the onset of severe malaria in Pcc-infected DBA/2 mice via a mechanism associated with preservation of liver function.
TNF Triggers Hepatic Failure in Pcc-Infected DBA/2 Mice.
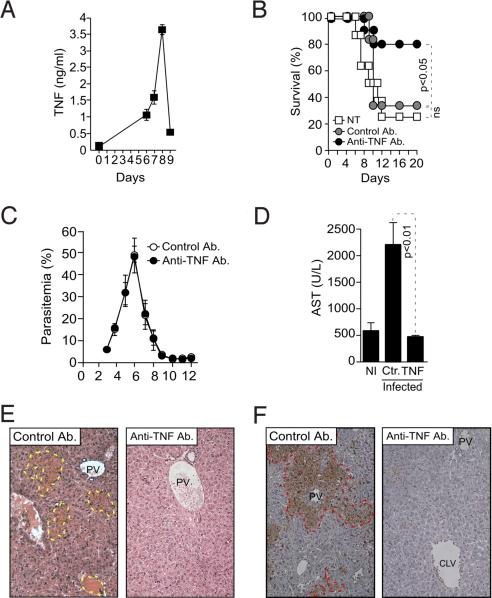
DBA/2 mice produced high levels of TNF in response to Pcc infection, reaching pick concentrations in plasma 8 days postinfection (3–4 ng/mL) and decreasing thereafter to basal levels in surviving mice (i.e., 25% of Pcc-infected mice) (Fig. 4A) (12). Administration of an anti-TNF-neutralizing Ab from day 5 to 9 postinfection reduced lethality to 25% vs. 75% and 66.7% in vehicle (PBS) or isotype-matched Ab-treated controls, respectively (Fig. 4B). There was no change in parasitemia in mice receiving the anti-TNF-neutralizing Ab, vs. control Ab (Fig. 4C). The protective effect of the anti-TNF-neutralizing Ab was associated with (i) decreased AST plasma concentration (a marker of hepatic failure) (Fig. 4D); (ii) decreased liver portal and central lobular vein necrosis (Fig. 4E); and (iii) inhibition of hepatocyte programmed cell death (Fig. 4F). These observations suggest TNF is critically involved in the pathogenesis of hepatic failure associated to Pcc-infection in DBA/2 mice.
Fig. 4.
Pcc-infected DBA/2 mice succumb to TNF-mediated hepatic failure. (A) Mean plasma TNF concentration ± SD in Pcc-infected DBA/2 mice (n = 5). (B) Percent survival of Pcc-infected DBA/2 mice treated with anti-TNF, isotype-matched control antibody, or not treated (NT) (n = 12 mice per group). (C) Mean parasitemia ± SD in Pcc-infected DBA/2 mice treated with an anti-TNF neutralizing antibody or an isotype-matched control antibody (n = 12 per group). (D) Mean plasma AST concentration ± SD in noninfected (NI; n = 4) and Pcc-infected DBA/2 mice treated with anti-TNF (n = 4) or isotype-matched control antibody (Ab; Ctr; n = 4). (E) Hematoxylin & eosin-stained liver sections, 8 days post-Pcc infection, in DBA/2 mice treated with neutralizing anti-TNF or isotype-matched control antibody (Ab). Images (200×) are representative of eight mice. Doted yellow line surrounds areas of necrosis/apoptosis. (F) Liver section from DBA/2 mice treated as in panel E stained by TUNEL. Dotted red line surrounds areas of necrosis/apoptosis. PV, portal vein; CLV, centrilobular vein. Images (200×) are representative of eight mice.
Free Heme Sensitizes Hepatocytes to Undergo TNF-Mediated Apoptosis.
We asked whether free heme interacts functionally with TNF to trigger hepatic failure following Pcc infection. At concentrations in the range of those detected in vivo, neither free heme (5 μM) nor TNF (5 ng/mL) were cytotoxic per se to primary mouse hepatocytes in vitro (Fig. 5A). However, combination of free heme with TNF was cytotoxic, i.e., >25% cell death (Fig. 5A), suggesting that free heme interacts functionally with TNF to trigger hepatocytes to undergo programmed cell death, presumably leading to hepatic failure in Pcc-infected DBA/2 mice.
Fig. 5.
Free heme sensitizes hepatocytes to TNF-mediated apoptosis. (A) Mean cytotoxicity of primary mouse hepatocytes exposed to heme (5μΜ for 1 h) plus mouse (m) or human (h) recombinant TNF (5 ng/mL for 16 h) ± SD (n = 6) in one out of three independent experiments. Cytotoxicity was assessed by crystal violet staining. (B) DNA staining (Hoechst 33342) of untreated (Ctrl), heme (40 μM for 1 h), and/or TNF (10 ng/mL for 6 h) treated Hepa1–6 cells (400×). Results are representative of three independent experiments. (C) Caspase-3 (C3), cleaved caspase-3 (Cl-C3), and α-tubulin (α-Tub) were detected by western blot in primary hepatocytes treated as in panel B. Results are representative of two independent experiments. (D) Hepa1–6 cells were transduced or not with HO-1 or LacZ Rec.Ad. and exposed to heme (40 μM for 1 h) alone or heme (40 μM for 1 h) plus TNF (ng/mL for 6 h). Results are expressed as mean ± SD from triplicates in one out of two independent experiments. TNF is expressed in ng/mL. (E) Hepa1–6 cells were treated as in panel D. Caspase-3 (C3), cleaved caspase-3 (Cl-C3), and α-tubulin (α-Tub) were detected by western blot. Results shown are representative of two independent experiments. (F) Liver sections were stained by TUNEL, 8 days post-Pcc infection in DBA/2 mice transduced with HO-1 or control LacZ Rec.Ad. Dotted red lines surround areas of necrosis/apoptosis. PV, portal vein. Images (200×) are representative of eight mice.
When exposed in vitro to free heme plus TNF, hepatocytes undergo of programmed cell death by apoptosis, as revealed by chromatin condensation, nucleus shrinking (Fig. 5B) and pro-caspase-3 processing (Fig. 5C), all of which are hallmarks of apoptosis. However, we cannot exclude that heme might also elicit other forms of programmed cell death such as necroptosis, as observed in other cell types (13). Transduction of hepatocytes in vitro with a HO-1 Rec.Ad. afforded cytoprotection against heme plus TNF-mediated apoptosis, i.e., average 73% reduction in cytotoxicity at maximal heme and TNF concentrations vs. nontransduced or LacZ Rec.Ad.-transduced controls (Fig. 5D). The cytoprotective effect of HO-1 was associated with inhibition of caspase-3 cleavage/activation (Fig. 5E). When transduced in vivo HO-1 suppressed hepatocytes apoptosis in Pcc-infected DBA/2 mice, as compared to nontransduced or LacZ Rec.Ad.-transduced control mice (Fig. 5F). Similar results were obtained using pO2 concentrations of 2%, which are closer to the physiologic steady state conditions in the mouse liver. Overall, these observations suggest that HO-1 prevents the free heme produced during Plasmodium infection from sensitizing host hepatocytes to undergo TNF-mediated apoptosis. This cytoprotective effect prevents the development of a lethal form of hepatic failure in Pcc-infected DBA/2 mice.
Free Heme and TNF Synergize to Trigger Oxidative Stress in Hepatocytes.
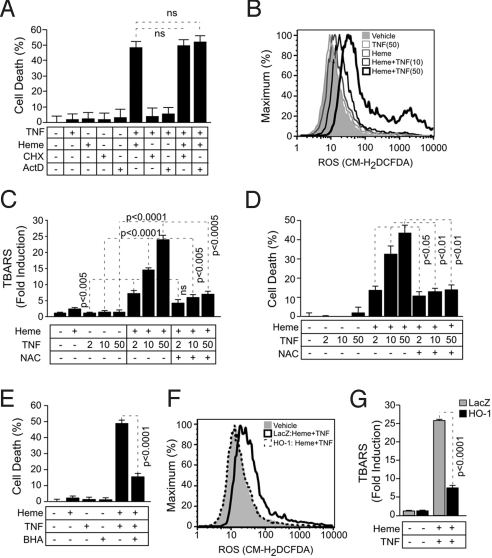
We tested whether the cytotoxic effect of free heme requires newly gene expression. Inhibition of mRNA synthesis by actinomycin D (ActD) or inhibition of protein synthesis by cycloheximide (CHX), did not interfere with the pro-apoptotic effect of heme plus TNF in hepatocytes, i.e., >45% cell death (Fig. 6A). Timing of TNF exposure in these assays was limited to 6 h, to avoid ActD or CHX from sensitizing hepatocytes to undergo TNF-mediated apoptosis (Fig. 6A). However, when exposure to TNF was prolonged to 12–24 h, both ActD and CHX sensitized hepatocytes to undergo TNF-mediated apoptosis (Fig. S2), confirming that these reagents blocked RNA and protein synthesis, respectively.
Fig. 6.
The antioxidant effect of HO-1 suppresses the cytotoxic effect of free heme plus TNF in hepatocytes. (A) Hepa1–6 cells were exposed (+) to heme (40 μM for 1 h) and/or (+) TNF (50 ng/mL for 6 h). When indicated, transcription was inhibited by ActD (5 μg/mL) and translation by CHX (5 μg/mL). Cytotoxicity was assessed by crystal violet staining. Results are expressed as mean ± SD from one out of two independent experiments, each in triplicate. Notice that ActD or CHX did not interfere with the pro-apoptotic effect of heme plus TNF. (B) Free radicals were measured by flow cytometry in Hepa1–6 cells exposed to vehicle, TNF (for 6 h), heme (40 μM for 1 h), or heme (40 μM for 1 h) plus TNF (for 6 h). (C) TBARS in Hepa1–6 cells treated as in panel A are expressed as mean ± SEM obtained from the mean values of three independent experiments, each in duplicate. NAC (10 mM for 4 h before heme and continuously thereafter). (D) Cytotoxicity in Hepa1–6 cells treated with heme plus TNF with or without NAC as in panel C. Results are expressed as mean ± SD from triplicates in one representative experiment out of two independent experiments, each in sixtuplicate. (E) Cytotoxicity in Hepa1–6 cells treated with heme (40 μM or 1 h) plus TNF (50 ng/mL for 6 h) with or without BHA (100 μM for 24 h before heme and continuously thereafter). (F) Free radicals were measured by flow cytometry in Hepa1–6 cells nontransduced (vehicle) or transduced with LacZ or HO-1 Rec.Ad. exposed to heme (40 μM for 1 h) plus TNF (50 ng/mL for 6 h). (G) TBARS in Hepa1–6 cells transduced with LacZ or HO-1 Rec.Ad., treated with heme plus TNF as in panel A, are expressed as mean ± SEM obtained from the mean values of three independent experiments, each in duplicate. TNF is expressed in ng/mL (panels C and D).
Accumulation of intracellular free radicals was negligible in untreated hepatocytes as well as in hepatocytes exposed in vitro to heme or TNF alone, as assessed by flow cytometry, using a broad range cell-permeable free radical probe (CM-H2DCFDA) (Fig. 6B). However, when exposed to heme and TNF, hepatocytes produced high levels of intracellular free radicals, a dose-response effect that increased with higher concentrations of TNF (Fig. 6B).
Neither TNF nor heme per se triggered the production of thiobarbituric acid reactive substances (TBARS), a lipid peroxidation derivative, in hepatocytes (Fig. 6C). However, exposure to heme plus TNF triggered an average 20-fold increase in TBARS, as compared to untreated control hepatocytes (Fig. 6C). This effect was dose-dependent in that higher TNF concentrations increased TBARS (Fig. 6C). That accumulation of TBARS is due to free radical production was confirmed by the observation that N-acetylcysteine (NAC), a glutathione precursor that acts as a broad antioxidant, reduced by an average 2.5- to 3.5-fold the levels of TBARS in hepatocytes exposed in vitro to heme plus TNF, as compared to control hepatocytes not treated with NAC (Fig. 6C). Similar results were obtained using pO2 concentrations of 2%, which as argued above are closer to the physiologic steady state conditions in the mouse liver. This data reveals that free heme acts as a pro-oxidant catalyst in hepatocytes, promoting oxidative stress, i.e., lipid peroxidation, in response to TNF.
HO-1 Negates the Pro-Oxidant Effect of Free Heme and Affords Protection Against Apoptosis.
When exposed in vitro to the antioxidant NAC, hepatocytes became resistant to heme plus TNF-mediated apoptosis, i.e., 4-fold decrease in cytotoxicity, as compared to control hepatocytes (Fig. 6D). A similar effect was observed using the lipid phase antioxidant butylated hydroxyanisole (BHA) (Fig. 6E), suggesting that lipid peroxidation is functionally involved in the pro-apoptotic effect of heme plus TNF. Transduction of hepatocytes in vitro with a HO-1 Rec.Ad. suppressed the accumulation of both intracellular free radicals (Fig. 6F) and TBARS (Fig. 6G) in response to heme plus TNF, as compared to control hepatocytes transduced with LacZ Rec.Ad. This suggests that HO-1 acts as an antioxidant to prevent hepatocyte apoptosis. Similar results were obtained using pO2 concentrations of 2%.
HO-1 Prevents the Onset of Severe Malaria Through Its Antioxidant Activity.
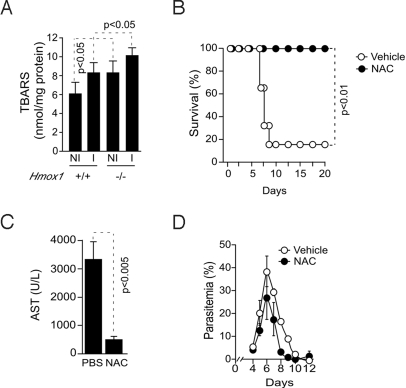
Pcc-infected Hmox1−/− BALB/c mice that developed hepatic failure (Figs. 1 and 2) accumulated higher levels of TBARS in the liver, as compared to Pcc-infected Hmox1+/+ BALB/c mice that did not develop hepatic failure (Fig. 7A). When treated with the antioxidant NAC, starting 4 days after Pcc infection, DBA/2 were protected against hepatic failure, i.e., 0% vs. 80% mortality in NAC vs. vehicle-treated Pcc-infected mice, respectively (Fig. 7B). The protective effect of NAC was associated with a 7-fold decrease in AST plasma concentration (Fig. 7C), demonstrating that this antioxidant prevents the development of hepatic failure in Pcc-infected DBA/2 mice. The protective effect of NAC was not due to modulation of parasitemia (Fig. 7D), suggesting that NAC affords tolerance to Pcc infection.
Fig. 7.
The antioxidant effect of HO-1 suppresses the onset of severe malaria. (A) TBARS in the liver of noninfected (NI) or Pcc-infected (I) wild-type (Hmox1+/+) or HO-1 deficient (Hmox1−/−) BALB/c mice, 5 days post-Pcc infection. Results are expressed as mean ± SD (n = 3 mice/group). (B) Survival of Pcc-infected DBA/2 mice receiving NAC (150 mg/kg) or vehicle (PBS) as control (n = 5–6 mice/group). (C) Mean plasma AST concentration ± SD in Pcc-infected DBA/2 mice receiving NAC (n = 4) or vehicle (PBS) (n = 4). (D) Mean parasitemia ± SD of Pcc-infected DBA/2 mice receiving NAC (n = 5) or vehicle (PBS) (n = 5).
Discussion
Survival of a Plasmodium-infected host is thought to rely almost exclusively on its ability to generate an effector immune response capable of limiting pathogen burden, i.e., a host defense strategy referred to as resistance to infection (3, 4). However, this immune response can contribute to the development of lethal forms of severe malaria (2). This suggests that resistance to Plasmodium infection can compromise the host's viability, a phenomenon observed in other types of infection, including polymicrobial infections leading to severe sepsis (14). Therefore, viability of a Plasmodium-infected host must involve additional defense strategies that do not aim at limiting pathogen burden, but instead aim at limiting the deleterious effects associated with the immune response targeting Plasmodium, i.e., a host defense strategy referred to as tolerance to infection (3, 4). The molecular mechanism(s) underlying tolerance to Plasmodium infection remain to be identified. As demonstrated hereby, expression of HO-1, outside the hematopoietic (immune) compartment, plays a central role in determining host tolerance to Plasmodium infection in mice (Fig. 1).
Production of protein-free heme is a pathological event inherent to the blood stage of Plasmodium infection, as illustrated hereby for Pcc infection (Fig. 3A) and as previously shown for infection with other Plasmodium strains in mice (5, 6). Perhaps more important is the notion that free heme plays a central role in the pathogenesis of experimental cerebral malaria in mice (5, 6). Our present data suggests that free heme also plays an important role in the pathogenesis of noncerebral forms of severe malaria in mice. This pathological effect is due to a unique biologic property of free heme, namely its ability to sensitize nonhematopoietic cells, i.e., hepatocytes, to undergo TNF-mediated apoptosis (Fig. 5). As shown hereby this cytotoxic effect plays a critical role in the development of tissue/organ injury, i.e., hepatic failure (Fig. 2), ultimately leading to death of Pcc-infected mice (Fig. 1).
The cytotoxic effects of TNF are revealed only when the expression of immediate-early TNF-responsive cytoprotective genes is artificially repressed (15, 16). To the best of our knowledge, there are no molecules that have been shown to act under pathophysiological conditions to override this protective mechanism and promote TNF cytotoxicity. Therefore, the finding that free heme acts in such a manner (Fig. 5) is unique, in that it provides not only evidence for the existence of a functional interaction between free heme and TNF in the pathogenesis of severe malaria, but suggests that the same pathophysiologic mechanism might apply to other inflammatory conditions.
The ability of free heme to sensitize hepatocytes to undergo TNF-mediated apoptosis is not suppressed when mRNA or protein synthesis are inhibited (Fig. 6A), suggesting that the cytotoxic effect of free heme acts independently of newly gene transcription and/or protein synthesis. Instead free heme acts as a potent pro-oxidant catalyst that fosters the production of free radicals in response to TNF, presumably via the participation of its Fe atom in the Fenton chemistry. In keeping with this notion, hepatocytes accumulate high levels of intracellular free radicals in response to heme plus TNF (Fig. 6B), leading to an unfettered oxidative stress response, i.e., lipid peroxidation (Fig. 6C). Antioxidants such as NAC (Fig. 6D) or BHA (Fig. 6E) suppress the pro-apoptotic effect of free heme plus TNF.
The finding that free heme acts as a pro-oxidant catalyst (Fig. 6B) to promote TNF-mediated apoptosis in hepatocytes (Fig. 5), led to the hypothesis that pharmacologic antioxidants might be used therapeutically (i.e., after Plasmodium infection) to suppress the pathogenesis of severe malaria. When administered to DBA/2 mice 4 days after Pcc infection, the antioxidant NAC prevents the development of hepatic failure and insures host survival (Fig. 7 B and C), a finding in keeping with previous reports suggesting that NAC might exert salutary effects in P. falciparum-infected individuals (17, 18).
In conclusion, we provide evidence for an unsuspected pathologic mechanism leading to the development of noncerebral forms of severe malaria. Namely, free heme produced during the blood stage of Plasmodium infection sensitizes nonhematopoietic cells, i.e., hepatocytes, to undergo TNF-mediated apoptosis. Presumably, this sequence of events is critically involved in the pathogenesis of noncerebral forms of severe malaria associated with the development of multi-organ injury (19), including hepatic failure (20, 21). The cytotoxic effect of free heme is suppressed when the heme-catabolizing enzyme HO-1 is expressed in hepatocytes, limiting the severity of hepatic disease irrespective of parasite burden and presumably therefore independently of the host immune response against Plasmodium. Identification of this host defense strategy against Plasmodium infection should help in the development of therapeutic approaches aimed at limiting the severity of malaria without the need to target Plasmodium.
Materials and Methods
Mouse Hepatocytes and Reagents.
Hepa1–6 cells were cultured as described in the SI Text. Primary mouse hepatocytes were isolated as described (22).
Cytotoxicity Assay.
Hepatocytes were seeded (96-well plates, 10 × 103 cells/well for Hepa1–6 cells, and 25 × 103 cells/well for primary hepatocytes; for 48 h), washed with PBS, and exposed for 1 h to heme in HBSS (Invitrogen). Control hepatocytes were washed with PBS and exposed to HBSS without heme. Hepatocytes were then washed in PBS and exposed to human (h) or mouse (m) recombinant TNF in DMEM, 10% FCS (Hepa1–6 cells), or 4% FCS (primary hepatocytes; Invitrogen). Viability was assessed by a crystal violet assay, as described (23). Immunofluorescence was performed as in SI Text. Flow cytometry was performed as described in SI Text.
Lipid Peroxidation.
Cellular thiobarbituric acid reactive substances were measured according to the manufacturer instructions (OXI-TEC; Alexis).
Mice and Parasites.
BALB/c, BALB/c.SCID, DBA/2, and BALB/c.Hmox1−/−.SCID mice were maintained under specific pathogen-free conditions, according to the Animal Care Committee of the “Instituto Gulbenkian de Ciência.” BALB/c Hmox1−/− mice were generated originally by Shaw-Fang Yet (24). Mice (6–8 weeks) were genotyped as described (6) and infected i.p. with 106 Pcc (AS)-infected RBC. Course of infection was monitored daily as described (25).
Bone Marrow Chimeras.
Hmox1+/+ and Hmox1−/− BALB/c mice (8–10 weeks) were lethally irradiated (900 rad, 2.35 min, 137Cs source) (Gammacell 2000; Mølsgaard Medical) and reconstituted 4 h thereafter with 5 × 106 total bone marrow cells from Hmox1+/+ and Hmox1−/− BALB/c mice (6 weeks). Chimerism was assessed 8–10 weeks thereafter by PCR as described (6, 26).
CO Exposure.
Plasma Cytokines.
Plasma TNF was measured by ELISA (R&D Systems). BBB permeability was assessed as described (6).
Heme.
Protein-bound and protein-free heme were measured in plasma, as described (6). Liver homogenates were centrifuged (for 3 min at 4 °C at 16,000× g), proteins were precipitated (10% trichloroacetic; vol/vol, for 1 h on ice), centrifuged (for 3 min at 4 °C at 14,000× g), and free heme was measured in the protein-free supernatant as described (6).
Histopathology.
Liver samples were harvested, fixed in 4% paraformaldehyde, embedded in paraffin, and stained with hematoxylin and eosin (6). Detection of fragmented DNA was carried out using the In Situ Cell Death Detection kit from Roche (TUNEL method). HO-1 staining was performed as described (27). In vivo treatments using anti-TNF mAb was performed as described in SI Text. Serum biochemistry was performed as described in SI Text. Quantitative real-time reverse transcription PCR (qRT-PCR). Expression of Hmox1 mRNA was assessed as described (26).
Recombinant Adenoviruses.
The LacZ, HO-1 Rec.Ad. were previously described (23). Mice were transduced with Rec.Ad. (i.p.; 4 × 108 pfu/mouse) 40 h before challenge with Pcc. Hepa1–6 cells were transduced with 103 pfu/cell, 48 h before treatment. Protein extraction and immunoblotting were performed as described in SI Text. Statistical analysis was performed as described in SI Text.
Supplementary Material
Acknowledgments.
We thank Thiago Carvalho (Instituto Gulbenkian de Ciência), Marcelo Bozza (Universidade Federal do Rio de Janeiro, Brasil), and Jonathan Howard (Institute for Genetics, Cologne, Germany) for critically reviewing of this manuscript; Ligia Gonçalves (Instituto Gulbenkian de Ciência) for invaluable help with isolation of primary mouse hepatocytes; Sílvia Cardoso (Instituto Gulbenkian de Ciência) for mouse breeding and genotyping; and all other members of the inflammation laboratory (Instituto Gulbenkian de Ciência) for critical input. This work was supported by “Fundação para a Ciência e Tecnologia,” Portugal Grants SFRH/BPD/6910/2001 and SFRH/BPD/34094/2006 (to E.S.), SFRH/BD/3106/2000 (to A.C.), PTDC/SAU-MII/71140/2006 and SFRH/BPD/21707/2005 (to A.F.), SFRH/BPD/21072/2004 (to G.S.), SFRH/BPD/25436/2005 (to R.L.), and POCTI/SAU-MNO/56066/2004 and PTDC/SAU-MII/65765/2006 (to M.P.S.), European Community 6th Framework Xenome Grant LSH-2005-1.2.5-1), and grants from the Gemi Fund (Linde Healthcare) (to M.P.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 15525.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903419106/DCSupplemental.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 3.Raberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318:812–814. doi: 10.1126/science.1148526. [DOI] [PubMed] [Google Scholar]
- 4.Schneider DS, Ayres JS. Two ways to survive infection: What resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira A, Balla J, Jeney V, Balla G, Soares MP. A central role for free heme in the pathogenesis of severe malaria: The missing link? J Mol Med. 2008;86:1097–1111. doi: 10.1007/s00109-008-0368-5. [DOI] [PubMed] [Google Scholar]
- 6.Pamplona A, et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med. 2007;13:703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 7.Balla J, et al. Endothelial-cell heme uptake from heme proteins: Induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci USA. 1993;90:9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh K, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 10.Soares MP, Bach FH. Heme oxygenase-1: From biology to therapeutic potential. Trends Mol Med. 2009;15:50–58. doi: 10.1016/j.molmed.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Newton CR, Warrell DA. Neurological manifestations of falciparum malaria. Ann Neurol. 1998;43:695–702. doi: 10.1002/ana.410430603. [DOI] [PubMed] [Google Scholar]
- 12.Seixas E, Oliveira P, Moura Nunes JF, Coutinho A. An experimental model for fatal malaria due to TNF-alpha-dependent hepatic damage. Parasitology. 2008;135:683–690. doi: 10.1017/S0031182008004344. [DOI] [PubMed] [Google Scholar]
- 13.Laird MD, Wakade C, Alleyne CH, Jr, Dhandapani KM. Hemin-induced necroptosis involves glutathione depletion in mouse astrocytes. Free Radic Biol Med. 2008;45:1103–1114. doi: 10.1016/j.freeradbiomed.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 15.Tartaglia LA, Rothe M, Hu YF, Goeddel DV. Tumor necrosis factor's cytotoxic activity is signaled by the p55 TNF receptor. Cell. 1993;73:213–216. doi: 10.1016/0092-8674(93)90222-c. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal BB. Signalling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 17.Treeprasertsuk S, et al. N-acetylcysteine in severe falciparum malaria in Thailand. Southeast Asian J Trop Med Public Health. 2003;34:37–42. [PMC free article] [PubMed] [Google Scholar]
- 18.Watt G, Jongsakul K, Ruangvirayuth R. A pilot study of N-acetylcysteine as adjunctive therapy for severe malaria. QJM. 2002;95:285–290. doi: 10.1093/qjmed/95.5.285. [DOI] [PubMed] [Google Scholar]
- 19.Clark IA, Alleva LM, Mills AC, Cowden WB. Pathogenesis of malaria and clinically similar conditions. Clin Microbiol Rev. 2004;17:509–539. doi: 10.1128/CMR.17.3.509-539.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guha M, Kumar S, Choubey V, Maity P, Bandyopadhyay U. Apoptosis in liver during malaria: Role of oxidative stress and implication of mitochondrial pathway. FASEB J. 2006;20:1224–1226. doi: 10.1096/fj.05-5338fje. [DOI] [PubMed] [Google Scholar]
- 21.Bhalla A, Suri V, Singh V. Malarial hepatopathy. J Postgrad Med. 2006;52:315–320. [PubMed] [Google Scholar]
- 22.Goncalves LA, Vigario AM, Penha-Goncalves C. Improved isolation of murine hepatocytes for in vitro malaria liver stage studies. Malar J. 2007;6:169. doi: 10.1186/1475-2875-6-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soares MP, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 24.Yet SF, et al. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest. 1999;103:R23–R29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seixas EM, Langhorne J. gammadelta T cells contribute to control of chronic parasitemia in Plasmodium chabaudi infections in mice. J Immunol. 1999;162:2837–2841. [PubMed] [Google Scholar]
- 26.Chora AA, et al. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest. 2007;117:438–447. doi: 10.1172/JCI28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDaid J, et al. Heme oxygenase-1 modulates the allo-immune response by promoting activation-induced cell death of T cells. FASEB J. 2005;19:458–460. doi: 10.1096/fj.04-2217fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.