Abstract
Maternal infection during pregnancy increases the risk of schizophrenia and other brain disorders of neurodevelopmental origin in the offspring. A multitude of infectious agents seem to be involved in this association. Therefore, it has been proposed that factors common to the immune response to a wide variety of bacterial and viral pathogens may be the critical link between prenatal infection and postnatal brain and behavioral pathology. More specifically, it has been suggested that the maternal induction of pro-inflammatory cytokines may mediate the neurodevelopmental effects of maternal infections. Here, we review recent findings from in vitro and in vivo investigations supporting this hypothesis and further emphasize the influence of enhanced anti-inflammatory cytokine signaling on early brain development. Disruption of the fetal brain balance between pro- and anti-inflammatory cytokine signaling may thus represent a key mechanism involved in the precipitation of schizophrenia-related pathology following prenatal maternal infection and innate immune imbalances.
Keywords: animal model, cytokines, fetus, infection, neurodevelopment, pregnancy, schizophrenia
Introduction
Disturbances directed at the maternal host during pregnancy can lead to direct physiological changes in the fetal environment, thereby influencing the normal course of prenatal brain development.1,2 This may have long-lasting consequences for subsequent brain and behavioral development and lead to the emergence of structural and functional brain abnormalities in adult life. Interference with normal early brain development is also implicated in the etiopathology of severe neuropsychiatric disorders, including schizophrenia.3,4 This disabling brain disorder is marked by impaired thinking, emotions, and behavior and affects approximately 1% of the population worldwide.5 Besides a strong genetic contribution,6 various environmental factors appear to increase the risk for schizophrenia and related disorders.7–9 Many of these factors operate at prenatal stages of life, ie, during the critical periods of central nervous system (CNS) development.
Epidemiological research over the last 2 decades has indicated that the risk for schizophrenia is enhanced in offspring exposed to viral or bacterial infections in utero (for recent reviews see Brown and Susser,10 Brown,11 Fatemi,12 and Patterson13). Following the initial report of increased incidence of schizophrenia after prenatal exposure to influenza virus during the second trimester of human pregnancy,14 many subsequent epidemiological studies have confirmed that maternal influenza infection during pregnancy represents a significant risk factor of schizophrenia in the offspring.15–17 However, because most of these epidemiological reports were based on retrospective research designs, several methodological limitations may have undermined the validity of the conclusions drawn from these reports.10,11 Such limitations may include imprecise measurements of the infectious exposure in the population studied, that is, exposure is defined on the basis of the dates of epidemics in the population or on the maternal recall of infection after pregnancy. This may be one of the reasons why several attempts have failed to find a significant association between maternal influenza infection during pregnancy and a higher incidence of schizophrenia and related disorders in the offspring.18–21
The establishment of prospective approaches has further advanced the research on prenatal environmental risk factors of neurodevelopmental disorders in general, and schizophrenia in particular.22,23 Most importantly, epidemiological studies using prospectively collected and quantifiable measurements have provided serologic evidence that maternal influenza infection during pregnancy increases the offspring's risk of schizophrenia 3- to 7-fold.24 Similarly, epidemiological studies involving clinical examination and serological testing have also confirmed a higher risk of schizophrenia and other psychosis-related disorders following prenatal exposure to rubella25 and toxoplasma gondii 26,27
The Prenatal Cytokine Hypothesis of Schizophrenia
Notably, the association between prenatal infection and higher risk of schizophrenia in the offspring does not appear to be limited to a single infectious pathogen. Besides infection with influenza,14–17,24 rubella,25 and toxoplasma gondii,26,27 a higher incidence of schizophrenia cases has also been reported after prenatal exposure to measles,28 polio,29 herpes simplex,30 and genital and/or reproductive infections.31 One implication is that factors common to the immune response to a multitude of infectious agents may be the critical mediators of the association between prenatal infection and risk of schizophrenia. This has led to the hypothesis that the induction of pro-inflammatory cytokines by the maternal immune system may play a key role in altering early brain development and increasing the risk of schizophrenia and related disorders in the resulting offspring, as first proposed by Gilmore and Jarskog.32
Cytokines are critical mediators of the early defense against a variety of infectious agents. These molecules belong to a class of low–molecular weight proteins secreted by various immune cells and other cell types in response to a number of environmental stimuli.33,34 Cytokines have wide-ranging roles in the innate and adaptive immune systems, where they assist in regulating the recruitment and activation of lymphocytes as well as in controlling immune cell differentiation and homeostasis. In addition, some cytokines possess direct effector mechanisms, including induction of cell apoptosis and inhibition of protein synthesis. Cytokines bind to specific receptors on the membrane of target cells, triggering signal transduction pathways that ultimately alter gene expression in the target cells. These molecules exhibit the attributes of pleiotropy, redundancy, synergy, antagonism, and cascade induction, which permit them to regulate cellular activity in a highly interactive and complex manner.
Different members of the cytokine family can be grouped according to their main production sites and functions in the peripheral immune system.33,34 For example, interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α are often classified as pro-inflammatory cytokines because of their critical roles in the early defense against infection and the initiation and/or progression of inflammation. On the other hand, IL-10 and transforming growth factor (TGF)-β are referred to as anti-inflammatory cytokines. They limit the production and biological activities of many pro-inflammatory molecules and are therefore fundamental to cytokine and immune cell homeostasis.
In the adult CNS, cytokines and their receptors are expressed by glial and neuronal cell types.35,36 In addition, many cytokines and cytokine receptors are constitutively expressed during fetal brain development both in rodents37–41 and in humans,42 suggesting essential roles for these molecules in the regulation and modulation of normal brain development. It is thus expected that abnormal levels of these molecules during critical periods of early brain development may adversely affect neurodevelopmental processes and contribute to a higher susceptibility for complex brain disorders of developmental origin such as schizophrenia.32,43,44
Sources of Fetal Cytokine Imbalances After Maternal Infection
There are several ways how maternal infection during pregnancy can lead to cytokine imbalances in the fetal environment. First, viral or bacterial pathogens invading the maternal host are recognized by peripheral immune cells via binding to various pattern-recognition receptors such as toll-like receptors (TLRs). Activation of TLR signaling leads to the rapid production and release of various cytokines and other mediators of inflammation in the peripheral maternal immune system.45,46 These maternally produced cytokines may then cross the placenta and enter the fetal circulation. However, it is still debatable whether the efficacy of cytokines for transplacental passage is limited to certain cytokine species, gestational stages, and physiological conditions.47 For example, IL-6 crosses the rat placenta in early/middle (gestation day [GD] 11–13) but not in late (GD17–19) gestation.48 Our own findings show that maternal viral-like immune activation in early/middle gestation in the mouse (GD9) elevates fetal brain IL-6 protein levels without concomitant increases in endogenous fetal IL-6 production.41 This suggests that transplacental passage of maternal IL-6 to the fetal system in early/middle gestation may account for increased levels of fetal IL-6 protein. Furthermore, Zaretsky et al49 found that IL-6 readily undergoes transplacental transfer in an ex vivo–isolated human placental perfusion model. Using the same model, IL-1β and TNF-α were shown to display only minimal transplacental transfer.49 Together, this indicates that IL-6 can efficiently cross the placenta in both inflammatory and non inflammatory conditions. TGF-β1 and granulocyte colony-stimulating factor (G-CSF) are 2 other cytokines with known capacity to cross the placenta.50–52 Whether other pro- and anti-inflammatory cytokines, chemokines, and interferons may efficiently cross the placenta is still a matter of debate.49,53–55
Production and secretion of cytokines by the placenta as such may constitute a second source for these molecules in the fetal system. Cytokines are normal regulatory components of the placenta and play an important role in maintaining the integrity of placental structures and functions.47 Cells of the placental barrier, including trophoblasts, uterine epithelial cells, and chorionic villi, express a variety of pattern-recognition receptors such as TLRs.56–60 These cells may thus mount additional cytokine responses upon maternal infection, which in turn may lead to altered cytokine levels in the fetal environment. It is important to notice, however, that there are several relevant distinctions between the rodent and primate placenta, in terms of both structure and function. The latter includes differences in placental endocrine and immunological functions, as well as differences in transplacental transfer of molecules (for a detailed review see Malassiné et al,61 Père,62 Moffett and Loke,63 and Battaglia64). This distinction has to be taken into account when translating experimental findings derived from rodents to the human condition.
Finally, the fetal system itself may be a source of cytokine production and secretion after maternal infection during pregnancy. However, this effect is critically influenced by the precise developmental stage of the fetus. The establishment of a functional immune system requires a sequential series of well-coordinated developmental events that begin early in fetal life.65 In most mammals, the fetal immune system is relatively poorly developed in early/middle pregnancy, and functional maturation is achieved only at late gestational and postnatal stages of development.66 As a consequence, the fetal cytokine reaction to maternal infection is also dependent on the precise gestation stage.41
Effects of Maternal/Fetal Cytokine Imbalances on Brain and Behavioral Development in Experimental Models
A number of animal models have been established in order to test the hypothesis of causality in the epidemiological link between maternal infection during pregnancy and higher risk of schizophrenia in the offspring. Based on the reported association between prenatal influenza infection and adult schizophrenia, Fatemi and colleagues have pioneered an animal model of prenatal exposure to human influenza virus in mice. In a series of experiments, these authors have demonstrated that maternal influenza infection in early/middle pregnancy (GD9 in the mouse species) leads to a variety of neuropathological signs in the offspring's brains postnatally, some of which are implicated in the neuropathology of schizophrenia and autism.67–70 Subsequently, the prenatal influenza model was applied to explore the long-term functional consequences on brain and behavior. These investigations revealed a number of behavioral and pharmacological abnormalities in adult mice born to influenza-infected mothers,71 many of which capture some critical behavioral and pharmacological dysfunctions associated especially with schizophrenia (reviewed in Meyer et al72,73 and Nawa and Takei74).
At least some of the behavioral changes induced by prenatal exposure to influenza virus in mice were likely attributable to the maternal immune response rather than direct viral effects on the developing fetus.71 This is because the virus was not detected in the fetal compartments after maternal infection.75 To test more specifically whether imbalances in maternal and/or fetal cytokines may be the critical mediators in the link between maternal infection and emergence of brain and behavioral pathology, several animal models have since been developed that are based on maternal exposure to cytokine-releasing agents. This includes maternal exposure to the bacterial endotoxin, lipopolysaccharide (LPS), and the synthetic analogue of double-stranded RNA, polyriboinosinic-polyribocytidilic acid (PolyI:C). LPS is recognized by the pattern-recognition receptors TLR2 and TLR4, whereas PolyI:C is recognized primarily by TLR3.76–78 Both immunogens are known to trigger the production and release of many pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, when administered to mammalian organisms.79–81 In addition, PolyI:C is a potent inducer of the type I interferons IFN-α and IFN-β.76,78 Therefore, while LPS exposure leads to a cytokine-associated innate immune response that is typically seen after infection with gram-negative bacteria,77 administration of PolyI:C mimics the acute phase response to viral infection.82
Maternal exposure to LPS and PolyI:C during pregnancy alters pro- and anti-inflammatory cytokine levels in the 3 relevant compartments of the maternal-fetal interface of rodents, namely the placenta, the amniotic fluid, and the fetus, including the fetal brain.41,44,83–87 Prenatal immune activation by LPS or PolyI:C exposure in rodents thus offers a valuable experimental tool to study the long-term consequences of fetal brain inflammation on subsequent brain and behavioral development.
As extensively reviewed elsewhere,72–74 a multitude of behavioral, cognitive, and psychopharmacological abnormalities has been detected in adult mice and rats following the prenatal exposure to LPS88–91 or PolyI:C.41,71,72,87,92–99 Many of the functional deficits are directly linked to some of the most critical endophenotypes of schizophrenia and other psychosis-related disorders.72,73,100,101 This includes impairments in prepulse inhibition102 and latent inhibition,103,104 enhanced sensitivity to dopamine-stimulating treatment with amphetamine105,106 or to NMDA-receptor blockade by dizocilpine,107 and working memory deficiency.108 Furthermore, the neuropathological effects of prenatal PolyI:C exposure in mice identified so far41,97,98,109 indicate that this model can also mimic some of the neuroanatomical and/or neurochemical abnormalities associated with schizophrenia, including disruption of hippocampal and prefrontal cortical GABAergic markers,110–114 reduced expression of the NMDA-receptor subunit NR1,115 and dopamine D1 receptors116 in the hippocampus and prefrontal cortex, respectively, as well as impaired postnatal neurogenesis.117 As outlined before, these effects emerge without concomitant signs of gross brain morphological changes, cell necrosis, or reactive gliosis in the preadolescent41 and adult brain.109 This is in agreement with the hypothesis that neurodevelopmental rather than neurodegenerative disturbances are central to the etiopathogenesis and disease process of schizophrenia.3,4
The long-term effects of prenatal PolyI:C exposure in rodents also mimic the characteristic maturational delay in disease onset of schizophrenia3,4 because the full spectrum of prenatal PolyI:C–induced behavioral, cognitive, and pharmacological abnormalities emerges only after the postpubertal stage of development.93,96,97,99 Notably, this dependency on postpubertal maturational processes is distinct from other prenatally acquired brain diseases with prenatal infectious etiologies, including autism spectrum disorders118 and mental retardation.119 Therefore, prenatal immune activation in rodents may be particularly suitable for the study of etiopathological and pathophysiological processes implicated in schizophrenia.
Importantly, our recent cross-fostering studies have provided evidence that at least some of the functional and structural brain abnormalities emerging after prenatal PolyI:C–induced immune challenge are likely to be attributable to prenatal but not postnatal maternal effects on the offspring.96,97 This is because multiple brain and behavioral abnormalities can emerge after prenatal PolyI:C exposure regardless of whether neonates are cross-fostered to immune-challenged or to control surrogate mothers.96,97 Putative changes in maternal behavior resulting from immunological stress during pregnancy are thus unlikely to mediate the major long-term behavioral effects of prenatal immune activation. Hence, inflammation-induced disruption of fetal brain development can predispose the offspring to the emergence of psychopathology in later life.
Relevance of Cytokine Specificity
Similar to their immunological roles in the peripheral immune system,33,34 distinct classes of cytokines may exert differing neurodevelopmental effects in the CNS. Support for this possibility is provided by numerous findings from in vitro culture systems. For example, among the variety of pro- and anti-inflammatory cytokines, IL-1β is the most capable in inducing the conversion of rat mesencephalic progenitor cells into a dopaminergic phenotype120,121 and IL-6 is highly efficacious in decreasing the survival of fetal brain serotonin neurons.122 In contrast, IL-1β and IL-6 (and to a lesser extent TNF-α) appear to have an equivalent capacity to negatively regulate the survival of fetal midbrain dopaminergic neurons at low to medium concentrations,122 whereas the same cytokines can promote survival of these cells at higher concentrations.123,124 A similar dependency on cytokine specificity and/or concentration has also been found in a recent in vitro study by Gilmore et al,40 who have demonstrated that TNF-α can disrupt cortical neuron's dendrite development at low concentration, while the same effects can be achieved by exposure of fetal cortical neurons to higher concentrations of IL-1β, IL-6, or TNF-α.
Recent findings from in vivo animal experimentation further support the hypothesis that cytokine specificity may play a critical role in the relationship between prenatal immune challenge and emergence of psychopathology and neuropathology in later life. We have recently compared the neuropathological consequences of prenatal immune activation by the viral mimic PolyI:C in wild-type mice and transgenic mice constitutively overexpressing IL-10 in macrophages.87 As already mentioned before, IL-10 is a cytokine with strong anti-inflammatory and immunosuppressive functions. It limits production and/or secretion and biological activities of many inflammatory molecules in both acute and chronic conditions.125,126 If the emergence of postnatal brain abnormalities after prenatal infection and/or inflammation were indeed accounted for by enhanced levels of pro-inflammatory cytokines in the fetal brain,32 then one would expect that enhanced expression of IL-10 at the maternal-fetal interface may attenuate the long-term consequences of prenatal exposure to pro-inflammatory cytokines.
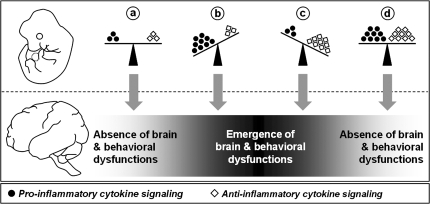
Our results confirmed this prediction by showing that enhanced levels of the anti-inflammatory cytokine IL-10 during prenatal development is sufficient to prevent the emergence of multiple behavioral and pharmacological abnormalities in the adult offspring after prenatal immune challenge by PolyI:C.87 However, in the absence of a discrete prenatal inflammatory stimulus, enhanced levels of IL-10 during prenatal development was also associated with specific behavioral abnormalities in the adult offspring.87 One important implication is that the development of normal adult brain functions may be critically influenced by the precise balance between pro- and anti-inflammatory cytokines in prenatal life (figure 1). According to this scheme, shifts of the balance toward either increased pro-inflammatory or anti-inflammatory cytokine species would precipitate adult behavioral pathology, whereas the concomitant induction of both cytokine classes in the fetal brain may nullify each other's long-term negative influences on brain and behavioral development (figure 1). This explanation is consistent with the antagonistic properties of distinct cytokine classes in the peripheral immune system33,126 and suggests that the effects of cytokines on early neurodevelopmental processes crucially depend on their specificity.
Fig. 1.
Hypothesized Model of the Modulation of Adult Brain and Behavioral Functions by Imbalances in Fetal Brain Cytokines. The pro-inflammatory and anti-inflammatory classes of cytokines are represented by black dots and white diamonds, respectively. (a) Brain and behavioral development is under the influence of a balance between pro- and anti-inflammatory cytokines in prenatal life. (b) A shift toward excess levels of pro-inflammatory cytokines (eg, by in utero exposure to infection) leads to the emergence of severe brain and behavioral abnormalities in adult life. (c) A shift toward enhanced anti-inflammatory signaling alone (eg, by genetically determined increases in IL-10 production) also precipitates adult brain and behavioral dysfunctions. (d) The concomitant induction of both pro- and anti-inflammatory cytokines (eg, after in utero infections in individuals with enhanced IL-10–mediated anti-inflammatory signaling) largely nullifies each other's long-term negative influences on brain and behavioral functions because the balance between the 2 cytokine classes is maintained. For a detailed discussion of the model see Meyer et al.87
To date, there is no direct evidence that other anti-inflammatory cytokines such as TGF-β may have similar protective effects against prenatal infection–induced brain and behavioral dysfunctions. However, because many anti-inflammatory and immunosuppressive molecules share similar signal transduction pathways,126,127 it may be expected that in addition to IL-10, enhanced expression of other anti-inflammatory cytokines may also be effective in attenuating or blocking the negative influences of prenatal immune challenge on normal brain and behavioral development.
Further evidence that the fetal system is highly sensitive to specific species of cytokines comes from a recent study by Smith et al,128 who examined several pro-inflammatory cytokines including IL-β, IL-6, IFN-γ, and TNF-α as potential mediators of the effects of maternal immune challenge on brain and behavioral development in the murine species. These authors demonstrated for the first time that prenatal administration with exogenous IL-6 can mimic the long-term consequences of prenatal PolyI:C exposure.128 Thus, adult mice born to mothers having been treated with IL-6 during mid-pregnancy displayed several behavioral abnormalities similar to offspring born to PolyI:C-treated mothers. Moreover, when IL-6 was eliminated from the maternal immune response by genetic interventions or with IL-6 blocking antibodies, maternal PolyI:C treatment during pregnancy was no longer efficient in inducing behavioral maldevelopment in the resulting offspring.128 Interestingly, prenatal exposure to IL-1β, IFN-γ, or TNF-α alone was insufficient to precipitate similar behavioral deficits in the adult animals, and co-administration of soluble IL-1β or IFN-γ receptor antagonist to pregnant dams did not prevent the behavioral deficits caused by prenatal PolyI:C exposure. This further supports the hypothesis that the relationship between prenatal immune activation and postnatal brain dysfunctions is critically dependent on the specificity of cytokine-associated immunological reactions. Specifically, it appears that the pro-inflammatory cytokine IL-6 assumes a key role in mediating the effects of maternal immune activation on fetal brain development.128
Interaction Between Cytokine Specificity and Fetal Developmental Windows
It is well known that the responsiveness and/or sensitivity of developing cells to many signaling cues, including cytokines, can vary considerably as neurodevelopment progresses. For example, while TNF-α is neurotrophic to dopaminergic ventral mesencephalic neurons during early fetal development, the same molecule can exert neurotoxic effects on these cells at later stages of fetal brain development.129 Similarly, embryonic cells cultured as progenitor neurospheres proliferate more robustly in response to basic fibroblast growth factor (bFGF) than to epidermal growth factor (EGF), whereas proliferation of postnatal and adult progenitor cells is enhanced more effectively by EGF than bFGF.130 In the context of maternal infection during pregnancy, this highlights that the eventual neurodevelopmental impact of abnormal maternal/fetal cytokine expression is likely to be determined also by the precise stage of brain development.
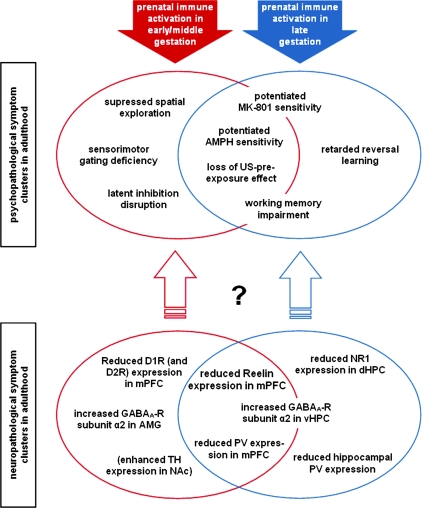
We have recently conducted a series of experiments designed to evaluate the influence of the timing of maternal immune challenge on the emergence of brain and behavioral dysfunctions in the resulting offspring.41,95,98 These experiments demonstrated that the precise times of prenatal immune activation is a critical determinant of the specificity of both the structural and the functional brain abnormalities in later life (for a recent review see Meyer et al73). We found that maternal immunological stimulation at different gestational times precipitates distinct psychopathological and neuropathological symptom clusters in the offspring (figure 2). Prenatal PolyI:C–induced immune challenge in early/middle gestation (GD9 in the mouse) leads to a pathological profile characterized by suppression in exploratory behavior,41,72 abnormalities in selective associative learning in the form of latent inhibition disruption, and abolition of the unconditioned stimulus (US) pre-exposure effect,72,95 impairments in sensorimotor gating in the form of reduced prepulse inhibition72,98 (see also Shi et al71 and Romero et al90), enhanced sensitivity to the indirect dopamine receptor agonist amphetamine and (to a lesser extent) the noncompetitive NMDA-receptor antagonist dizocilpine,97,98 as well as deficiency in spatial working memory when the demand on temporal retention is high.72 On the other hand, prenatal immune challenge in late gestation (GD17 in the mouse species) leads to a partially overlapping symptom profile involving the emergence of perseverative behavior in the form of retarded reversal learning,41 spatial working memory impairments even when the demand on temporal retention is low,98 potentiated response to amphetamine and dizocilpine,98 and abolition of the US pre-exposure effect95 (figure 2). Hence, some of the identified pathological traits are clearly restricted to the symptom cluster associated with prenatal immune activation in early/middle or late gestation (eg, latent inhibition deficiency and retardation in reversal learning), whereas others are common to both symptom clusters (eg, potentiation of amphetamine sensitivity; see figure 2). This suggests that the developmental vulnerability of specific forms of postnatal brain dysfunction to prenatal exposure to cytokine-releasing agents varies across different gestational stages.73
Fig. 2.
Emergence of Distinct Psychopathological and Neuropathological Symptom Clusters in Adulthood After Prenatal Polyriboinosinic-Polyribocytidilic Acid (PolyI:C)–Induced Immune Activation in Early/Middle (Red) and Late (Blue) Gestation in the Mouse. The diagram illustrates the identified structural and functional brain abnormalities that are characteristic of the symptom profiles associated with prenatal PolyI:C exposure in early/middle and late gestation. Some of the psychopathological and neuropathological traits are clearly restricted to the symptom cluster associated with prenatal immune activation in early/middle or late gestation, whereas others are common to both symptom clusters. The exact correspondence between the distinct neuropathological and psychopathological symptom clusters remains to be determined. The neuropathological effects presented in brackets denote that they were studied only after prenatal PolyI:C–induced immune activation in early/middle gestation so far. AMG, amygdala; AMPH, amphetamine; D1R/D2R, dopamine D1/D2 receptor; dHPC, dorsal hippocampus; GABAA-R, gamma-amino-butyric acid (A) receptor; mPFC, medial prefrontal cortex; MK-801, dizocilpine; NAc, nucleus accumbens; PV, Parvalbumin; TH, tyrosine hydroxylase; US, unconditioned stimulus; vHPC, ventral hippocampus.
According to one hypothesis, the temporal dependency of the link between prenatal immune challenge and postnatal brain pathology may be explained by infection-mediated interference with specific spatiotemporal events in the course of fetal brain development.73 Prenatal immune activation at distinct times of gestation may thus lead to dissociable long-term psychopathology and neuropathology because it affects distinct neurodevelopmental programs in fetal life.73
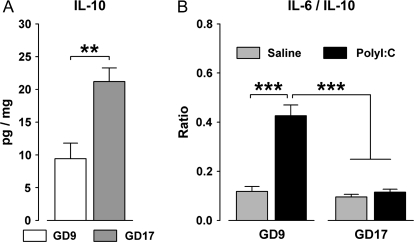
However, the precise cytokine-associated inflammatory response to infection in both the maternal and the fetal compartments is influenced by the precise stage of gestation.131,132 For example, pregnant rats in late gestation display a diminished pro-inflammatory cytokine response to infection with the bacterial endotoxin LPS compared with pregnant rats in middle gestation.133 Rats near the term of pregnancy also display an attenuated febrile response to exogenous pyrogen, which may result from an increased production of antipyretic/cryogenic cytokines such as the soluble IL-1 receptor antagonist (sIL-1ra).134,135 This raises the possibility that the dissociable long-term brain and behavioral effects of prenatal immune challenge at distinct gestational times73 may be accounted for by differences in cytokine specificity in the fetal brain. This idea is also in keeping with the hypothesis that the association between prenatal immune challenge (in early/middle gestation) and the emergence of behavioral dysfunctions in adulthood is critically dependent on the precise pro-inflammatory vs anti-inflammatory cytokine reactions taking place at the maternal-fetal interface87,128 (see also figure 1). For example, the basal fetal brain levels of the anti-inflammatory cytokine IL-10 increase as prenatal development progresses (figure 3A). Maternal exposure to acute immunological stimulation is thus less efficient in shifting the fetal brain balance toward increased pro-inflammatory cytokines in late gestation compared with early/middle gestation (figure 3B). As a consequence, the fetal brain in late gestation may be less susceptible to the disrupting effects of high levels of pro-inflammatory cytokines after maternal infection because of enhanced anti-inflammatory signaling by IL-10. Further investigations are therefore clearly warranted in order to evaluate whether the identified differences between the long-term brain and behavioral effects of prenatal immune activation at distinct gestational times (figure 2) may be attributable to variations in the specificity/intensity of the cytokine-associated immunological reactions, the neurodevelopmental stage of the fetus, or a combination of both.
Fig. 3.
(A) Basal Protein Levels (pg/mg) of the Anti-Inflammatory Cytokine IL-10 Are Increased in the Fetal Brains of Mice Derived From Pregnant Mothers in Late Gestation (Gestation Day 17, GD17) Compared With Fetuses of Mothers in Early/Middle Gestation (GD9). Pregnant mice were killed by decapitation, and the fetal brains were dissected as described in Meyer et al.41,72 Levels of IL-10 were measured using a multiplexed particle–based flow cytometric cytokine as described previously.41,72 **P < .01, based on one-way analysis of variance (ANOVA) [F(1,21) = 13.8]; N(GD9 fetuses) = 10, N(GD17 fetuses) = 13. Fetuses derived from 4 mothers in each gestation period. (B) Acute maternal immunological stimulation by the viral mimic polyriboinosinic-polyribocytidilic acid (PolyI:C) (2 mg/kg, intravenously) increases the fetal brain ratio of IL-6/IL-10 in fetuses derived from mothers in early/middle gestation (GD9), but not in fetuses of mothers in late gestation (GD17). The former effect indicates a shift in the fetal brain balance toward increased pro-inflammatory cytokines in early/middle gestation. Pregnant mice were killed by decapitation 2 h after maternal vehicle (saline) or PolyI:C treatment, and the fetal brains were dissected as described previously.41,72 Levels of IL-6 and IL-10 were measured using a multiplexed particle–based flow cytometric cytokine assay as described in Meyer et al.41,72 ***P < .01, based on Fisher's post hoc comparisons following a significant gestation day × maternal treatment interaction in the 2 × 2 ANOVA [F(1,36) = 17.1, P < .001]; N(GD9-saline fetuses) = 8, N(GD9-PolyI:C fetuses) = 7, N(GD17-saline fetuses) = 13, N(GD17-PolyI:C fetuses) = 12. Fetuses derived from 3 mothers in each gestation period/treatment condition.
The issue as to whether there may be a time window with maximal vulnerability for prenatal infection–induced abnormalities in brain and behavioral development in humans remains a matter of debate. Many of the initial retrospective epidemiological studies found a significant association between maternal viral infection during pregnancy and a higher incidence of schizophrenia in the progeny only when the maternal host was infected in the second trimester of human pregnancy.14–17 However, several findings from epidemiological studies using prospectively collected and quantifiable serologic samples indicate that there has been a somewhat excessive emphasis on second trimester infections. For example, there is serologic evidence that influenza infection in early gestation (ie, in the first trimester of human pregnancy) is associated with the highest risk for schizophrenia in the offspring.24 This thus challenges the prevailing view that influenza infection during the second trimester of pregnancy may confer the maximal risk for the offspring to develop schizophrenia and related disorders in adulthood.14–17 In addition, there is evidence that periconceptional infections can also increase the risk of schizophrenia in the offspring,31 pointing to effects on early fetal brain development similar to what has been implicated in the association between prenatal exposure to rubella and increased risk for schizophrenia.25 As highlighted above, the findings from our recent experimental examinations of the critical time window hypothesis in a mouse model of prenatal immune activation by the viral mimic PolyI:C suggest that prenatal infection in both early/middle and late pregnancy is efficacious in causing subsequent brain abnormalities.41,73,95,98 Importantly, this animal model indicates that prenatal immune challenge in early/middle gestation (GD9) may have a more extensive impact on brain and behavioral symptom profiles relevant to schizophrenia in comparison with infection occurring in late gestation (GD17).73 Early/middle pregnancy (GD9) and late pregnancy (GD17) in the mouse roughly correspond to the middle/end of the first trimester and to the middle of the second trimester of human pregnancy, respectively, with respect to developmental biology and percentage of gestation from mice to human.136,137 Hence, the experimental data obtained in mouse models of prenatal immune challenge added further weight to the hypothesis that the first rather than the second trimester of human pregnancy may be a time window with maximal vulnerability for prenatal infection–induced interference with normal brain development and subsequent risk for schizophrenia in later life.
Alternative Mechanisms to Fetal Brain Cytokine Imbalances
Numerous experimental findings underline the importance of abnormal fetal expression of cytokines in the precipitation of neurodevelopmental defects relevant to the emergence of schizophrenia-like brain and behavioral pathology. However, there are several alternative (but not mutually exclusive) mechanisms whereby prenatal exposure to infection can bring about changes in brain and behavioral development.
First, exposure to infection and the subsequent cytokine-associated inflammatory reactions leads to the activation of the hypothalamic-pituitary-adrenal (HPA) axis by stimulating the release of corticotropin-releasing factor from the hypothalamus and of adrenocorticotropic hormone from the pituitary gland; this results eventually in an increase of glucocorticoid levels in the peripheral bloodstream.138 The cytokine-mediated effects on glucocorticoid secretion may be of special interest because it has been suggested that prenatal physiological stress triggered by high glucocorticoid levels can interfere with fetal neurodevelopment as well as the subsequent functioning of the HPA axis in adults.139,140
Second, it is well established that peripheral cytokine elevation in response to infection induces a set of behavioral and physiological changes collectively referred to as sickness behavior.141 In rodents, sickness behavior typically includes fever, malaise, and reduced exploratory and social investigation, as well as decreased food and water intake, accompanied usually by weight loss. The effects of immune challenge on weight loss may be particularly relevant because prenatal malnutrition has also been implicated as a risk factor in schizophrenia,142,143 and recent animal models have highlighted the effects of prenatal protein deprivation upon adult brain functions.144
Third, even though there is considerable evidence that many of the reported brain and behavioral dysfunctions emerging after prenatal exposure to infection are likely attributable to indirect effects via changes in maternal/fetal immunological parameters such as cytokines, certain infectious pathogens may exert their detrimental effects on fetal brain development directly by gaining access to the fetal brain. For example, it has been well documented that rubella virus can cross the placenta and enter the fetal brain.12,145 Besides induction of inflammatory responses, rubella viruses may induce a variety of other effects that are detrimental to normal fetal brain development, including inhibition of mitosis and myelination.146,147 In addition to rubella virus, specific influenza virus strains may undergo transplacental transfer. For example, infection of pregnant mice with the influenza A/WSN/33 strain results in the invasion and persistence of the virus in the offspring's brain both at prenatal and at postnatal stages of life.148 Offspring born to influenza A/WSN/33-infected mothers have been shown to display abnormal gene expression patterns in the CNS,149 indicating that direct viral effects of the offspring may contribute to the emergence of adult neuropathology following prenatal viral infection. In contrast, influenza virus of the A/NWS/33 strain does not cross the placenta and enter the fetal circulation following maternal infection.75 Despite the lack of transplacental transfer, the offspring born to A/NWS/33-infected mothers show schizophrenia-like behavioral and pharmacological abnormalities when they reach adulthood.71 This clearly illustrates that the strain and/or type of the infectious pathogen is also a critical factor in the association between prenatal exposure to infection and emergence of schizophrenia-related brain and behavioral abnormalities in the offspring.
Gene-Environment Interactions and Epigenetic Factors
It is now widely accepted that schizophrenia results from aberrations in neurodevelopmental processes caused by an interaction between environmental and genetic factors preceding the onset of clinical symptoms.4 A variety of susceptibility genes for schizophrenia have already been identified.6 However, it remains largely unknown to date how genetic and environmental factors may interact with each other to modulate the vulnerability for this disorder.150 Notably, most offspring having been exposed to prenatal infection do not develop schizophrenia in humans. This suggests that if in utero exposure to infection plays a role in the etiology of this disorder, then it probably does so by interacting with other factors, including genetically determined factors. Therefore, elucidating the contribution of the genetic background would be highly relevant in the study of the prenatal infectious etiologies of schizophrenia and related disorders.
The combination of prenatal immune activation models with genetic animal models of schizophrenia151,152 may represent a fruitful approach to identify critical interactions between genetic- and infection-associated environmental factors, and to evaluate their modulatory influence on the vulnerability for schizophrenia-like brain disorders. As outlined in detail before, most of the current animal models of prenatal immune activation have been designed to mimic, in an accentuated fashion, the specific immunological events associated with an infection, leading to robust alterations in brain and behavior relevant to schizophrenia. However, these models can be modified in such a way that the maternal infectious manipulation is less severe and thus only leads to a restricted pathological phenotype in the offspring.72 This modification would allow studying how genetic and prenatal infectious factors may interact to increase or reduce the vulnerability for schizophrenia-like brain abnormalities. One clear possibility to study such gene-environment interactions in experimental models of prenatal immune activation would be to explore and compare the effects of prenatal infection in wild-type animals and genetically modified animals.
One may first consider examinating the impact of genes that are directly involved in innate and acquired immunity. Some of the identified genetic risk factors of schizophrenia include promoter polymorphisms of pro-inflammatory153,154 and anti-inflammatory cytokines,155 as well as human leukocyte antigens and alleles.156 The precise immune-related genetic background of the maternal host may influence the liability to certain infections, or result in an excessive or inappropriate inflammatory response in the maternal periphery and thereafter in the fetal system. This may in turn determine the impact of prenatal infection on early neurodevelopmental processes and subsequent brain and behavioral development. Recent investigations in mice designed to examine immunological gene-environment interactions have already provided evidence that the association between prenatal immune challenge and emergence of schizophrenia-like behavioral and pharmacological dysfunctions is critically influenced by the anti-inflammatory87 and pro-inflammatory128 genetic background of the infected host.
Another model worth considering is the examination of genes that have been identified as major genetic susceptibility factors of schizophrenia, including neuregulin-1(NRG1), catechol-O-methyltransferase (COMT), and disrupted in schizophrenia-1 (DISC1)6. It is likely that many of these genes as such play only minor roles in infectious or inflammatory processes. Nevertheless, disruption of neurodevelopmental mechanisms by abnormal expression of these genes may act synergistically with prenatal infection/inflammation to increase the risk for schizophrenia.
In addition to gene-environment interactions, there is a growing interest in the role of epigenetic mechanisms in the pathophysiology of schizophrenia and related disorders.157–159 Epigenetic regulation of gene expression refers to mechanisms that modulate gene activity without altering the DNA code, and this includes DNA methylation, histone modifications, and other chromatin-remodeling events. Such epigenetic mechanisms are now recognized to be crucially involved in many neurodevelopmental processes and adult brain functions.160 Recent evidence suggests that cytokines can also exert multiple influences on epigenetic mechanisms involved in the development of the CNS, including regulation of the epigenetic control of neural stem cell differentiation and fetal astrocyte development.161,162 This highlights the possibility that abnormal maternal/fetal cytokine expression may also affect critical epigenetic mechanism involved in normal early brain development, thereby contributing to neurodevelopmental disturbances following prenatal exposure to infection. However, this hypothesis has not yet been substantiated by direct experimental evidence and thus needs to be subjected to further examination.
Conclusions and Outlook
There is considerable evidence derived from in vitro and in vivo experimentation supporting the hypothesis that cytokine imbalances at the maternal-fetal interface may be critically involved in mediating the effects of prenatal infection on brain and behavioral development, thereby increasing the risk of brain disorders of neurodevelopmental origin such as schizophrenia. Specifically, the disruption of the cytokine balance in the fetal brain toward excess pro-inflammatory cytokines leads to multiple behavioral abnormalities in later life, whereas blocking specific pro-inflammatory cytokines or enhancing anti-inflammatory cytokine signaling during acute maternal immune challenge attenuates the effects of prenatal infection and/or inflammation on fetal brain development. In addition, abnormal brain and behavioral development can also occur following a shift toward excess anti-inflammatory cytokines as such in the fetal brain. This suggests that relative shifts between distinct cytokine classes may determine the ultimate neurodevelopmental impact of these molecules in prenatal infectious conditions and innate immune imbalances. Furthermore, intricate interactions between specific cytokine abnormalities and fetal developmental windows need to be taken into consideration in order to identify the various neurodevelopmental effects attributed to pro- and anti-inflammatory cytokine species.
Undoubtedly, disruption of the fetal brain cytokine balance may not readily account for all cases of schizophrenia. However, this mechanism may be particularly relevant for individuals developing the disorder following in utero exposure to infection and in individuals with innate immune imbalances. Within this context, disruption of the fetal brain cytokine balance may represent an integrative mechanism of how maternal infection during pregnancy can negatively influence normal fetal neurodevelopment and predispose the organism to long-lasting changes in subsequent brain maturation and behavioral development.
Several important issues still remain to be addressed in future experimental analyses of the association between maternal infection during pregnancy and higher risk of schizophrenia in the offspring. Specifically, it will be highly relevant to (1) explore the effects of maternal infection during pregnancy on mal-development of the CNS in the offspring at both prenatal and postnatal stages of life; (2) investigate the nature and extent of potential gene-environment interactions in the link between prenatal immune challenge and emergence of postnatal brain dysfunction; (3) evaluate whether imbalances in maternal/fetal cytokine expression may precipitate abnormal epigenetic regulation of genes critically involved in early brain development; (4) investigate the efficacy of preconceptional maternal vaccination to reduce the susceptibility to prenatal infection–induced abnormalities in brain and behavioral development in the offspring; and (5) to establish and evaluate early preventive strategies in order to reduce the risk of brain disorders following in utero exposure to infection, including acute anti-inflammatory interventions in pregnant mothers exposed to infection and therapeutic interventions in postnatal life in order to halt or limit the disease process in the offspring. For technical and ethical reasons, experimental investigation of these issues in humans is essentially impossible. Therefore, animal models of prenatal immune activation will be indispensable for experimental analyses of neuroimmunological and pathophysiological mechanisms underlying the link between prenatal infection and postnatal brain dysfunctions, as well as for the exploration of preventive interventions designed to reduce the incidence of severe brain disorders following prenatal exposure to infection.
Funding
Swiss Federal Institute of Technology (ETH) Zurich; Swiss National Science Foundation; National Centre of Competence in research: Neural Plasticity and Repair, funded by the Swiss National Science Foundation.
Acknowledgments
We are extremely grateful to Dr Andrea Engler for running the cytokine assays (figure 3) and to Natalie Aeschbach-Jones for her editorial support.
References
- 1.Rees S, Harding R. Brain development during fetal life: influences of the intra-uterine environment. Neurosci Lett. 2004;361:111–114. doi: 10.1016/j.neulet.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Rees S, Inder T. Fetal and neonatal origins of altered brain development. Early Hum Dev. 2005;81:753–761. doi: 10.1016/j.earlhumdev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 4.Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- 5.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 7.McDonald C, Murray RM. Early and late environmental factors for schizophrenia. Brain Res Rev. 2000;31:130–137. doi: 10.1016/s0165-0173(99)00030-2. [DOI] [PubMed] [Google Scholar]
- 8.Dean K, Murray RM. Environmental risk factors for psychosis. Dialogues Clin Neurosci. 2005;7:69–80. doi: 10.31887/DCNS.2005.7.1/kdean. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opler MG, Susser ES. Fetal environment and schizophrenia. Environ Health Perspect. 2005;113:1239–1242. doi: 10.1289/ehp.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown AS, Susser ES. In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev. 2002;8:51–57. doi: 10.1002/mrdd.10004. [DOI] [PubMed] [Google Scholar]
- 11.Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fatemi SH. Neuropsychiatric Disorders and Infection. London, UK: Martin Dunitz-Taylor & Francis Group; 2005. [Google Scholar]
- 13.Patterson PH. Neuroscience. Maternal effects on schizophrenia risk. Science. 2007;318:576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- 14.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 15.Wright P, Takei N, Rifkin L, Murray RM. Maternal influenza, obstetric complications, and schizophrenia. Am J Psychiatry. 1995;152:1714–1720. doi: 10.1176/ajp.152.12.1714. [DOI] [PubMed] [Google Scholar]
- 16.Stöber G, Franzek E, Beckmann H, Schmidtke A. Exposure to prenatal infections, genetics and the risk of systematic and periodic catatonia. J Neural Transm. 2002;109:921–929. doi: 10.1007/s007020200075. [DOI] [PubMed] [Google Scholar]
- 17.Limosin F, Rouillon F, Payan C, Cohen JM, Strub N. Prenatal exposure to influenza as a risk factor for adult schizophrenia. Acta Psychiatr Scand. 2003;107:331–335. doi: 10.1034/j.1600-0447.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 18.Crow TJ, Done DJ. Prenatal exposure to influenza does not cause schizophrenia. Br J Psychiatry. 1992;161:390–393. doi: 10.1192/bjp.161.3.390. [DOI] [PubMed] [Google Scholar]
- 19.Selten JP, Slaets JP. Evidence against maternal influenza as a risk factor for schizophrenia. Br J Psychiatry. 1994;164:674–676. doi: 10.1192/bjp.164.5.674. [DOI] [PubMed] [Google Scholar]
- 20.Morgan V, Castle D, Page A, et al. Influenza epidemics and incidence of schizophrenia, affective disorders and mental retardation in Western Australia: no evidence of a major effect. Schizophr Res. 1997;26:25–39. doi: 10.1016/S0920-9964(97)00033-9. [DOI] [PubMed] [Google Scholar]
- 21.Mino Y, Oshima I, Tsuda T, Okagami K. No relationship between schizophrenic birth and influenza epidemics in Japan. J Psychiatr Res. 2000;34:133–138. doi: 10.1016/s0022-3956(00)00003-0. [DOI] [PubMed] [Google Scholar]
- 22.Susser ES, Schaefer CA, Brown AS, Begg MD, Wyatt RJ. The design of the prenatal determinants of schizophrenia study. Schizophr Bull. 2000;26:257–273. doi: 10.1093/oxfordjournals.schbul.a033451. [DOI] [PubMed] [Google Scholar]
- 23.Brown AS, Schaefer CA, Wyatt RJ, et al. Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophr Bull. 2000;26:287–295. doi: 10.1093/oxfordjournals.schbul.a033453. [DOI] [PubMed] [Google Scholar]
- 24.Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 25.Brown AS, Cohen P, Harkavy-Friedman J, et al. A.E. Bennett Research Award. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry. 2001;49:473–486. doi: 10.1016/s0006-3223(01)01068-x. [DOI] [PubMed] [Google Scholar]
- 26.Brown AS, Schaefer CA, Quesenberry CP, Jr, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- 27.Mortensen PB, Nørgaard-Pedersen B, Waltoft BL, et al. Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biol Psychiatry. 2007;61:688–693. doi: 10.1016/j.biopsych.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 28.Torrey EF, Rawlings R, Waldman IN. Schizophrenic births and viral diseases in two states. Schizophr Res. 1988;1:73–77. doi: 10.1016/0920-9964(88)90043-6. [DOI] [PubMed] [Google Scholar]
- 29.Suvisaari J, Haukka J, Tanskanen A, Hovi T, Lonnqvist J. Association between prenatal exposure to poliovirus infection and adult schizophrenia. Am J Psychiatry. 1999;156:1100–1102. doi: 10.1176/ajp.156.7.1100. [DOI] [PubMed] [Google Scholar]
- 30.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58:1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- 31.Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am J Psychiatry. 2006;163:927–929. doi: 10.1176/ajp.2006.163.5.927. [DOI] [PubMed] [Google Scholar]
- 32.Gilmore JH, Jarskog LF. Exposure to infection and brain development: cytokines in the pathogenesis of schizophrenia. Schizophr Res. 1997;24:365–367. doi: 10.1016/s0920-9964(96)00123-5. [DOI] [PubMed] [Google Scholar]
- 33.Curfs JH, Meis JF, Hoogkamp-Korstanje JA. A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev. 1997;10:742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borish LC, Steinke JW. Cytokines and chemokines. J Allergy Clin Immunol. 2003;111:S460–S475. doi: 10.1067/mai.2003.108. [DOI] [PubMed] [Google Scholar]
- 35.Hopkins SJ, Rothwell NJ. Cytokines and the nervous system. I: expression and recognition. Trends Neurosci. 1995;18:83–88. [PubMed] [Google Scholar]
- 36.Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9:259–275. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 37.Burns TM, Clough JA, Klein RM, Wood GW, Berman NE. Developmental regulation of cytokine expression in the mouse brain. Growth Factors. 1993;9:253–258. doi: 10.3109/08977199308991585. [DOI] [PubMed] [Google Scholar]
- 38.Pousset F. Developmental expression of cytokine genes in the cortex and hippocampus of the rat central nervous system. Dev Brain Res. 1994;81:143–146. doi: 10.1016/0165-3806(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 39.Mehler MF, Kessler JA. Hematolymphopoietic and inflammatory cytokines in neural development. Trends Neurosci. 1997;20:357–365. doi: 10.1016/s0166-2236(96)01045-4. [DOI] [PubMed] [Google Scholar]
- 40.Gilmore JH, Jarskog LF, Vadlamudi S, Lauder JM. Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology. 2004;29:1221–1229. doi: 10.1038/sj.npp.1300446. [DOI] [PubMed] [Google Scholar]
- 41.Meyer U, Nyffeler M, Engler A, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mousa A, Seiger A, Kjaeldgaard A, Bakhiet M. Human first trimester forebrain cells express genes for inflammatory and anti-inflammatory cytokines. Cytokine. 1999;11:55–60. doi: 10.1006/cyto.1998.0381. [DOI] [PubMed] [Google Scholar]
- 43.Patterson PH. Maternal infection: window on neuroimmune interactions in fetal brain development and mental illness. Curr Opin Neurobiol. 2002;12:115–118. doi: 10.1016/s0959-4388(02)00299-4. [DOI] [PubMed] [Google Scholar]
- 44.Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11:47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto M, Funami K, Oshiumi H, Seya T. Toll-like receptor 3: a link between toll-like receptor, interferon and viruses. Microbiol Immunol. 2004;48:147–154. doi: 10.1111/j.1348-0421.2004.tb03500.x. [DOI] [PubMed] [Google Scholar]
- 46.Kawai T, Akira S. Innate immune recognition of viral infection. Nature Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 47.Jonakait GM. The effects of maternal inflammation on neuronal development: possible mechanisms. Int J Dev Neurosci. 2007;25:415–425. doi: 10.1016/j.ijdevneu.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Dahlgren J, Samuelsson AM, Jansson T, Holmäng A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res. 2006;60:147–151. doi: 10.1203/01.pdr.0000230026.74139.18. [DOI] [PubMed] [Google Scholar]
- 49.Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 2004;103:546–550. doi: 10.1097/01.AOG.0000114980.40445.83. [DOI] [PubMed] [Google Scholar]
- 50.Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. Maternal rescue of transforming growth factor-beta 1 null mice. Science. 1994;264:1936–1938. doi: 10.1126/science.8009224. [DOI] [PubMed] [Google Scholar]
- 51.Lennard SN, Stewart F, Allen WR. Transforming growth factor beta 1 expression in the endometrium of the mare during placentation. Mol Reprod Dev. 1995;42:131–140. doi: 10.1002/mrd.1080420202. [DOI] [PubMed] [Google Scholar]
- 52.Calhoun DA, Gersting JA, Lunøe M, Du Y, Christensen RD. Transfer of recombinant human granulocyte colony stimulating factor (rhG-CSF) from the maternal to the fetal circulation is not dependent upon a functional G-CSF-receptor. Placenta. 2001;22:609–612. doi: 10.1053/plac.2001.0682. [DOI] [PubMed] [Google Scholar]
- 53.Waysbort A, Giroux M, Mansat V, Teixeira M, Dumas JC, Puel J. Experimental study of transplacental passage of alpha interferon by two assay techniques. Antimicrob Agents Chemother. 1993;37:1232–1237. doi: 10.1128/aac.37.6.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reisenberger K, Egarter C, Vogl S, Sternberger B, Kiss H, Husslein P. The transfer of interleukin-8 across the human placenta perfused in vitro. Obstet Gynecol. 1996;87:613–616. doi: 10.1016/0029-7844(95)00473-4. [DOI] [PubMed] [Google Scholar]
- 55.Aaltonen R, Heikkinen T, Hakala K, Laine K, Alanen A. Transfer of proinflammatory cytokines across term placenta. Obstet Gynecol. 2005;106:802–807. doi: 10.1097/01.AOG.0000178750.84837.ed. [DOI] [PubMed] [Google Scholar]
- 56.Holmlund U, Cebers G, Dahlfors AR, et al. Expression and regulation of the pattern recognition receptors toll-like receptor-2 and toll-like receptor-4 in the human placenta. Immunology. 2002;107:145–151. doi: 10.1046/j.1365-2567.2002.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428–436. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 59.Abrahams VM, Mor G. Toll-like receptors and their role in the trophoblast. Placenta. 2005;26:540–547. doi: 10.1016/j.placenta.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 60.Krikun G, Lockwood CJ, Abrahams VM, Mor G, Paidas M, Guller S. Expression of toll-like receptors in the human decidua. Histol Histopathol. 2007;22:847–854. doi: 10.14670/HH-22.847. [DOI] [PubMed] [Google Scholar]
- 61.Malassiné A, Frendo JL, Evain-Brion D. A comparison of placental development and endocrine functions between the human and mouse model. Hum Reprod Update. 2003;9:531–539. doi: 10.1093/humupd/dmg043. [DOI] [PubMed] [Google Scholar]
- 62.Père MC. Materno-foetal exchanges and utilisation of nutrients by the foetus: comparison between species. Reprod Nutr Dev. 2003;43:1–15. doi: 10.1051/rnd:2003002. [DOI] [PubMed] [Google Scholar]
- 63.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 64.Battaglia FC. Placental transport: a function of permeability and perfusion. Am J Clin Nutr. 2007;85:591S–597S. doi: 10.1093/ajcn/85.2.591S. [DOI] [PubMed] [Google Scholar]
- 65.Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. 2000;55:688–697. doi: 10.1034/j.1398-9995.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 66.Holsapple MP, West LJ, Landreth KS. Species comparison of anatomical and functional immune system development. Birth Defects Res B Dev Reprod Toxicol. 2003;68:321–334. doi: 10.1002/bdrb.10035. [DOI] [PubMed] [Google Scholar]
- 67.Fatemi SH, Sidwell R, Akhter P, et al. Human influenza viral infection in utero increases nNOS expression in hippocampi of neonatal mice. Synapse. 1998;9:84–88. doi: 10.1002/(SICI)1098-2396(199805)29:1<84::AID-SYN8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 68.Fatemi SH, Sidwell R, Kist D, et al. Differential expression of synaptosome-associated protein 25 kDa [SNAP-25] in hippocampi of neonatal mice following exposure to human influenza virus in utero. Brain Res. 1998;800:1–9. doi: 10.1016/s0006-8993(98)00450-8. [DOI] [PubMed] [Google Scholar]
- 69.Fatemi SH, Emamian ES, Kist D, et al. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry. 1999;4:145–154. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- 70.Fatemi SH, Earle J, Kanodia R, et al. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 73.Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13:241–256. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- 74.Nawa H, Takei N. Recent progress in animal modeling of immune inflammatory processes in schizophrenia: implication of specific cytokines. Neurosci Res. 2006;56:2–13. doi: 10.1016/j.neures.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 75.Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int J Dev Neurosci. 2005;23:299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 76.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kB by toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 77.Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23:301–304. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- 78.Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol Rev. 2007;220:214–224. doi: 10.1111/j.1600-065X.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 79.Kimura M, Toth LA, Agostini H, Cady AB, Majde JA, Krueger JM. Comparison of acute phase responses induced in rabbits by lipopolysaccharide and double-stranded RNA. Am J Physiol. 1994;267:R1596–R605. doi: 10.1152/ajpregu.1994.267.6.R1596. [DOI] [PubMed] [Google Scholar]
- 80.Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2004;287:R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- 81.Cunningham C, Campion S, Teeling J, Felton L, Perry VH. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C) Brain Behav Immun. 2007;21:490–502. doi: 10.1016/j.bbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 82.Traynor TR, Majde JA, Bohnet SG, Krueger JM. Intratracheal double-stranded RNA plus interferon-gamma: a model for analysis of the acute phase response to respiratory viral infections. Life Sci. 2004;74:2563–2576. doi: 10.1016/j.lfs.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 83.Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- 84.Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 85.Gayle DA, Beloosesky R, Desai M, Amidi F, Nuñez SE, Ross MG. Maternal LPS induces cytokines in the amniotic fluid and corticotropin releasing hormone in the fetal rat brain. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1024–R1029. doi: 10.1152/ajpregu.00664.2003. [DOI] [PubMed] [Google Scholar]
- 86.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 87.Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry. 2008;13:208–221. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- 88.Borrell J, Vela JM, Arévalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats: implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;6:204–221. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- 89.Fortier ME, Joober R, Luheshi GN, Boksa P. Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. J Psychiatr Res. 2004;38:335–345. doi: 10.1016/j.jpsychires.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 91.Romero E, Ali C, Molina-Holgado E, Castellano B, Guaza C, Borrell J. Neurobehavioral and immunological consequences of prenatal immune activation in rats. Influence of antipsychotics. Neuropsychopharmacology. 2007;32:1791–1804. doi: 10.1038/sj.npp.1301292. [DOI] [PubMed] [Google Scholar]
- 92.Zuckerman L, Weiner I. Post-pubertal emergence of disrupted latent inhibition following prenatal immune activation. Psychopharmacology. 2003;169:308–313. doi: 10.1007/s00213-003-1461-7. [DOI] [PubMed] [Google Scholar]
- 93.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- 94.Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 95.Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun. 2006;20:378–388. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 96.Meyer U, Schwendener S, Feldon J, Yee BK. Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Exp Brain Res. 2006;173:243–257. doi: 10.1007/s00221-006-0419-5. [DOI] [PubMed] [Google Scholar]
- 97.Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology. 2008;33:441–456. doi: 10.1038/sj.npp.1301413. [DOI] [PubMed] [Google Scholar]
- 98.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2007.09.012. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 99.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 100.Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 101.Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 102.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 103.Weiner I. The “two-headed” latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology. 2003;169:257–297. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- 104.Lubow RE. Construct validity of the animal latent inhibition model of selective attention deficits in schizophrenia. Schizophr Bull. 2005;31:139–153. doi: 10.1093/schbul/sbi005. [DOI] [PubMed] [Google Scholar]
- 105.Laruelle M, Abi-Dargham A, van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 107.Lahti AC, Weiler MA, Michaelidis BAT, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- 108.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 109.Nyffeler M, Meyer U, Yee BK, Feldon J, Knuesel I. Maternal immune activation during pregnancy increases limbic GABA-A receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience. 2006;143:51–62. doi: 10.1016/j.neuroscience.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 110.Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;11:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- 111.Hashimoto T, Volk DW, Eggan SM, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hashimoto T, Arion D, Unger T, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fatemi SH. Reelin glycoprotein: structure, biology and roles in health and disease. Mol Psychiatry. 2005;10:251–257. doi: 10.1038/sj.mp.4001613. [DOI] [PubMed] [Google Scholar]
- 114.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 115.Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- 116.Okubo Y, Suhara T, Suzuki K, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 117.Reif A, Fritzen S, Finger M, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 118.Courchesne E, Pierce K, Schumann CM, et al. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 119.Dierssen M, Ramakers GJ. Dendritic pathology in mental retardation: from molecular genetics to neurobiology. Genes Brain Behav. 2006;5(suppl 2):48–60. doi: 10.1111/j.1601-183X.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 120.Ling ZD, Potter ED, Lipton JW, Carvey PM. Differentiation of mesencephalic progenitor cells into dopaminergic neurons by cytokines. Exp Neurol. 1998;149:411–423. doi: 10.1006/exnr.1998.6715. [DOI] [PubMed] [Google Scholar]
- 121.Potter ED, Ling ZD, Carvey PM. Cytokine-induced conversion of mesencephalic-derived progenitor cells into dopamine neurons. Cell Tissue Res. 1999;296:235–246. doi: 10.1007/s004410051285. [DOI] [PubMed] [Google Scholar]
- 122.Jarskog LF, Xiao H, Wilkie MB, Lauder JM, Gilmore JH. Cytokine regulation of embryonic rat dopamine and serotonin neuronal survival in vitro. Int J Dev Neurosci. 1997;15:711–776. doi: 10.1016/s0736-5748(97)00029-4. [DOI] [PubMed] [Google Scholar]
- 123.Kushima Y, Hama T, Hatanaka H. Interleukin-6 as a neurotrophic factor for promoting the survival of cultured catecholaminergic neurons in a chemically defined medium from fetal and postnatal rat midbrains. Neurosci Res. 1992;13:267–280. doi: 10.1016/0168-0102(92)90039-f. [DOI] [PubMed] [Google Scholar]
- 124.Akaneya Y, Takahashi M, Hatanaka H. Interleukin-1 beta enhances survival and interleukin-6 protects against MPP+ neurotoxicity in cultures of fetal rat dopaminergic neurons. Exp Neurol. 1995;136:44–52. doi: 10.1006/exnr.1995.1082. [DOI] [PubMed] [Google Scholar]
- 125.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 126.Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 127.Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002;13:413–421. doi: 10.1016/s1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 128.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Doherty GH. Developmental switch in the effects of TNFalpha on ventral midbrain dopaminergic neurons. Neurosci Res. 2007;57:296–305. doi: 10.1016/j.neures.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 130.Zhu G, Mehler MF, Mabie PC, Kessler JA. Developmental changes in progenitor cell responsiveness to cytokines. J Neurosci Res. 1999;56:131–145. doi: 10.1002/(sici)1097-4547(19990415)56:2<131::aid-jnr3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 131.Sargent IL. Maternal and fetal immune responses during pregnancy. Exp Clin Immunogenet. 1993;10:85–102. [PubMed] [Google Scholar]
- 132.Entrican G. Immune regulation during pregnancy and host-pathogen interactions in infectious abortion. J Comp Pathol. 2002;126:79–94. doi: 10.1053/jcpa.2001.0539. [DOI] [PubMed] [Google Scholar]
- 133.Simrose RL, Fewell JE. Body temperature response to IL-1 beta in pregnant rats. Am J Physiol. 1995;269(5 pt 2):R1179–R1182. doi: 10.1152/ajpregu.1995.269.5.R1179. [DOI] [PubMed] [Google Scholar]
- 134.Fofie AE, Fewell JE. Influence of pregnancy on plasma cytokines and the febrile response to intraperitoneal administration of bacterial endotoxin in rats. Exp Physiol. 2003;88:747–754. doi: 10.1113/eph8802594. [DOI] [PubMed] [Google Scholar]
- 135.Fofie AE, Fewell JE, Moore SL. Pregnancy influences the plasma cytokine response to intraperitoneal administration of bacterial endotoxin in rats. Exp Physiol. 2005;90:95–101. doi: 10.1113/expphysiol.2004.028613. [DOI] [PubMed] [Google Scholar]
- 136.Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- 137.Kaufman MH. The Atlas of Mouse Development. London, UK: Academic Press; 2003. [Google Scholar]
- 138.Haddad JJ, Saadé NE, Safieh-Garabedian B. Cytokines and the neuro-immune-endocrine interactions: a role for the hypothalamic–pituitary–adrenal revolving axis. J Neuroimmunol. 2002;133:1–19. doi: 10.1016/s0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- 139.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;2:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 140.Koenig JI, Kirkpatrick B, Lee P. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology. 2002;27:309–318. doi: 10.1016/S0893-133X(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 141.Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 142.Susser E, Neugebauer R, Hoek HW, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 143.Brown AS, Susser ES, Butler PD, Andrews RR, Kaufmann CA, Gorman JM. Neurobiological plausibility of prenatal nutritional deprivation as a risk factor for schizophrenia. J Nerv Ment Dis. 1996;184:71–85. doi: 10.1097/00005053-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 144.Palmer AA, Printz DJ, Butler PD, Dulawa SC, Printz MP. Prenatal protein deprivation in rats induces changes in prepulse inhibition and NMDA receptor binding. Brain Res. 2004;996:193–201. doi: 10.1016/j.brainres.2003.09.077. [DOI] [PubMed] [Google Scholar]
- 145.Whitley RJ, Stagno S. Perinatal infections. In: Scheld WM, Whitley RJ, Durack DT, editors. Infections of the Central Nervous System. 2nd ed. New York, NY: Lippincott-Raven Press; 1997. pp. 223–253. [Google Scholar]
- 146.Boué JG, Boué A. Effects of rubella virus infection on the division of human cells. Am J Dis Child. 1969;118:45–48. doi: 10.1001/archpedi.1969.02100040047008. [DOI] [PubMed] [Google Scholar]
- 147.Rorke LB. Nervous system lesions in the congenital rubella syndrome. Arch Otolaryngol. 1973;98:249–251. doi: 10.1001/archotol.1973.00780020259007. [DOI] [PubMed] [Google Scholar]
- 148.Aronsson F, Lannebo C, Paucar M, Brask J, Kristensson K, Karlsson H. Persistence of viral RNA in the brain of offspring to mice infected with influenza A/WSN/33 virus during pregnancy. J Neurovirol. 2002;8:353–357. doi: 10.1080/13550280290100480. [DOI] [PubMed] [Google Scholar]
- 149.Asp L, Beraki S, Aronsson F, et al. Gene expression changes in brains of mice exposed to a maternal virus infection. Neuroreport. 2005;16:1111–1115. doi: 10.1097/00001756-200507130-00016. [DOI] [PubMed] [Google Scholar]
- 150.Tsuang MT, Bar JL, Stone WS, Faraone SV. Gene-environment interactions in mental disorders. World Psychiatry. 2004;3:73–83. [PMC free article] [PubMed] [Google Scholar]
- 151.Chen J, Lipska BK, Weinberger DR. Genetic mouse models of schizophrenia: from hypothesis-based to susceptibility gene-based models. Biol Psychiatry. 2006;59:1180–1188. doi: 10.1016/j.biopsych.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 152.O'Tuathaigh CM, Babovic D, O'Meara G, Clifford JJ, Croke DT, Waddington JL. Susceptibility genes for schizophrenia: characterisation of mutant mouse models at the level of phenotypic behaviour. Neurosci Biobehav Rev. 2007;31:60–78. doi: 10.1016/j.neubiorev.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 153.Boin F, Zanardini R, Pioli R, Altamura CA, Maes M, Gennarelli M. Association between -G308A tumor necrosis factor alpha gene polymorphism and schizophrenia. Mol Psychiatry. 2001;6:79–82. doi: 10.1038/sj.mp.4000815. [DOI] [PubMed] [Google Scholar]
- 154.Zanardini R, Bocchio-Chiavetto L, Scassellati C, et al. Association between IL-1beta -511C/T and IL-1RA (86bp)n repeats polymorphisms and schizophrenia. J Psychiatr Res. 2003;37:457–462. doi: 10.1016/s0022-3956(03)00072-4. [DOI] [PubMed] [Google Scholar]
- 155.Chiavetto LB, Boin F, Zanardini R, et al. Association between promoter polymorphic haplotypes of interleukin-10 gene and schizophrenia. Biol Psychiatry. 2002;51:480–484. doi: 10.1016/s0006-3223(01)01324-5. [DOI] [PubMed] [Google Scholar]
- 156.Wright P, Nimgaonkar VL, Donaldson PT, Murray RM. Schizophrenia and HLA: a review. Schizophr Res. 2001;47:1–12. doi: 10.1016/s0920-9964(00)00022-0. [DOI] [PubMed] [Google Scholar]
- 157.Dong E, Agis-Balboa RC, Simonini MV, Grayson DR, Costa E, Guidotti A. Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia. Proc Natl Acad Sci U S A. 2005;102(35):12578–12583. doi: 10.1073/pnas.0505394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 159.Veldic M, Kadriu B, Maloku E, et al. Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizophr Res. 2007;91:51–61. doi: 10.1016/j.schres.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Feng J, Fouse S, Fan G. Epigenetic regulation of neural gene expression and neuronal function. Pediatr Res. 2007;61:58R–63R. doi: 10.1203/pdr.0b013e3180457635. [DOI] [PubMed] [Google Scholar]
- 161.Takizawa T, Nakashima K, Namihira M, et al. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev Cell. 2001;1:749–758. doi: 10.1016/s1534-5807(01)00101-0. [DOI] [PubMed] [Google Scholar]
- 162.Taga T, Fukuda S. Role of IL-6 in the neural stem cell differentiation. Clin Rev Allergy Immunol. 2005;28:249–256. doi: 10.1385/CRIAI:28:3:249. [DOI] [PubMed] [Google Scholar]