Abstract
The essential Caenorhabditis elegans gene rfl-1 encodes one subunit of a heterodimeric E1-activating enzyme in the Nedd8 ubiquitin-like protein conjugation pathway. This pathway modifies the Cullin scaffolds of E3 ubiquitin ligases with a single Nedd8 moiety to promote ligase function. To identify genes that influence neddylation, we used a synthetic screen to identify genes that, when depleted with RNAi, enhance or suppress the embryonic lethality caused by or198ts, a temperature-sensitive (ts) mutation in rfl-1. We identified reproducible suppressor and enhancer genes and employed a systematic specificity analysis for each modifier using four unrelated ts embryonic lethal mutants. Results of this analysis highlight the importance of specificity controls in identifying genetic interactions relevant to a particular biological process because 8/14 enhancers and 7/21 suppressors modified lethality in other mutants. Depletion of the strongest specific suppressors rescued the early embryonic cell division defects in rfl-1(or198ts) mutants. RNAi knockdown of some specific suppressors partially restored Cullin neddylation in rfl-1(or198ts) mutants, consistent with their gene products normally opposing neddylation, and GFP fusions to several suppressors were detected in the cytoplasm or the nucleus, similar in pattern to Nedd8 conjugation pathway components in early embryonic cells. In contrast, depletion of the two strongest specific enhancers did not affect the early embryonic cell division defects observed in rfl-1(or198ts) mutants, suggesting that they may act at later times in other essential processes. Many of the specific modifiers are conserved in other organisms, and most are nonessential. Thus, when controlled properly for specificity, modifier screens using conditionally lethal C. elegans mutants can identify roles for nonessential but conserved genes in essential processes.
UBIQUITIN-mediated proteolysis regulates many biological processes (Nandi et al. 2006). In the early Caenorhabditis elegans embryo, these include oocyte maturation, cell cycle progression, cell polarization, and cell fate patterning, all of which require the timely destruction of maternally expressed proteins (Bowerman and Kurz 2006; Greenstein and Lee 2006). One C. elegans protein targeted for proteolysis early in embryogenesis is MEI-1, the AAA-ATPase subunit of the microtubule-severing complex called katanin (Mains et al. 1990; Dow and Mains 1998; Srayko et al. 2000; Kurz et al. 2002; Pintard et al. 2003a; Xu et al. 2003). Katanin is a heterodimer of two subunits called p60 and p80 in vertebrates and MEI-1 and MEI-2 in C. elegans. Katanin in C. elegans is required for proper assembly and function of the small, barrel-shaped meiotic spindles (Albertson and Thomson 1993; McNally et al. 2006) and must be degraded after meiotic divisions to permit assembly of the much larger first mitotic spindle in the one-cell zygote. In mutants that fail to degrade katanin after the completion of meiosis, the first mitotic spindle is fragmented and mis-oriented, cytokinesis is defective, and the embryos die without hatching (Dow and Mains 1998; Srayko et al. 2000; Kurz et al. 2002).
The katanin subunit MEI-1 is targeted for poly-ubiquitylation and proteolytic destruction by a Cullin-based E3 ligase (Kurz et al. 2002). This complex includes the Cullin scaffolding protein CUL-3 and a substrate-specific adaptor called MEL-26 that binds to CUL-3 through a BTB domain and to MEI-1 through a MATH domain (Pintard et al. 2003b). Cullin 3-based E3 ligases in mammals also utilize substrate-specific adaptor proteins that, like MEL-26, have both a Cullin-binding BTB/POZ domain and another protein–protein interaction domain that binds to the substrate (Geyer et al. 2003; Cullinan et al. 2004; Angers et al. 2006). While MEI-1/Katanin downregulation by the CUL-3/MEL-26 E3 ligase is essential at most growth temperatures, a mel-26 null mutation is viable at the low growth temperature of 15° (Lu and Mains 2007). This bypass of mel-26 at 15° depends at least in part on the anaphase-promoting complex and its targeting of MEI-1 for proteolytic degradation (Lu and Mains 2007). Phosphorylation by the kinase MBK-2 primes MEI-1 for proteolysis (Quintin et al. 2003; Stitzel et al. 2007) and also promotes the downregulation of MEI-1 by the anaphase-promoting complex (Lu and Mains 2007).
CUL-3 is the only C. elegans Cullin thus far identified that requires modification by the ubiquitin-like protein Nedd8 (Bowerman and Kurz 2006). In contrast, C. elegans CUL-2 is required for progression through meiosis and for the localized degradation of cell fate determinants in one-cell-stage embryos (Liu et al. 2004; Sonneville and Gonczy 2004), but neddylation-defective mutants do not exhibit these early defects (Bowerman and Kurz 2006). Cullin neddylation is mediated by the Nedd8 protein conjugation pathway, which begins with a heterodimeric E1-activating enzyme consisting of ULA-1 and RFL-1 (Uba3p in budding yeast) and also includes the E2-conjugating enzyme UBC-12 (Jones and Candido 2000; Srayko et al. 2000; Kurz et al. 2002) and the E3 ligase DCN-1 (Kurz et al. 2005).
The downregulation of MEI-1/katanin by the CUL-3/MEL-26 E3 ligase requires a balance of both CUL-3 neddylation, which is mediated by the Nedd8 conjugation pathway, and deneddylation, which is mediated by the conserved COP-9 Signalosome (Pintard et al. 2003a). Other Cullin-based E3 ubiquitin ligases also require a balance of neddylation and deneddylation (Lyapina et al. 2001; Schwechheimer et al. 2001; Bornstein et al. 2006; Hetfeld et al. 2008). Deneddylation may modulate activation of the E3 ligase and thereby prevent the premature degradation of substrate adaptor proteins that also can become poly-ubiquitylated and degraded as a result of E3 ligase function.
To identify additional factors that influence neddylation, and the downregulation of MEI-1/katanin after the completion of meiosis in C. elegans, we report here our use of RNA interference (RNAi) to reduce gene functions in a temperature-sensitive (ts) neddylation-defective mutant, rfl-1(or198ts). The discovery of RNAi and its systemic properties in C. elegans have made it possible to systematically target C. elegans genes for depletion by feeding worms bacterial strains that express double-strand RNAs corresponding to C. elegans gene sequences (Fire et al. 1998; Timmons et al. 2001; Feinberg and Hunter 2003; Baugh et al. 2005; Lehner et al. 2006; van Haaften et al. 2006). Furthermore, chemical mutagenesis screens have identified temperature-sensitive mutations in many essential C. elegans genes, which can be used for synthetic screens by choosing intermediate-growth temperatures that sensitize the genetic background and also optimize visual scoring of embryonic viability. Recently, genomewide RNAi screens have been used to identify C. elegans genes that, when reduced in function, restore viability to temperature-sensitive, embryonic-lethal mutants (Labbe et al. 2006; O'Rourke et al. 2007). Because a loss of suppressor function restores mutant viability, the suppressors may negatively regulate either the wild-type gene product or the process that requires the wild-type gene product.
Here we report our identification of C. elegans genes that, when reduced in function by feeding RNAi, reproducibly suppressed or enhanced rfl-1(or198ts) embryonic lethality. Most suppressors were specific for rfl-1(or198ts), while specific enhancement was less common. Many of the rfl-1-specific suppressors and enhancers are conserved but appear nonessential. GFP fusions to several specific suppressors exhibit localization patterns that resemble those known for neddylation pathway components, and depletion of some of these partially restored CUL-3 neddylation in rfl-1(or198ts) mutants. In addition to identifying possible roles for conserved genes in cullin neddylation, we report the first quantitative analysis of specificity for both the enhancement and the suppression of a conditionally lethal mutant in C. elegans. Our results highlight the importance of testing genetic modifiers of conditionally lethal mutants for locus specificity.
MATERIALS AND METHODS
C.elegans strains and culture:
Strains were cultured according to standard procedures (Brenner 1974). Temperature-sensitive mutants were maintained at 15°, and GFP-expressing strains were maintained at room temperature. Isolation of transgenic worms was performed with the microparticle bombardment method as previously described (Praitis 2006; O'Rourke et al. 2007).
RNAi screening and quantification of embryonic viability:
Methods used for RNAi screening and quantification of embryonic viability in this study were those described in detail in O'Rourke et al. (2007), with the following modifications. For scoring enhancement, genes that previously had been identified as lethal when targeted by RNAi, as reported in large-scale screens, were not given candidate enhancer status (Kamath and Ahringer 2003; Simmer et al. 2003) To quantify embryonic viability of embryos at 23.5°, the broods of 7–10 gravid adult worms were analyzed. For all quantitative analysis, experiments were repeated at least four times and the average viability was determined. For enhancement, we report the percentage of dead embryos (using the average of the replicates). For suppression, we report the percentage of viable larvae, calculated again by taking the average of the replicates. The total number of progeny counted, the percentage viability or lethality, and the standard deviations used to generate Figure 3 and data for wild-type (N2 strain) embryonic viability are included in the supporting information, Table S1.
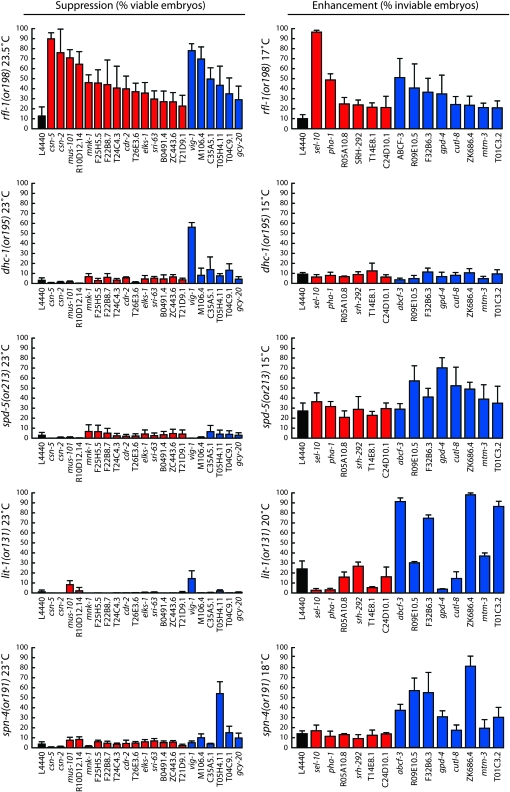
Figure 3.—
Specificity of modifiers for rfl-1(or198ts) embryonic lethality. (Left) Percentage embryonic viability (hatching) for suppression. (Right) Percentage embryonic lethality for enhancement. Identity of conditionally lethal mutations and growth temperatures are shown at the left of the y-axes. Blue bars indicate nonspecific modifiers; red bars indicate specific modifiers.
Embryo extract preparation and immunoblotting:
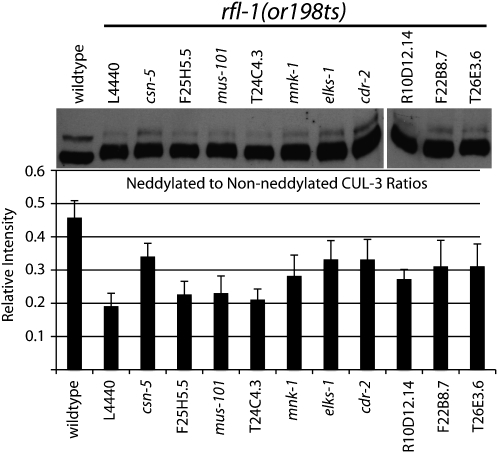
For Western blotting analysis of CUL-3 neddylation, worms were raised at 15° until the young adult stage and then shifted to 26° for 5 hr before embryo extract preparation. Embryo extracts were prepared by hypochlorite treatment of young adult worms following feeding RNAi. After bleaching, embryos were washed several times in M9 buffer and then snap frozen in liquid nitrogen. Embryos were then resuspended in 1 volume of Laemmli 3X sample buffer and boiled for 5 min at 95°. Samples were then refrozen in liquid nitrogen and briefly boiled once again before sample loading. Standard procedures were used for SDS–PAGE and Western blotting. Rabbit anti-CUL-3 (Pintard et al. 2003a) was used at a 1:2000 dilution in 4% milk and Tris-buffered saline plus 0.02% Tween-20. Anti-Rabbit secondary antibodies conjugated to peroxidase (Santa Cruz Biotechnology) were used at a concentration of 1:5000. Densitometry measurements of bands were carried out in Adobe Photoshop CS3. Integrated intensity was determined by multiplying the mean intensity (or average gray value) in each band, in a scanned and inverted image, by the number of pixels in the band. Integrated intensity of the neddylated band was divided by the integrated intensity of the non-neddylated band to determine relative intensity ratios. The graph in Figure 5 shows the average relative intensity of three separate exposures, and the error bars indicate ±SD.
Figure 5.—
Neddylated CUL-3 levels in suppressed rfl-1(or198ts) embryo extracts. Embryo extracts were prepared from rfl-1(or198ts) embryos and wild-type embryos following a 5-hr shift to 26° and then loaded on an SDS–PAGE gel. After transferring the proteins to a membrane, the membrane was probed with an affinity-purified CUL-3 antibody. Lower band of ∼82 kDa corresponds to the un-neddylated CUL-3, and upper band of ∼85 kDa corresponds to the neddylated CUL-3. Some enrichment of the neddylated CUL-3 band, relative to the un-neddylated band, is seen in seven of the samples. The bar graph shows the ratio of the integrated intensity of the neddylated CUL-3 band to the non-neddylated CUL-3 band (labeled as relative intensity) for each of the samples (see materials and methods).
Molecular biology:
For all pie-1 driven, N-terminal GFP constructs (rfl-1 suppressors), genes were amplified using Pfu Turbo polymerase (Stratagene) from a cDNA library (Invitrogen), with the exception of R10D12.14, which was amplified from N2 genomic DNA. PCR products were subsequently ligated into pGEM-T or pGEM-T-easy shuttle vectors (Promega). Inserted genes were sequenced at the University of Oregon sequencing facility prior to cleavage and ligation into pSO26 (pSO26 is described in O'Rourke et al. (2007).
To construct N-terminal GFP and tdTomato (Shaner et al. 2004) fusions for sel-10, C24D10.1, and rfl-1, recombineering was used, as described in protocol #3 available at http://recombineering.ncifcrf.gov/Protocol.asp (Warming et al. 2005). We used the following fosmid clones available from GeneService (http://www.geneservice.co.uk): WRM0610aC12 (sel-10), WRM0633dC (C24D10.1), and WRM066dF09 (rfl-1). We used pSO26 as a template for amplifying GFP (O'Rourke et al. 2007). For the gap repair step, we used pPUB (Sarov et al. 2006) and designed primers to allow for inclusion of DNA sequence up to the next open reading frame (start or stop codon) from the gene of interest. To construct the N-terminal tdTomato∷RFL-1 fusion, we used the pAA64 Vector to amplify the fluorescent protein-encoding gene. At least two independent transgenic lines for each construct were examined, and a representative line is shown in Figures 6 and 7. All fusion proteins are expressed from extrachromosomal arrays as determined by segregation analysis.
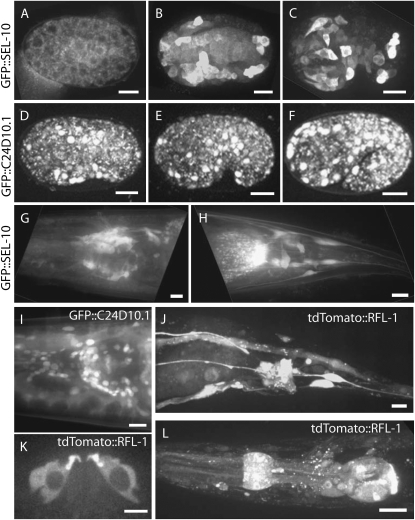
Figure 6.—
Expression of pie-1 promoter-driven GFP fusions to suppressor-encoded proteins in early embryos from transgenic strains (see materials and methods). Frames are from single optical sections at the indicated cell cycle stage from spinning-disk confocal time-lapse videomicrographs (see materials and methods). Bar, 10 μm.
Figure 7.—
Spinning-disk confocal projected Z-stack images of GFP and tdTomato fusion proteins driven by endogenous promoters (see materials and methods). GFP∷SEL-10 expression (A–C, G, H). (A) Approximately 50-cell stage embryos. (B) Bean-stage embryos. (C) Comma-stage embryos. (G) Expression in head and (H) expression in tail neurons of adult worms. GFP∷C24D10.1 expression (D–F, I). Bean stage (D), comma stage (E), 1.5-fold stage embryos (F), and head of adult around pharynx (I). (J–L) tdTomato∷RFL-1. (J) Tail neurons and intestine in adults. (K) Vulval VulB2 cells in adult. (L) Larval head. Anterior is to the left. Bars, 10 μm.
GFP imaging:
Imaging of GFP and tdTomato fusion protein localization was done by mounting embryos or whole worms on M9 + 3% agarose pads on microscope slides and overlayed with a coverslip. Time-lapse videos were obtained on a spinning disk Leica DMI 4000B microscope using a Leica 63X/1.40-0.60 HCX Plan Apo oil objective, fitted with a Hamamatsu EM-CCD Digital Camera. Images in Figure 7 are projected stacks that include optical sections through the entire embryo or worm at a spacing of 0.5 μm for embryos and of 1 μm for worms. Images of GFP∷C24D10.1 in Figure 7, D–F, were taken with a Leica 40X/1.25-0.75 Plan Apo oil objective, and all other images were taken with the Leica 63X described above. Data were recorded using Velocity software and videos, and images were adjusted for contrast in ImageJ and adjusted for levels with Adobe Photoshop. ImageJ was used to obtain pixel intensity ratios of GFP∷RFL-1 in nuclei and cytoplasm of unenhanced images (Figure S1). Measurements were taken by selecting a circular area slightly smaller than the nuclei and obtaining the average intensity values for each nuclei compared to the average intensity values for a region of the cytoplasm. Background pixel intensities from the area surrounding the embryo were subtracted from nuclei and cytoplasmic values.
DIC imaging:
Cellular phenotypic analysis for suppression was performed by incubating rfl-1(or198ts) L1 stage larvae at 20°, which was followed by a shift to 23° once the larvae reached the L4 stage (∼60 hr at 20°). The incubation of rfl-1(or198ts) mutants at 20° through L4 stage allowed for slightly increased brood size and a decrease in the number of sterile adults. To image the embryos at a controlled temperature, we utilized a temperature-controlled microscope stage, equipped with an HEC-400 heat exchanger (20/20 Technology) and a BC-110 bionomic controller (20/20 Technology). To calibrate the apparatus, we used an Omega HH12 temperature probe to obtain the temperature of a M9 + 3% agarose pad placed on a sapphire inset metallic microscope slide (20/20 Technology) while on the microscope stage with the light source on. We adjusted the Bionomic controller until a temperature of 23° was achieved. Use of the temperature-controlled stage prevented optimal focus of the condenser; DIC image quality was reduced but sufficient to score phenotypes.
Following incubation at 23° for 12-hr embryos were mounted on microscope slides and immediately transferred to the microscope stage. Images were recorded every 5 sec using a Dage MT1 VE1000 digital camera and Scion Image or ImageJ software. Contrast and levels were adjusted using Adobe Photoshop. Spindle angle measurements were done using the ImageJ angle tool. Spindle angles at cytokinesis were measured 1 min (12 frames) following the first appearance of cytokinetic furrow. The same procedure was used to image embryos for enhancement of cellular phenotypes except incubation of mutant worms were grown at a constant temperature throughout development (17°, 18°, or 20°). The microscope stage temperature was calibrated to these lower temperatures for imaging as described above.
RESULTS
A sensitized genetic background:
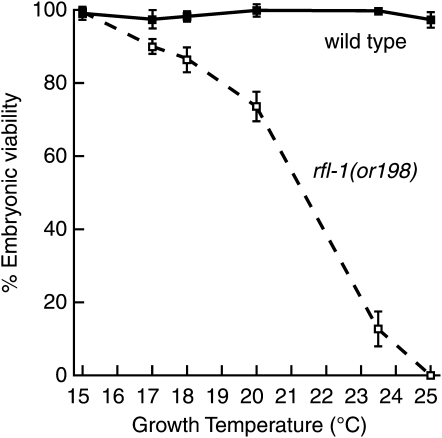
To identify semipermissive temperatures that would optimize our ability to identify enhancers and suppressors of rfl-1(or198ts) embryonic lethality, we examined the viability of embryos produced by homozygous rfl-1(or198ts) hermaphrodite worms raised to adulthood at six different temperatures ranging from 15° to 25° (Figure 1; see materials and methods). We chose 18° (86.7% hatching) to screen for enhancers of embryonic lethality and 23.5° (12.7% hatching) to screen for suppressors. These growth conditions optimized our ability to detect modifiers by initially scoring qualitatively for changes in embryonic lethality by using stereomicroscopes and 48-well agar plates with worm cultures (see materials and methods). At temperatures ≥24°, most rfl-1(or198ts) larvae matured into either sterile or fertile adults with ruptured vulvae and produced very few or no progeny (data not shown), precluding use of higher temperatures to achieve a lower background level of embryonic hatching for suppressor screening.
Figure 1.—
Temperature vs. embryonic viability for rfl-1(or198ts) and wild type (N2). L1 larvae were raised to adulthood at the indicated temperatures on L4440 empty-vector control bacteria. Progeny were scored as hatched larvae or dead embryos (n > 200 except for 25° where n = 7 was due to sterile phenotypes at this temperature; see materials and methods).
Quantifying embryonic viability to identify reproducible enhancers and suppressors:
To screen for modifiers, we used an RNAi feeding library of 16,757 bacterial strains, each capable of inducibly expressing double-stranded RNA corresponding to C. elegans gene sequences, although some of the bacterial strains produce dsRNAs that correspond to two genes due to updated gene annotations (Kamath and Ahringer 2003). We screened a total of 14,045 genes for suppression and 8192 genes for enhancement of rfl-1(or198ts) embryonic lethality after the mutant larvae matured into adults while feeding on the dsRNA-expressing bacterial strains at the semipermissive temperatures. Our initial qualitative screen yielded 248 candidate enhancers and 388 candidate suppressors. We then consolidated and systematically rescreened each candidate enhancer and suppressor dsRNA using the same qualitative scoring method, reducing the number of candidate enhancers and suppressors to 101 and 104, respectively (see O'Rourke et al. 2007 for a description of the qualitative screening method).
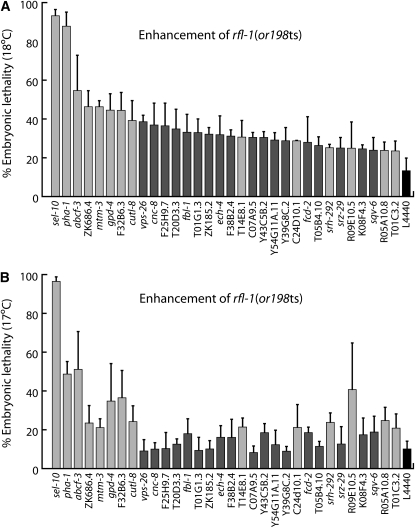
To quantify suppression and enhancement, we compared the embryonic viability of broods from enhanced or suppressed young adults and from control young adults raised to adulthood on bacteria carrying the feeding RNAi vector without an insert (hereafter referred to as empty-vector control broods). Thirty-two of the candidate enhancer genes, when reduced in function by RNAi, increased embryonic lethality in the broods of rfl-1(or198ts) mutants by at least 1.8-fold at 18°, compared to empty-vector control broods (Figure 2A). We chose this arbitrary and relatively nonstringent cutoff point because strong enhancers were less common than strong suppressors (see below), and we did not want to exclude potentially interesting genes entirely on the basis of strong enhancement of embryonic lethality. To verify that depletion of these enhancer loci themselves did not cause embryonic lethality, we depleted each of these 32 genes using feeding RNAi and wild-type worms (Table S1). None of the enhancer genes were strongly required for embryonic viability under our conditions, with 5.7% embryonic lethality being the most penetrant essential requirement that we observed. We also tested each of the 32 enhancers with rfl-1(or198ts) mutants raised at 17°, at which temperature 90% (SD ± 4%) of unenhanced mutant embryos hatched. We found that 14 of the 32 enhancers still increased embryonic lethality by ≥2-fold at 17° (Figure 2B). We limited further analysis to these 14 enhancers that reproducibly acted at both semipermissive temperatures (see Table S2 for modifier gene identities).
Figure 2.—
Enhancement of rfl-1(or198ts) embryonic lethality. L1 larvae were raised to adulthood at (A) 18° and (B) 17° with RNAi-mediated depletion of 32 candidate enhancer genes. L4440 empty-vector negative controls are at far right. Light-gray bars indicate loci that enhance embryonic lethality by at least twofold at 17° (these genes were selected for further analysis). Error bars indicate 1 SE.
A similar quantitative brood analysis identified 21 suppressors that, when reduced in function by RNAi, consistently increased the viability of embryos from hermaphrodites raised at 23.5° by ≥1.8-fold, compared to worms raised on the empty-vector control Escherichia coli (see Table S2 for gene identities). Depletion of 13 of the suppressors restored viability to >38% hatching (3-fold over the control); depletion of the strongest suppressor, csn-5, restored viability to 90% hatching. We chose an arbitrary and more restrictive cutoff of 3-fold suppression, compared to 1.8-fold and 2-fold for enhancers at 17° and 18°, respectively, because suppressors tended to be both stronger and more specific.
Specificity of suppressors and enhancers:
To test whether the modifiers that we identified specifically influenced rfl-1 function, or if depleting them can nonspecifically influence multiple conditionally mutant loci, we used four different temperature-sensitive, embryonic-lethal mutants that to our knowledge are not defective in functions related to rfl-1 or ubiquitin-mediated proteolysis: lit-1(or131ts), spn-4(or191ts), dhc-1(or195ts), and spd-5(or213ts). The lit-1 gene encodes a MAP Kinase that modulates Wnt signaling (Meneghini et al. 1999); spn-4 encodes a protein with an RNA-binding motif that regulates cell fate patterning in the early embryo (Gomes et al. 2001); dhc-1 encodes the heavy chain of the minus-end-directed microtubule motor dynein (Hamill et al. 2002); and spd-5 encodes a coiled-coil protein required for centrosome maturation (Hamill et al. 2002). For each of these mutants, we used growth temperatures that gave nearly complete embryonic viability for testing enhancement or nearly complete embryonic lethality for testing suppression (O'Rourke et al. 2007). We quantified the effects of depleting modifier genes on embryonic viability with these four additional mutants and compared the results to those obtained with rfl-1(or198ts) (Figure 3).
We found that while enhancers were often nonspecific, most of the suppressors were specific for rfl-1. Reducing the function of 8 of the 14 enhancers increased embryonic lethality by at least 2-fold in one or more of the four other conditionally mutant strains. Depletions of the remaining six enhancer loci specifically increased rfl-1(or198ts) embryonic lethality by between 2.1- and 9.6-fold (21% and 96.4% lethality), but reducing their function did not increase embryonic lethality by ≥2-fold in any of the four other conditional mutants that we tested (Figure 3 and Table S1). In contrast, of the 21 reproducible suppressor loci, 14 were specific for rfl-1(or198ts): reducing their function failed to suppress embryonic lethality by >2-fold in at least three of the four unrelated conditional mutants that we tested (Figure 3). All 14 specific suppressors failed to raise viability to ≥10% in any of the four unrelated mutants, while background viabilities on the empty-vector control ranged from 1.2% for lit-1(or131ts) as the lowest of the four to 3.7% for spn-4(or191ts) as the highest.
Two of the specific and most penetrant rfl-1(or198ts) suppressors were csn-2 and csn-5, which encode components of the COP-9 signalosome and have been shown previously to suppress rfl-1(or198ts) embryonic lethality when reduced in function (Pintard et al. 2003a). These were the only two signalosome components included in the 14,045 gene set that we tested for suppression, confirming our ability to identify functionally important suppressors.
Modifier depletion and cell division defects in rfl-1(or198ts) mutant embryos:
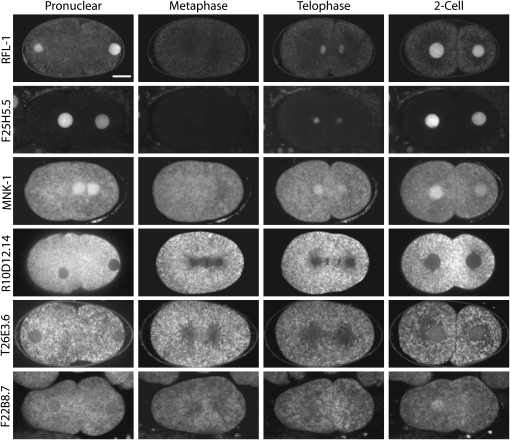
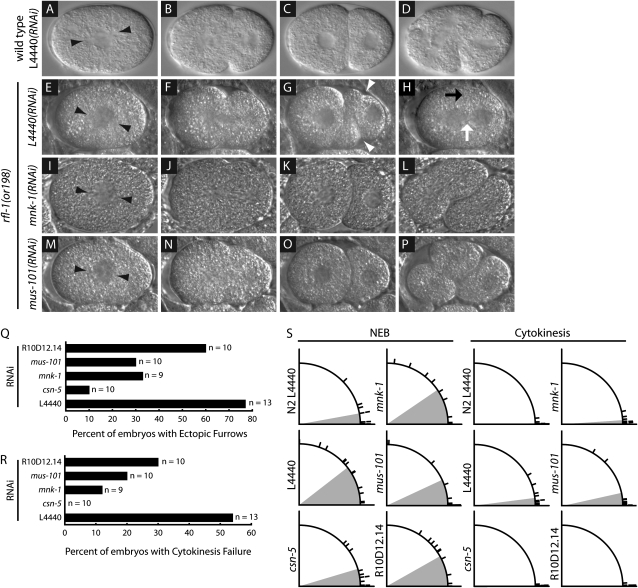
We next used DIC time-lapse videomicroscopy to examine live embryos at the first mitotic division in both suppressed and unsuppressed rfl-1(or198ts) mutants. In unsuppressed embryos from rfl-1(or198ts) mutants grown at the fully restrictive temperature of 25°, the failure to degrade MEI-1/katanin leads to defects in mitotic spindle orientation, ectopic membrane furrows, and a failure to complete cytokinesis (Kurz et al. 2002). In unsuppressed embryos, produced by rfl-1(or198ts) mutant worms raised at 23° and fed bacteria carrying the empty vector, 54% of the embryos failed to complete cytokinesis, 77% had ectopic cleavage furrow(s) following cytokinesis, and the first mitotic spindle was mis-oriented in comparison to the wild type (Figure 4 and Table S3). Depletion of the strongest suppressor, csn-5, almost completely rescued the cytokinesis and ectopic furrowing defects: 0% and 10%, respectively, of the embryos exhibited these defects, and the spindle orientation defect was also suppressed (Figure 4 and Table S3). Depletion of the specific suppressors mnk-1, mus-101, and R10D12.14 produced less complete but still substantial suppression of these defects in early stage mutant embryos (Figure 4 and Table S3). We conclude that these specific suppressors influence the same early embryonic processes that require rfl-1 and that the suppression of these cell division defects may account at least in part for the increased viability of the suppressed mutant embryos.
Figure 4.—
Suppression of early embryonic cell division defects in rfl-1(or198ts) mutants raised at 20° and then shifted to 23° for 7–12 hr prior to imaging (see materials and methods). (A–P) Frames from time-lapse DIC videomicrographs at nuclear envelope breakdown (A, E, I, M), during cytokinesis 1 min after first appearance of a cleavage furrow (B, F, J, N), and at the two-cell stage after the completion of cytokinesis (C, G, K, O). Black arrowheads indicate centrosome position; white arrowheads indicate ectopic membrane furrows; white arrow indicates nuclei of AB cell and P1 cell advancing toward each other after cytokinesis failure; black arrow indicates regression of cytokinetic furrow. Temperature-controlled stage prevented use of optimal DIC optics (see materials and methods). (Q) Quantification of ectopic cleavage furrows in suppressed and control embryos. (R) Quantitation of cytokinesis failure in suppressor RNAi-treated and control RNAi-treated embryos. (Q and R) Number of embryos scored for each condition. (S) Angle of mitotic spindle at Nuclear Envelope Breakdown (NEB) and at cytokinesis (1 min following appearance of cleavage furrow). Hatch marks indicate individual data points, and shading indicates mean angle of all data points. Top left panels under “NEB” and “Cytokinesis” are wild-type (N2) embryos; all other panels are rfl-1(or198ts) embryos.
The synthetic embryonic lethality caused by depletion of specific enhancers in rfl-1(or198ts) mutants raised at a semipermissive temperature could result from further compromising essential processes in the early embryo that require rfl-1 or from deleterious interactions that occur later in development. We therefore used DIC time-lapse videomicroscopy to examine the first mitotic division in enhanced and unenhanced mutant embryos, focusing on the two most penetrant and specific enhancer genes, sel-10 and pha-1. At 17° on empty-vector RNAi-expressing bacteria, 0/15 unenhanced rfl-1(or198ts) embryos exhibited cytokinesis failures, while 2/15 had ectopic cleavage furrows. At 18°, 2/15 empty-vector RNAi-fed embryos exhibited cytokinesis failure and 5/15 had ectopic furrows. While both sel-10(RNAi) and pha-1(RNAi) dramatically increased the rate of lethality at these temperatures, we observed no increase in cytokinesis failures, ectopic furrowing, or spindle orientation defects at either 17° or 18° (Table 1). This lack of enhancement of early cell division defects suggests that the synthetic lethal interaction(s) may occur at a later stage in embryonic development or were not detected by our methods if they occur in the early embryo.
TABLE 1.
Modifier depletion does not enhance early embryonic cell division defects in rfl-1(or198ts) mutants
| Genotype | No. of embryos with ectopic furrows | No. of embryos with cytokinesis failure |
|---|---|---|
| rfl-1(or198); empty-vector RNAi 17° | 2/15 | 0/15 |
| rfl-(or198); sel-10(RNAi) 17° | 2/11 | 0/11 |
| rfl-1(or198); pha-1(RNAi) 17° | 2/11 | 0/11 |
| rfl-1(or198); empty-vector RNAi 18° | 5/15 | 2/15 |
| rfl-(or198); sel-10(RNAi)18° | 5/13 | 0/13 |
| rfl-1(or198); pha-1(RNAi) 18° | 3/13 | 1/13 |
To ask whether rfl-1 has functional requirements later in embryogenesis, beyond the first few cell divisions, we performed temperature upshift experiments after the completion of early cell divisions at the permissive temperature of 15°. After shifting 16- to 50-cell-stage rfl-1(or198ts) embryos to 26°, we found that 18/42 embryos (48.2%) failed to hatch, while in a control experiment with no temperature upshift, only 3/51 embryos (5.8%) failed to hatch, indicating that rfl-1 does have additional essential requirements later in embryonic development. We examined the terminal phenotypes of the unhatched, upshifted rfl-1(or198ts) and found that 16/18 unenhanced and unhatched rfl-1(or198ts) embryos arrested after elongation to or beyond the threefold stage (data not shown). These results are consistent with a previous study that identified requirements for the neddylation pathway components ned-8, ubc-12, and ula-1 during postembryonic epidermal development and reported partially penetrant late-stage embryonic arrest after RNAi-mediated depletion of ubc-12 and ula-1 (Jones and Candido 2000).
We next examined the terminal phenotypes of the unhatched synthetically lethal embryos using DIC microscopy. After sel-10 depletion at 18°, we found that 25/28 (89%) of the enhanced mutant embryos failed to hatch, and many of the unhatched embryos appeared to arrest after little if any elongation (data not shown), a more severe phenotype than we observed after upshifts of unenhanced rfl-1(or198ts) embryos to 26° (see above). Other rfl-1(or198ts) embryos enhanced by sel-10 depletion arrested after a variable amount of elongation (data not shown). We conclude that the synthetic-lethal interactions observed after enhancer depletion could result from interactions that occur after the early embryonic cell division processes known to require rfl-1. However, we have not determined precisely where or when in embryogenesis the synthetic-lethal interactions occur.
Suppressor depletions partially restore CUL-3 neddylation in rfl-1(or198ts) embryos:
Neddylation of CUL-3 requires rfl-1 and other neddylation pathway components (Pintard et al. 2003a). We therefore asked if the rfl-1 suppressors influence CUL-3 neddylation after isolating embryonic cell extracts from unsuppressed and suppressed rfl-1(or198ts) mutants shifted to the nonpermissive temperature of 26° for 5 hr prior to sample collection (Figure 5). In comparison to wild type, we observed decreased CUL-3 neddylation in rfl-1(or198ts) mutant embryos, and partial restoration of neddylation after depletion of the signalosome component CSN-5, as expected (see Introduction). We observed partial restoration of CUL-3 neddylation reproducibly after depletion of six of the nine specific suppressors: most clearly with elks-1, C54D10.1/cdr-2, and F22B8.7, and more modestly but consistently with mnk-1, R10D12.14, and T26E3.6 (Figure 5 and data not shown). Thus these suppressor gene products may oppose, directly or indirectly, CUL-3 neddylation in wild-type embryos (see discussion). We also attempted to detect MEI-1/katanin and RFL-1 proteins but were unable to reproducibly analyze their levels in our extracts with available antibodies.
Suppressor and enhancer protein localization overlap with RFL-1:
To gain further insight into how the specific modifiers might influence rfl-1-dependent processes, we constructed N-terminal GFP fusions to rfl-1 and to five of the specific suppressors, all driven by the maternal pie-1 promoter, and isolated transgenic lines for all six fusions (see materials and methods). We also constructed fusions to other suppressors but were unable to obtain germline-expressing transgenic strains for all constructs. Four of the five suppressors for which we did obtain germline expression (mnk-1, R10D12.14, F22B8.7, T26E3.6) are among the six that, when depleted by RNAi, partially restored CUL-3 neddylation (see above). As observed for other Nedd8 pathway components (Kurz et al. 2002; Pintard et al. 2003a,b), we detected both cytoplasmic and enriched nuclear localization of the GFP∷RFL-1 fusion protein in live embryos, using spinning disk confocal microscopy (Figure 6; see materials and methods). Fusion constructs for both F25H5.5 (an ortholog of the human protein CLASPIN) and MNK-1 (a conserved kinase) displayed nuclear enrichment in early embryonic cells. GFP fusions to F22B8.7 (an uncharacterized conserved iron-sulfur domain-containing protein) and T26E3.6 (related to fibrillin) exhibited diffuse cytoplasmic and nuclear localization patterns. GFP∷R10D12.14 (a protein of unknown function; see discussion) localized to the cytoplasm and was largely excluded from nuclear and mitotic spindle regions. In addition, GFP∷R10D12.14 was cortically enriched near cytokinetic furrows and at cell boundaries in oocytes (Figure 6 and data not shown). While these are very general localization patterns, most of the suppressor proteins, including four that influenced CUL-3 neddylation, exhibited localization patterns similar at least in part to those observed for RFL-1, NED-8, and CSN-5, consistent with the possibility that some suppressor proteins could directly oppose CUL-3 neddylation.
We also examined the expression and localization of GFP fusions to the proteins encoded by the two most specific enhancers, SEL-10 (an F-box substrate adaptor for SCF-type E3 ligases) and C24D10.1 (an uncharacterized protein tyrosine phosphatase), using recombineering to construct GFP translational fusions regulated by native promoter and other noncoding sequences (see materials and methods). Embryonic expression of the other strong enhancer, pha-1, has been reported previously on the basis of GFP fusion protein studies that detected cytoplasmic expression in most later-stage embryonic cells (Fay et al. 2004). We did not detect any expression of GFP∷SEL-10 in early embryos, but we did detect cytoplasmic expression in some cells beginning at about the 50-cell stage (Figure 7A). In many cells throughout the embryo we detected higher levels of cytoplasmic expression beginning at about the bean stage (Figure 7B), after the completion of most embryonic cell divisions. In larvae and adults, we observed expression in head and tail neurons and in unidentified cells along the entire length of the body (Figure 7, G and H). For the fusion GFP∷C24D10.1, we again did not detect any expression in early embryos but first detected expression beginning at approximately the bean stage of embryogenesis, predominantly in nuclei and in many cells throughout the embryo (Figure 7, D and E). In larvae and adults, we observed GFP∷C24D10.1 in the nuclei of many cells throughout the head.
The lack of early embryonic expression for the two most penetrant enhancers could be due to transgene silencing in the maternal germline, a frequent outcome for maternally expressed genes in transgenic C. elegans strains (Kelly and Fire 1998). However, we did detect presumably zygotic expression of the GFP fusions to these proteins in later-stage embryos, and we wanted to compare these later expression patterns to RFL-1. We therefore used recombineering to produce an N-terminal-tagged tdTomato∷RFL-1 fusion driven by the rfl-1 promoter and generated a transgenic line that expresses this fusion (see materials and methods). We again did not observe any early embryonic expression, presumably because of germline silencing. However, we did detect strong cytoplasmic expression in larval stages and in adults in both head and tail neurons and in vulval epithelial cells and intestinal cells (Figure 7), consistent with previous studies of a rfl-1∷GFP promoter fusion as a transcriptional reporter (Hunt-Newbury et al. 2007). To summarize, although the two strongest specific rfl-1(or198ts) enhancers are expressed in later-stage embryos, we did not detect any RFL-1 expression in later-stage embryos. For this reason, and because we did not detect any maternal expression of the two enhancer GFP fusions, we do not know in which cells RFL-1 might interact with SEL-10 or C24D10.1 during embryogenesis, assuming such interactions are responsible for the enhanced embryonic lethality that we observe. It is possible that such interactions do occur in later-stage embryos but involve undetected low levels of RFL-1, or maternal expression of the two enhancers. Finally, the expression of RFL-1 in vulval epithelial cells may help to explain the frequent vulval ruptures observed in young adult rfl-1(or198ts) hermaphrodites raised at restrictive temperatures (data not shown) and in vulval defects observed after depletions of other neddylation pathway components (Jones and Candido 2000).
To further investigate the requirements for the RFL-1 modifiers that we identified, we asked if depletion of the suppressor gene products can alter the localization of GFP∷RFL-1 and if depletion of rfl-1 or other neddylation pathway components can change the localization of the GFP suppressor protein fusions in early embryonic cells. We did not detect changes in localization for any of the suppressor fusions following rfl-1, nedd-8, and ula-1 depletions (data not shown). Similarly, no change in GFP∷RFL-1 localization was observed upon depletion of the suppressor genes mus-101, R10D12.14, mnk-1, F25H5.5, T26E3.6, or F22B8.7 (data not shown). We also did not observe any change in early embryonic GFP∷RFL-1 localization upon depletion of sel-10, pha-1, or C24D10.1, a result consistent with our finding that sel-10 and pha-1 depletion does not enhance the early embryonic phenotypes in rfl-1 mutants.
Finally, we also examined GFP∷RFL-1 expression in transgenic embryos after depleting the neddylation pathway components ula-1, nedd-8, and ubc-12 (Figure S1). We did not observe changes in RFL-1 localization after depletion of either nedd-8 or ubc-12, but we did observe a loss of GFP∷RFL-1 nuclear enrichment following ula-1 depletion. ULA-1 and RFL-1/UBA-3 form the E1-activating enzyme complex for the Nedd8 conjugation pathway, with RFL-1 being the catalytic AAA-ATPase subunit (Jones and Candido 2000). A comparison of pixel intensities showed a nuclear/cytoplasmic ratio of 1.3 after ula-1 depletion, compared to 5.7 in wild-type embryos (n = 6 embryos for each analysis and then averaged). Using Western blots, we detected similar levels of GFP∷RFL-1 in extracts from ULA-1-depleted worms and wild-type worms (data not shown), suggesting that ULA-1 is required for RFL-1 nuclear enrichment independent of any changes in protein levels. To our knowledge, such a requirement for ULA-1 orthologs has not been reported in other organisms. Perhaps the heterodimerization of these two proteins allows for regulation of nuclear localization that is not observed when other neddylation pathway components are depleted.
DISCUSSION
To identify factors that influence neddylation and proteolytic regulation in C. elegans, we used a high-throughput RNAi screen to identify suppressors and enhancers of the embryonic lethality associated with or198ts, a temperature-sensitive, embryonic-lethal mutation in the C. elegans gene rfl-1, which encodes a component of the ubiquitin-like Nedd8 protein conjugation pathway. Using ts mutations in other essential loci, we tested the modifiers to determine if they were specific for rfl-1(or198ts) embryonic lethality. We found that many of the enhancers and suppressors were nonspecific, with specific suppressors being more common. Depletion of the rfl-1-specific suppressors partially rescued the embryonic cell division defects associated with loss of rfl-1 function. Furthermore, we found similar subcellular distributions of several suppressor proteins and RFL-1, also consistent with the suppressors having RFL-1-related roles in the early embryo. Importantly, depletion of several suppressors partially restored CUL-3 neddylation in rfl-1(or198ts) mutants, suggesting that these suppressors may normally oppose neddylation. We also identified two highly penetrant and specific enhancers of rfl-1(or198ts) embryonic lethality. While their depletion did not detectably enhance early embryonic cell division defects in mutant embryos, later requirements for rfl-1 may explain their synthetic lethality. Finally, most of the modifiers do not themselves appear to be essential. Thus, when controlled for specificity, high-throughput synthetic screens that combine RNAi and conditionally lethal C. elegans mutations can be used to identify possible roles for nonessential genes in essential processes.
Synthetic screening with RNAi and temperature-sensitive, embryonic-lethal C.elegans mutants—controlling for specificity:
While some large-scale synthetic screens using RNAi and temperature-sensitive C. elegans mutants have addressed the issue of modifier specificity (Fraser 2004; Lehner et al. 2006; O'Rourke et al. 2007), relatively few such studies have been reported thus far. We report here for the first time a systematic and quantitative analysis of specificity for both enhancers and suppressors of a conditional embryonic-lethal C. elegans mutant.
We found that while many rfl-1 enhancers were nonspecific, most suppressors were specific. Of the 21 suppressors, 71% were specific in that their depletion did not restore embryonic viability to other conditionally embryonic-lethal mutants grown at semipermissive temperatures. In contrast, only 43% (6/14) of enhancers were specific, and only 14% (2 of 14) strongly enhanced embryonic lethality (Figures 2 and 3 and Table S1 and Table S2) Indeed, enhancers identified in modifier screens with other conditional mutants also exhibit, with very few exceptions, a high degree of nonspecificity when tested with multiple conditionally lethal mutants (M. Dorfman and S. O'Rourke, unpublished data). We conclude that most examples of synthetic embryonic lethality result from additive and unrelated defects, rather than from the two genes influencing the same biological process or pathway. Nevertheless, some genes that, when depleted, enhance conditional embryonic lethality in more than one mutant background could represent functional links among the different mutant loci.
Synthetic lethal screens in budding yeast have been powerful tools for the discovery of new gene functions (Tong et al. 2004; Ooi et al. 2006), and it is interesting to compare the specificity observed in synthetic lethal C. elegans screens with synthetic knockout screens done with budding yeast. For example, one yeast study analyzed 132 nonessential genes (Tong et al. 2004). Each of these 132 viable knockout mutants was mated with ∼4700 additional viable knockout mutants to produce double mutants. A total of ∼4000 synthetic lethal interactions were identified, with an average of 34 interactions/mutant. Of the interactions found, 27% were with pairs of genes known to be in the same or related genetic pathways. These findings suggest a substantial degree of specificity for synthetic lethal interactions in yeast, and thus the interactions are relatively likely to reflect participation in a common process. By contrast, one study in C. elegans found that over one-half of the genes on chromosome III, when reduced in function by feeding RNAi, significantly enhanced the embryonic lethality of a hypomorphic mutation in the transcription factor gene dpl-l (Fraser 2004). Similarly, we found that many of the strong enhancers, when depleted by RNAi, enhanced embryonic lethality with multiple conditional mutants.
This high degree of nonspecificity, compared to the synthetic-lethal screens in budding yeast, could reflect the added genetic complexity of the cellular and developmental processes in a multicellular organism. Alternatively, the different degrees of specificity observed in yeast and worms may simply reflect the different kinds of mutant alleles used for screening. The yeast synthetic-lethal screens used double mutants made with viable deletion alleles. In contrast, the synthetic screens in C. elegans have used partial inactivation of essential genes, usually by growing temperature-sensitive mutants at intermediate temperatures to sensitize genetic backgrounds, and RNAi to deplete the expression of other genes that may or may not themselves be essential. Perhaps temperature-sensitive mutants grown at just-viable temperatures are particularly vulnerable to nonspecific synthetic lethality caused by the disruption of unrelated processes. It would be interesting to use genomewide feeding RNAi screens to detect synthetic lethality in fit, fertile C. elegans strains that are homozygous for deletion mutations in nonessential genes. Conversely, it would be interesting to know if temperature-sensitive mutations in essential budding yeast genes are also prone to high levels of nonspecific enhancement and suppression when other genes are reduced in function.
While nonspecific enhancement of embryonic lethality is especially common, suppression of embryonic lethality by RNAi knockdown of other C. elegans loci also is frequently nonspecific. In a genomewide RNAi screen for suppressors of a temperature-sensitive dynein heavy chain mutant, dhc-1(or195ts), 49 genes were identified that, when depleted by feeding RNA, restored dhc-1(or195ts) embryonic viability by threefold or more (O'Rourke et al. 2007). However, depletion of 57% of the dhc-1(or195ts) suppressors also significantly suppressed embryonic lethality for at least one of two other mutants with temperature-sensitive mutations in essential loci unrelated in function to dhc-1. As we report here, 29% of the rfl-1(or198ts) suppressors were similarly nonspecific. For at least some of the nonspecific dhc-1(or195ts) suppressor genes, their depletion has been reported by others to increase glycerol production, which may nonspecifically stabilize temperature-sensitive proteins and thereby increase embryonic viability at semipermissive temperatures (Lamitina et al. 2006; O'Rourke et al. 2007). Clearly, it is important to carefully assess the specificity of both enhancer and suppressor interactions that influence the degree of lethality associated with temperature-sensitive mutations in essential C. elegans genes.
Known roles for specific modifier genes:
The six suppressors that when depleted partially restored CUL-3 neddylation in rfl-1 mutants are all conserved, but how they might act is not apparent. Their influence on neddylation could be direct and of importance to other processes that involve neddylation, or it could be indirect. F22B8.7 encodes an uncharacterized conserved iron-sulfur domain-containing protein; T26E3.6, a fibrillin-related protein; cdr-2, a glutathione S-transferase-like protein (Dong et al. 2005); mnk-1, a conserved kinase; and elks-1, a Rab GTPase-interacting protein (Deken et al. 2005). The strong rfl-1 suppressor R10D12.14 is highly conserved only in nematodes and is one of six suppressors that, when depleted, partially restored CUL-3 neddylation in rfl-1(or198ts) mutants. This gene is at least partially essential but its specific cellular requirements remain unknown (Piano et al. 2002). It encodes a protein with a GYF domain (poly-proline interaction motif) and also was identified as a binding partner of dynein light chain (DLC-1) in a yeast two-hybrid screen of metazoan-specific C. elegans genes (Li et al. 2004). While the link to dynein might reflect roles in cell division, the GFP∷R10D12.14 fusion protein that we examined was largely excluded from nuclei and mitotic spindles and was mostly cytoplasmic, with some cortical enrichment in early embryonic cytokinesis furrows and in oocytes. Moreover, how binding to dynein light chain might relate to neddylation is not obvious. Regardless of the functional significance of the dynein light chain interaction, our analysis suggests that this protein of unknown function directly or indirectly opposes CUL-3 neddylation. It will be interesting to learn if any of these suppressors prove to influence neddylation in other model systems.
Another strong specific suppressor, called mus-101, encodes a widely conserved 1227-amino-acid protein containing six copies of the BRCA1 carboxyl-terminal (BRCT) repeat (Yamamoto et al. 2000; Holway et al. 2005). The mus-101 gene is essential but feeding RNAi results in only partially penetrant embryonic lethality (Maeda et al. 2001; Holway et al. 2005). BRCT domains are commonly found in proteins involved in DNA metabolism and have been implicated in mediating protein–protein interactions between other BRCT domain-containing proteins (Manke et al. 2003; Yu et al. 2003). Some proteins with BRCT repeats, including BRCA1, have roles in mitosis as well as more established roles in DNA replication and repair. For example, BRCA1 binds to tubulin and localizes to centrosomes and spindle microtubules (Hsu and White 1998), and high levels of BRCA1 are maintained throughout mitosis, while the protein is ubiquitinated and degraded during G1 and S phase (Choudhury et al. 2004). Furthermore, in both mammalian and Xenopus cells, BRCA1 is required for spindle pole assembly and the centrosomal accumulation of TPX2 (Joukov et al. 2006). Perhaps the BRCA repeat-protein-encoded mus-101 influences cell division such that it can restore viability when depleted in rfl-1 mutants. Such a role might bypass requirements for neddylation, consistent with no influence of mus-101 depletion on CUL-3 neddylation.
The two strongest specific enhancer genes were sel-10 and pha-1. The sel-10 gene encodes an F-box protein and has been implicated as a substrate adaptor in E3 ligases that control the ubiquitin-mediated degradation of LIN-12/Notch, SEL-12/Presenilin, and the sex-determining proteins FEM-1 and FEM-3 (Hubbard et al. 1997; Wu et al. 2001; Jager et al. 2004). There are no identified requirements for Cullin neddylation in the ubiquitin-mediated degradation of SEL-10 targets, but our findings raise this possibility. The pha-1 gene encodes a novel protein that has been shown to function redundantly with class B SynMuv genes, such as lin-35/Rb and efl-1, to influence pharyngeal morphogenesis (Fay et al. 2004). Furthermore, reducing the function of both pha-1 and any one of four ubiquitin ligases—ubc-18, C27A12.6, C27A12.7, and ari-1—results in pharyngeal morphogenesis defects and partially penetrant early larval lethality (Qiu and Fay 2006). ARI-1 has been shown to interact with CSN-5 by yeast two-hybrid screening, and inactivation of csn-5 also enhances pharyngeal defects in some mutant backgrounds (D. Fay, personal communication). Our results suggest that neddylation may be important for proper proteolytic regulation of pha-1- and/or lin-35/Rb-dependent processes. While the F-box class of ubiquitin E3 ligases that may regulate pha-1 and lin-35 are not known to require neddylation, our results suggest that neddylation may promote their function.
Identifying requirements for nonessential genes:
While mutational studies and genomewide RNAi screens in C. elegans have identified thousands of genes that either are essential or have visible requirements, ∼70% of the known and predicted genes in C. elegans have no known requirements (Kemphues 2005), even though at least half have homologs in other animal phyla (Lander et al. 2001). Identifying roles for conserved but nonessential genes in essential processes is an important frontier of genetic research in model organisms, as many such genes could be relevant to human disease processes. Moreover, nonessential genes that influence essential processes may be valuable drug targets: altering their function may allow for modification of an essential process without causing deleterious side effects that result from targeting more pleiotropic and essential disease genes. In our screen for modifiers of the essential C. elegans gene rfl-1, 11 of the 16 specific suppressors and all 6 of the specific enhancers that we identified appear to be nonessential. Thus, when controlled for specificity, modifier screens that use RNAi and conditional mutations in essential C. elegans genes may prove useful in identifying roles in essential processes for nonessential but conserved genes.
Acknowledgments
We thank I. Cruxent for help with RNAi screening; M. Price and J. Canman for microscopy assistance; L. Pintard for kindly providing the CUL-3 antibody used in this study; and D. Greenstein, T. Herman, and a reviewer for helpful comments on the manuscript. This work was supported by a National Institutes of Health (NIH) Training Grant (to M.D.), by the Fundação para a Ciência e a Tecnologia (to J.-E.G.), and by NIH grant GM058017 (to B.B.).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.104885/DC1.
References
- Albertson, D. G., and J. N. Thomson, 1993. Segregation of holocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans. Chromosome Res. 1 15–26. [DOI] [PubMed] [Google Scholar]
- Angers, S., C. J. Thorpe, T. L. Biechele, S. J. Goldenberg, N. Zheng et al., 2006. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 8 348–357. [DOI] [PubMed] [Google Scholar]
- Baugh, L. R., J. C. Wen, A. A. Hill, D. K. Slonim, E. L. Brown et al., 2005. Synthetic lethal analysis of Caenorhabditis elegans posterior embryonic patterning genes identifies conserved genetic interactions. Genome Biol. 6 R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein, G., D. Ganoth and A. Hershko, 2006. Regulation of neddylation and deneddylation of cullin1 in SCFSkp2 ubiquitin ligase by F-box protein and substrate. Proc. Natl. Acad. Sci. USA 103 11515–11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowerman, B., and T. Kurz, 2006. Degrade to create: developmental requirements for ubiquitin-mediated proteolysis during early C. elegans embryogenesis. Development 133 773–784. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury, A. D., H. Xu and R. Baer, 2004. Ubiquitination and proteasomal degradation of the BRCA1 tumor suppressor is regulated during cell cycle progression. J. Biol. Chem. 279 33909–33918. [DOI] [PubMed] [Google Scholar]
- Cullinan, S. B., J. D. Gordan, J. Jin, J. W. Harper and J. A. Diehl, 2004. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell. Biol. 24 8477–8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deken, S. L., R. Vincent, G. Hadwiger, Q. Liu, Z. W. Wang et al., 2005. Redundant localization mechanisms of RIM and ELKS in Caenorhabditis elegans. J. Neurosci. 25 5975–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, J., M. O. Song and J. H. Freedman, 2005. Identification and characterization of a family of Caenorhabditis elegans genes that is homologous to the cadmium-responsive gene cdr-1. Biochim. Biophys. Acta 1727 16–26. [DOI] [PubMed] [Google Scholar]
- Dow, M. R., and P. E. Mains, 1998. Genetic and molecular characterization of the Caenorhabditis elegans gene, mel-26, a postmeiotic negative regulator of mei-1, a meiotic-specific spindle component. Genetics 150 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay, D. S., X. Qiu, E. Large, C. P. Smith, S. Mango et al., 2004. The coordinate regulation of pharyngeal development in C. elegans by lin-35/Rb, pha-1, and ubc-18. Dev. Biol. 271 11–25. [DOI] [PubMed] [Google Scholar]
- Feinberg, E. H., and C. P. Hunter, 2003. Transport of dsRNA into cells by the transmembrane protein SID-1. Science 301 1545–1547. [DOI] [PubMed] [Google Scholar]
- Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver et al., 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391 806–811. [DOI] [PubMed] [Google Scholar]
- Fraser, A., 2004. Towards full employment: using RNAi to find roles for the redundant. Oncogene 23 8346–8352. [DOI] [PubMed] [Google Scholar]
- Geyer, R., S. Wee, S. Anderson, J. Yates and D. A. Wolf, 2003. BTB/POZ domain proteins are putative substrate adaptors for cullin 3 ubiquitin ligases. Mol. Cell 12 783–790. [DOI] [PubMed] [Google Scholar]
- Gomes, J. E., S. E. Encalada, K. A. Swan, C. A. Shelton, J. C. Carter et al., 2001. The maternal gene spn-4 encodes a predicted RRM protein required for mitotic spindle orientation and cell fate patterning in early C. elegans embryos. Development 128 4301–4314. [DOI] [PubMed] [Google Scholar]
- Greenstein, D., and L. A. Lee, 2006. Oocyte-to-embryo transition: kinase cabal plots regime change. Curr. Biol. 16 R93–R95. [DOI] [PubMed] [Google Scholar]
- Hamill, D. R., A. F. Severson, J. C. Carter and B. Bowerman, 2002. Centrosome maturation and mitotic spindle assembly in C. elegans require SPD-5, a protein with multiple coiled-coil domains. Dev. Cell 3 673–684. [DOI] [PubMed] [Google Scholar]
- Hetfeld, B. K., A. Peth, X. M. Sun, P. Henklein, G. M. Cohen et al., 2008. The COP9 signalosome-mediated deneddylation is stimulated by caspases during apoptosis. Apoptosis 13 187–195. [DOI] [PubMed] [Google Scholar]
- Holway, A. H., C. Hung and W. M. Michael, 2005. Systematic, RNA-interference-mediated identification of mus-101 modifier genes in Caenorhabditis elegans. Genetics 169 1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, L. C., and R. L. White, 1998. BRCA1 is associated with the centrosome during mitosis. Proc. Natl. Acad. Sci. USA 95 12983–12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard, E. J., G. Wu, J. Kitajewski and I. Greenwald, 1997. sel-10, a negative regulator of lin-12 activity in Caenorhabditis elegans, encodes a member of the CDC4 family of proteins. Genes Dev. 11 3182–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt-Newbury, R., R. Viveiros, R. Johnsen, A. Mah, D. Anastas et al., 2007. High-throughput in vivo analysis of gene expression in Caenorhabditis elegans. PLoS Biol. 5 e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager, S., H. T. Schwartz, H. R. Horvitz and B. Conradt, 2004. The Caenorhabditis elegans F-box protein SEL-10 promotes female development and may target FEM-1 and FEM-3 for degradation by the proteasome. Proc. Natl. Acad. Sci. USA 101 12549–12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D., and E. P. Candido, 2000. The NED-8 conjugating system in Caenorhabditis elegans is required for embryogenesis and terminal differentiation of the hypodermis. Dev. Biol. 226 152–165. [DOI] [PubMed] [Google Scholar]
- Joukov, V., A. C. Groen, T. Prokhorova, R. Gerson, E. White et al., 2006. The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell 127 539–552. [DOI] [PubMed] [Google Scholar]
- Kamath, R. S., and J. Ahringer, 2003. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30 313–321. [DOI] [PubMed] [Google Scholar]
- Kelly, W. G., and A. Fire, 1998. Chromatin silencing and the maintenance of a functional germline in Caenorhabditis elegans. Development 125 2451–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues, K., 2005. Essential genes. WormBook Dec 24 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz, T., L. Pintard, J. H. Willis, D. R. Hamill, P. Gonczy et al., 2002. Cytoskeletal regulation by the Nedd8 ubiquitin-like protein modification pathway. Science 295 1294–1298. [DOI] [PubMed] [Google Scholar]
- Kurz, T., N. Ozlu, F. Rudolf, S. M. O'Rourke, B. Luke et al., 2005. The conserved protein DCN-1/Dcn1p is required for cullin neddylation in C. elegans and S. cerevisiae. Nature 435 1257–1261. [DOI] [PubMed] [Google Scholar]
- Labbe, J. C., A. Pacquelet, T. Marty and M. Gotta, 2006. A genomewide screen for suppressors of par-2 uncovers potential regulators of PAR protein-dependent cell polarity in Caenorhabditis elegans. Genetics 174 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamitina, T., C. G. Huang and K. Strange, 2006. Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. Proc. Natl. Acad. Sci. USA 103 12173–12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander, E. S., L. M. Linton, B. Birren, C. Nusbaum, M. C. Zody et al., 2001. Initial sequencing and analysis of the human genome. Nature 409 860–921. [DOI] [PubMed] [Google Scholar]
- Lehner, B., C. Crombie, J. Tischler, A. Fortunato and A. G. Fraser, 2006. Systematic mapping of genetic interactions in Caenorhabditis elegans identifies common modifiers of diverse signaling pathways. Nat. Genet. 38 896–903. [DOI] [PubMed] [Google Scholar]
- Li, S., C. M. Armstrong, N. Bertin, H. Ge, S. Milstein et al., 2004. A map of the interactome network of the metazoan C. elegans. Science 303 540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., S. Vasudevan and E. T. Kipreos, 2004. CUL-2 and ZYG-11 promote meiotic anaphase II and the proper placement of the anterior-posterior axis in C. elegans. Development 131 3513–3525. [DOI] [PubMed] [Google Scholar]
- Lu, C., and P. E. Mains, 2007. The C. elegans anaphase promoting complex and MBK-2/DYRK kinase act redundantly with CUL-3/MEL-26 ubiquitin ligase to degrade MEI-1 microtubule-severing activity after meiosis. Dev. Biol. 302 438–447. [DOI] [PubMed] [Google Scholar]
- Lyapina, S., G. Cope, A. Shevchenko, G. Serino, T. Tsuge et al., 2001. Promotion of NEDD-CUL1 conjugate cleavage by COP9 signalosome. Science 292 1382–1385. [DOI] [PubMed] [Google Scholar]
- Maeda, I., Y. Kohara, M. Yamamoto and A. Sugimoto, 2001. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr. Biol. 11 171–176. [DOI] [PubMed] [Google Scholar]
- Mains, P. E., K. J. Kemphues, S. A. Sprunger, I. A. Sulston and W. B. Wood, 1990. Mutations affecting the meiotic and mitotic divisions of the early Caenorhabditis elegans embryo. Genetics 126 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manke, I. A., D. M. Lowery, A. Nguyen and M. B. Yaffe, 2003. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science 302 636–639. [DOI] [PubMed] [Google Scholar]
- McNally, K., A. Audhya, K. Oegema and F. J. McNally, 2006. Katanin controls mitotic and meiotic spindle length. J. Cell Biol. 175 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghini, M. D., T. Ishitani, J. C. Carter, N. Hisamoto, J. Ninomiya-Tsuji et al., 1999. MAP kinase and Wnt pathways converge to downregulate an HMG-domain repressor in Caenorhabditis elegans. Nature 399 793–797. [DOI] [PubMed] [Google Scholar]
- Nandi, D., P. Tahiliani, A. Kumar and D. Chandu, 2006. The ubiquitin-proteasome system. J. Biosci. 31 137–155. [DOI] [PubMed] [Google Scholar]
- Ooi, S. L., X. Pan, B. D. Peyser, P. Ye, P. B. Meluh et al., 2006. Global synthetic-lethality analysis and yeast functional profiling. Trends Genet. 22 56–63. [DOI] [PubMed] [Google Scholar]
- O'Rourke, S. M., M. D. Dorfman, J. C. Carter and B. Bowerman, 2007. Dynein modifiers in C. elegans: light chains suppress conditional heavy chain mutants. PLoS Genet. 3 e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano, F., A. J. Schetter, D. G. Morton, K. C. Gunsalus, V. Reinke et al., 2002. Gene clustering based on RNAi phenotypes of ovary-enriched genes in C. elegans. Curr. Biol. 12 1959–1964. [DOI] [PubMed] [Google Scholar]
- Pintard, L., T. Kurz, S. Glaser, J. H. Willis, M. Peter et al., 2003. a Neddylation and deneddylation of CUL-3 is required to target MEI-1/Katanin for degradation at the meiosis-to-mitosis transition in C. elegans. Curr. Biol. 13 911–921. [DOI] [PubMed] [Google Scholar]
- Pintard, L., J. H. Willis, A. Willems, J. L. Johnson, M. Srayko et al., 2003. b The BTB protein MEL-26 is a substrate-specific adaptor of the CUL-3 ubiquitin-ligase. Nature 425 311–316. [DOI] [PubMed] [Google Scholar]
- Praitis, V., 2006. Creation of transgenic lines using microparticle bombardment methods. Methods Mol. Biol. 351 93–107. [DOI] [PubMed] [Google Scholar]
- Qiu, X., and D. S. Fay, 2006. ARI-1, an RBR family ubiquitin-ligase, functions with UBC-18 to regulate pharyngeal development in C. elegans. Dev. Biol. 291 239–252. [DOI] [PubMed] [Google Scholar]
- Quintin, S., P. E. Mains, A. Zinke and A. A. Hyman, 2003. The mbk-2 kinase is required for inactivation of MEI-1/katanin in the one-cell Caenorhabditis elegans embryo. EMBO Rep. 4 1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov, M., S. Schneider, A. Pozniakovski, A. Roguev, S. Ernst et al., 2006. A recombineering pipeline for functional genomics applied to Caenorhabditis elegans. Nat. Methods 3 839–844. [DOI] [PubMed] [Google Scholar]
- Schwechheimer, C., G. Serino, J. Callis, W. L. Crosby, S. Lyapina et al., 2001. Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIRI in mediating auxin response. Science 292 1379–1382. [DOI] [PubMed] [Google Scholar]
- Shaner, N. C., R. E. Campbell, P. A. Steinbach, B. N. Giepmans, A. E. Palmer et al., 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22 1567–1572. [DOI] [PubMed] [Google Scholar]
- Simmer, F., C. Moorman, A. M. van der Linden, E. Kuijk, P. V. van den Berghe et al., 2003. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 1 E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneville, R., and P. Gonczy, 2004. Zyg-11 and cul-2 regulate progression through meiosis II and polarity establishment in C. elegans. Development 131 3527–3543. [DOI] [PubMed] [Google Scholar]
- Srayko, M., D. W. Buster, O. A. Bazirgan, F. J. McNally and P. E. Mains, 2000. MEI-1/MEI-2 katanin-like microtubule severing activity is required for Caenorhabditis elegans meiosis. Genes Dev. 14 1072–1084. [PMC free article] [PubMed] [Google Scholar]
- Stitzel, M. L., K. C. Cheng and G. Seydoux, 2007. Regulation of MBK-2/Dyrk kinase by dynamic cortical anchoring during the oocyte-to-zygote transition. Curr. Biol. 17 1545–1554. [DOI] [PubMed] [Google Scholar]
- Timmons, L., D. L. Court and A. Fire, 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263 103–112. [DOI] [PubMed] [Google Scholar]
- Tong, A. H., G. Lesage, G. D. Bader, H. Ding, H. Xu et al., 2004. Global mapping of the yeast genetic interaction network. Science 303 808–813. [DOI] [PubMed] [Google Scholar]
- van Haaften, G., R. Romeijn, J. Pothof, W. Koole, L. H. Mullenders et al., 2006. Identification of conserved pathways of DNA-damage response and radiation protection by genome-wide RNAi. Curr. Biol. 16 1344–1350. [DOI] [PubMed] [Google Scholar]
- Warming, S., N. Costantino, D. L. Court, N. A. Jenkins and N. G. Copeland, 2005. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33 e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, G., S. Lyapina, I. Das, J. Li, M. Gurney et al., 2001. SEL-10 is an inhibitor of notch signaling that targets notch for ubiquitin-mediated protein degradation. Mol. Cell. Biol. 21 7403–7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L., Y. Wei, J. Reboul, P. Vaglio, T. H. Shin et al., 2003. BTB proteins are substrate-specific adaptors in an SCF-like modular ubiquitin ligase containing CUL-3. Nature 425 316–321. [DOI] [PubMed] [Google Scholar]
- Yamamoto, R. R., J. M. Axton, Y. Yamamoto, R. D. Saunders, D. M. Glover et al., 2000. The Drosophila mus101 gene, which links DNA repair, replication and condensation of heterochromatin in mitosis, encodes a protein with seven BRCA1 C-terminus domains. Genetics 156 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X., C. C. Chini, M. He, G. Mer and J. Chen, 2003. The BRCT domain is a phospho-protein binding domain. Science 302 639–642. [DOI] [PubMed] [Google Scholar]