Abstract
Constitutive expression of Hsp27 has been demonstrated in vertebrate embryos, especially in developing skeletal and cardiac muscle. Results of several previous studies have indicated that Hsp27 could play a role in the development of these tissues. For example, inhibition of Hsp27 expression has been reported to cause defective development of mammalian myoblasts in vitro and frog embryos in vivo. In contrast, transgenic mice lacking Hsp27 develop normally. Here, we examined the distribution of Hsp27 protein in developing and adult zebrafish and effects of suppressing Hsp27 expression using phosphorodiamidate morpholino oligonucleotides (PMO) on zebrafish development. Consistent with our previous analysis of hsp27 messenger RNA expression, we detected the protein Hsp27 in cardiac, smooth, and skeletal muscle of both embryonic and adult zebrafish. However, embryos lacking detectable Hsp27 after injection of antisense hsp27 PMO exhibited comparable heart beat rates to that of control embryos and cardiac morphology was indistinguishable in the presence or absence of Hsp27. Loss of Hsp27 also had no effect on the structure of the skeletal muscle myotomes in the developing embryo. Finally, embryos injected with antisense hsp27 and scrambled control PMO displayed equal motility. We conclude that Hsp27 is dispensable for zebrafish morphogenesis but could play a role in long-term maintenance of heart and muscle tissues.
Keywords: Hsp27, Hsp70, Muscle, Heart, Zebrafish
Introduction
Heat shock proteins play essential roles in all stages of an organism’s life. Well-known functions of heat shock proteins include folding of nascent proteins and refolding of proteins damaged as a consequence of thermal injury and other stresses. Other vital functions include participation as cofactors in receptor-mediated signaling (Pearl and Prodromou 2006), scaffolding proteins for signaling elements (Rane et al. 2003), maintenance of cellular reduction/oxidation (redox) states (Arrigo 2001), and stabilization of cytoskeletal arrays (for representative review, see Mounier and Arrigo 2002). High-molecular-weight heat shock proteins use the energy of adenosine triphosphate (ATP) to modify or stabilize the conformation of other proteins, thereby preventing misfolding or restoring normal folding of target proteins. In contrast, small heat shock proteins (sHsps) lack ATPase activity but can undergo phosphorylation-dependent transition between high-molecular-weight oligomers and smaller subunits (Lambert et al. 1999).
Hsp27, also known as HspB1, is one of the most widely expressed and distributed small heat shock protein. Expression of Hsp27 is upregulated in response to sublethal injury by epithelial (Kiriyama et al. 2001; Bonham et al. 2003), neuronal (Costigan et al. 1998; Dodge et al. 2006), muscle (Knoll et al. 1994; van de Klundert et al. 1998), and other cell types, and orthologs of human Hsp27 are found in mammals, birds, fish, and amphibians (Gernold et al. 1993; Norris et al. 1997; Kawazoe et al. 1999; Mao et al. 2005; Tuttle et al. 2007). Altered expression and regulation of Hsp27 have been demonstrated in nerve and muscle tissues as a consequence of injury (Yoshida et al. 1999; Sakamoto et al. 2000), disease (Clemen et al. 2005), and aging (Chung and Ng 2006; Yamaguchi et al. 2007). A variety of functions for Hsp27 in injured cells have been proposed, including chaperone function (Jakob et al. 1993), modulation of apoptotic signaling cascades (Garrido et al. 1999; Charette et al. 2000; Pandey et al. 2000; Rane et al. 2003), and the regulation of cellular redox and glutathione levels (Arrigo 2001; Escobedo et al. 2004). In addition, Hsp27 can interact with cytoskeletal elements and stabilize cytoskeletal arrays in injured cells (Lavoie et al. 1993a, b). Many of these functions are also performed by other heat shock proteins, and there is evidence that small heat shock proteins may act, at least in part, as a cochaperone for high-molecular-weight heat shock proteins such as Hsp70 (for review, see Haslbeck et al. 2005).
In addition to stress-induced expression, hsp27, along with genes for a number of related small heat shock proteins, is expressed in some tissues in the absence of stress, including all types of muscle cells (Dillmann 1999; Benndorf and Welsh 2004). The role of constitutively expressed Hsp27 is not completely understood. Upregulation of hsp27 expression is seen in differentiating myoblasts in vitro (Davidson and Morange 2000; Ito et al. 2001), as well as developing muscle cells of murine and amphibian embryos (Gernold et al. 1993; Tuttle et al. 2007). Similarly, we and others have reported muscle-cell-specific expression of Hsp27 in myotomes of developing zebrafish embryo (Mao et al. 2005; Marvin et al. 2008). Antisense hsp27 messenger RNA (mRNA) expression also inhibits development of murine myoblasts (Davidson and Morange 2000) and induces cell death of embryonic stem cells (Mehlen et al. 1997) in vitro. Recently, knockdown of Hsp27 expression using phosphorodiamidate morpholino oligonucleotides (PMO) was reported to cause defects in heart formation and myofibril architecture in both skeletal and cardiac muscle of developing frog embryos (Brown et al. 2007). Together, these results suggest that Hsp27 may be required for differentiation of muscle cells. In contrast, Drosophila embryos lacking a homolog of human Hsp27 develop normally and, surprisingly, loss of Hsp27 does not alter the resistance of flies to heat shock or oxidative injury (Hao et al. 2007). The Drosophila homolog of Hsp27 lacks phosphorylated serines conserved among vertebrate homologs of Hsp27 (Ingolia and Craig 1982) and displays a constitutive localization to the nucleus of cells under control conditions (Marin and Tanguay 1996) not typical of vertebrate Hsp27. Therefore, it is not clear that direct comparisons between the roles of vertebrate and Drosophila Hsp27 are appropriate. However, normal development of mice lacking Hsp27 as a result of gene disruption has also been reported (Huang et al. 2007). Thus, there are currently conflicting data regarding the role of Hsp27 during embryogenesis.
In the present study, we have examined cellular and physiological consequences of transient Hsp27 knockdown in zebrafish embryos using injection of antisense hsp27 PMO. We have also produced an antiserum suitable for immunolocalization and immunoblotting detection of zebrafish Hsp27. Data presented below demonstrate that skeletal muscle morphogenesis and function in zebrafish embryos lacking detectable Hsp27 are indistinguishable from that of embryos injected with a nonspecific control PMO. Assessment of cardiac beat frequency and morphology also failed to show an aberrant phenotype in the absence of Hsp27. Constitutive and stress-induced expression of Hsp70 was unaffected by the loss of Hsp27 in our experiments. However, Hsp27 was expressed in both embryonic and adult muscle tissues as well as a small number of other locations. These data reveal that Hsp27 plays a dispensable role in the morphogenesis of zebrafish and provides a new perspective on the functional significance of Hsp27 in vivo. Specifically, our findings are consistent with a role for Hsp27 in the maintenance of muscle tissue homeostasis.
Materials and methods
Zebrafish husbandry and experimental stress Adult zebrafish, Danio rerio, were reared and maintained as described previously (Mao et al. 2005). Embryos were collected from wild-type crosses and maintained at 26°C in embryo medium as described by Westerfield (1993). Embryos were heat-shocked at 36 h postfertilization (hpf) in a circulating water bath (IsoTemp 2150, Fisher Scientific, Pittsburgh, PA, USA) in parafilm-sealed 35-mm Petri dishes for 30 min at 39°C and allowed to recover at 26°C for 14 h before collection. Protocols for the use of animals in these experiments were approved by the Washington State University Animal Care and Use Committee and were in accord with National Institute of Health standards established by the Guidelines for the Care and Use of Experimental Animals.
Production of antiserum detecting zebrafish Hsp27 A complementary DNA coding for zebrafish Hsp27 (Mao et al. 2005) was cloned into the prokaryotic vector pET45b (Novagen, Madison, WI, USA) and expressed in Escherichia coli, strain BL21. The 6-His-tagged fusion protein was purified using Talon metal affinity resin (Clontech Laboratories, Palo Alto, CA, USA) and used to generate polyclonal Hsp27 antiserum (αHsp27) as described by Monteville et al. (2003). Pre-immune and αHsp27 sera were tested for recognition of purified recombinant protein and zebrafish Hsp27 expressed in NIH3T3 fibroblasts (Mao et al. 2005) by Western blotting and indirect immunolocalization, respectively (data not shown).
SDS-polyacrylamide gel electrophoresis and Western blotting For expression analysis during development, pools of ten or more embryos were chilled and sonicated on ice in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (62.5 mM Tris/HCl (pH 6.8), 2% SDS, 0.01% bromophenol blue, 10% glycerol) and samples were cleared by centrifugation at 10,000×g for 10 min at 4°C. For tissue analysis (Fig. 6), adult male zebrafish were euthanized in ice water and dissected to obtain select tissues. Tissue pieces were immersed in SDS-PAGE sample buffer containing 2.5 mM ethylenediaminetetraacetic acid (EDTA) and a 1:200 dilution of Protease Inhibitor Cocktail (SigmaAldrich P8340). Protein concentrations were measured using a Bio-Rad DC Protein Assay Kit. After adding β-mercaptoethanol to a final concentration of 4%, proteins were separated using 4% acrylamide stacking and 12% running gels. Resolved proteins were transferred to membranes (NitroBind, 0.22 μm, GE Water & Process Technologies, Trevose, PA, USA) and stained with Ponceau S to confirm uniform loading and transfer of proteins. Immunoblotting was conducted using the αHsp27 serum described above, an anti-Hsp70 antibody (rabbit polyclonal SPA-812, Stressgen, Inc.), or an antisarcomeric actin antibody (SigmaAldrich, clone 5C5) and peroxidase-labeled secondary antibodies. Blots were developed and analyzed as described previously (Mao et al. 2005). Each experiment was performed at least three times.
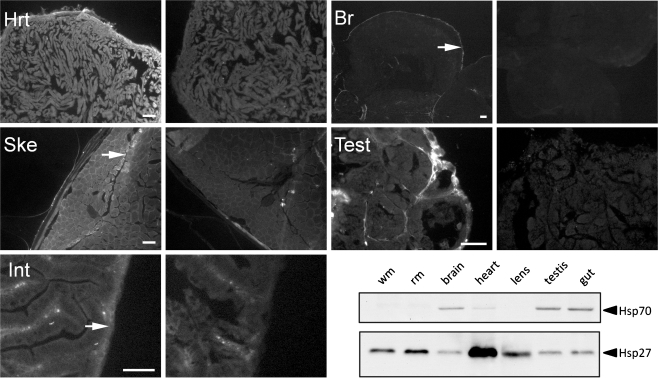
Fig. 6.
Hsp27 immunolocalization in unstressed adult zebrafish tissues. Hsp27 was detected throughout the heart myocardium (Hrt) and skeletal muscle, especially in slow-twitch muscle and at the boundary between external slow and internal fast-twitch muscle (arrow, Ske). Hsp27 was detected within the smooth muscle surrounding the intestinal tract (arrow, Int) and in the lining of the brain tissues (arrow, Br). Hsp27 was also detected at the margin of seminiferous tubules in the testis (Test). Right-hand panels are secondary antibody-only controls of each tissue type. Scale bars are 50 μm. A Western blot probed for Hsp70 and Hsp27 expression in white, or fast-twitch, muscle (wm), red, or slow-twitch, muscle (rm), brain, heart, lens, testis, and gut tissues is shown for comparison
Phosphorodiamidate morpholino oligonucleotide microinjections PMO were obtained from GeneTools, Inc. (Corvallis, OR, USA). PMO sequences were: Hsp27 antisense (hsp27i), 5’GTTTTGAAGAGTTGTT TTTCGGCTC3’, and scrambled control (scr), 5’GGAGCTTAGTGATGTTCTGTTCTTT3’. The hsp27i PMO used in our studies is complementary to the −11–36 bp of the 5’-untranslated region of the zebrafish hsp27 mRNA sequence. The scrambled morpholino has the identical nucleotide composition in a random order. A BLASTn search of over 37 million annotated zebrafish nucleotide sequences identified only one sequence (hsp27/hspB1) with significant homology to hsp27i. PMO were dissolved in either sterile distilled water (see Figs. 1c and 3) or injection buffer (0.4 mM MgSO4, 0.6 mM CaCl2, 0.7 mM KCl, 58 mM NaCl, 25 mM HEPES pH 7.1) and aliquots were frozen at −80°C. Zebrafish embryos were injected as described by Liu et al. (2007) at the one- to four-cell stage using a Narishige coarse micromanipulator and MMPI-2 pneumatic pressure regulator (Applied Scientific Instruments, Inc., Eugene, OR, USA). Needles were pulled from borosilicate capillary tubes (1.00 × 0.78 mm × 10 cm, Sutter Instruments Co., Novato, CA, USA) using a Sutter Instruments pipette puller (Model P-87). Injection volumes (assessed by injecting solutions into mineral oil and calculating the volume of droplets) were approximately 1.8 nl. In preliminary studies, injection of both hsp27i and scr PMO at a concentration of 1 mM caused equivalent embryo toxicity, manifested as reduced size at 24 hpf, abnormal appearance, heart and trunk muscle development, and white patches of dead cells, particularly in the developing brain (data not shown). Injection of the hsp27i PMO at a concentration of 0.02 mM or less had little or no effect on Hsp27 expression (Fig. 1b). Injection of hsp27i PMO at concentrations of 0.1 and 0.15 mM inhibited Hsp27 expression without affecting development or behavior of embryos, relative to solvent or scr-PMO-injected embryos (see “Results”).
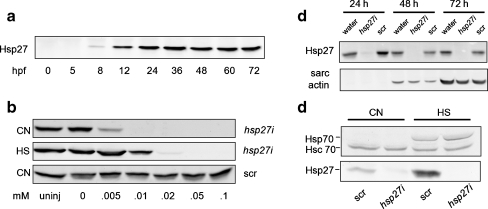
Fig. 1.
Western blot detection of Hsp27 and Hsp70 in zebrafish embryos. a Developmental time course of Hsp27 expression. Numbers are time of sample harvest in hours postfertilization (hpf). b Concentration effects of scrambled (scr) and antisense hsp27 (hsp27i) PMO injection on Hsp27 expression. Numbers represent injection concentration in millimolar. A sample from uninjected embryos (uninj) is also shown. c Effects of 0.15-mM hsp27i PMO injection on Hsp27 expression in embryos of varying ages. The expression of sarcomeric (sarc) actin is shown for comparison. d Effects of 0.1 mM hsp27i PMO injection on expression of constitutive Hsc70 and inducible Hsp70 in control (CN) and heat-shocked (HS) embryos. Samples were harvested at 36 hpf. All lanes were loaded with equal amounts (30 μg) of total embryo protein
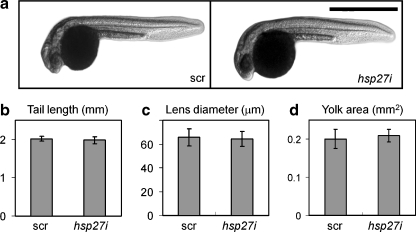
Fig. 3.
Morphology of zebrafish embryos is not altered by Hsp27 knockdown. a Overall morphology of 24-hpf embryos after injection at the one to two cell stages with 0.15-mM scrambled (scr) or antisense hsp27 (hsp27i) PMO. Scale bar = 1 mm. b Tail length of embryos measured from the tip of the tail to the yolk mass (arrows, a). c Lens diameter. d Two-dimensional area of the yolk. Values were obtained from 20 or more embryos in each group collected in a total of three independent trials
Fluorescent labeling of embryos To visualize myofibrils, embryos were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 2 h at room temperature. Whole embryos were stained with rhodamine-labeled phalloidin (0.1 μg/ml) diluted in lysis buffer (20 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM ethylene glycol tetraacetic acid, 0.5% Triton X-100, pH 7.3) overnight at 4°C, then rinsed twice for 1 h in PBS containing 0.5% Tween 20 (PBST). Embryos were mounted on slides with Mowiol containing an antifade agent (DABCO, Sigma Chemical Co.). To visualize cardiac myofibrils, tails were removed from labeled embryos and the thorax was mounted in agarose. Images of embryos were obtained using a Zeiss LSM 510M confocal microscope (Carl Zeiss Inc., Thornwood, NY, USA) and a ×20 1.2 NA glycerol immersion objective. A through-focus series of images in the Z dimension was collected for each embryo at 3-μm intervals. For detection of Hsp27 in situ, whole embryos or dissected adult tissues were fixed for 30 min in 4% formaldehyde in PBST, permeabilized for >2 h in lysis buffer and then frozen in 100% OCT by immersion in liquid nitrogen. Twelve- to 15-µm-thick cryosections were cut using a Reichert-Jung cryotome (Cryocut model 1800) and placed on chrome-gelatin-subbed coverslips. Cryosections were fixed for an additional 10 min in 4% formaldehyde and then probed with αHsp27 serum and fluorescein-isothiocyanate-labeled secondary antibody. Fluorescent phalloidin was used as a counterstain. Images of cryosections were obtained using a Zeiss Axiovert 200M and an ORCA AG cooled charge-coupled device camera (Hamamatsu Photonics, Bridgewater, NJ, USA). Different exposure times were used to image fluorescein- and rhodamine-labeled probes, but images were otherwise obtained using a single set of camera and illumination parameters for all embryos or tissue preparations. Postprocessing was conducted using Axiovision 4.1 (Carl Zeiss, Inc.) to set the background image intensity to black. This manipulation was applied simultaneously and equivalently to all images.
Motility assay and heart beat measurements Single batches of fertilized embryos were collected and injected with scr or hsp27i PMO at the one to two cell stages. Embryos were manually dechorionated at 50 hpf and placed in a 10-cm Petri dish containing embryo medium (Westerfield 1993) in a temperature-controlled chamber at 28.5°C. Movement was initiated by touching the embryo on the side and recorded using a digital camcorder (Panasonic Model PU-GS32). Videos were digitized using Window Movie Maker version 5.1 (Microsoft Corp.) at 30 frames per second and saved as AVI files, then converted to an image series using Quicktime Player Pro for Windows version 7.5.5 (Apple Inc.). Images were imported at six frames per second into ImageJ version 1.37 (Wayne Rasband, National Institute of Health, Bethesda, MD, USA) for further analysis. The X and Y coordinates of each embryo in each frame of videos were marked with a digital stylus and exported to Microsoft Excel. Excel was used to calculate the total distance between embryo positions in sequential frames. Average maximal rate of movement for each embryo was calculated by averaging the three highest velocity measurements from each swim.Heart rates were measured by counting heartbeats during a 15-s interval for 50–52-hpf embryos. The heartbeats were counted by eye using a dissecting microscope (SMZ-2T, Nikon, USA). Data were obtained from a total of 30 embryos in each test group in three independent trials.
Myotome area measurements For analysis of myotome area, the myotome found dorsal to the caudal tip of the developing gut (the 12th myotome) was selected for analysis because it could be reliably identified in high-magnification confocal images of embryonic trunk regions. The area of the 12th myotome for each embryo was measured using ImageJ.
Statistical analysis Average values resulting from our quantitative analyses are presented with the calculated standard deviation or standard error of the mean as indicated. Average values measured for scr-PMO-injected embryos were compared to values measured from hsp27i-PMO-injected embryos, using Student’s T test or Z test where indicated. Calculated p values of less than or equal to 0.05 were considered an indication of statistically significant differences in average values.
Results
Hsp27 expression during zebrafish embryogenesis
We previously demonstrated expression of zebrafish hsp27 mRNA embryos as early as 8 hpf, with maximal expression observed at 24 hpf. Figure 1a shows a Western blot of equal amounts of total protein isolated from embryos at different ages. A single band at the predicted size of zebrafish Hsp27 (22 kDa) is detected on blots probed with αHsp27 serum in samples harvested as early as 8 hpf, consistent with our previous analysis of hsp27 mRNA expression. Densitometry values (not shown) indicated that Hsp27 protein levels increased approximately sevenfold between 9 and 24 hpf. Unlike hsp27 mRNA expression levels that declined starting at 36 hpf, Hsp27 protein levels remained constant between 24 hpf and hatching at 72 hpf (Fig. 1a), although reduced expression, as a proportion of total protein, was seen by 1 week postfertilization (data not shown).
Morpholino knockdown of Hsp27 in zebrafish embryos
To investigate the potential roles for Hsp27 in morphogenesis, we injected zebrafish embryos at the one to two cell stages with antisense Hsp27 (hsp27i) or scrambled control (scr) PMO. Figure 1b shows effects of injecting PMO at varying initial concentrations on Hsp27 expression, evaluated by Western blotting. Injection of hsp27i PMO abrogated detectable expression of Hsp27 in embryos at concentrations >0.05 mM. In contrast, injection of scr PMO at all tested concentrations had no effect on Hsp27 expression (Fig. 1b).
Figure 1c demonstrates that injection of hsp27i PMO at a concentration of 0.15 mM inhibits expression of Hsp27 at all developmental time points examined in the present study. In contrast, no difference in Hsp27 protein expression is detected when comparing scr-PMO- and water-injected embryos at all time points. Figure 1c also shows immunoblotting to detect sarcomeric actin in samples obtained from embryos injected with hsp27i PMO, scr PMO, or water. There is no apparent difference in sarcomeric actin expression levels at any developmental time point between embryos expressing and lacking Hsp27, indicating that loss of Hsp27 does not affect muscle development at the level of sarcomeric actin expression.
Figure 1d examines if Hsp27 knockdown using 0.1 mM hsp27i PMO affects expression of the high-molecular-weight heat shock protein, Hsp70. Hsp70 has two isoforms in zebrafish, constitutively expressed Hsc70 and stress-upregulated Hsp70. Hsc70 expression (lower band) was unaltered after Hsp27 knockdown in both unstressed embryos and embryos recovering from heat shock. Additionally, Hsc70 protein expression is unaltered by heat shock, consistent with previous reports (Evans et al. 2005). As expected, inducible Hsp70 protein expression (upper band) is upregulated in embryos recovering from heat shock. The knockdown of Hsp27 had no effect on the expression of Hsp70 after heat shock.
Immunofluorescent detection of Hsp27 in 50-hpf embryos
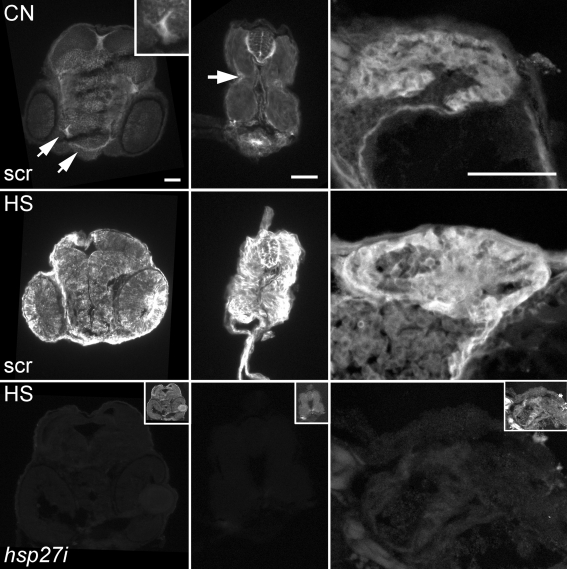
Figure 2 shows cryosections of 50-hpf zebrafish embryos probed with αHsp27 serum and fluorescent secondary antibodies. Embryos were injected with equal concentrations (0.1 mM) and volumes of scrambled (scr; top and middle rows) or hsp27i PMO (bottom row) at the one to two cell stages. Consistent with published data on hsp27 mRNA expression (Mao et al. 2005; Marvin et al. 2008), embryos injected with scr PMO exhibit ubiquitous low levels of Hsp27 expression and higher levels in the developing facial muscles (top left, arrows, and inset). Localization of Hsp27 within cryosections of the tail of scr-PMO-injected embryos (top center) also indicates ubiquitous low-level expression throughout developing myotomes and higher expression in a subset of myocytes on the outer edge of the myotome. This is most noticeable near the developing lateral line of the embryo where a subset of myocytes exhibits a high level of Hsp27 expression (top center, arrow). These cells were positively identified as myocytes by counterstaining with fluorescent phalloidin, a probe for F-actin filaments (data not shown). Finally, under control conditions, the highest level of Hsp27 fluorescence was observed in the heart (top right). Scrambled-PMO-injected embryos heat-shocked (30 min at 37°C) at 36 hpf and allowed to recover for 14 h showed global increases in Hsp27 expression in all tissues, including the trunk muscles, heart, brain, spinal cord, and eye (center row). In contrast, hsp27i-PMO-injected embryos processed for Hsp27 immunolocalization after an identical heat shock and recovery period display almost no fluorescence using the same imaging parameters (bottom row). This negative control experiment further demonstrates the effectiveness of the hsp27i PMO and the utility of our αHsp27 serum for immunofluorescence localization of Hsp27.
Fig. 2.
Immunolocalization of Hsp27 in 50-hpf zebrafish embryos. All images were obtained with identical illumination and camera settings. Top row: Hsp27 expression is detected in the head (left), trunk (middle), and heart (right) of 0.1 mM scrambled (scr)-PMO-injected embryos under control conditions (CN). Hsp27 is most highly expressed in craniofacial muscles (arrows, left, and inset), in myocytes near the developing lateral line of the trunk (center, arrow), and in the heart (right). Middle row: Hsp27 expression increases in all tissues of 0.1 mM scr-PMO-injected embryos after heat shock (HS). Bottom row: Hsp27 immunostaining of embryos injected with 0.1 mM antisense hsp27 (hsp27i) PMO and heat-shocked. The inset images in the bottom row (not to scale) were digitally enhanced to show the section morphology. Images show the dorsal surface up for head and trunk images and down for heart images. Scale bars are 50 μm
Zebrafish morphological development is normal in the absence of Hsp27
Previous studies have concluded that Hsp27 could play a role in regulating differentiation or survival of some vertebrate embryonic cell types. However, comparison of the overall morphology of zebrafish developed after injection with equal volumes of 0.15 mM scr and hsp27i PMO did not reveal changes in the appearance of resulting embryos (Fig. 3a). To provide a quantitative assessment of the morphology of these embryos, we measured the length of the tail (Fig. 3b), the diameter of the lens (Fig. 3c), and the two-dimensional (2-D) area of the yolk mass, an indirect measure of overall metabolism (Fig. 3d) of 24-hpf embryos. No significant differences in these measured values were detected (p > 05).
Morphology and function of cardiac and skeletal muscles are unaltered by knockdown of Hsp27
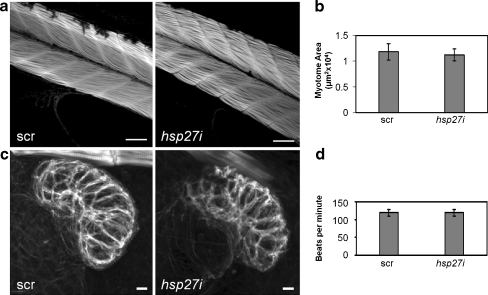
Hsp27 is highly expressed in the skeletal and cardiac muscle tissues (Fig. 2) and previous reports have indicated that Hsp27 knockdown using phosphorodiamidate morpholino oligonucleotides can disrupt development of these tissues in frogs (Brown et al. 2007). Here, we have examined effects of Hsp27 knockdown on morphology and functional characteristics of these tissues in zebrafish embryos. Figure 4a shows representative images, centered on the 12th myotome, of skeletal muscle in 50-hpf embryos stained with fluorescent phalloidin. No overall morphological difference in skeletal muscle architecture is seen when comparing 0.1 mM hsp27i and scr-PMO-injected embryos. We also measured the 2-D area of the 12th myotome in 30 scr- and 30 hsp27i-PMO-injected embryos (Fig. 4b). Analysis of these values revealed no statistically significant difference (p ≥ 0.05) between these groups of embryos.
Fig. 4.
Striated muscle architecture and heart rate in 50-hpf embryos are not altered by Hsp27 knockdown. a Phalloidin staining of skeletal muscle in embryos. These images are centered on the 12th myotome. b Area of the 12th myotome measured from 30 embryos injected with 0.1 mM scrambled (scr) or antisense hsp27 (hsp27i) PMO in a total of three independent trials. c Z-series projections of F-actin in hearts of embryos injected with hsp27i or scr PMO. Images are representative of four projections in each group. d Heart beat frequency measured from 40 scr and 40 hsp27i-PMO-injected embryos. No significant differences in myotome area or heart rate (p ≥ 0.05) were detected. Error bars are standard deviations. Scale bars are 50 μm (myotomes) and 10 μm (hearts)
Figure 4c shows confocal Z-series projections of phalloidin fluorescence in hearts of 50-hpf scr- and hsp27i-PMO-injected embryos. The thickened myocardium of the ventricle is the most obvious structure. These images are representative of four Z-series projections generated for embryos in each group. Although in this example the scr-PMO-injected embryo heart appears slightly larger and more brightly stained, this difference was not observed consistently. Importantly, these images are representative in showing no difference in overall morphology of the ventricle or in myofilament prevalence, organization, or thickness. As an assay of function, we also analyzed heart beat rate in 40 scr and 40 hsp27i-PMO-injected embryos at 50 hpf in a total of three independent experiments (Fig. 4d). Analysis of these values revealed no significant difference in the heart beat rates between these groups of embryos (p > 05).
Swimming velocity of zebrafish larvae is unaltered by Hsp27 knockdown
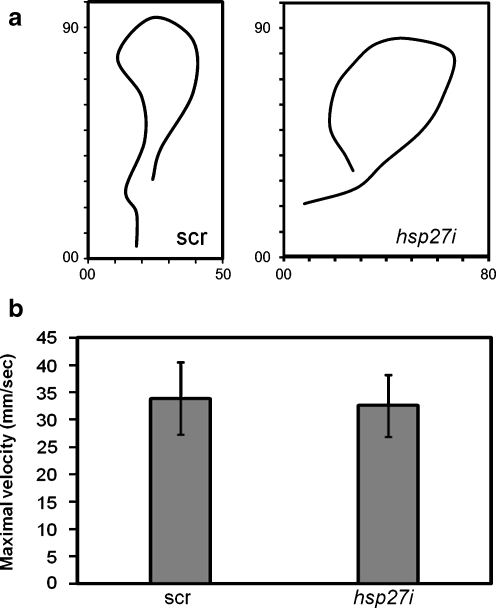
As an indirect measure of both skeletal muscle and motor neuron function, we assayed swimming motility during the startle response of dechorionated embryos at 50 hpf, the earliest developmental time point when robust swimming behavior was observed in uninjected controls (data not shown). Figure 5a shows representative paths of movement of a scr and an hsp27i-PMO-injected embryo. No overt changes in parameters such as distance or directional persistence are observed. Figure 5b shows a quantitative analysis of the average maximal swimming velocity displayed by 0.1 mM PMO-injected embryos (n > 30 for each group). No difference in average value was detected (p ≥ 0.05) when comparing scr and hsp27i-PMO-injected embryos.
Fig. 5.
Motility of 50-hpf embryos is not altered by Hsp27 knockdown. a Traces of motility by embryos injected with 0.1 mM scrambled (scr) or antisense hsp27 (hsp27i) PMO. Traces are representative of at least 30 embryos per group measured in three independent trials. Axes show x (horizontal) and y (vertical) image coordinates. b Mean maximal velocity of hsp27i and scr-PMO-injected embryos in millimeter per second. No statistically significant (p ≥ 0.05) difference was detected. Error bars indicate standard deviations
Hsp27 is persistently expressed in adult zebrafish tissues
Because Hsp27 appears to play no role in the morphogenesis of zebrafish tissues, we considered whether its expression might be involved in maintenance of zebrafish tissue homeostasis over longer periods. To begin to test this hypothesis, we conducted immunolocalization and immunoblotting studies of Hsp27 expression in tissues of unstressed adult zebrafish (Fig. 6). Immunolocalization of Hsp27 in tissue cryosections was most evident in ventricular myocardium (Hrt) and was somewhat more evident in peripheral muscle fibers than interior ones (Fig. 6, Hrt). Skeletal muscle cryosections also showed relatively high levels of Hsp27 (Fig. 6, Ske). The highest level of expression in this tissue was consistently observed in an outer lateral wedge of muscle previously shown in a number of fish species, including zebrafish, to consist of slow-twitch, or red, muscle (Greer-Walker and Pull 1975). Intriguingly, the greatest level of expression in skeletal muscle sections was detected in a subset of myocytes found at the border between the slow- and fast-twitch muscle tissues (Ske, arrow). Hsp27 is also detected, albeit at much lower levels, in a layer of tissue found at the outer lining of the intestinal tract (Int, arrow). Counterstaining with fluorescent phalloidin indicated that this layer contains smooth muscle tissue (data not shown). Internal structures of the adult zebrafish brain are largely devoid of Hsp27 expression. However, the outer epithelial lining of the adult brain displayed a thin layer of Hsp27-positive fluorescence (Br, arrow). Finally, Hsp27 was localized in cells surrounding seminiferous tubules in the testis (Fig. 6, Test). However, no Hsp27-expressing cells were observed within the tubule structures. To confirm these overall expression patterns within the adult zebrafish, we analyzed Hsp27 expression levels in adult zebrafish tissues using Western blots. Immunoblotting of Hsp70 was performed for comparison. Hsp27 expression was highest in heart tissues, skeletal muscle, and the lens but was also detected at lower levels in all tissues examined. Hsp27 expression was also consistently higher in red muscle than in white muscle. Interestingly, Hsp70 was also constitutively expressed in adult tissues, with the highest levels of expression detected in the brain, testis, and intestinal tissue, whereas little or no Hsp70 expression was detected in the heart, lens, or skeletal muscles.
Discussion
Hsp27 is not required for development of zebrafish under control conditions
Hsp27 expression is upregulated in muscle and some other tissues of the developing vertebrate embryo in the absence of stress (Gernold et al. 1993; Norris et al. 1997; Kawazoe et al. 1999; Mao et al. 2005; Tuttle et al. 2007). Results of previous in vitro studies have indicated that Hsp27 expression could be required for differentiation of myoblasts and other embryonic cell types (Mehlen et al. 1997; Davidson and Morange 2000); however, in vivo data addressing this hypothesis are limited and conflicting. Huang et al. (2007) showed no effects of hsp27 gene deletion on mouse development, while Brown et al. (2007) have reported that transient knockdown of Hsp27 causes cardia bifida and myofibril defects in frogs. We have previously shown that zebrafish share with other vertebrates the expression of hsp27 mRNA in cardiac and skeletal muscle early in development and the ability of zebrafish Hsp27 to protect cultured cells from thermal injury (Mao et al. 2005). In the present study, we have conducted functional studies of Hsp27 in zebrafish using an hsp27i PMO to inhibit expression during embryogenesis. Like results published previously for both mice and Xenopus (Brown et al. 2007; Huang et al. 2007), the global pattern of embryonic development was unaltered by the loss of Hsp27 in zebrafish. The present and two previous studies appear to provide a consensus that Hsp27 is dispensable for overall vertebrate developmental and thus that results obtained from in vitro studies of the role of stress proteins during development should be viewed with caution. One interpretation of available literature is that differentiation may be more difficult for cells in vitro than in vivo. In support of this view, oxidative stress in cultured cells has been well documented (Halliwell 2003). Expression of Hsps may promote survival and function of cells during differentiation in vitro, with implications for bioengineering of tissues and organs.
Cardia bifida and defects in myofibril organization were demonstrated in Xenopus embryos lacking Hsp27 as a result of morpholino depletion (Brown et al. 2007). Development of the zebrafish heart is regulated by a network of transcription factors that share both sequence and functional homology with those involved in the development of hearts in other vertebrates, including frogs, mice, and humans (Weinstein and Fishman 1996). Additionally, like hearts of other vertebrates, the zebrafish heart develops from bilateral heart fields that migrate toward and fuse at the embryonic midline to form the primitive heart tube, and defects in these morphogenic movements result in cardia bifida in zebrafish embryos (Chen et al. 1996; Yelon et al. 1999; Trinh and Stainier 2004; Wang et al. 2005; Matsui et al. 2007). Similar to studies of mice lacking Hsp27 as a result of gene deletion (Huang et al. 2007), our studies demonstrate that these events do not require the expression of Hsp27 in zebrafish embryos under control conditions. Additionally, unlike results from studies of frog embryos, myofibril architecture in heart and skeletal muscle were not detectably different between hsp27i- and scr-PMO-injected zebrafish embryos. Hsp27 has also been shown to play a role in the development and protection of the emerging motor neuron system in vitro (Williams et al. 2006). However, our results showed no difference in motility of zebrafish embryos lacking Hsp27 versus controls. Because swimming requires proper function of both the muscle and nervous systems, these results indicate that not only the skeletal muscle but also motor neurons were functionally intact. One possible interpretation of the available data is that mammals and zebrafish, but not frogs, express proteins that can compensate for the loss of Hsp27. Humans and mice have genes for ten related small heat shock proteins (Fontaine et al. 2003), and zebrafish have genes for 15 such proteins (Elicker and Hutson 2007). In mammals, expression of the related αB crystallin in skeletal and cardiac muscle tissues has been well documented (see Inaguma et al. (1993), for example), and these proteins have some common functionality (Jakob et al. 1993). We and others have reported that αB crystallin is not detected in zebrafish muscle tissues (Posner et al. 1999; Mao and Shelden 2006). However, mRNAs for four other sHsps (Hspb7, Hspb8, Hspb9, Hspb11) are detected in somites of zebrafish embryos. In cardiac tissues, hspb7 and hspb12 mRNA are expressed through at least 48 h of development (Marvin et al. 2008). The number of potentially complementary proteins expressed in amphibians has not been determined, but it is conceivable that frogs and other amphibians exhibit greater reliance on Hsp27 during development than zebrafish and mice. Alternatively, interpretation of previous results may have been complicated by issues related to well-documented morpholino toxicity (Heasman 2002). To address these issues, the present study employed antiserum detecting zebrafish Hsp27 to determine the minimal PMO concentration effective in reducing Hsp27 expression in zebrafish. In addition, we compared results of our experiments using hsp27i PMO with a control PMO containing identical nucleotides in a scrambled order.
Our present study has several important limitations. Our studies may have lacked the sensitivity required for analysis of subtle changes in heart or skeletal muscle function, so we cannot dismiss the possibility of a unique role for Hsp27 in zebrafish embryogenesis. Our studies also did not address possible roles for Hsp27 during later stages of development, such as sexual maturation, and did directly address the performance of these tissues after stress- or injury-induced damage. These issues are under consideration in our laboratory. However, taken together, the expression of Hsp27 in metabolically active muscle tissues, coupled with the absence of morphological defects in embryos lacking Hsp27 during development most strongly support the view that Hsp27 has a role in protecting myocytes against mechanical or oxidative stress, rather than having a direct role in modulating morphogenesis or organization of the myofilament system.
The expression pattern of Hsp27 supports a role in maintenance of tissue homeostasis
Although Hsp27 expression patterns have been widely examined in several mammalian species, previous studies of Hsp27 expression in zebrafish and frogs have been limited to analysis of hsp27 mRNA distribution patterns (Mao and Shelden 2006; Tuttle et al. 2007; Marvin et al. 2008) or gene reporter constructs in zebrafish embryos and larvae (Wu et al. 2008). For example, hsp27 mRNA expression was detected in developing heart, craniofacial muscle and lens tissues of embryos (Marvin et al. 2008). In trunks of 48-hpf zebrafish embryos, hsp27 mRNA was elevated in superficial skeletal muscles. This region contains developing slow-twitch muscle, leading Marvin et al. to suggest that Hsp27 expression might be specifically elevated in slow-twitch muscle fibers. Our results confirm that Hsp27 protein expression is elevated in zebrafish embryos, compared to surrounding tissues, in cardiac and craniofacial muscles and a subset of muscle fibers found at the lateral margin of embryonic trunk and tail muscle tissues. However, our antibody also detected ubiquitous lower-level Hsp27 expression, not seen in previous studies, in embryos raised under control conditions. The widespread expression of Hsp27 protein detected by immunofluorescence in zebrafish embryos in the present study, particularly in the developing brain and spinal cord, was unexpected and initially led us to consider whether this pattern could result from nonspecific binding of the αHsp27 serum used in our studies. However, our Western blotting studies corroborate the relative changes in Hsp27 expression seen by immunostaining during development, after heat shock, and in adult tissues. In addition, little or no staining is detected in embryos by immunofluorescence after knockdown of Hsp27 in our studies. These data indicate that there is significant constitutive expression of Hsp27 in most tissues of the zebrafish embryo.
Only a few studies have examined Hsp27 expression as a function of muscle fiber type. In mammals, Hsp27 and related αB crystallin protein or mRNA levels are generally higher in muscle tissues containing predominately slow-twitch muscle than in those containing mostly fast-twitch muscle (Inaguma et al. 1993; Huey et al. 2007; Ishihara et al. 2008). However, the opposite has also been reported (Kim et al. 2004). In addition, Hsp27 expression in predominately fast-twitch muscle has been shown to vary as a function of age, with declining expression correlating positively with increased age (Gupte et al. 2008). The present study demonstrates that Hsp27 protein expression is higher in lateral slow-twitch muscle fibers than more central fast-twitch muscles in both 50-hpf embryos and, for the first time, in adult zebrafish muscle tissues. These results suggest that Hsp27 expression and function in slow-twitch muscle fibers has been conserved between fish and mammals and that zebrafish may therefore be a useful model in which to address the functional significance of this expression. However, some differences can also be observed. For example, some studies have shown that Hsp70 is also highly expressed in mammalian slow-twitch muscle tissues (Tupling et al. 2007; Ishihara et al. 2008), but our present results are consistent with previous reports (Blechinger et al. 2002) showing that significant constitutive expression of Hsp70 is not detected in zebrafish muscle tissues. In addition, both slow- and fast-twitch muscle tissues in mammals express significant levels of the related αB crystallin, but αB crystallin expression is detected only in the lens of zebrafish (Posner et al. 1999; Mao and Shelden 2006). Presently, it is unclear whether Hsp27 plays a more significant role in muscle tissues of zebrafish than mammals or if other heat shock proteins found in fish species perform the roles played by Hsp70 and αB crystallin in mammals.
Expression patterns of Hsp27 and Hsp70 are distinct in zebrafish under control conditions
Finally, recent attention has been focused on the potential role of Hsp27 as cochaperone for high-molecular-weight Hsp70. For example, several studies have shown that Hsp27 and other small heat shock proteins can prevent irreversible unfolding of damaged proteins in vitro (Haslbeck et al. 2005; Bryantsev et al. 2007) and in cultured cells (Bryantsev et al. 2007) and that Hsp27 can synergistically promote recovery of cells when coexpressed with Hsp70 (Riordan et al. 2004). This function would presumably require the spatial and temporal coexpression and colocalization of both proteins. Consistent with this hypothesis, both Hsp70 and Hsp27 expression levels are ubiquitously induced in zebrafish embryos following sublethal thermal injury (Adam et al. 2000; Mao and Shelden 2006). However, Western blots presented here indicate that only the testis, intestinal tissues, and brain express both Hsp27 and Hsp70 at detectable levels in the absence of stress. In addition, Hsp27 expression is strikingly higher in tissues lacking detectable Hsp70 than in those in which Hsp70 is readily detected. Our data therefore have implications for understanding both regulation and function of Hsp27 and Hsp70 expression patterns in zebrafish. We suggest that the function of Hsp27 in zebrafish muscle tissues, at least under control conditions, does not require Hsp70 coexpression. These data also have some implication for understanding the regulation of Hsp27 expression. For example, the lack of protein coexpression under control conditions suggests differential regulation of gene expression in the absence of stress. Therefore, transcription of hsp27 under control conditions may reflect activity of tissue-specific factors as opposed to general stress-responsive regulation. These conclusions also suggest that Hsp27 performs a function in muscle tissues that may be distinct from that involved in stress protection.
Summary
Our studies indicate that Hsp27 is not required for the differentiation of muscle cells or the establishment of normal cell architecture, including that of muscle cells, during development. However, our results confirm earlier studies of hsp27 mRNA distribution suggesting that Hsp27 is preferentially expressed in skeletal and cardiac muscle cells in the zebrafish and demonstrate that expression in these tissues persists throughout the life of the animal. These data seem to support the view that Hsp27 promotes the integrity of muscle cell architecture or survival under conditions of mechanical strain or oxidative stress. The similarities in expression pattern and functional significance of Hsp27 observed in zebrafish in the present study and in mammalian systems indicate that zebrafish may be a useful model with which to establish the interrelationships between members of the small heat shock protein family.
Acknowledgements
We thank Amy Nickel for assistance with image analysis, Dr. Li Mao for help with zebrafish rearing, and Dr. Maria B. Chechanova for plasmid production and recombinant Hsp27 expression and purification. We also thank Drs. Deborah Stenkamp (University of Idaho) and Michael J. Welsh (University of Michigan Medical School) for critical reading of this manuscript. This work was supported by a grant from the NSF (IOS-0818993) and a USDA special grant administered by the UI/WSU Aquaculture research program awarded to E. A. S, and by the National Research Initiative of the USDA Cooperative State Research, Education, and Extension Service, grant number 2006-35201-16553, awarded to M. E. K.
Footnotes
Tucker and Ustyugov contributed equally to this work.
References
- Adam A, Bartfai R, Lele Z, Krone PH, Orban L. Heat-inducible expression of a reporter gene detected by transient assay in zebrafish. Exp Cell Res. 2000;256:282–290. doi: 10.1006/excr.2000.4805. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. Hsp27: novel regulator of intracellular redox state. IUBMB Life. 2001;52:303–307. doi: 10.1080/152165401317291156. [DOI] [PubMed] [Google Scholar]
- Benndorf R, Welsh MJ. Shocking degeneration. Nat Genet. 2004;36:547–548. doi: 10.1038/ng0604-547. [DOI] [PubMed] [Google Scholar]
- Blechinger SR, Evans TG, Tang PT, Kuwada JY, Warren JT, Jr., Krone PH. The heat-inducible zebrafish hsp70 gene is expressed during normal lens development under non-stress conditions. Mech Dev. 2002;112:213–215. doi: 10.1016/S0925-4773(01)00652-9. [DOI] [PubMed] [Google Scholar]
- Bonham RT, Fine MR, Pollock FM, Shelden EA. Hsp27, Hsp70, and metallothionein in MDCK and LLC-PK1 renal epithelial cells: effects of prolonged exposure to cadmium. Toxicol Appl Pharmacol. 2003;191:63–73. doi: 10.1016/S0041-008X(03)00226-6. [DOI] [PubMed] [Google Scholar]
- Brown DD, Christine KS, Showell C, Conlon FL. Small heat shock protein Hsp27 is required for proper heart tube formation. Genesis. 2007;45:667–678. doi: 10.1002/dvg.20340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryantsev AL, Kurchashova SY, Golyshev SA, et al. Regulation of stress-induced intracellular sorting and chaperone function of Hsp27 (HspB1) in mammalian cells. Biochem J. 2007;407:407–417. doi: 10.1042/BJ20070195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol. 2000;20:7602–7612. doi: 10.1128/MCB.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JN, Haffter P, Odenthal J, et al. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123:293–302. doi: 10.1242/dev.123.1.293. [DOI] [PubMed] [Google Scholar]
- Chung L, Ng YC. Age-related alterations in expression of apoptosis regulatory proteins and heat shock proteins in rat skeletal muscle. Biochim Biophys Acta. 2006;1762:103–109. doi: 10.1016/j.bbadis.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Clemen CS, Fischer D, Roth U, et al. Hsp27-2D-gel electrophoresis is a diagnostic tool to differentiate primary desminopathies from myofibrillar myopathies. FEBS Lett. 2005;579:3777–3782. doi: 10.1016/j.febslet.2005.05.051. [DOI] [PubMed] [Google Scholar]
- Costigan M, Mannion RJ, Kendall G, et al. Heat shock protein 27: developmental regulation and expression after peripheral nerve injury. J Neurosci. 1998;18:5891–5900. doi: 10.1523/JNEUROSCI.18-15-05891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson SM, Morange M. Hsp25 and the p38 MAPK pathway are involved in differentiation of cardiomyocytes. Dev Biol. 2000;218:146–160. doi: 10.1006/dbio.1999.9596. [DOI] [PubMed] [Google Scholar]
- Dillmann WH. Small heat shock proteins and protection against injury. Ann N Y Acad Sci. 1999;874:66–68. doi: 10.1111/j.1749-6632.1999.tb09225.x. [DOI] [PubMed] [Google Scholar]
- Dodge ME, Wang J, Guy C, Rankin S, Rahimtula M, Mearow KM. Stress-induced heat shock protein 27 expression and its role in dorsal root ganglion neuronal survival. Brain Res. 2006;1068:34–48. doi: 10.1016/j.brainres.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Elicker KS, Hutson LD. Genome-wide analysis and expression profiling of the small heat shock proteins in Zebrafish. Gene. 2007;403:60–69. doi: 10.1016/j.gene.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo J, Pucci AM, Koh TJ. HSP25 protects skeletal muscle cells against oxidative stress. Free Radic Biol Med. 2004;37:1455–1462. doi: 10.1016/j.freeradbiomed.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Evans TG, Yamamoto Y, Jeffery WR, Krone PH. zebrafish Hsp70 is required for embryonic lens formation. Cell Stress Chaperones. 2005;10:66–78. doi: 10.1379/CSC-79R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine JM, Rest JS, Welsh MJ, Benndorf R. The sperm outer dense fiber protein is the 10th member of the superfamily of mammalian small stress proteins. Cell Stress Chaperones. 2003;8:62–69. doi: 10.1379/1466-1268(2003)8<62:TSODFP>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. Faseb J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- Gernold M, Knauf U, Gaestel M, Stahl J, Kloetzel PM. Development and tissue-specific distribution of mouse small heat shock protein hsp25. Dev Genet. 1993;14:103–111. doi: 10.1002/dvg.1020140204. [DOI] [PubMed] [Google Scholar]
- Greer-Walker M, Pull G. A survey of red and white muscle in marine fish. J Fish Biol. 1975;7:295–300. doi: 10.1111/j.1095-8649.1975.tb04602.x. [DOI] [Google Scholar]
- Gupte AA, Bomhoff GL, Geiger PC. Age-related differences in skeletal muscle insulin signaling: the role of stress kinases and heat shock proteins. J Appl Physiol. 2008;105:839–848. doi: 10.1152/japplphysiol.00148.2008. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress in cell culture: an under-appreciated problem? FEBS Lett. 2003;540:3–6. doi: 10.1016/S0014-5793(03)00235-7. [DOI] [PubMed] [Google Scholar]
- Hao X, Zhang S, Timakov B, Zhang P. The Hsp27 gene is not required for Drosophila development but its activity is associated with starvation resistance. Cell Stress Chaperones. 2007;12:364–372. doi: 10.1379/CSC-308.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Heasman J. Morpholino oligos: making sense of antisense? Dev Biol. 2002;243:209–214. doi: 10.1006/dbio.2001.0565. [DOI] [PubMed] [Google Scholar]
- Huang L, Min JN, Masters S, Mivechi NF, Moskophidis D. Insights into function and regulation of small heat shock protein 25 (HSPB1) in a mouse model with targeted gene disruption. Genesis. 2007;45:487–501. doi: 10.1002/dvg.20319. [DOI] [PubMed] [Google Scholar]
- Huey KA, McCall GE, Zhong H, Roy RR. Modulation of HSP25 and TNF-alpha during the early stages of functional overload of a rat slow and fast muscle. J Appl Physiol. 2007;102:2307–2314. doi: 10.1152/japplphysiol.00021.2007. [DOI] [PubMed] [Google Scholar]
- Inaguma Y, Goto S, Shinohara H, Hasegawa K, Ohshima K, Kato K. Physiological and pathological changes in levels of the two small stress proteins, HSP27 and alpha B crystallin, in rat hind limb muscles. J Biochem (Tokyo) 1993;114:378–384. doi: 10.1093/oxfordjournals.jbchem.a124184. [DOI] [PubMed] [Google Scholar]
- Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara A, Fujino H, Nagatomo F, Takeda I, Ohira Y. Gene expression levels of heat shock proteins in the soleus and plantaris muscles of rats after hind limb suspension or spaceflight. J Physiol Sci. 2008;58:413–417. doi: 10.2170/physiolsci.RP000808. [DOI] [PubMed] [Google Scholar]
- Ito H, Kamei K, Iwamoto I, Inaguma Y, Kato K. Regulation of the levels of small heat-shock proteins during differentiation of C2C12 cells. Exp Cell Res. 2001;266:213–221. doi: 10.1006/excr.2001.5220. [DOI] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Kawazoe Y, Tanabe M, Nakai A. Ubiquitous and cell-specific members of the avian small heat shock protein family. FEBS Lett. 1999;455:271–275. doi: 10.1016/S0014-5793(99)00900-X. [DOI] [PubMed] [Google Scholar]
- Kim NK, Joh JH, Park HR, Kim OH, Park BY, Lee CS. Differential expression profiling of the proteomes and their mRNAs in porcine white and red skeletal muscles. Proteomics. 2004;4:3422–3428. doi: 10.1002/pmic.200400976. [DOI] [PubMed] [Google Scholar]
- Kiriyama MT, Oka M, Takehana M, Kobayashi S. Expression of a small heat shock protein 27 (HSP27) in mouse skin tumors induced by UVB-irradiation. Biol Pharm Bull. 2001;24:197–200. doi: 10.1248/bpb.24.197. [DOI] [PubMed] [Google Scholar]
- Knoll R, Arras M, Zimmermann R, Schaper J, Schaper W. Changes in gene expression following short coronary occlusions studied in porcine hearts with run-on assays. Cardiovasc Res. 1994;28:1062–1069. doi: 10.1093/cvr/28.7.1062. [DOI] [PubMed] [Google Scholar]
- Lambert H, Charette SJ, Bernier AF, Guimond A, Landry J. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J Biol Chem. 1999;274:9378–9385. doi: 10.1074/jbc.274.14.9378. [DOI] [PubMed] [Google Scholar]
- Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993;268:3420–3429. [PubMed] [Google Scholar]
- Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- Liu Q, Frey RA, Babb-Clendenon SG, et al. Differential expression of photoreceptor-specific genes in the retina of a zebrafish cadherin2 mutant glass onion and zebrafish cadherin4 morphants. Exp Eye Res. 2007;84:163–175. doi: 10.1016/j.exer.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Shelden EA. Developmentally regulated gene expression of the small heat shock protein Hsp27 in zebrafish embryos. Gene Expr Patterns. 2006;6:127–133. doi: 10.1016/j.modgep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Mao L, Bryantsev AL, Chechenova MB, Shelden EA. Cloning, characterization, and heat stress-induced redistribution of a protein homologous to human hsp27 in the zebrafish Danio rerio. Exp Cell Res. 2005;306:230–241. doi: 10.1016/j.yexcr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Marin R, Tanguay RM. Stage-specific localization of the small heat shock protein Hsp27 during oogenesis in Drosophila melanogaster. Chromosoma. 1996;105:142–149. doi: 10.1007/BF02509495. [DOI] [PubMed] [Google Scholar]
- Marvin M, O, Rourke D, Kurihara T, Juliano CE, Harrison KL, Hutson LD. Developmental expression patterns of the zebrafish small heat shock proteins. Dev Dyn. 2008;237:454–463. doi: 10.1002/dvdy.21414. [DOI] [PubMed] [Google Scholar]
- Matsui T, Raya A, Callol-Massot C, Kawakami Y, Oishi I, Rodriguez-Esteban C, Belmonte JC. Miles-apart-mediated regulation of cell–fibronectin interaction and myocardial migration in zebrafish. Nat Clin Pract Cardiovasc Med 4 Suppl. 2007;1:S77–S82. doi: 10.1038/ncpcardio0764. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Mehlen A, Godet J, Arrigo AP. Hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem. 1997;272:31657–31665. doi: 10.1074/jbc.272.50.31657. [DOI] [PubMed] [Google Scholar]
- Monteville MR, Yoon JE, Konkel ME. Maximal adherence and invasion of INT 407 cells by Campylobacter jejuni requires the CadF outer-membrane protein and microfilament reorganization. Microbiology. 2003;149:153–165. doi: 10.1099/mic.0.25820-0. [DOI] [PubMed] [Google Scholar]
- Mounier N, Arrigo A. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperone. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:ACASHS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CE, Brown MA, Hickey E, Weber LA, Hightower LE. Low-molecular-weight heat shock proteins in a desert fish (Poeciliopsis lucida): homologs of human Hsp27 and Xenopus Hsp30. Mol Biol Evol. 1997;14:1050–1061. doi: 10.1093/oxfordjournals.molbev.a025711. [DOI] [PubMed] [Google Scholar]
- Pandey P, Farber R, Nakazawa A, et al. Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 2000;19:1975–1981. doi: 10.1038/sj.onc.1203531. [DOI] [PubMed] [Google Scholar]
- Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- Posner M, Kantorow M, Horwitz J. Cloning, sequencing and differential expression of alphaB-crystallin in the zebrafish, Danio rerio. Biochim Biophys Acta. 1999;1447:271–277. doi: 10.1016/s0167-4781(99)00155-4. [DOI] [PubMed] [Google Scholar]
- Rane MJ, Pan Y, Singh S, et al. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem. 2003;278:27828–27835. doi: 10.1074/jbc.M303417200. [DOI] [PubMed] [Google Scholar]
- Riordan M, Garg V, Thulin G, Kashgarian M, Siegel NJ. Differential inhibition of HSP72 and HSP25 produces profound impairment of cellular integrity. J Am Soc Nephrol. 2004;15:1557–1566. doi: 10.1097/01.ASN.0000127996.42634.2B. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Urushidani T, Nagao T. Translocation of HSP27 to sarcomere induced by ischemic preconditioning in isolated rat hearts. Biochem Biophys Res Commun. 2000;269:137–142. doi: 10.1006/bbrc.2000.2233. [DOI] [PubMed] [Google Scholar]
- Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6:371–382. doi: 10.1016/S1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- Tupling AR, Bombardier E, Stewart RD, Vigna C, Aqui AE. Muscle fiber type-specific response of Hsp70 expression in human quadriceps following acute isometric exercise. J Appl Physiol. 2007;103:2105–2111. doi: 10.1152/japplphysiol.00771.2007. [DOI] [PubMed] [Google Scholar]
- Tuttle AM, Gauley J, Chan N, Heikkila JJ. Analysis of the expression and function of the small heat shock protein gene, hsp27, in Xenopus laevis embryos. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:112–121. doi: 10.1016/j.cbpa.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Klundert FA, Gijsen ML, den IPR, Snoeckx LH, Jong WW. alpha B-crystallin and hsp25 in neonatal cardiac cells—differences in cellular localization under stress conditions. Eur J Cell Biol. 1998;75:38–45. doi: 10.1016/s0171-9335(98)80044-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhong T, Qian L, Dong Y, Jiang Q, Tan L, Song H. Wortmannin induces zebrafish cardia bifida through a mechanism independent of phosphoinositide 3-kinase and myosin light chain kinase. Biochem Biophys Res Commun. 2005;331:303–308. doi: 10.1016/j.bbrc.2005.03.145. [DOI] [PubMed] [Google Scholar]
- Weinstein BM, Fishman MC. Cardiovascular morphogenesis in zebrafish. Cardiovasc Res. 1996;31(Spec No):E17–E24. [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio) Eugene: Univ. of Oregon Press; 1993. [Google Scholar]
- Williams KL, Rahimtula M, Mearow KM. Heat shock protein 27 is involved in neurite extension and branching of dorsal root ganglion neurons in vitro. J Neurosci Res. 2006;84:716–723. doi: 10.1002/jnr.20983. [DOI] [PubMed] [Google Scholar]
- Wu YL, Pan X, Mudumana SP, Wang H, Kee PW, Gong Z. Development of a heat shock inducible gfp transgenic zebrafish line by using the zebrafish hsp27 promoter. Gene. 2008;408:85–94. doi: 10.1016/j.gene.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Arai H, Katayama N, Ishikawa T, Kikumoto K, Atomi Y. Age-related increase of insoluble, phosphorylated small heat shock proteins in human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2007;62:481–489. doi: 10.1093/gerona/62.5.481. [DOI] [PubMed] [Google Scholar]
- Yelon D, Horne SA, Stainier DY. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol. 1999;214:23–37. doi: 10.1006/dbio.1999.9406. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Aki T, Harada K, Shama KM, Kamoda Y, Suzuki A, Ohno S. Translocation of HSP27 and MKBP in ischemic heart. Cell Struct Funct. 1999;24:181–185. doi: 10.1247/csf.24.181. [DOI] [PubMed] [Google Scholar]