Abstract
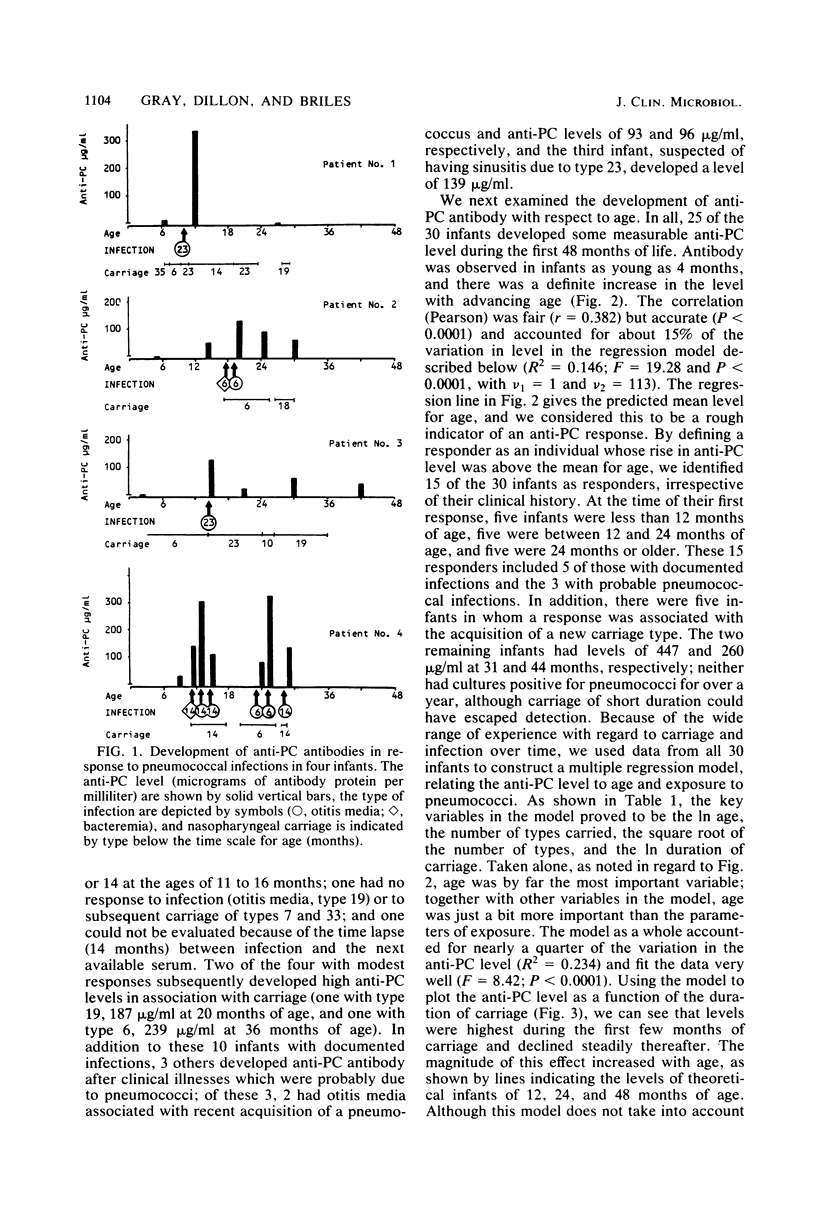
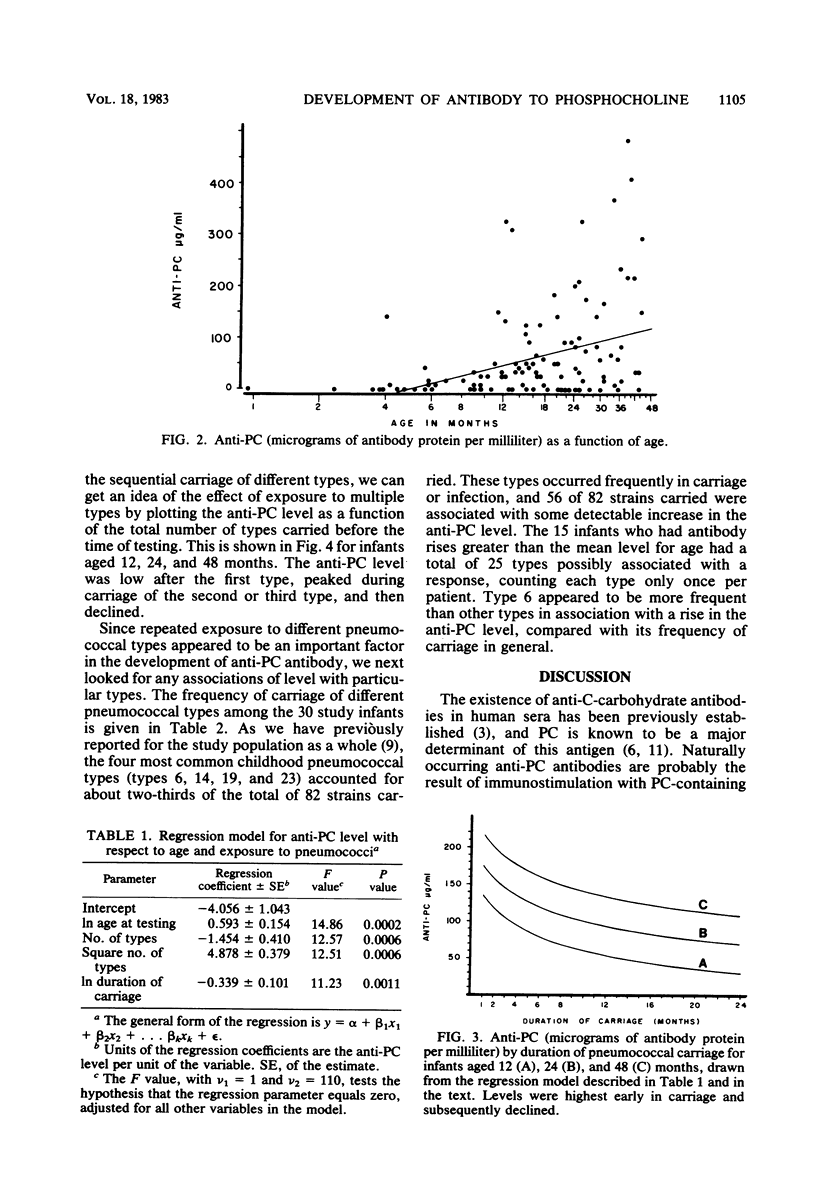
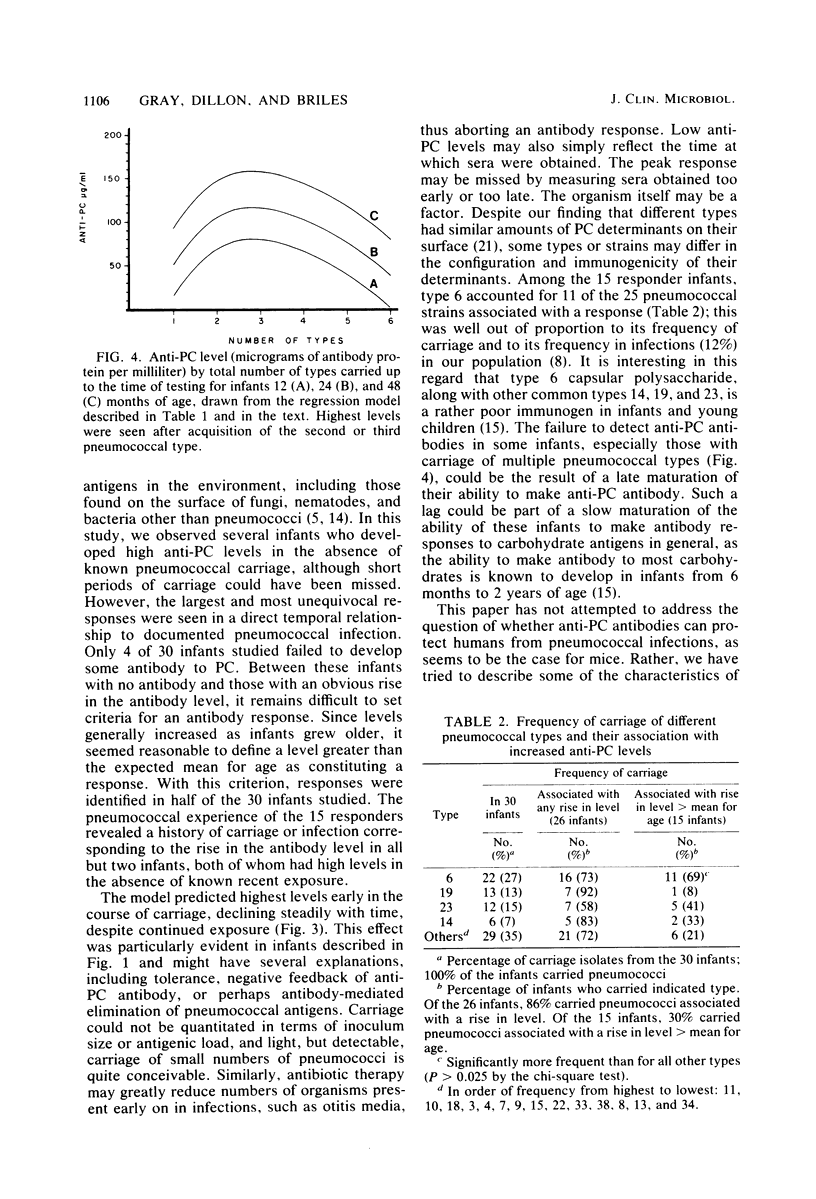
The pneumococci possess, in addition to type-specific capsular polysaccharides, a number of antigens common to the species. Antibodies to phosphocholine (PC), a major determinant of the C-carbohydrate, have been shown to protect mice from experimental pneumococcal infection, but little is known of the role of anti-PC antibodies in humans or the extent to which anti-PC levels are affected by carriage or infection. We examined 115 sera from 30 infants, who were followed prospectively from birth through 4 years of age, for the presence of immunoglobulin M antibody to PC, using a solid-phase radioimmunoassay method. Infants were found to develop antibody to PC in response to pneumococcal carriage and infection, and nearly all infants developed some antibody. Antibody levels increased with age. By using a regression model including both age and nasopharyngeal carriage of pneumococci, anti-PC levels were found to be highest after exposure to two or three different types of pneumococci; levels were highest soon after acquisition of pneumococci and declined thereafter.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au C. C., Eisenstein T. K. Nature of the cross-protective antigen in subcellular vaccines of Streptococcus pneumoniae. Infect Immun. 1981 Jan;31(1):160–168. doi: 10.1128/iai.31.1.160-168.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrian R. Pneumococcal vaccine: development and prospects. Am J Med. 1979 Oct;67(4):547–549. doi: 10.1016/0002-9343(79)90222-5. [DOI] [PubMed] [Google Scholar]
- Bornstein D. L., Schiffman G., Bernheimer H. P., Austrian R. Capsulation of pneumococcus with soluble C-like (Cs) polysaccharide. I. Biological and genetic properties of Cs pneumococcal strains. J Exp Med. 1968 Dec 1;128(6):1385–1400. doi: 10.1084/jem.128.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Nahm M., Schroer K., Davie J., Baker P., Kearney J., Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981 Mar 1;153(3):694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. R., Crandall C. A. A phosphorylcholine idiotype related to TEPC 15 in mice infected with Ascaris suum. J Immunol. 1976 Apr;116(4):1105–1109. [PubMed] [Google Scholar]
- Brundish D. E., Baddiley J. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem J. 1968 Dec;110(3):573–582. doi: 10.1042/bj1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray B. M., Converse G. M., 3rd, Dillon H. C., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980 Dec;142(6):923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- Gray B. M., Converse G. M., 3rd, Dillon H. C., Jr Serotypes of Streptococcus pneumoniae causing disease. J Infect Dis. 1979 Dec;140(6):979–983. doi: 10.1093/infdis/140.6.979. [DOI] [PubMed] [Google Scholar]
- Gray B. M., Converse G. M., 3rd, Huhta N., Johnston R. B., Jr, Pichichero M. E., Schiffman G., Dillon H. C., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: antibody response to nasopharyngeal carriage of types 3, 19, and 23. J Infect Dis. 1981 Oct;144(4):312–318. doi: 10.1093/infdis/144.4.312. [DOI] [PubMed] [Google Scholar]
- Leon M. A., Young N. M. Specificity for phosphorylcholine of six murine myeloma proteins reactive with Pneumococcus C polysaccharide and beta-lipoprotein. Biochemistry. 1971 Apr 13;10(8):1424–1429. doi: 10.1021/bi00784a024. [DOI] [PubMed] [Google Scholar]
- Loda F. A., Collier A. M., Glezen W. P., Strangert K., Clyde W. A., Jr, Denny F. W. Occurrence of Diplococcus pneumoniae in the upper respiratory tract of children. J Pediatr. 1975 Dec;87(6 Pt 2):1087–1093. doi: 10.1016/s0022-3476(75)80120-x. [DOI] [PubMed] [Google Scholar]
- Potter M. Antigen-binding myeloma proteins in mice. Ann N Y Acad Sci. 1971 Dec 31;190:306–321. doi: 10.1111/j.1749-6632.1971.tb13543.x. [DOI] [PubMed] [Google Scholar]
- Sell S. H., Wright P. F., Vaughn W. K., Thompson J., Schiffman G. Clinical studies of pneumococcal vaccines in infants. I. Reactogenicity and immunogenicity of two polyvalent polysaccharide vaccines. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S97–107. doi: 10.1093/clinids/3.supplement_1.s97. [DOI] [PubMed] [Google Scholar]
- Thompson H. C., Eisenstein T. K. Biological properties of an immunogenic pneumococcal subcellular preparation. Infect Immun. 1976 Mar;13(3):750–757. doi: 10.1128/iai.13.3.750-757.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J., Forman C., Gray B. M., Briles D. E. Protection of mice from infection with Streptococcus pneumoniae by anti-phosphocholine antibody. Infect Immun. 1982 Apr;36(1):184–188. doi: 10.1128/iai.36.1.184-188.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


