Abstract
Mercury distribution in the oceans is controlled by complex biogeochemical cycles, resulting in retention of trace amounts of this metal in plants and animals. Inter- and intra-specific variations in mercury levels of predatory pelagic fish have been previously linked to size, age, trophic position, physical and chemical environmental parameters, and location of capture; however, considerable variation remains unexplained. In this paper, we focus on differences in ecology, depth of occurrence, and total mercury levels in 9 species of commercially important pelagic fish (Thunnus obesus, T. albacares, Katsuwonus pelamis, Xiphias gladius, Lampris guttatus, Coryphaena hippurus, Taractichthys steindachneri, Tetrapturus audax, and Lepidocybium flavobrunneum) and in numerous representatives (fishes, squids, and crustaceans) of their lower trophic level prey sampled from the central North Pacific Ocean. Results indicate that total mercury levels of predatory pelagic fishes and their prey increase with median depth of occurrence in the water column and mimic concentrations of dissolved organic mercury in seawater. Stomach content analysis results from this study and others indicate a greater occurrence of higher-mercury containing deeper-water prey organisms in the diets of the deeper-ranging predators, X. gladius, T. obesus, and L. guttatus. While present in trace amounts, dissolved organic mercury increases with depth in the water column suggesting that the mesopelagic habitat is a major entry point for mercury into marine food webs. These data suggest that a major determinant of mercury levels in oceanic predators is their depth of forage.
Keywords: depth of forage, marine pelagic predators, North Pacific Ocean, mercury bioaccumulation, mesopelagic zone
Mercury is a trace element distributed throughout the earth's atmosphere, biosphere, and geosphere. With numerous redox states, mercury is readily transformed by a suite of physical and biologically mediated reactions, which results in global biogeochemical cycling and trace retention in plants and animals. Mercury enters food webs following abiotic or biotic (i.e., bacterially mediated) methylation (1); after microbial uptake and subsequent consumer uptake, organic forms of mercury (primarily methylmercury) readily bind to proteins and bioaccumulate in higher trophic level organisms (2).
The presence of organic mercury in commercially important pelagic fishes (e.g., tunas, billfishes, and sharks) has long captured the interest of scientists, public health officials, and the general public (3, 4). Industrial activities have increased mercury emissions over past decades (5, 6), and growing awareness of the negative health impacts of mercury have forced many organizations (e.g., United States Environmental Protection Agency, Food and Drug Administration, and United Nations Environmental Program) to issue advisories and consider mercury emission controls. Thus, a comprehensive understanding of the environmental and ecological factors controlling mercury bioaccumulation in pelagic fishes is crucial for medical advisors and resource managers alike.
Variations in fish mercury levels have previously been linked to an assortment of synergistic factors such as location of capture (7, 8), trophic level (9, 10), environmental parameters (e.g., pH, temperature, algal concentrations) (11), and perhaps most commonly, size (12). Despite extensive measurements of mercury in both the environment and biota, it is still not known why, irrespective of size, some fish species have elevated mercury concentrations and others do not.
Biogeochemical studies detailing the movement of mercury between air, land, and ocean reservoirs have offered insight into where mercury may be distributed in the marine environment (13). In the Pacific Ocean specifically, vertical profiles show that methylated mercury species are usually below limits of detection in open ocean surface waters whereas subthermocline, low-oxygen deeper intermediate waters are sites for enhanced mercury methylation (14–17). Monomethylmercury (CH3Hg) is the organic form of mercury that bioaccumulates in food webs and is toxic at elevated levels (1); therefore, if oxygen depletion is coupled with enhanced methylation rates in open ocean waters (16), it suggests that via the microbial loop (18), low oxygen deeper open ocean waters containing higher levels of bioavailable mercury will transfer elevated concentrations to animals both living at depth and predators foraging at depth. In support of this hypothesis, results of studies examining mercury levels in apex predatory seabirds suggest that the presence of mesopelagic prey in their diet may have contributed to elevated mercury levels (19, 20). Additionally, Monteiro et al. (21) found a positive mercury gradient with depth of occurrence in small epi- and mesopelagic fishes from the North Atlantic. However, these studies did not look concurrently at a diversity of predators and prey.
To better understand the mechanisms governing mercury bioaccumulation in open ocean animals, we examined mercury contents in predators and their lower trophic level prey in an ecological food web context. Using marine animals collected from waters surrounding Hawaii in the central North Pacific Ocean, we examined the hypothesis that animal mercury levels vary as a function of depth of occurrence in the water column. Ingestion of mercury from food has been confirmed as the dominant uptake pathway for fish, thus recording the integrated feeding behavior of the consumer (22, 23). Mercury levels were examined in 9 predatory pelagic fish species with distinct foraging behaviors and a representative collection of their prey items (fishes, cephalopods, and crustaceans comprising 56 taxa), inhabiting a large depth continuum.
Lastly, stomach content analysis of predators was used in the present study to corroborate satellite/recapture tagging studies that have demonstrated differences in vertical water column utilization among various pelagic fishes (24, 25), and augment diet data from published studies. By selecting predators with known foraging behaviors and prey with distinct vertical distributions it is likely that trophic transfers of mercury will be more clearly observed. The present approach is comprehensive in its coupling of analyses in predators and prey concurrently and provides information on food web dynamics and the factors governing mercury distributions in oceanic top predators.
Results and Discussion
Predators were designated as being either “shallow-ranging” (i.e., swimming/foraging generally above the thermocline) {Coryphaena hippurus [0–50 m (26)], Thunnus albacares [0–100 m (27)], Tetrapturus audax [0–100 m (28, 29)]; Katsuwonus pelamis [0–300 m (30, 31)]} or “deep-ranging” (i.e., with the ability to swim/forage below the thermocline) {Taractichthys steindachneri [300–500+ m (32, 33)], T. obesus [0–800 m (24, 34)], Lampris guttatus [25–700 m (25)], Lepidocybium flavobrunneum [200–885 m (35, 36)], Xiphias gladius [0–1,000 m (37)]} based on tagging data from Hawaiian waters. Using literature-derived depth distributions (see Table S1 for complete details), prey organisms were placed into 1 of 5 ecological depth categories created with respect to physical and ecological factors most likely to influence mercury content of the organisms (16, 17), namely day-time depth of occurrence. Briefly, “epipelagic” prey remain within the upper 200 m of the water column, while “upper-mesopelagic” and “lower-mesopelagic” prey are separated into diel vertical migrators and nonmigrators, where prey have median day-time depths between 200–600 m and 600–1,000 m, respectively.
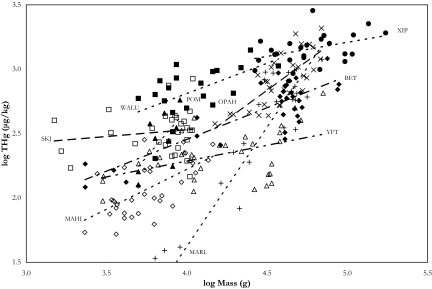
Predator mercury concentrations varied widely (Fig. 1). Results from a generalized linear model (GLM) found that location (latitude and longitude as continuous variables) and sex did not have a significant effect on mercury concentrations but species and size did (P < 0.05). Fish size was designated a continuous variable within the GLM, while sex and species were categorical variables and fixed factors. The slopes of the mercury-size relationship were all positive, indicating that THg (total mercury, which includes all organic and inorganic species) concentrations increase with mass (Fig. 1) but the slopes varied as indicated by the significant interaction term between species and size (P < 0.05). All other interaction terms were not significant. Fish lengths were converted to ages (years) using published von Bertalanffy growth curves from the central North Pacific Ocean. Linear regressions examining the relationship between age and mercury concentration were significantly positive (P < 0.05) for 5 predators (age and growth not available for L. guttatus, T. steindachneri, L. flavobruneum, and T. audax). Age explained more variance in mercury than size. Inter- and intra-specific mercury variation has been attributed to size or age in numerous marine and freshwater studies (7, 10, 38, 39). The present results confirm these trends.
Fig. 1.
Comparison of regressions of log-transformed THg concentrations (μg/kg) as a function of log-transformed body mass (g) for 9 predators. Broadbill Swordfish (Xiphias gladius), XIP (solid circles) (n = 24): THg = 0.22(mass) + 2.11, r2 = 0.18; Bigeye Tuna (Thunnus obesus), BET (solid diamonds) (n = 32): THg = 0.49(mass) + 0.47, r2 = 0.71; Moonfish or Opah (Lampris guttatus), OPAH (X's) (n = 31): THg = 0.92(mass) − 1.30, r2 = 0.56; Skipjack Tuna (Katsuwonus pelamis), SKJ (open squares) (n = 29): THg = 0.11(mass) + 2.11, r2 = 0.02; Yellowfin Tuna (T. albacares), YFT (open triangles) (n = 34): THg = 0.25(mass) + 1.30, r2 = 0.22; Common Dolphinfish or Mahi-mahi (Coryphaena hippurus), MAHI (open diamonds) (n = 33): THg = 0.63(mass) − 0.28, r2 = 0.39; Striped Marlin (Tetrapturus audax), MARL (crosses) (n = 30): THg = 1.85(mass) − 5.75, r2 = 0.84; Sickle Pomfret (Taractichthys steindachneri), POM (solid triangles) (n = 9): THg = 1.42(mass) − 3.02, r2 = 0.40; and Escolar (Lepidocybium flavobrunneum), WALU (solid squares) (n = 20): THg = 0.47(mass) + 0.91, r2 = 0.31.
Mean THg concentrations for the 6 predator species in this study are similar to values previously reported for fishes from the central North Pacific Ocean (5, 40). More importantly, the relative rankings of THg concentrations by species in this study are in agreement with previous work (40).
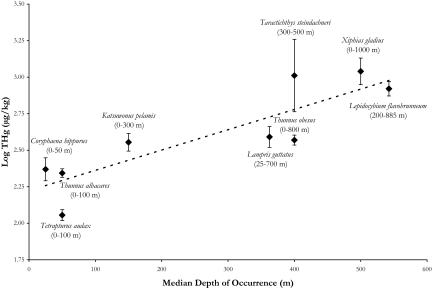
Although size and age were important determinants of mercury concentrations considerable interspecific variation in mercury concentrations remained (Fig. 1). Depth was not included in the GLM because each species was represented by a single median depth and the variables were redundant. By regressing predator median depth of occurrence with size standardized mercury concentration we explained 76% of the residual variation (P < 0.05) (Fig. 2), showing a clear trend of increasing predator mercury concentrations with increasing median depth of occurrence. Conclusions of this statistical analysis are exemplified by the shallow-dwelling species T. albacares and T. audax, (yellowfin tuna and striped marlin, respectively), which are bigger and lower in mercury contents than the deeper-dwelling species T. steindachneri and L. flavobrunneum (sickle pomfret and escolar, respectively). We clearly show that the depth at which these predators forage directly influences their mercury concentrations.
Fig. 2.
Log-transformed mean THg concentrations (μg/kg) at the mean log(mass) of 4.24 or approximately 17.4 kg plotted as a function of median depth of occurrence for 9 species of pelagic fishes. Species names are given along with their depth ranges and standard error bars are shown. The overall regression of THg concentrations as a function of median depth of occurrence is significantly positive [Log THg = 0.0014(median depth of occurrence) + 2.2217; P < 0.05, r2 = 0.76].
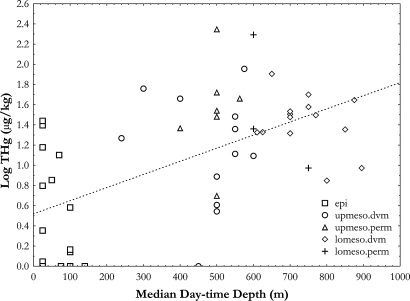
Log-transformed mean THg concentrations of prey organisms varied significantly with prey median day-time depths (i.e., the depth at which an animal spends the majority of its' time) as determined by ecological depth categories; the epipelagic prey group had significantly lower mean THg concentrations than all 4 of the remaining deeper-dwelling prey groups (ANOVA, P < 0.05). In addition, log-transformed THg concentrations and median day-time depths of prey organisms in this study were significantly positively correlated (P < 0.05, r2 = 0.34) (Fig. 3). This general trend of increasing THg concentrations with increasing depth of occurrence in the prey suggests that the depth- related trend for the predators results from deeper-ranging species having access to deeper-living prey organisms with greater mercury concentrations.
Fig. 3.
Graphical relationship between log-transformed mean THg values (μg/kg) and median day-time depth (m) for all prey organisms per ecological depth category (epi = epipelagic prey, lomeso.dvm = lower-mesopelagic migrants, lomeso.perm = lower-mesopelagic nonmigrants, upmeso.dvm = upper-mesopelagic migrants, upmeso.perm = upper-mesopelagic nonmigrants). Equation for global regression line is Log(THg) = 0.0013(median day-time depth) + 0.5191, P < 0.05, r2 = 0.34.
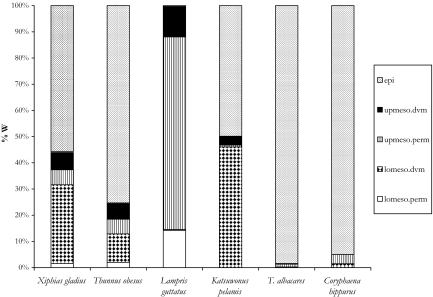
Relative to their shallow-water counterparts, the diets of the deep-ranging predators consisted of a greater numeric and gravimetric occurrence of mesopelagic prey items with day-time depths >200 m (Fig. 4). Differences in the relative importance of epi- and mesopelagic prey groups between shallow and deep-ranging predators are in agreement with conclusions from past diet studies (41–45) and evidence from tagging studies showing separation in vertical habitat utilization (24, 25, 27, 29, 31, 34, 35, 37), which are in turn explained by species-specific physiological capabilities. For example, it is well known that vascular countercurrent heat exchangers in scombrids allow tunas to access deeper colder waters while maintaining a body temperature above ambient levels (46). K. pelamis is the 1 exception; a shallow-ranging predator with a referenced depth range of 0–300 m, individuals from this study ingested a relatively high number of lower-mesopelagic prey items, almost all of which were the mesopelagic squid Sthenoteuthis oualaniensis. This squid has been found in epipelagic waters at night (47, 48) and it is likely that K. pelamis feeds on this and other deep-migrating cephalopods at night. Relative to similar-sized specimens of other shallow-ranging species (C. hippurus and T. albacares) in this study K. pelamis did display elevated mean mercury concentrations (Fig. 1).
Fig. 4.
Predatory diet composition based on identifiable prey items according to ecological depth groupings (epi, epipelagic; up.dvm, upper mesopelagic migrators; up.perm, upper mesopelagic nonmigrators; lo.dvm, lower mesopelagic migrators; lo.perm, lower mesopelagic migrators) (see Results and Discussion section of text for details). Diet is expressed as a function of the total gravimetric contribution of the prey (%W).
Ultimately, because a predator obtains its' mercury from food, evidence that deeper-dwelling prey organisms have elevated mercury concentrations means that vertical differences in prey selection accumulated over the lifetime of a predator are crucial to mercury burdens.
In the pelagic realm, an understanding of the environments where bacterial communities convert inorganic mercury to bioavailable methylmercury is critical to tracing subsequent food web movement and accumulation patterns. The present data show that upper and lower-mesopelagic prey organisms whose day-time depths coincided with depleted oxygen waters [∼600–1,000 m in the central North Pacific Ocean (49)] had elevated mercury concentrations relative to epipelagic prey organisms (Fig. 3). These results provide preliminary evidence to confirm biogeochemical studies hypothesizing enhanced mercury methylation with depth in the open ocean (14–17). Vertical profiles from the North Pacific show elevated levels of total-methylmercury (i.e., the sum of monomethylmercury and dimethylmercury) in intermediate, deeper open ocean waters, suggesting that particulate organic carbon transport and remineralization are linked to the production of organic mercury at depth (50). Similar data from open waters of the Mediterranean Sea link maximal total-methylmercury concentrations with depths of greatest oxygen consumption (51). Future work that is able to distinguish between bioavailable monomethylmercury and nonbioavailable dimethylmercury species with depth would provide further confirmation for our hypothesis.
Conclusions
After considering variability in age and size, increasing predatory mercury concentrations were clearly explained by increasing depth of occurrence. Prey mercury levels were also significantly positively correlated with day-time depth of occurrence. In agreement with established dietary studies, stomach content results from this study showed a greater relative contribution of deeper-dwelling, higher mercury containing prey in deeper-ranging predators compared to shallow-ranging. Thus, vertical differences in foraging behaviors over the lifetime of a pelagic predator are likely to be directly responsible for total mercury burdens.
Differences in vertical habitat utilization by co-occurring species of commercially important pelagic fishes have formed the basis of numerous management questions (52). Understanding the trophic ecology of these economically exploited predators is crucial if they are to be efficiently managed for the sustainable use of future generations. Results from this study may directly benefit fishery managers using ecosystem-based management strategies by offering predictive value to defining large-scale trophic links and the flow of organic matter and trace elements within marine pelagic ecosystems. Results of this study also provide the fish-consuming public with information about the mercury contents of popularly eaten marine fish species. Finally, our data support recent conclusions that the main source of methylmercury in the open ocean is from the deep water column (50, 51) and not export from coastal regions (13) or the euphotic zone (53).
Materials and Methods
Sampling Methods.
Prey samples were collected using mid-water trawls (0–650 m) in April through May of 2007 and 2008 at Cross Seamount near Hawaii (located ≈295 km south of Oahu at 18°45′ N, 158°15′ W). Mixed zooplankton samples (1–2 mm size) were collected from Station ALOHA (22.5° N, 158° W) in the central North Pacific Ocean with a 1-m2 plankton net with 202-μm mesh using oblique tows from the surface to 175 m depth (54).
Predator stomach and tissue samples were primarily collected by trained fishery observers of NOAA's Hawaii Observer Program, working on commercial vessels operating in the central North Pacific Ocean. Observers recorded species, forklength, sex, and date. Predator samples were also collected onshore from recreational boat captains in a similar manner. L. guttatus specimens captured in the central North Pacific Ocean were sampled from a local seafood wholesaler. T. audax, T. steindachneri, and L. flavobruneum were sampled directly from the Honolulu Fish Auction, as in Kaneko and Ralston (40). Catch locations of predators were all within a section of the central North Pacific Ocean bounded by 35 and 10° N latitude, and 190 and 215° W longitude.
Stomach Content Analysis.
Using standard stomach content analysis protocols (55) predator stomachs were analyzed for diet composition relative to prey vertical habitat. The percent contribution of the total weight of the prey for each ecological prey category was summarized for each predator (% W).
Analytical Methods.
Predator and prey white muscle tissue samples were analyzed for total mercury (THg) using atomic absorption spectrophotometry (Direct Mercury Analyzer, Milestone) (56, 57). Drying temperatures and decomposition times were chosen based on Milestone's recommendations for fish tissue. Methylmercury on average comprises more than 95% of all mercury present in fish tissue (58), thus THg measurements in this study serve as a viable proxy for methylmercury levels. All concentrations are reported on a wet-weight basis.
Primary and daily calibration of the instrument was performed using prepared aqueous standards over both the low (0–20 ng Hg) and high (20–1,000 ng Hg) working ranges. Method blanks and standard reference materials (SRM) (TORT-2 (0.27 ± 0.06 mg/kg Hg) for the low working range; ERM-CE464 (5.24 ± 0.10 mg/kg Hg) for the high working range) were analyzed at the end of every 10 samples to assess instrument accuracy and assure calibration curve stability. Prepared aqueous mercury standards were also used during analysis to verify the working calibration curve. To assess precision, 2 replicate analyses were performed at the beginning of every 10 samples; if the relative percent difference was within 5%, THg values for the remaining samples were used. To prevent carryover, blanks were analyzed between samples expected to have high THg concentrations.
Supplementary Material
Acknowledgments.
We thank J. Pitz, A. Asato, and S. Bailey for assistance with THg analyses; K. Busscher, observers of the PIRO Longline Observer Program, E. Grabowski, P. Lethaby, B. Takenaka, M. Lee, R. Domokos, R/V Oscar Elton Sette Crew, M. Musyl, D. Curran, S. Lee, and L. De Forest for sample collection; R. Young, V. Allain, and C. Sanchez for stomach content assistance; A. Taylor, A. Nielsen, J. Polovina, and D. Kobayashi for statistical help; and G. Ravizza, M. McManus, B. Fry, R. Mason, B. Graham, N. Ralston, and J. Sibert for helpful criticisms. This work was supported by University of Hawaii Sea Grant College Program Development Award RFM-27PD, the State of Hawaii Department of Health Chemical Terrorism Laboratory, and Cooperative Agreement NA17RJ1230 between the Joint Institute for Marine and Atmospheric Research and the National Oceanic and Atmospheric Administration Pelagic Fisheries Research Program. A subset of the data was supported by National Oceanic and Atmospheric Administration Awards NA05NMF4521112 and NA06NMF4520222 to PacMar.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900711106/DCSupplemental.
References
- 1.Morel F, Kraepiel A, Amyot M. The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst. 1998;29:543–566. [Google Scholar]
- 2.Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer AM. In: Ecotoxicology of Mercury: Handbook of Ecotoxicology. 2nd Ed. Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr, editors. Boca Raton, FL: CRC; 2003. pp. 409–463. [Google Scholar]
- 3.Peterson C, Klawe W, Sharp G. Mercury in tunas: A review. Fish Bull. 1973;71:603–613. [Google Scholar]
- 4.Rasmussen RS, Nettleton JA, Morrissey MT. A review of mercury in seafood special focus on tuna. J Aquat Food Prod Tech. 2005;14:71–100. [Google Scholar]
- 5.Kraepiel AM, Keller K, Chin HB, Malcolm EG, Morel FM. Sources and variations of mercury in tuna. Environ Sci Technol. 2003;37:5551–5558. doi: 10.1021/es0340679. [DOI] [PubMed] [Google Scholar]
- 6.Lindberg S, et al. A synthesis of progress and uncertainties in attributing the sources of mercury deposition. Ambio. 2007;36:19–32. doi: 10.1579/0044-7447(2007)36[19:asopau]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Rivers JB, Pearson JE, Shultz CD. Total and organic mercury in marine fish. Bull Environ Contam Toxicol. 1972;8:257–266. doi: 10.1007/BF01684554. [DOI] [PubMed] [Google Scholar]
- 8.Colaco A, Bustamante P, Fouquet Y, Sarradin PM, Serrao-Santos R. Bioaccumulation of Hg, Cu, and Zn in the Azores triple junction hydrothermal vent fields food web. Chemosphere. 2006;65:2260–2267. doi: 10.1016/j.chemosphere.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Power M, Klein GM, Guiguer KRRA, Kwan MKH. Mercury accumulation in the fish community of a sub-Arctic lake in relation to trophic position and carbon sources. J Appl Ecol. 2002;39:819–830. [Google Scholar]
- 10.Cai Y, Rooker JR, Gill GA, Turner JP. Bioaccumulation of mercury in pelagic fishes from the Northern Gulf of Mexico. Can J Fish Aquat Sci. 2007;64:458–469. [Google Scholar]
- 11.Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD. Algal blooms reduce the uptake of toxic methylmercury in freshwater food webs. Proc Natl Acad Sci USA. 2002;99:4419–4423. doi: 10.1073/pnas.072531099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boush GM, Thieleke JR. Total mercury content in yellowfin and bigeye tuna. Bull Environ Contam Toxicol. 1983;30:291–297. doi: 10.1007/BF01610135. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald WF, Lamborg CH, Hammerschmidt CR. Marine biogeochemical cycling of mercury. Chem Rev. 2007;107:641–662. doi: 10.1021/cr050353m. [DOI] [PubMed] [Google Scholar]
- 14.Gill GA, Fitzgerald WF. Vertical mercury distributions in the oceans. Geochim Cosmochim Acta. 1988;52:1719–1728. [Google Scholar]
- 15.Mason RP, Fitzgerald WF. Alkylmercury species in the Equatorial Pacific. Nature. 1990;347:457–459. [Google Scholar]
- 16.Mason RP, Fitzgerald WF. Mercury speciation in open ocean waters. Water Air Soil Poll. 1991;56:779–789. [Google Scholar]
- 17.Mason RP, Fitzgerald WF. The distribution and biogeochemical cycling of mercury in the Equatorial Pacific Ocean. Deep Sea Res Part I. 1993;40:1897–1924. [Google Scholar]
- 18.Mason RP, Reinfelder JR, Morel FMM. The uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ Sci Technol. 1996;30:1835–1845. [Google Scholar]
- 19.Monteiro LR, Furness RW. Accelerated increase in mercury contamination in North Atlantic mesopelagic food chains as indicated by time series of seabird feathers. Environ Toxicol Chem. 1997;16:2489–2493. [Google Scholar]
- 20.Thompson DR, Furness RW, Monteiro LR. Seabirds as biomonitors of mercury inputs to epipelagic and mesopelagic marine food chains. Sci Tot Environ. 1998;213:307–315. [Google Scholar]
- 21.Monteiro LR, Costa V, Furness RW, Santos RS. Mercury concentrations in prey fish indicate enhanced bioaccumulation in mesopelagic environments. Mar Ecol Prog Ser. 1996;141:21–25. [Google Scholar]
- 22.Spry DJ, Wiener JG. Metal bioavailability and toxicity to fish in low-alkalinity lakes: A critical review. Environ Poll. 1991;71:243–304. doi: 10.1016/0269-7491(91)90034-t. [DOI] [PubMed] [Google Scholar]
- 23.Hall BD, Bodaly RA, Fudge RJP, Rudd JWM, Rosenberg DM. Food as the dominant pathway of methylmercury uptake by fish. Water Air Soil Poll. 1997;100:13–24. [Google Scholar]
- 24.Musyl MK, et al. Vertical movements of bigeye tuna (Thunnus obesus) associated with islands, buoys, and seamounts near the main Hawaiian Islands from archival tagging data. Fish Oceanog. 2003;12:152–169. [Google Scholar]
- 25.Polovina JJ, Hawn D, Abecassis M. Vertical movement and habitat of opah (Lampris guttatus) in the Central North Pacific recorded with pop-up archival tags. Mar Biol. 2007;153:257–267. [Google Scholar]
- 26.Palko BJ, Beardsley GL, Richards W. Synopsis of the biological data on dolphinfishes, Coryphaena hippurus and Coryphaena equiselis. NMFS Circ. 1982:443. NOAA Technical Reports. [Google Scholar]
- 27.Brill RW, et al. Horizontal movements and depth distribution of large adult yellowfin tuna (Thunnus albacares) near the Hawaiian Islands, recorded using ultrasonic telemetry: Implications for the physiological ecology of pelagic fishes. Mar Biol. 1999;133:395–408. [Google Scholar]
- 28.Sippel TJ, Davie PS, Holdsworth JC, Block BA. Striped marlin (Tetrapturus audax) movements and habitat utilization during a summer and autumn in the Southwest Pacific Ocean. Fish Oceanogr. 2007;16:459–472. [Google Scholar]
- 29.Brill R, et al. Vertical and horizontal movements of striped marlin (Tetrapturus audax) near the Hawaiian Islands, determined by ultrasonic telemetry, with simultaneous measurement of oceanic currents. Mar Biol. 1993;117:567–576. [Google Scholar]
- 30.Barkley RA, Neill WH, Gooding RM. Skipjack tuna, Katsuwonus pelamis, habitat based on temperature and oxygen requirements. Fish Bull. 1978;76:653–662. [Google Scholar]
- 31.Schaefer KM, Fuller DW. Vertical movement patterns of skipjack tuna (Katsuwonus pelamis) in the Eastern Equatorial Pacific Ocean, as revealed with archival tags. Fish Bull. 2007;105:379–389. [Google Scholar]
- 32.Nakano H, Okazaki M, Okamoto H. Analysis of catch depth by species for tuna longline fisheries based on catch by branch lines. Bull Nat Res Inst Far Seas Fish. 1997;34:43–62. [Google Scholar]
- 33.Boggs CH. Depth, capture time, and hooked longevity of longline-caught pelagic fish: timing bites of fish with chips. Fish Bull. 1992;90:642–658. [Google Scholar]
- 34.Schaefer KM, Fuller DW. Movements, behavior, and habitat selection of bigeye tuna (Thunnus obesus) in the Eastern Equatorial Pacific, ascertained through archival tags. Fish Bull. 2002;100:765–788. [Google Scholar]
- 35.Kerstetter DW, Rice PH, Snodgrass D, Prince ED. Behavior of an escolar Lepidocybium flavobrunneum in the windward passage as determined by popup satellite archival tagging. Gulf Caribb Res. 2007;20:97–102. [Google Scholar]
- 36.Nakamura I, Parin NV. FAO species catalogue, Vol. 15: Snake mackerels and cutlassfishes of the world (Families Gempylidae and Trichiuridae) FAO Fisheries Synopsis. 1993 [Google Scholar]
- 37.Carey FG, Robison BH. Daily patterns in the activities of swordfish, Xiphias gladius, observed by acoustic telemetry. Fish Bull. 1981;79:277–292. [Google Scholar]
- 38.Yamashita Y, Omura Y, Okazaki E. Total mercury and methylmercury levels in commercially important fishes in Japan. Fish Sci. 2005;71:1029–1035. [Google Scholar]
- 39.Kojadinovic J, Potier M, Le Corre M, Cosson RP, Bustamante P. Mercury content in commercial pelagic fish and its risk assessment in the Western Indian Ocean. Sci Tot Environ. 2006;366:688–700. doi: 10.1016/j.scitotenv.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Kaneko JJ, Ralston NVC. Selenium and mercury in pelagic fish in the central North Pacific near Hawaii. Biol Trace Elem Res. 2007;119:242–254. doi: 10.1007/s12011-007-8004-8. [DOI] [PubMed] [Google Scholar]
- 41.Moteki M, Arai M, Tsuchiya K, Okamoto H. Composition of piscine prey in the diet of large pelagic fish in the Eastern tropical Pacific Ocean. Fish Sci. 2001;67:1063–1074. [Google Scholar]
- 42.Markaida U, Hochberg FG. Cephalopods in the diet of swordfish (Xiphias gladius) caught off the west coast of Baja California, Mexico. Pac Sci. 2005;59:25. [Google Scholar]
- 43.King JE, Ikehara I. Comparative study of food of bigeye and yellowfin tuna in the Central Pacific. Fish Bull. 1956;57:61–81. [Google Scholar]
- 44.Olson RJ, Galvan-Magana F. Food habits and consumption rates of common dolphinfish (Coryphaena hippurus) in the Eastern Pacific Ocean. Fish Bull. 2002;100:279–298. [Google Scholar]
- 45.Hida TS. Food of tunas and dolphins (Pisces: Scombridae and Coryphaenidae) with emphasis on the distribution and biology of their prey Stolephorus buccaneeri (Engraulidae) Fish Bull. 1973;71:135–143. [Google Scholar]
- 46.Carey FG, Teal JM. Heat conservation in tuna fish muscle. Proc Natl Acad Sci USA. 1966;56:1461–1469. doi: 10.1073/pnas.56.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clarke MR. Review of the systematics and ecology of oceanic squids. Adv Mar Biol. 1966;4:91–300. [Google Scholar]
- 48.Young RE. A brief review of the biology of the oceanic squid, Symplectoteuthis oualaniensis (Lesson) Comp Biochem Phys. 1975;52B:141–143. doi: 10.1016/0305-0491(75)90129-7. [DOI] [PubMed] [Google Scholar]
- 49.Riser SC, Johnson KS. Net production of oxygen in the subtropical ocean. Nature. 2008;451:323–325. doi: 10.1038/nature06441. [DOI] [PubMed] [Google Scholar]
- 50.Sunderland EM, Krabbenhoft DP, Moreau JW, Strode SA, Landing WM. Mercury sources, distribution, and bioavailability in the North Pacific Ocean: Insights from data and models. Glob Biogeochem Cycles. 2009;23 doi: 10.1029/2008GB003425. [Google Scholar]
- 51.Cossa D, Averty B, Pirrone N. The origin of methylmercury in Mediterranean waters. Limnol Oceanogr. 2009;54:837–844. [Google Scholar]
- 52.Brill R, Lutcavage M. Understanding environmental influences on movements and depth distributions of tunas and billfishes can significantly improve population assessments. Am Fish Soc Symp. 2001;25:179–198. [Google Scholar]
- 53.Monperrus M, et al. Mercury methylation, demethylation and reduction rates in coastal and marine surface waters of the Mediterranean Sea. Mar Chem. 2007;107:49–63. [Google Scholar]
- 54.Hannides CCS, Popp BN, Landry MR, Graham BS. Quantification of zooplankton trophic position in the North Pacific Subtropical Gyre using stable nitrogen isotopes. Limnol Oceanog. 2009;54:50–61. [Google Scholar]
- 55.Drazen J, Buckley T, Hoff G. The feeding habits of slope dwelling macrourid fishes in the eastern North Pacific. Deep Sea Res Part I. 2001;48:909–935. [Google Scholar]
- 56.Costley CT, et al. Determination of mercury in environmental and biological samples using pyrolysis atomic absorption spectrometry with gold amalgamation. Anal Chim Acta. 2000;405:179–183. [Google Scholar]
- 57.Cizdziel JV, Hinners TA, Heithmar EM. Determination of total mercury in fish tissues using combustion atomic absorption spectrometry with gold amalgamation. Water Air Soil Poll. 2002;135:355–370. [Google Scholar]
- 58.Bloom NS. On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci. 1992;49:1131–1140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.