Abstract
Filamentous fungi are indispensable biotechnological tools for the production of organic chemicals, enzymes, and antibiotics. Most of the strains used for industrial applications have been—and still are—screened and improved by classical mutagenesis. Sexual crossing approaches would yield considerable advantages for research and industrial strain improvement, but interestingly, industrially applied filamentous fungal species have so far been considered to be largely asexual. This is also true for the ascomycete Trichoderma reesei (anamorph of Hypocrea jecorina), which is used for production of cellulolytic and hemicellulolytic enzymes. In this study, we report that T. reesei QM6a has a MAT1-2 mating type locus, and the identification of its respective mating type counterpart, MAT1-1, in natural isolates of H. jecorina, thus proving that this is a heterothallic species. After being considered asexual since its discovery more than 50 years ago, we were now able to induce sexual reproduction of T. reesei QM6a and obtained fertilized stromata and mature ascospores. This sexual crossing approach therefore opens up perspectives for biotechnologically important fungi. Our findings provide a tool for fast and efficient industrial strain improvement in T. reesei, thus boosting research toward economically feasible biofuel production. In addition, knowledge of MAT-loci and sexual crossing techniques will facilitate research with other Trichoderma spp. relevant for agriculture and human health.
Keywords: biofuels, Hypocrea jecorina, mating type, female sterility, cellulase
The increasing awareness for the limited supply of fossil fuels, accompanied by the recent rise of energy costs and an imminent climate change has recently led to increased research efforts toward development of biofuels (1). As one of the most prolific cellulase producers, Trichoderma reesei represents an ideal model system to study the regulation of plant cell wall degrading enzymes, which play an important role in conversion of cellulosic waste material into glucose, which is then fermented to bioethanol by yeast. Using this approach, lignocellulosic biomass from agricultural byproducts or even municipal solid waste can become an environmentally compatible energy source for the future. However, the efficiency of the respective enzyme mixtures still needs considerable improvement to render this process economically feasible (2, 3).
The enzyme producer T. reesei is unique among industrially applied fungi, because it is solely known from the single wild-type isolate QM6a, which was originally isolated from the Solomon islands in World War II because of its degradation of canvas and garments of the US army (4). All strains used nowadays in biotechnology and basic research have been derived from this one isolate. Presently, strain development in this fungus is a major focus of industrial research. Besides the commonly used classical mutagenesis approaches using UV light or mutagenic chemicals, a broad array of genetic engineering techniques and DNA-mediated transformation systems have been developed for T. reesei to improve the enzyme production capacity of QM6a-derived strains. However, similar to other industrially important fungi, classical genetic approaches using sexual crossings, as have been established for the model fungi Aspergillus nidulans or Neurospora crassa, are unavailable.
The genus Trichoderma/Hypocrea contains several hundred species, some of which only occur as teleomorphs, i.e., in their sexual form, whereas others have so far only been observed as asexually propagating anamorphs (5). In the last decade, the use of DNA-based molecular phylogenetic approaches has succeeded in the identification of anamorph-teleomorph relationships for several fungi (including Trichoderma spp.) that were so far believed to occur only in an asexual form. However, only few of these could be mated under laboratory conditions (6). Using gene sequence analysis, Kuhls et al. (7) found that T. reesei is indistinguishable from the the pantropical ascomycete Hypocrea jecorina. However, despite this in silico evidence, attempts to cross T. reesei with wild-type strains of H. jecorina failed, giving rise to the assumption that QM6a would be an asexual clonal lineage of H. jecorina.
Filamentous ascomycete fungi can have two mating types, MAT1-1 and MAT1-2, and these MAT loci occupy the same chromosomal location but lack sequence similarity and are thus termed “idiomorphs” rather than alleles (8). The genes necessary for signal transduction and the formation of sexual reproduction structures for both mating types are present in each genome, but are strictly regulated by the respective MAT locus. Ascomycete fungi are haploid during their vegetative life cycle and can either have a heterothallic or a homothallic sexual cycle. Heterothallic fungi need a compatible strain carrying the opposite MAT idiomorph for sex (9). Crossing experiments using single ascospore isolates of H. jecorina wild-type isolates showed a typical bipolar segregation for mating type, suggesting it to be a heterothallic species (10).
The aim of this study was to (re)address the question whether the industrial workhorse T. reesei QM6a is really an asexual clonal line. We identified the MAT1-2 mating type locus of QM6a and cloned and characterized the opposite MAT1-1 locus of a purified, sexually compatible wild-type isolate of H. jecorina. Thereby we could also confirm at a molecular level that this species is heterothallic. Our crossing approach also works for strains engineered for enhanced cellulase production. Further, we developed a technique for sexual crossing of T. reesei QM6a, the ancestor of all industrially used strains of this species, and we could consequently show that QM6a can be successfully crossed with a H. jecorina strain of MAT1-1 mating type. The presence of a sexual cycle provides an invaluable tool for classical genetic analyses, and our findings therefore will form the basis to greatly facilitate genetic work and industrial strain improvement with this fungus. In the following, we will use the name of the anamorph T. reesei for strain QM6a and mutant strains derived from it, and the name of the teleomorph H. jecorina for strains known to propagate sexually.
Results
Analysis of the Mating Type of T. reesei QM6a.
We screened the T. reesei genome database v2.0 (11) for the presence of a mating type locus. Characterization of mutants led to the identification of the mat1-2-1 gene of the Neurospora crassa MAT1–2 idiomorph, which encodes a protein with a high mobility group (HMG) domain, as the main regulator of sexual development in MAT1-2 strains. In N. crassa, Podospora anserina, and Gibberella fujikuroi, the mating type idiomorphs contain an equal set of genes (9). Tblastn searches of the T. reesei genome database with the mat1-2-1 homologs of these fungi revealed that the sequenced strain QM6a has a MAT1-2 mating type locus with an intact single ORF encoding an HMG-domain protein (Fig. 1). The corresponding T. reesei gene, mat1-2-1, denominated following the nomenclature of mating type genes of filamentous ascomycetes suggested by Turgeon and Yoder (12), was found on scaffold 6 and has protein ID 124341 in T. reesei genome database v2.0. The finding of a typical MAT1-2 locus is indicative of a heterothallic sexual lifestyle and therefore successful mating of QM6a would require a strain of the opposite MAT1-1 mating type.
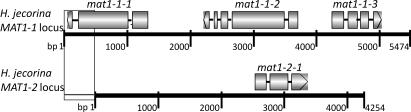
Fig. 1.
Mating type idiomorphs of H. jecorina. The MAT1-1 locus has a length of ≈5.5 kb and contains three genes, mat1-1-1, mat1-1-2, mat1-1-3, and MAT1-2 has a length of ≈4.3 kb and contains one gene, mat1-2-1. The exons of genes are shown as boxes, connected by lines that represent the introns. The 3′-end of mat1-1-1, present in strains of both mating types, is marked with a gray box. The exon/intron structures are drawn to scale.
Isolation of Both Mating Types from H. jecorina.
To study the potential for sexual reproduction of T.reesei QM6a, elucidation of the H. jecorina MAT1-1 mating type was essential. To find the MAT1-1 locus of H. jecorina, we made use of single ascospore cultures of H. jecorina CBS999.97. This strain was reported to be able to form mature stromata (fruiting bodies) on agar plates under laboratory conditions (10). Stromata, the sexual reproduction structures of Hypocrea spp. (13) are macroscopical structures consisting of hyphal mass that is pigmented at the surface, into which the actual fruiting bodies, the perithecia, are embedded. In these perithecia, the asci containing the ascospores, derived from the sexual recombination process, grow, mature, and are ejected upon maturation. Lieckfeldt and coworkers (10) had reported that H. jecorina isolate CBS999.97 produced stromata without the apparent presence of a mating partner on agar plates, which indicated that this isolate was either homothallic or a vegetatively compatible mixture of both mating types. Because the results of our genome analysis pointed toward a heterothallic lifestyle of T. reesei/H. jecorina, we tried to obtain strains with a MAT1-1 and MAT1-2 mating type from the ascospore progeny of H. jecorina CBS999.97. The strain was cultivated under daylight conditions and produced stromata in a regular pattern on the whole agar plate (Fig. S1A). Ascospores were collected from the lids of the Petri dishes (Fig. 2J), and single ascospore cultures were purified. In contrast to other ascomycota, which can produce stromata independently of a sexual stimulus and need the mating partner only for fertilization of the perithecia, H. jecorina cultures derived from single ascospores did not produce stromata anymore when they were grown alone on agar plates (Fig. S1B). Instead, in crossing experiments, they formed fertilized stromata in approximately half of the crosses at the interaction zones, indicating a bipolar segregation of mating type (Fig. S1 C and D). This confirmed that H. jecorina is indeed heterothallic, and we now had purified strains with a MAT1-1 and MAT1-2 mating type, which we used for further molecular biological studies to elucidate mating in H. jecorina. In the following, we will refer to the respective strains, derived from isolate CBS999.97, as H. jecorina MAT1–1 and MAT1–2. It should be noted that formation of fruiting bodies was only observed in cultures that were incubated in the presence of light, whereas no stromata were formed in cultures cultivated in constant darkness, indicating that light is essential for the sexual cycle in H. jecorina.
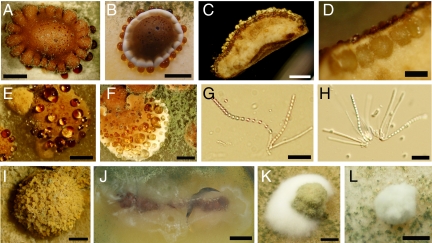
Fig. 2.
Morphological characteristics of H. jecorina fruiting bodies. (A–F) Stromata (A and B) with perithecia embedded in the upper surface (C and D) and droplets of liquid forming on their surface (E and F). (G–J) Asci containing 16 bipart ascospores (G and H). Ascospores are either squeezed out of perithecia as a yellow substance under dry conditions (I) or shot to the lid of Petri dishes where they become visible as a white haze (J). (K and L) Unfertilized fruiting bodies. [Scale bars: 2 mm (A–C, E, I, K, L); 0.5 mm (D); 1 mm (F); 30 μm (G and H); 1 cm (J).]
Analysis of the MAT-Loci of H. jecorina.
To clone and analyze the MAT1-1 locus from purified H. jecorina MAT1–1 strains, we first needed to determine the borders and size of the T. reesei MAT1-2 locus, to be able to design primers binding to its conserved flanking regions because MAT-idiomorphs occupy the same genomic region in the respective strains. We therefore compared genomes of N. crassa and Gibberella species for genes flanking the MAT-loci and searched for the corresponding orthologs in the T. reesei genome database. The respective genes encode a putative DNA lyase (T. reesei protein ID 59147) and a conserved hypothetical protein (T. reesei protein ID 76930). In the genome databases of the mycoparasitic fungi Trichoderma atroviride IMI206040 and Trichoderma virens Gv29–8, which also turned out to have a MAT1-2 mating type, the same orthologous proteins were detected flanking the MAT-loci. From the H. jecorina MAT1–1 and MAT1–2 strains the MAT-loci of both mating types were consequently amplified (all primers used in this study are listed in Table S1). The obtained 10.7- and 9.4-kb (MAT1-2) PCR fragments were subjected to restriction digestions, using three different enzymes, which gave identical patterns for T. reesei QM6a and H. jecorina MAT1–2, respectively, but different patterns for the MAT1–1 strain. The results indicated that there are no major differences at the MAT1-2 locus between the sexually and asexually propagating strains (Fig. S2). Further, sequencing of the mat1-2-1 gene revealed identical sequences for QM6a and H. jecorina MAT1–2. The complete MAT1-1 locus was also sequenced and deposited in DDBJ/EMBL/GenBank, accession number FJ599756. It has a total length of 5.5 kb—compared with 4.2 kb of the MAT1-2 locus—and contains three genes of which the exact ORFs, including exon/intron structures and 5′- and 3′-untranslated regions were assessed by RACE-PCR and RT-PCR (Fig. 1). In analogy to other heterothallic ascomycetes (9), mat1-1-1 encodes an alpha-domain protein (380 aa), mat1-1-2 an A2-domain protein (434 aa), and mat1-1-3 a HMG protein (205 aa). Further, we detected that 486 bp of the 3′-end of mat1-1-1 are present in both idiomorphs, but this is unlikely to be a functional gene in the MAT1-2 locus because no translational start point could be detected for this fragment and the MAT1-2 sequence adjacent to the shared region contains several stop codons in all reading frames.
Mating Types of a Worldwide Collection of H. jecorina Isolates.
Having assessed the sequences of both mating type loci from H. jecorina, we designed mating type-specific primers for diagnostic PCR. We studied the distribution of mating types among a collection of H. jecorina isolates from different geographical locations. Of the available 27 H. jecorina isolates, 13 had a MAT1-1 locus and 14 a MAT1-2 locus (Table 1). Based on the equal distribution of mating types, it can be assumed that these populations propagate sexually in their original habitats.
Table 1.
Mating types of H. jecorina isolates
| H. jecorina isolate* | Mating type | Origin |
|---|---|---|
| South America | ||
| G.J.S. 86–404† | MAT1–1 | French Guiana |
| G.J.S. 86–408† | MAT1–2 | French Guiana |
| G.J.S. 89–7† | MAT1–2 | Brazil, Para |
| G.J.S. 86–410† | MAT1–1 | French Guiana |
| G.J.S. 88–6† | MAT1–2 | Brazil, Para |
| G.J.S. 97–177† | MAT1–2 | Brazil, Para |
| G.J.S. 97–178† | MAT1–1 | Brazil, Para |
| AYR 2896,DAOM 220850, 220846† | MAT1–2 | French Guiana |
| CTR 72–94† | MAT1–1 | French Guiana |
| G.J.S. 84–473,DAOM 220886† | MAT1–1 | French Guiana |
| G.J.S. 87–7,CBS 836.91 | MAT1–2 | French Guiana |
| G.J.S. 86–403† | MAT1–1 | French Guiana |
| G.J.S. 97–38,CBS 999.97 | MAT1–1/MAT1–2 | French Guiana |
| Macronesia | ||
| G.J.S. 85–249† | MAT1–1 | Indonesia, Celebes |
| G.J.S. 85–229,DAOM 220794† | MAT1–2 | Indonesia, Celebes |
| G.J.S. 85–230† | MAT1–2 | Indonesia, Celebes |
| G.J.S. 85–236† | MAT1–2 | Indonesia, Celebes |
| G.J.S. 85–238† | MAT1–2 | Indonesia, Celebes |
| CBS 881.96 | MAT1–1 | Papua New Guinea |
| Micronesia | ||
| G.J.S. 93–23† | MAT1–2 | New Caledonia |
| G.J.S. 93–24† | MAT1–1 | New Caledonia |
| G.J.S. 93–22† | MAT1–2 | New Caledonia |
| CBS 383.78(= QM6a)† | MAT1–2 | Solomon Islands |
| Central America | ||
| G.J.S. 96–401 | MAT1–2 | Puerto Rico |
| G.J.S. 95–82 | MAT1–1 | Puerto Rico |
| G.J.S. 95–2081 | MAT1–1 | Puerto Rico |
| G.J.S. 95–2082 | MAT1–1 | Puerto Rico |
| G.J.S. 95–123 | MAT1–1 | Puerto Rico |
*G.J.S., collection of G. J. Samuels, USDA Beltsville, USA; CBS, Centraalbureau voor Schimmelcultures, Leiden, The Netherlands; CTR, collection of C.T. Rogerson, Botanical Museum of New York, USA; DAOM, Canadian Fungal Culture Collection, Ottawa. Canada.
Morphology of H. jecorina Sexual Reproduction Structures.
Morphological characteristics of the sexual reproduction structures of H. jecorina are shown in Fig. 2. H. jecorina stromata are highly variable in size, shape and color. The size can range from a diameter of 3–4 mm up to 2 cm. Stromata have a dark brown pigmentation and perithecia are embedded into their upper surface. Large droplets of liquid can form on and around the stromata during maturation of the fruiting bodies. After 1–2 weeks, mature ascospores are either squeezed out of the perithecia as a yellow substance or, depending on the humidity, ascospores are shot to the lid of the agar plate where they can be seen as a white haze. The asci contain 16-part ascospores, as has been described for Hypocrea spp. (13). Occasionally, undifferentiated early forms in fruiting body development can be found on unfertilized H. jecorina plates. They appear as fluffy structures and later on become covered with asexual conidia.
Sexual Development of T. reesei QM6a.
After the finding that T. reesei QM6a has a MAT1-2 locus, mating experiments were set up between strains QM6a and H. jecorina MAT1–1 using different growth conditions and taking into account the requirement of light for sexual reproduction in H. jecorina (see above). We were able to obtain stromata after 7–10 days at the respective interaction zones of T. reesei QM6a and H. jecorina MAT1–1 in all cases, independent of the growth medium or the temperature used (Fig. 3A). Microscopical analysis confirmed that the stromata were fertilized, the fruiting bodies had the same appearance as upon mating between H. jecorina MAT1–1 and MAT1–2 (Fig. 3 B and C), the frequency of ascospore germination was completely normal. To confirm that sexual recombination had occurred, a T. reesei mutant strain containing a single copy of the hygromycin-phosphotransferase gene hph (14) was used for crossing experiments. Approximately 50% of the progeny were resistant to hygromycin B, hence confirming that mating between the two strains had occurred. As expected, no formation of stromata was detected upon mating of QM6a with MAT1-2 strains (Fig. 3D).
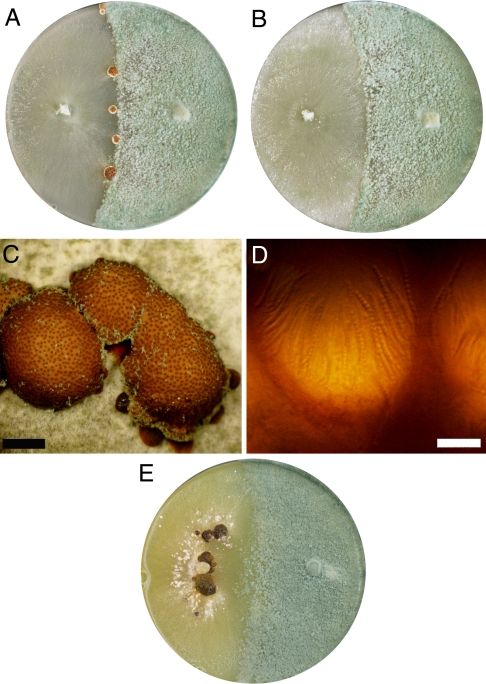
Fig. 3.
Mating with T. reesei QM6a. (A) H. jecorina MAT1–1 was crossed with QM6a on agar plates, and the formation of stromata was observed at the interaction zone of the fungal colonies. (B) No stromata were formed upon mating of QM6a with MAT1-2 strains. (C) The stromata have a typical appearance for H. jecorina and perithecia, which can be seen as small dark dots, are embedded into their upper surface. (Scale bar: 2 mm.) (D) Fertilized perithecia contain asci with 16 part-ascospores. (Scale bar: 2 mm.) (E) In crossings of T. reesei QM6a with the unpurified H. jecorina CBS999.97 isolate, often no stromata were formed.
It should be noted that in crossings of T. reesei QM6a with the unpurified H. jecorina CBS999.97 strain, also often no stromata were observed at the interaction zone (Fig. 3E), suggesting that sexual reproduction occurred preferably between H. jecorina wild-type strains in the unpurified isolate than between CBS999.97 and QM6a. This could possibly be due to a lower or absent expression of genes related to the sexual cycle in QM6a, which might impair the efficient induction of the sexual cycle between CBS999.97 and QM6a.
Mating of Strains Engineered for Enhanced Cellulase Production.
Having identified a mating partner for T. reesei QM6a, we now tested whether two strains mutated for enhanced cellulase production, RUT-C30 and QM9414, and a uridine auxotrophic strain frequently used for genetic transformation, TU-6, had maintained the ability to mate with a MAT1-1 strain. Although the common ancestor of these strains is QM6a, strains RUT-C30 and QM9414 were obtained independently during different mutation programs. We could confirm that crossing indeed was successful with all of them, despite the harsh treatments used during mutagenesis of these strains and hence that this method is feasible for use with industrial mutants to engineer superior production strains.
Conversion of Mating Types in H. jecorina and T. reesei.
To be able to cross industrial strains—all of them are derived from QM6a and thus have a MAT1-2 locus—it would be convenient to be able to switch their mating type. We therefore proposed to replace the MAT1-2 locus of T. reesei QM6a with the entire MAT1-1 locus amplified from the respective CBS999.97-derived strain. As a first step, it was necessary to assess whether it is possible at all to change the mating type in H. jecorina. We used the H. jecorina MAT1–2 strain that was isolated in this study and transformed it with a linear 10.7-kb fragment of the MAT1-1 locus including 3.3 kb and 1.9 kb of the upstream and downstream flanking regions, respectively, in a cotransformation with a DNA-fragment that contains a hygromycin B resistance cassette (15). Hygromycin B-resistant transformants were checked by crossing them with H. jecorina MAT1–1, H. jecorina MAT1–2, and T. reesei QM6a. Thereby, transformants were obtained that indeed had their mating type converted from MAT1-2 to MAT1-1 (which was confirmed by PCR) and therefore now formed fruiting bodies upon contact with H. jecorina MAT1–2 and T. reesei QM6a but not with H. jecorina MAT1–1. Replacement of MAT1-2 with MAT1-1 was also attempted in T. reesei QM6a and QM9414. The MAT1-1 locus was successfully integrated into the genome of these strains and consequently a switched mating behavior was achieved, i.e., fruiting body formation occurred with H. jecorina MAT1–2. However, none of the respective strains was able to develop fruiting bodies upon mating with the native T. reesei QM6a. We thus hypothesize that T. reesei QM6a is able to act as male mating partner, but that QM6a cannot produce the female fruiting bodies and is thus female sterile.
Genes Involved in Sexual Development.
To possibly find an explanation for the observed female sterility, we analyzed the genome of T. reesei QM6a (http://genome.jgi-psf.org/Trire2/Trire2.home.html) for genes with a reported role in sexual development in Aspergillus, Neurospora, or yeasts (16, 17). Although putative orthologs for the respective genes in Aspergillus and Neurospora were mostly detected, we could not find them for N. crassa asd-1 and asd-3 (ascus development-1 and -3; NCU05598.3 and NCU05597.3): These genes are in immediate vicinity to each other in the N. crassa genome (http://www.broad.mit.edu/annotation/genome/neurospora/Home.html), but unfortunately the respective genomic region is not syntenic to T. reesei, and thus we cannot test whether these genes have been specifically lost in T. reesei QM6a but might be present in H. jecorina strains. However, because N. crassa mutants in these genes are still able to initiate sexual development (18), we consider it unlikely that these genes are the cause for the female sterility of T. reesei.
Discussion
Sexual reproduction is the prevalent reproduction form of eukaryotes. It is essential for the long-term population persistence of most eukaryotic species despite the fact that it requires more time and energy and is sometimes even termed less efficient than asexual reproduction (19). The evolutionary benefits that promote sexual reproduction are DNA maintenance and repair during meiosis as well as the production of increased genetic variation among offspring, which allows more efficient natural selection and elimination of deleterious mutations (19). Fungi differ from this general scheme insofar because they exhibit a huge variation in their reproduction strategies. Although most basidiomycota are obligatory sexual, and sexual development is common in ascomycota, sexuality has never been observed in other groups, most of them combined in the deuteromycota (fungi imperfecti), and they reproduce by production of asexual spores. The advent of molecular biological methods in the analysis of fungal taxonomy and population biology has, however, shown that many of these “asexual” fungi (anamorphs) show genetic evidence of recombination and corresponding sexual forms (teleomorphs) have been identified based on genomic sequence patterns (20, 21).
Fungi comprise some of the most important organisms used in biotechnology for the production of enzymes and secondary metabolites (22). Although many of the fungi applied in industry are considered to reproduce asexually and fungi of medical or agricultural relevance molecular biological studies have been performed to establish anamorph-teleomorph relationships, this information has not been available so far for any biotechnologically important species. The lack of sexual recombination in these fungi is a major limiting factor for industrial strain improvement by classical strain crossing approaches. In the case of recombinant fungal strains containing resistance markers against antibiotics, the possibility of crossing would allow for straightforward removal of marker genes and hence considerably facilitate work with those fungi.
Attempts to successfully mate T. reesei QM6a with wild-type isolates of H. jecorina had previously failed despite considerable efforts, although its teleomorph H. jecorina was determined by in silico analysis more than 10 years ago. Therefore, in agreement with the “slow decline” hypothesis (23), this strain was regarded to represent a lineage having lost fertility (7). An alternative hypothesis, suggested by Ekelund and Ronn (24), which states that there may be no need for sexual development if the natural habitat provides constant environmental conditions to which the organism is adapted, can be rejected for T. reesei QM6a because the habitats of sexually propagating H. jecorina strains such as CBS999.97 show similar characteristics as the Solomon islands from which QM6a was isolated. Therefore, the so far clandestine sexual development of T. reesei may be due to inappropriate cultivation conditions or the lack of compatible mating partners under experimental conditions. The availability of a (partially) functional sexual cycle in T. reesei is of utmost significance for industrial applications and classical genetics—also for other members of the genus, which includes Trichoderma species involved in plant symbiosis (25), biocontrol, and mycoparasitism (26): In the genomes of T. atroviride (http://genome.jgi-psf.org/Triat1) and T. virens (http://genome.jgi-psf.org/Trive1), which are used in agriculture as biological control agents, a MAT1-2 mating type locus is also present. Although the sequenced strains propagate asexually under laboratory conditions, for these species, the corresponding teleomorphs, H. atroviridis and H. virens, have already been described. The identification of the second mating type of a member of the genus Hypocrea/Trichoderma provides us with a means to investigate important questions for other Hypocrea spp.: What is the impact of sexual recombination of strains on the biocontrol efficiency of Trichoderma/Hypocrea spp.? Further, similar issues can now be addressed in opportunistic human pathogenic species of the genus Hypocrea, H. orientalis/T. longibrachiatum (27–29): Does sexual development play a role in virulence (30)? Because of the considerable antibiotic resistance (29, 31) and potential lethality of the strains analyzed so far, detailed investigations on their mechanism of virulence are indispensable for developing appropriate treatment strategies.
To facilitate crossing of industrial strains, we attempted to alter the mating type of T. reesei QM6a from MAT1-2 to MAT1-1. Changes in the reproductive mode of fungi have already been carried out in different species. Targeted gene deletion of MAT-locus genes in the homothallic Gibberella zeae rendered this fungus heterothallic (32). For Cochliobolus heterostrophus, conversion from heterothallic to homothallic lifestyle, albeit with reduced fertility, was achieved (33). In N. crassa, transformants with a replaced mating-type locus from MAT-A (MAT1-1) to MAT-a (MAT1-2) grew normally and were fertile upon crossing with MAT-A strains, but transformant strains with an ectopic integration of the MAT-a mating type locus resulted in a vegetative incompatibility reaction (34). Direct switching of a fungal strain from MAT1-2 to MAT1-1 by genetic engineering had not been reported previously. We were able to successfully change the mating type in H. jecorina MAT1–2 and in T. reesei QM6a, because the obtained transformants, containing the MAT1-1 locus, could be crossed with the original MAT1–2 strain. However, upon crossing with T. reesei QM6a, only the H. jecorinaMAT1–2→MAT1–1, but not the respective QM6aMAT1–2→MAT1–1 transformants, were able to produce fruiting bodies. We conclude from these findings, that QM6a is—independent of its mating type—not able to produce female reproduction structures and is thus female sterile.
Several mutations causing female sterility have been reported (35), but there are two possibilities why these mutations could have occurred to T. reesei QM6a: First, after its collection from the Solomon islands, QM6a was subcultivated and maintained for decades under laboratory conditions, which could have caused the loss of female fertility. However, the theory of Leslie and Klein (36) provides an alternative explanation: They suggest that in a heterothallic haploid ascomycete capable of sexual and asexual reproduction, loss-of-meiosis mutations accumulate during vegetative reproduction, which result in female sterile mutants. For these mutants, the advantage not to spend metabolic resources for production of female structures, which may never be fertilized, can even be beneficial during asexual reproduction (35). Based on the relative frequency of female fertile strains, an estimation of the relative amounts of sexual and asexual reproduction occurring in a given population is possible (36). The extent of female sterility within a population varies widely. It is tempting to speculate that female sterility of T. reesei QM6a led to efficient asexual growth, prevalence in its natural habitat, and eventually to its isolation.
Nevertheless, such a possibly beneficial mutation is a major issue in direct application of our study to industrial strain improvement, but can be circumvented with only minor additional efforts. From crossings of H. jecorina MAT1–1 with industrial strains, the progeny could be screened for the capability of sexual development and retaining of production efficiency. At the same time, elimination of unintended random mutations in the industrial mutants detrimental to, for example, growth, by complementation with the wild-type background can be beneficial even without further crossings with other production strains. For identification of altered loci in this isolate and to consequently overcome this deficiency by genetic engineering, a comparison of the complete genome sequence of QM6a with that of the fertile H. jecorina strains will be essential.
With respect to crossing of strains engineered for enhanced production efficiency, a further issue should be considered: Repeat induced point (RIP) mutation, which efficiently detects and mutates duplicated sequences and only acts during the sexual cycle (for a review see ref. 37) and was therefore not relevant in T. reesei so far. Now this process should be considered in strain improvement using our crossing approach, especially when working with strains bearing multiple copies of certain genes. Although the introduction of multiple cellulase genes or regulators is not frequently used anymore, for example additional alleles bearing point mutations or genes duplicated during strain improvement might be subject to RIP. Reduction in duplicated genes in T. reesei (11) points at a functional RIP mechanism. Hence, effects similar to N. crassa, for which this process has been studied in detail, can be expected. However, because it has been shown for N. crassa that deletion of a single gene (rid-1) (38), which has a homolog in T. reesei, is sufficient to abolish RIP, this mechanism should not significantly interfere with application of crossing approaches to industrial strain improvement with T. reesei.
Materials and Methods
Microbial Strains and Culture Conditions.
Fungal strains QM6a (13631; ATCC), G.J.S. 97–38 (CBS999.97; obtained from the Centraalbureau of Schimmelcultures, Utrecht, The Netherlands), QM9414 (26921; ATCC), RutC30 (56765; ATCC), and TU6 (MYA-256; ATCC) were maintained at 28 °C on malt extract agar plates, supplemented with 10 mM uridine for TU-6 (39), and 0.1% Triton X-100 for single spore isolation, and stock cultures were kept at −80 °C. The most favorable conditions for induction of sexual development turned out to be malt extract agar (3% wt/vol; Merck), 20–22 °C incubation temperature in a SANYO MIR-154 incubator (Sanyo Europe Ltd) set to a 12-h light–dark cycle or in daylight—both light conditions gave equal results. Sexual development was also analyzed upon growth on potato dextrose agar (PDA; Difco, BD Biosciences) and Mandels Andreotti medium (40) with glucose (1% wt/vol; Merck) as a carbon source. Single spore cultures were obtained by inoculating malt extract agar plates containing 0.1% Triton X-100 with a diluted spore solution and picking cultures grown from single spores after 1–2 days of incubation at 28 °C.
Microscopic Analyses.
For microscopic analysis of samples, we used differential interference contrast optics on an inverted T300 microscope (Nikon) and a M420 Photomacroscope (Wild; Leica). Images were captured with a Nikon DXM1200F digital camera and digitally processed using Photoshop CS3 (Adobe).
Manipulation of Nucleic Acids and Analytical PCR.
Strains were grown on malt extract agar plates overlaid with cellophane (#700–2101; VWR). Mycelia were ground to a fine powder in liquid nitrogen, and DNA was isolated as described in refs. 41 and 42, and for RNA, the guanidinium thiocyanate method (43) was used. Mating-type loci were amplified using primers in the conserved up- and downstream regions and the Long Template Expand PCR kit (Roche). Analytical PCR of the mating types of H. jecorina isolates was carried out using the GoTaq system (Promega). RACE-PCR was carried out as described by Seidl and coworkers (44), using the Creator SMART cDNA library construction kit (Clontech) followed by PCR with adapter primers from the kit and gene specific primers. Primers used in this study are listed in Table S1. PCR fragments of the MAT-loci were digested with PstI, HindIII, and SacI (Fermentas). DNA sequencing of a PCR fragment comprising the MAT1-1 locus of CBS999.97 was carried out at Eurofins MWG Operon (Ebersberg, Germany).
Transformation of H. jecorina.
Protoplast preparation and DNA-mediated transformation of T. reesei/H. jecorina strains was essentially done as described in ref. 39. The 10.7-kb MAT1-1 fragment was used for a cotransformation together with the circular plasmid pRLMex30 (15). After transformation protoplasts were stabilized and regenerated on malt extract medium containing d-sorbitol (1 M) and 50 μg/mL hygromycin B. Colonies were transferred to malt extract agar and purified by single spore isolation on selection medium.
Supplementary Material
Acknowledgments.
This work was supported by Austrian Science Fund Grant P-20004 (to M.S.) and Hertha Firnberg Program Fellowship T390-B03 (to V.S.). M.S. is recipient of an APART Fellowship (Grant 11212) of the Austrian Academy of Sciences at the Institute of Chemical Engineering, Vienna University of Technology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession no. FJ599756) (MAT1–1 locus) or are available at the Trichoderma reesei genome database v2.0 (http://genome.jgi-psf.org/Trire2/Trire2.home.html) under protein ID 124341 (mat1-2-1).
This article contains supporting information online at www.pnas.org/cgi/content/full/0904936106/DCSupplemental.
References
- 1.Farrell AE, et al. Ethanol can contribute to energy and environmental goals. Science. 2006;311:506–508. doi: 10.1126/science.1121416. [DOI] [PubMed] [Google Scholar]
- 2.Patel-Predd P. Overcoming the hurdles to producing ethanol from cellulose. Environ Sci Technol. 2006;40:4052–4053. [PubMed] [Google Scholar]
- 3.Sticklen MB. Plant genetic engineering for biofuel production: Towards affordable cellulosic ethanol. Nat Rev Genet. 2008;9:433–443. doi: 10.1038/nrg2336. [DOI] [PubMed] [Google Scholar]
- 4.Reese ET. History of the cellulase program at the U.S. army Natick Development Center. Biotechnol Bioeng Symp. 1976:9–20. [PubMed] [Google Scholar]
- 5.Druzhinina IS, Kopchinskiy AG, Kubicek CP. The first 100 Trichoderma species characterized by molecular data. Mycoscience. 2006;47:55–64. [Google Scholar]
- 6.Samuels GJ. Trichoderma: Systematics, the sexual state, and ecology. Phytopathology. 2006;96:195–206. doi: 10.1094/PHYTO-96-0195. [DOI] [PubMed] [Google Scholar]
- 7.Kuhls K, et al. Molecular evidence that the asexual industrial fungus Trichoderma reesei is a clonal derivative of the ascomycete Hypocrea jecorina. Proc Natl Acad Sci USA. 1996;93:7755–7760. doi: 10.1073/pnas.93.15.7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metzenberg RL, Glass NL. Mating type and mating strategies in Neurospora. Bioessays. 1990;12:53–59. doi: 10.1002/bies.950120202. [DOI] [PubMed] [Google Scholar]
- 9.Poggeler S. Mating-type genes for classical strain improvements of ascomycetes. Appl Microbiol Biotechnol. 2001;56:589–601. doi: 10.1007/s002530100721. [DOI] [PubMed] [Google Scholar]
- 10.Lieckfeldt E, Kullnig CM, Samuels GJ, Kubicek CP. Sexually competent, sucrose- and nitrate-assimilating strains of Hypocrea jecorina (Trichoderma reesei) from South American soils. Mycologia. 2000;92:374–380. [Google Scholar]
- 11.Martinez D, et al. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina) Nat Biotechnol. 2008;26:553–560. doi: 10.1038/nbt1403. [DOI] [PubMed] [Google Scholar]
- 12.Turgeon BG, Yoder OC. Proposed nomenclature for mating type genes of filamentous ascomycetes. Fungal Genet Biol. 2000;31:1–5. doi: 10.1006/fgbi.2000.1227. [DOI] [PubMed] [Google Scholar]
- 13.Samuels GJ, Petrini O, Manguin S. Morphological and macromolecular characterization of Hypocrea schweinitzii and its Trichoderma anamorph. Mycologia. 1994;86:421–435. [Google Scholar]
- 14.Druzhinina IS, Schmoll M, Seiboth B, Kubicek CP. Global carbon utilization profiles of wild-type, mutant, and transformant strains of Hypocrea jecorina. Appl Environ Microbiol. 2006;72:2126–2133. doi: 10.1128/AEM.72.3.2126-2133.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mach RL, Schindler M, Kubicek CP. Transformation of Trichoderma reesei based on hygromycin B resistance using homologous expression signals. Curr Genet. 1994;25:567–570. doi: 10.1007/BF00351679. [DOI] [PubMed] [Google Scholar]
- 16.Borkovich KA, et al. Lessons from the genome sequence of Neurospora crassa: Tracing the path from genomic blueprint to multicellular organism. Microbiol Mol Biol Rev. 2004;68:1–108. doi: 10.1128/MMBR.68.1.1-108.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galagan JE, et al. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature. 2005;438:1105–1115. doi: 10.1038/nature04341. [DOI] [PubMed] [Google Scholar]
- 18.Nelson MA, Merino ST, Metzenberg RL. A putative rhamnogalacturonase required for sexual development of Neurospora crassa. Genetics. 1997;146:531–540. doi: 10.1093/genetics/146.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aanen DK, Hoekstra RF. In: Sex in Fungi—Molecular Determination and Evolutionary Implications. Heitman J, Kronstad JW, Taylor JW, Casselton L, editors. Washington, DC: ASM Press; 2007. pp. 527–534. [Google Scholar]
- 20.Gow NA. Fungal genomics: Forensic evidence of sexual activity. Curr Biol. 2005;15:R509–R511. doi: 10.1016/j.cub.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 21.Taylor J, Jacobson D, Fisher M. The evolution of asexual fungi: Reproduction, speciation and classification. Annu Rev Phytopathol. 1999;37:197–246. doi: 10.1146/annurev.phyto.37.1.197. [DOI] [PubMed] [Google Scholar]
- 22.Adrio JL, Demain AL. Fungal biotechnology. Int Microbiol. 2003;6:191–199. doi: 10.1007/s10123-003-0133-0. [DOI] [PubMed] [Google Scholar]
- 23.Dyer PS, Paoletti M. Reproduction in Aspergillus fumigatus: Sexuality in a supposedly asexual species? Med Mycol. 2005;43(Suppl 1):S7–S14. doi: 10.1080/13693780400029015. [DOI] [PubMed] [Google Scholar]
- 24.Ekelund F, Ronn R. If you don't need change, maybe you don't need sex. Nature. 2008;453:587. doi: 10.1038/453587a. [DOI] [PubMed] [Google Scholar]
- 25.Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- 26.Benitez T, Rincon AM, Limon MC, Codon AC. Biocontrol mechanisms of Trichoderma strains. Int Microbiol. 2004;7:249–260. [PubMed] [Google Scholar]
- 27.Walsh TJ, et al. Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect. 2004;10(Suppl 1):48–66. doi: 10.1111/j.1470-9465.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 28.Druzhinina IS, et al. Alternative reproductive strategies of Hypocrea orientalis and genetically close but clonal Trichoderma longibrachiatum, both capable of causing invasive mycoses of humans. Microbiology. 2008;154:3447–3459. doi: 10.1099/mic.0.2008/021196-0. [DOI] [PubMed] [Google Scholar]
- 29.Kredics L, et al. Clinical importance of the genus Trichoderma. Rev Acta Microbiol Immunol Hung. 2003;50:105–117. doi: 10.1556/AMicr.50.2003.2-3.1. [DOI] [PubMed] [Google Scholar]
- 30.Hsueh YP, Heitman J. Orchestration of sexual reproduction and virulence by the fungal mating-type locus. Curr Opin Microbiol. 2008;11:517–524. doi: 10.1016/j.mib.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kratzer C, Tobudic S, Schmoll M, Graninger W, Georgopoulos A. In vitro activity and synergism of amphotericin B, azoles and cationic antimicrobials against the emerging pathogen Trichoderma spp. J Antimicrob Chemother. 2006;58:1058–1061. doi: 10.1093/jac/dkl384. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Lee T, Lee YW, Yun SH, Turgeon BG. Shifting fungal reproductive mode by manipulation of mating type genes: Obligatory heterothallism of Gibberella zeae. Mol Microbiol. 2003;50:145–152. doi: 10.1046/j.1365-2958.2003.03694.x. [DOI] [PubMed] [Google Scholar]
- 33.Yun SH, Berbee ML, Yoder OC, Turgeon BG. Evolution of the fungal self-fertile reproductive life style from self-sterile ancestors. Proc Natl Acad Sci USA. 1999;96:5592–5597. doi: 10.1073/pnas.96.10.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang S, Staben C. Directed replacement of mt A by mt a-1 effects a mating type switch in Neurospora crassa. Genetics. 1994;138:75–81. doi: 10.1093/genetics/138.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hornok L, Waalwijk C, Leslie JF. Genetic factors affecting sexual reproduction in toxigenic Fusarium species. Intl J Food Microbiol. 2007;119:54–58. doi: 10.1016/j.ijfoodmicro.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Leslie JF, Klein KK. Female fertility and mating type effects on effective population size and evolution in filamentous fungi. Genetics. 1996;144:557–567. doi: 10.1093/genetics/144.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galagan JE, Selker EU. RIP: The evolutionary cost of genome defense. Trends Genet. 2004;20:417–423. doi: 10.1016/j.tig.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Freitag M, Williams RL, Kothe GO, Selker EU. A cytosine methyltransferase homologue is essential for repeat-induced point mutation in Neurospora crassa. Proc Natl Acad Sci USA. 2002;99:8802–8807. doi: 10.1073/pnas.132212899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gruber F, Visser J, Kubicek CP, de Graaff LH. The development of a heterologous transformation system for the cellulolytic fungus Trichoderma reesei based on a pyrG-negative mutant strain. Curr Genet. 1990;18:71–76. doi: 10.1007/BF00321118. [DOI] [PubMed] [Google Scholar]
- 40.Mandels M, Andreotti R. Problems and challenges in the cellulose to cellulase fermentation. Proc Biochem. 1978;13:6–13. [Google Scholar]
- 41.Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 42.Schmoll M, Zeilinger S, Mach RL, Kubicek CP. Cloning of genes expressed early during cellulase induction in Hypocrea jecorina by a rapid subtraction hybridization approach. Fungal Genet Biol. 2004;41:877–887. doi: 10.1016/j.fgb.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 44.Seidl V, Huemer B, Seiboth B, Kubicek CP. A complete survey of Trichoderma chitinases reveals three distinct subgroups of family 18 chitinases. FEBS J. 2005;272:5923–5939. doi: 10.1111/j.1742-4658.2005.04994.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.