Summary
A group of retinal interneurons known as horizontal cells has recently been shown to exhibit a variety of unique biological properties, as compared with other nerve cells, that challenge many long-standing assumptions in the fields of neural development and cancer biology. These features include their unusual migratory behavior, their unique morphological plasticity, and their propensity to divide at a relatively late stage during development. Here, we review these novel features, discuss their relevance for other cell types, outline open questions in our understanding of horizontal cell development and consider their implications.
Introduction
The retina, a highly specialized extension of the brain, plays host to the first stages of visual perception. Visual stimuli from the world around us are projected upon the retina, converted into neural signals and transmitted to higher visual centers of the brain via the optic nerve. Despite this complex physiological function, the retina, which is composed of only seven cell types and resides as a thin neuronal sheet in the back of eye, is a deceptively simple structure (Box 1). By studying how the retina develops, we can gain a more thorough understanding of its mature architecture and circuitry and of how these underlie the mechanisms of visual perception, but also come to appreciate the defects in this developmental program that arise as a consequence of genetic mutations that lead to retinal disease.
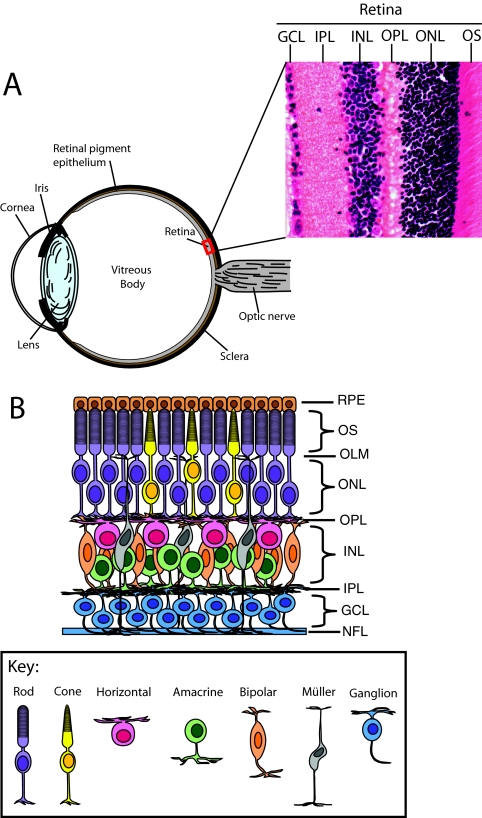
Box 1. Retinal architecture
In contrast to the thousands of neuronal cell types in the mature brain, the retina is composed of only six neuronal cell types and one glial cell type, which are derived from a common progenitor cell population and precisely organized into a discrete laminar structure at the back of the eye (A,B). The light-sensing photoreceptors (PR), the rods and cones, are localized to the outer nuclear layer (ONL), the outermost region of the neural retina adjacent to the retinal pigment epithelium (RPE). Rods are highly sensitive PRs that are essential for vision under scotopic conditions (i.e. in low light), whereas cones are responsible for vision under photopic conditions (i.e. in bright light) and underlie our visual acuity and color vision. They form specialized apical extensions at the outer limiting membrane (OLM) of the retina, the outer segments (OS), which contain the light-sensitive photopigments and the visual transduction machinery that enable the PRs to generate a neural signal in response to light. This signal is then transferred to retinal neurons residing within the inner nuclear layer (INL). Bipolar cells transmit this signal radially from the outer retina to the ganglion cell layer (GCL). Signaling from the PRs to the bipolar cells is modulated within the outer plexiform layer (OPL) by inhibitory interneurons positioned within the INL, the horizontal cells. Within the inner retina, the bipolar cells make synapses with the amacrine cells and the ganglion cells. There is a variety of different amacrine cell types, each modulating retinal function through unique circuitry and signaling mechanisms within the inner plexiform layer (IPL). Visual information converges upon the dendrites of different retinal ganglion cell types and is transmitted to the visual centers in the brain via their axons, which initially course beneath the inner surface of the retina in the nerve fiber layer (NFL), collecting at the optic nerve head to form the optic nerve. The primary glial cell population of the retina, the Müller glia, extend across the entire thickness of the retina and provide essential structural and functional support to the neuronal population. For illustrative purposes, a schematic of the human eye is shown in A, but the Hematoxylin and Eosin staining to the right illustrates the cytoarchitecture of an adult mouse retina.
More generally, the retina can be thought of as a `stripped down' version of the brain, and for this reason it is often utilized as a model system to elucidate fundamental questions regarding the central nervous system (CNS) at large. In terms of neurodevelopment, the retina is a particularly attractive model. Developmental mechanisms that result in a highly specialized and intricately organized neuronal structure composed of precise numbers and ratios of cell types, such as the retina, are very likely to be conserved in other neural contexts. The elucidation of the developmental pathways that function in the retina therefore holds much promise for ultimately understanding some of the mysteries of the brain. Furthermore, owing to it being a non-essential tissue, the retina is an ideal system in which to perform genetic loss-of-function studies, which facilitates a decoding of the genomic control of neurodevelopment. In this review, we focus specifically on new and exciting results pertaining to the development of one type of retinal neuron, the horizontal cell.
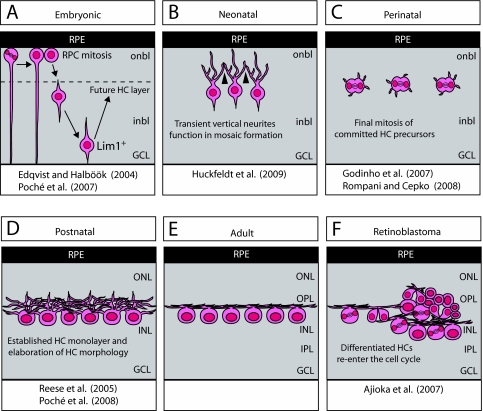
Retinal horizontal cells (HCs), which were initially characterized by Cajal in 1893 (Cajal, 1893), are, together with amacrine cells, retinal interneurons that lie within the inner nuclear layer (INL) (Fig. 1). HCs function in modulating signaling between photoreceptors and bipolar cells (Box 1). HCs, like all other retinal cell types, are derived from a common retinal progenitor cell population (Turner et al., 1990; Wetts and Fraser, 1988). According to the generally accepted view, these retinal progenitor cells translocate to the future outer limiting membrane (OLM) of the developing retina (the ventricular surface), where they undergo mitosis and subsequently exit the cell cycle as specific, committed retinal progenitor cells. This is followed by their migration to the appropriate retinal stratum and by the initiation of a differentiation program that results in a morphologically and functionally specific cell type [for a review of these various developmental processes, see Sernagor et al. (Sernagor et al., 2006)]. Recent studies of HCs, however, have uncovered new levels of complexity in HC precursor migration, mitosis and differentiation that challenge this linear developmental scheme (Fig. 2). Upon exiting the cell cycle, mouse and chick HCs do not migrate directly to their final resting place, but rather bypass their prospective layer before they change trajectory and return to the future HC stratum, which lies adjacent to the outer plexiform layer (OPL). Furthermore, HCs do not appear to require apical-basal connections during radial migration, but rather migrate freely. These characteristics of migratory HCs may utilize mechanisms that are conserved in other freely migrating neurons, such as cortical interneurons (Nadarajah et al., 2002). Another interesting feature of HCs is their remarkable morphological plasticity, which is evident in the dramatic changes in cell polarity and dendritic morphology that take place when these cells are mispositioned or when their neighbor relationships are altered. Even more intriguingly, in chicken and zebrafish retinas, committed HC progenitors undergo mitosis at a relatively late stage in retinal development to give rise exclusively to HCs. This observation may indicate a generally utilized mechanism of neuronal layer formation, possibly employed elsewhere in the CNS, whereby mitosis is confined to a particular laminar position and facilitates the rapid and efficient connection of synaptic partners. Finally, new data that indicate that fully differentiated mouse HCs have the ability to give rise to metastatic retinoblastoma challenge widely held assumptions about the origins of tumor cells as being derived from progenitor/stem-like cells. This finding should prompt a reassessment of certain therapies aimed at inducing tumor cell differentiation as a means to halt tumor progression. In this review, we discuss each of these discoveries in turn, outline some as yet unanswered questions regarding their significance and consider their relevance to CNS development in general.
Fig. 1.
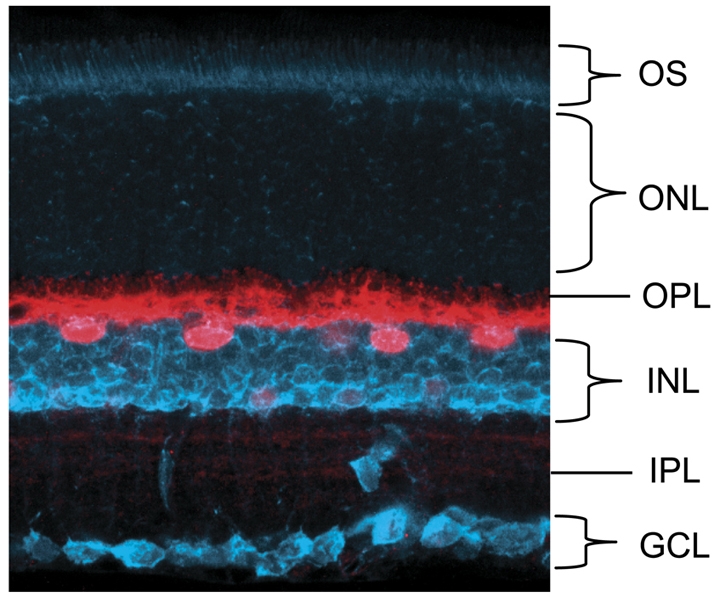
Horizontal cells in the retina. An adult mouse retina, with horizontal cells (HCs) labeled with an antibody against calbindin (red). This marker labels both the HC somata and their lateral processes within the outer plexiform layer. Neurotrace (blue) identifies all cells within the retina, thereby revealing its laminar cytoarchitecture. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; OS, outer segment.
Fig. 2.
Unusual features of HCs. (A) During embryonic development, retinal progenitor cells (RPCs) undergo mitosis near the outer limiting membrane and subsequently migrate to their appropriate retinal layer. HC precursors do not migrate directly to the prospective HC layer, but rather bypass this layer and migrate basally to the ganglion cell layer (GCL) before changing direction to migrate apically towards the HC layer. This second phase of HC migration has been shown to depend on Lim1. (B) Upon the completion of HC migration, the HCs are organized in a non-random spatial arrangement (mosaic) within the outer retina. HC spacing has been shown to be regulated by homotypic repulsive interactions mediated by transient, apically directed neurites (arrowheads). (C) Zebrafish and chick retinae contain committed progenitor cells that divide to produce only HCs, and in the zebrafish do so within the HC stratum. (D,E) Subsequent to cell cycle exit, homotypic interactions restrain dendritic overlap, as these processes stratify and form synaptic contacts with their afferents in the outer plexiform layer (OPL). (F) In a mouse model of retinoblastoma, fully differentiated HCs re-enter the cell cycle and give rise to aggressively metastatic tumors. ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; onbl, outer neuroblastic layer; inbl, inner neuroblastic layer; RPE, retinal pigment epithelium.
Transcriptional control of HC fate determination and differentiation
As indicated by retroviral lineage-tracing studies (Turner and Cepko, 1987), all seven retinal cell types are derived from a common, multipotent retinal progenitor cell (RPC). It is now well established that the process of retinal cell fate determination is, to a large extent, guided by combinatorial transcription factor activity such that RPCs exist in various states of `competency' that change over time (Cepko et al., 1996). These transcription factors probably regulate the expression of cell surface receptors or components of signal transduction cascades, which enables the cell to respond to extrinsic cues that further refine fate decisions and differentiation (Belliveau and Cepko, 1999; Cepko et al., 1996; Hatakeyama and Kageyama, 2004).
Over the past several years, much has been learned regarding the identity and function of transcription factors that specify mouse retinal cell fates, including HCs, and many excellent reviews of these data currently exist (reviewed by Cayouette et al., 2006; Cook, 2003; Livesey and Cepko, 2001; Ohsawa and Kageyama, 2008). Here, we briefly discuss data relating to HC specification, as many of the unique properties of HCs that are discussed in this review are probably the consequence of genetic pathways that are downstream of these factors.
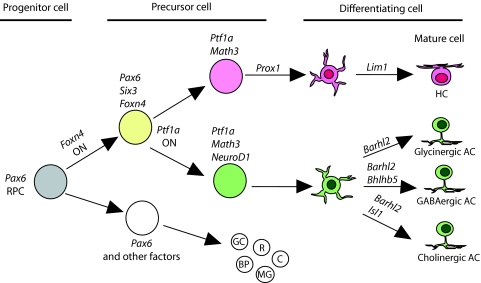
HCs and amacrine cells share molecular mechanisms that are important for their determination and differentiation, and a fairly well-defined fate determination pathway has emerged (Fig. 3). The expression of the neural bHLH transcription factors neurogenic differentiation 1 (NeuroD; Neurod1 - Mouse Genome Informatics) and mouse atonal homolog 3 (Math3; Neurod4) in embryonic RPCs coincides with the birth of amacrine cells and HCs. In NeuroD/Math3 double-knockout mice, amacrine cell numbers are severely reduced, and there is a corresponding increase in retinal ganglion cells (Hatakeyama et al., 2001; Inoue et al., 2002). Oddly, the misexpression of NeuroD or of Math3 alone does not result in an increase in amacrine cell production, but rather in the induction of photoreceptor development (Hatakeyama and Kageyama, 2004; Inoue et al., 2002). These data suggest that although NeuroD and Math3 are required for RPCs to adopt an amacrine cell fate, other factors must also play a role. Indeed, the homeodomain transcription factor paired box gene 6 (Pax6) has been shown to be such a factor. When Pax6 is misexpressed with either NeuroD or Math3, this results in the exclusive production of amacrine cells (Hatakeyama and Kageyama, 2004; Inoue et al., 2002). These data indicate that the combinatorial activity of bHLH and homeodomain transcription factors is required for amacrine cell fate specification. By contrast, when Pax6 is misexpressed along with Math3, a greater percentage of HCs (∼14% increase) as compared with amacrine cells (∼7% increase) is produced, which suggests that Math3 promotes HC fate over amacrine cell fate and that NeuroD and Math3 are not functionally equivalent (Inoue et al., 2002).
Fig. 3.
Transcriptional control of HC and amacrine cell development. The transcription factor Foxn4 lies at the top of the genetic hierarchy that regulates HC and amacrine cell fate determination in mice and probably also in other vertebrates. Downstream of Foxn4, the transcription factor Ptf1a reinforces the decision towards either amacrine or HC fate. The transcription factor Prox1 steers a subset of Ptf1a+ cells towards the HC fate, whereas transcription factors Math3 and NeuroD direct a separate cohort towards the amacrine cell fate. Further downstream in the amacrine cell developmental pathway, the transcription factors Barhl2, Bhlhb5 and Isl1 specify subtypes of amacrine cell. See text for details on the other factors. HC, horizontal cell; AC, amacrine cell; BP, bipolar cell; MG, Müller glial cell; GC, ganglion cell; R, rod photoreceptor; C, cone photoreceptor; RPC, retinal progenitor cell.
Recently, three additional transcription factors have been discovered to be important for amacrine cell and HC specification. The winged helix/forkhead transcription factor forkhead box N4 (Foxn4) was shown to be essential for the generation of both cell types. Foxn4-null mice exhibit a complete loss of HCs and a severe reduction in amacrine cells (Li et al., 2004). Math3 and NeuroD expression is downregulated in these mutants, which indicates that they are downstream of Foxn4. However, it is important to note that their expression was not completely abolished, which points to a Foxn4-independent mechanism that functions in a subset of cells as also regulating Math3 and NeuroD expression. It is possible that this residual expression of Math3 and NeuroD might explain why the loss of amacrine cells is incomplete in Foxn4-null mice.
Another transcription factor essential for amacrine and HC development is the bHLH factor pancreas specific transcription factor 1a (Ptf1a). Ptf1a-null mice have a phenotype that is almost identical to that of Foxn4-null mice and in which all HCs and most amacrine cells are missing (Fujitani et al., 2006). Importantly, Foxn4 is expressed prior to Ptf1a, and Foxn4 expression is properly initiated in the Ptf1a-null mice, whereas Ptf1a expression is lost in Foxn4-null mice (Fujitani et al., 2006; Li et al., 2004). This indicates that Ptf1a is probably downstream of Foxn4 in regulating amacrine and HC fate determination. However, Math3 and NeuroD are not downregulated in the Ptf1a mutants. Based on this result, it would appear that Math3 and NeuroD are downstream of Foxn4 but upstream of (and possibly regulate) Ptf1a. This interpretation is, however, likely to be invalid, despite the finding that Ptf1a mutant mice exhibit a loss of HCs (Fujitani et al., 2006), as HC development is unperturbed in Math3/NeuroD mutants (Inoue et al., 2002). Rather, it seems likely that Ptf1a and Math3/NeuroD function in parallel pathways, both of which are downstream of Foxn4. The further analysis of Math3/NeuroD mutant mice might clarify this issue.
The homeodomain transcription factor prospero-related homeobox 1 (Prox1) lies even further downstream in the Foxn4-Ptf1a pathway, as indicated by its lack of expression in both Foxn4- and Ptf1a-null retinae (Fujitani et al., 2006; Li et al., 2004). Prox1-null mice display a complete loss of HCs, whereas the amacrine cell population appears unaffected (Dyer et al., 2003). Furthermore, the forced expression of Prox1 results in the production of HCs (Dyer et al., 2003). Thus, it appears that whereas the Foxn4 pathway confers upon RPCs the ability to differentiate into an amacrine cell or HC, Prox1 expression biases a subset of these cells to solely adopt an HC fate.
The above-mentioned studies are interesting in light of Pax6 conditional knockout experiments, which show that Pax6 confers the multipotent nature of RPCs. Pax6 has, however, been shown to be dispensable for amacrine cell development, as these mice show a striking phenotype in which amacrine cells are generated exclusively. In Pax6 conditional knockout mice, NeuroD expression persists. This does not necessarily explain why amacrine cells are still generated in these mutants, as NeuroD was shown to require co-expression with Pax6 in order to generate amacrine cells (Inoue et al., 2002; Marquardt et al., 2001). A possible explanation is that sine oculis-related homeobox 3 (Six3), another homeodomain transcription factor expressed in RPCs, might exhibit partial functional redundancy with Pax6 and cooperate with NeuroD in generating amacrine cells. Consistent with this idea, Six3 expression is unaltered in the Pax6 conditional mutants, and forced co-expression of Six3 and NeuroD leads to the generation of amacrine cells (Inoue et al., 2002; Marquardt et al., 2001).
Upon fate determination, amacrine cell and HC precursors subsequently migrate, differentiate and acquire their appropriate morphologies, while each expressing a cell type-specific repertoire of genes that is essential for their function in visual perception. The control of amacrine cell differentiation is a striking example of this specificity, as up to 26 morphologically distinct types of amacrine cell reside in the mouse retina (MacNeil and Masland, 1998). Presumably, each of these activates a unique terminal differentiation program that is under some level of transcriptional control. Very few transcription factors, however, have been identified that participate in the creation of this diversity. In the case of mouse amacrine cells, the Bar-class homeodomain transcription factor Barhl2 was shown to be expressed in post-mitotic amacrine cells and to specify glycinergic, GABAergic and cholinergic amacrine cells (Ding et al., 2009; Mo et al., 2004), whereas the bHLH transcription factor Bhlhb5 (Bhlhe22) was shown to be required for the specification of GABAergic amacrine cells (Feng et al., 2006). Additionally, the LIM-homeodomain transcription factor Islet1 (Isl1) has been implicated in cholinergic amacrine cell differentiation (Elshatory et al., 2007).
In the mouse retina, there is only one identified HC type, an axon-bearing HC, although most other vertebrate retinae possess two or three types of HCs (Peichl and Gonzalez-Soriano, 1994). Notably, the axons of such axon-bearing HCs do not transmit neural signals; rather, the axon provides metabolic support for a functionally independent `axon terminal system', which, in the mouse, subserves lateral inhibition between the rod photoreceptors. The HC dendritic arbor fulfils the same function exclusively for the cone photoreceptors. In the chick retina, the HC population is composed of three cell types (H1, H2 and H3), each characterized by a distinct morphology (Genis-Galvez et al., 1979). The H1, or `brush-shaped', HC type is axon bearing, whereas the H2 `stellate' and H3 `candelabrum-shaped' HC types are without axons. A recent report (Suga et al., 2009) provides data that indicate that the transcription factor Isl1 drives the differentiation of the H2 cell type. When Isl1 is exogenously expressed in H1 cells, which normally express LIM homeobox protein 1 (Lim1; Lhx1), Lim1 appears to be downregulated, and a larger proportion of cells with the H2 morphology are observed at the expense of H1 cells. The expression of a dominant-negative form of Lim1 did not grossly affect subtype specification, but H1 axons increased in length and were straighter than those of control H1 cells. Taken together, these data suggest that combinatorial transcriptional programs indeed function in regulating later aspects of HC development, including subtype specification and morphogenesis.
Following this overview of the transcriptional control of amacrine cell and HC specification, we are now in a position to review the new and intriguing studies that have uncovered certain unusual properties of HC development that are potentially conserved in other parts of the CNS. These aspects of HC development might themselves prove to be downstream of the transcription factors that confer HC fate determination.
HCs exhibit a novel mode of neuronal migration
Upon exit from the cell cycle, a differentiating HC must migrate from the site of its final mitosis to become positioned within the future HC stratum. It was previously thought that, subsequent to cell cycle exit, retinal neurons migrate directly to their appropriate laminar position. Given the early birth of retinal HCs and the relatively underdeveloped neural retina at these early stages, this targeted positioning of newly born HCs hardly seemed remarkable. The study of developing chicken HCs, however, has revealed that these cells actually bypass the prospective HC stratum and continue to migrate towards the ganglion cell layer, where they coalesce before translocating back to their final location adjacent to the OPL (Edqvist and Hallbook, 2004) (Fig. 2A). Subsequent studies have shown that mouse HCs behave similarly, and furthermore that the transcription factor Lim1 is crucial for the translocation back to the HC stratum. It had been shown previously that migrating HCs are immunopositive for Lim1 (Liu et al., 2000), implicating Lim1 in this process. By employing a retinal-specific knockout approach in mice, it was shown that upon loss of Lim1 function, mutant HCs remain positioned within the innermost parts of the INL, adjacent to the inner plexiform layer (IPL) (Poché et al., 2007). These mispositioned Lim1-mutant cells express molecular markers that are consistent with an HC identity, which argues against a change in cell fate. Rather, these data indicate that Lim1 regulates target genes that are required for HC migration.
A major obstacle to a full understanding of the developmental genetic program of HC migration is the fact that currently, no downstream targets of Lim1 are known. In the future, it will be necessary to perform gene profiling experiments on wild-type and Lim1-null HCs with the goal of identifying genes that are controlled either directly or indirectly by Lim1 and that function in HC migration. Nevertheless, retina-wide gene profiling experiments have provided several promising HC-specific candidate genes. By performing a serial analysis of gene expression (SAGE), in which retinal cDNAs expressed at multiple time points were partially sequenced and identified, and mRNA in situ hybridization, it was revealed that septin 4 (Sept4) is specifically expressed in developing and mature mouse HCs (Blackshaw et al., 2004). The septins are a family of polymerizing GTP-binding proteins that interact with the actin and microtubule cytoskeleton and have been implicated in various cellular processes, including cytoskeletal and cell membrane dynamics, exocytosis, cytokinesis and vesicle transport (reviewed by Spiliotis and Nelson, 2006; Weirich et al., 2008). Interestingly, the bi-directional migration of chicken HCs was found to be an at least partially actin-dependent event (Edqvist and Hallbook, 2004). Although speculative, these data indicate that Lim1 might regulate septin gene expression or certain aspects of septin function. The same retinal SAGE analysis uncovered a second HC-specific gene called binder of Rho GTPase 4 (Borg4; Cdc42ep4) (Blackshaw et al., 2004). Borgs are a family of Cdc42 GTPase effector proteins that can bind septins, thereby altering septin filament organization within the cell (Joberty et al., 2001; Sheffield et al., 2003). Thus, HCs might employ a cellular mechanism whereby borg-septin interactions regulate certain aspects of HC cytoskeletal dynamics. Whether such a mechanism functions downstream of Lim1 in HC migration remains to be determined. Septin loss-of-function studies could be informative in this regard, but are hindered by a large degree of functional redundancy among septin family members (Peng et al., 2002; Kinoshita, 2008). Additionally, the cellular phenotype of Lim1-null HCs is not yet fully defined. The live imaging of fluorescently labeled HCs within acutely isolated retinal tissue might provide the level of detail necessary to gain insight into the cellular manifestation of Lim1 loss-of-function mutations, which might ultimately be correlated to the loss of activity of candidate Lim1-regulated genes.
It is not yet clear whether other retinal neuron populations undergo bi-directional migration, and it will be fascinating to determine how many other neuronal populations of the CNS migrate in this fashion. Interestingly, interneuron migration in the developing cerebral cortex (Nadarajah et al., 2002; Noctor et al., 2004), as well as the nucleogenesis of precerebellar neurons (Kawauchi et al., 2006), have been shown to exhibit heterogeneity in their migratory pattern, with neurons migrating towards the ventricular surface before changing trajectory toward their appropriate layer. Whether the above-mentioned modes of neuronal migration have anything in common with HC migration remains to be elucidated; however, it is interesting to note that Lim1 is also expressed in various locations throughout the developing CNS (R.A.P., unpublished). Once we gain a further understanding of the role of Lim1 in HC migration, an analogous role for Lim1 in the regulation of neuronal positioning elsewhere within the CNS may be uncovered.
It will also be useful to determine whether other Lhx family members are expressed in migratory retinal neurons. From our studies on the role of Lim1 in HC laminar positioning, one can speculate that other Lhx family members, expressed in various retinal cell types, might also regulate cell type-specific retinal neuron migration or positioning. Indeed, Lhx2, Lhx3, Lhx4 and Lhx9 have been reported to be expressed within the adult retina (Cheng et al., 2005; Zhang et al., 2006), whereas Lhx4 is expressed in differentiating bipolar cells (Blackshaw et al., 2004). Although bipolar cells do not undergo free migration, but rather translocate their nuclei through a radial process before differentiating their dendritic and axonal arbors from an immature neuroepithelial morphology (Morgan et al., 2006), Lhx4 could still play a role in this transformation. Detailed temporal and spatial expression profiles and loss-of-function analyses for these Lhx family members should shed light on this issue.
Striking plasticity in HC morphology
In addition to the discovery that mouse HCs migrate bi-directionally, the Lim1 conditional mutants provide evidence that mature HC morphology is highly plastic and susceptible to local environmental cues. In the absence of Lim1 function, mouse HCs that fail to migrate back to the OPL (and hence remain in the inner portion of the INL) stratify within the IPL (Poché et al., 2007). Importantly, these cells express the Lim1-lacZ knockin allele, which is an HC-specific marker. Thus, it was suggested that the fate of Lim1-null cells is properly specified as HCs, but that they exhibit a morphology similar to that of amacrine cells. This aberrant morphological differentiation might be caused by local environmental cues within the inner parts of the retina. Alternatively, these results could implicate Lim1 in additionally regulating an intrinsic program that directs HC morphogenesis. In either case, the post-mitotic HC appears relatively plastic with respect to its polarity and dendritic differentiation, consistent with other recent studies that demonstrate that the dendritic patterning of the HC is itself immensely plastic and instructed by afferent innervation (Raven et al., 2007).
HCs employ a novel method of mosaic formation
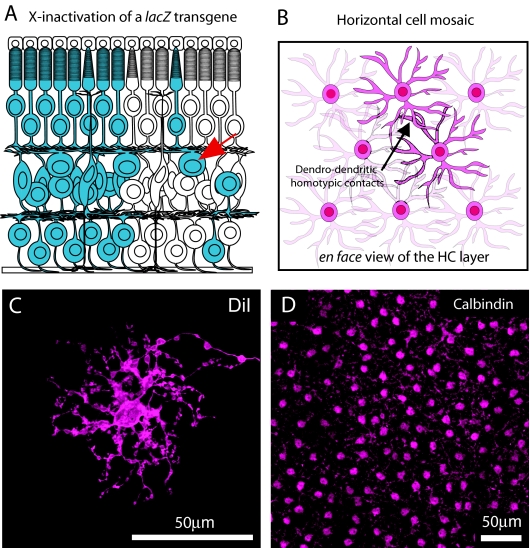
Sensory neurons of the same cell type are often organized in a non-random manner, otherwise known as a mosaic, with their cell bodies spaced regularly. Such regular distributions of nerve cells are seen in both invertebrates and vertebrates and are particularly well studied within the vertebrate retina, including the HCs (Gan and Macagno, 1995; Grueber et al., 2003; Jan and Jan, 2003; Reese, 2008b), but exactly how such cellular patterning becomes established is debated (Reese, 2008a). Earlier lineage-tracing studies that examined the progeny of single RPCs (Fekete et al., 1994; Holt et al., 1988; Turner and Cepko, 1987; Turner et al., 1990; Wetts and Fraser, 1988) as well as studies that examined the distribution of clonally related cells in Mus musculus/Mus caroli inter-specific chimeras (Williams and Goldowitz, 1992a; Williams and Goldowitz, 1992b), have shown that single RPCs must be multipotent and capable of producing all retinal cell types. Most of these clonally related cells were aligned as radial columns of cells, indicating that nearly all of them migrate radially to reach their appropriate laminar positions within the retina. A few RPCs, however, have been observed to be tangentially dispersed from their clonal columns of origin and are restricted to the fates of the earlier-generated minority cell types (cones, HCs, some types of amacrine cells and retinal ganglion cells). The full significance of this tangential dispersion became apparent only later, through the analysis of mosaic transgenic mice bearing an X-linked lacZ reporter gene (Fig. 4A) (Reese et al., 1995; Reese et al., 1999). Using this approach, in which 50% of each cell type is labeled as a consequence of X-inactivation, it was possible to estimate the frequency of the occurrence of tangential dispersion for any given cell type. Rather than this being a rare or ectopic event, tangential dispersion was found to be a universal feature for particular types of cells. Furthermore, by examining the emergence of this dispersion in such X-inactivation mosaic mice, these minority cell types were observed to disperse around the time of their differentiation rather than dispersion being linked to the earlier birth of these cells. This indicates that the dispersion is not simply a passive consequence of the later genesis of the majority cell types, as though these later-born rods, bipolar cells and Müller glia cells simply pushed the earlier-born cells out of their clonal columns (Reese et al., 1995; Reese et al., 1999). Rather, these results suggest that some active cellular interaction between neighboring cells drives this tangential dispersion, which led to the proposal that tangential dispersion is driven by homotypic interactions between differentiating cells, and that this is one of the mechanisms by which regularity in retinal mosaics is established during development (Reese and Galli-Resta, 2002).
Fig. 4.
HCs are non-randomly distributed. (A) The tangential dispersion of immature HCs, as observed in X-inactivation lacZ-mosaic mice, was proposed to be the means by which HCs (arrow) and other regularly spaced cell types establish regularity in their patterned distributions. Cells in blue are all derived from the same retinal progenitor cell, revealing the dispersion of particular types of cell from their clonal column of origin. This process is thought to be driven by homotypic interactions between neighboring cells. (B) Viewed from the surface, the HC somata exhibit regular spacing within their stratum in the mature retina. Their dendrites overlap one another (arrow), so that a single cone photoreceptor innervates multiple HCs. (C) A single HC labeled with DiI (within a retinal flat-mount) illustrates HC dendritic morphology. (D) A retinal flat-mount labeled with an antibody against calbindin, which highlights the HC somata and processes and reveals the non-random distribution of these cells across the retina.
The suggestion that such homotypic interactions might be mediated via dendritic interactions during differentiation is supported by a study in which the cytoskeleton of developing HCs was destabilized by molecularly or pharmacologically interfering with microtubule-binding proteins such as Tau (Mapt) or Map2 (Mtap2) (Galli-Resta et al., 2002). In these retinae, regular cellular spacing is disrupted when the interference occurs during a critical developmental window during which mosaic regularity is known to increase (Raven et al., 2005a). Furthermore, recent studies that have analyzed the HC mosaic in different strains of mice with a 2-fold difference in total HC number, or in mice with a genetically engineered reduction in normal HC number, have shown that the dendritic area of HCs varies inversely with HC density (Reese et al., 2005; Poché et al., 2008). From these data, it was concluded that mouse HCs employ homotypic cues to restrict dendritic growth, but how such dendritic interactions might mediate intercellular spacing remained unclear. One other unresolved question stemmed from the fact that mature mouse HC dendritic arbors exhibit extensive overlap with neighboring cells (Fig. 4B-D) (Reese et al., 2005). Thus, it remained difficult to envisage how such homotypic signaling might be propagated through dendro-dendritic contacts, assuming that they interact with one another at the tips of their dendrites. Recent studies on dopaminergic amacrine cells have suggested a role for the Down syndrome cell adhesion molecule (Dscam) in regulating dendritic self-avoidance (Fuerst et al., 2008). Whether Dscam or a related molecule might also play a role in regulating dendritic self-avoidance in HCs remains to be seen, but it is difficult to envisage it playing a role in homotypic repulsion between neighboring HCs, given their extensive dendritic overlap: the dendritic fields of five to seven neighboring HCs overlie one another on the retinal surface. This degree of retinal `coverage' is approximated when the dendritic radius approaches the average intercellular spacing. Rather, it suggests that active dendritic extension at the tips could be constrained by contact with the neighboring somata of homotypic cells, but what might mediate this signaling remains to be established.
Very recently, an elegant study that employed multi-photon time-lapse imaging of embryonic and early postnatal retinae has brought a new level of insight into HC mosaic formation and has resolved some of these issues (Huckfeldt et al., 2009). The authors prepared flat-mounts of acutely isolated retinae from a transgenic mouse line in which HCs are labeled with green fluorescent protein (GFP) driven by the Gad67 (Gad1) promoter (Chattopadhyaya et al., 2004). This transgene allows for high-resolution imaging of HC processes throughout development, and, therefore, discrete morphological changes become readily apparent. Upon analyzing late embryonic retinae, before HCs have completed their migration to the OPL, one or two apical and basal processes were observed that extended from the somata of GFP+ HCs as they migrated radially towards their prospective layer. Also, tangential HC movements, in which somata squeezed through thick, lateral neurites, were seen near the level of the eventual HC layer, confirming the interpretation of previous results from clonal analyses that proposed that discrete cells located outside of their clonal columns must have moved tangentially (see also Cook and Chalupa, 2000).
At early postnatal stages, as the HCs settle into their prospective stratum, the vertical neurites were observed to become more numerous and branched, while also exhibiting dynamic extension and retraction every one to two hours (Huckfeldt et al., 2009). Unexpectedly, these radially oriented neurites were confined to distinct columnar territories, and neurites from adjacent cells appeared to be in close physical contact, but displayed minimal overlap (Huckfeldt et al., 2009). These non-overlapping neuritic domains also coincided with an increase in intercellular spacing between neighboring HCs, suggesting that transient vertical neurites, rather than lateral dendro-dendritic contacts, participate in the homotypic repulsive interactions that drive mosaic formation (Fig. 2B). To test this idea directly, HCs were targeted for ablation with an infrared laser during the period when the HC mosaic is known to increase in regularity and packing efficiency (Raven et al., 2005b), and the behavior of adjacent HCs was monitored in live retinal explants. As predicted, HCs that surround the ablated region were found to extend vertical neurites apically and laterally into the space once occupied by the ablated HCs. Although this resulted in HC bodies also moving towards the ablated region, none was observed as far into the ablated field as their dendrites, but longer imaging sessions might uncover such repopulation. Nevertheless, this study has provided the first direct observations that link the morphological development of a retinal cell type with the emergence of a patterned distribution upon the retina.
One important, although as yet unexplored, implication of the above-mentioned study is that the vertical neurites might mediate homotypic interactions between HCs as early as embryonic day 17 (E17), before these cells have migrated back to their future stratum, which raises the possibility that the Lim1-dependent migration to the HC stratum might be coupled to mosaic formation. For instance, the same apically directed processes that are important for cell spacing and mosaic formation might simultaneously function in targeting the HC apically towards its appropriate layer.
It will be interesting to determine whether other CNS cell types employ a cellular mechanism of mosaic formation that is similar to that of the HCs. Even though the free, radial migration (i.e. during which the migrating cells lack any contact with either the apical or basal surface) observed during mouse HC migration is similar to that of cortical interneurons, it is not clear whether the molecular mechanisms controlling these processes are conserved (Nadarajah et al., 2002; Nadarajah et al., 2001). The short, lateral neuronal movement observed in mouse HCs is more reminiscent of mouse cortical neurons that undergo nucleokinesis, in which the nucleus moves though a dilated neurite that extends laterally (Bellion et al., 2005; Schaar and McConnell, 2005; Solecki et al., 2004), but whether any type of cortical neuron spaces itself out through homotypic interactions to achieve a quasi-regular distribution remains to be seen. Indeed, the determinants of neuronal positioning within the brain constitute a largely neglected field of inquiry, although tools have recently become available for addressing such questions of patterning and regularity in three dimensions (Eglen et al., 2008).
In the following sections, we move away from HC migration and mosaic formation and address recent exciting findings regarding the control of HC proliferation as it relates to retinal cancer as well as to normal development.
HCs have a propensity for cell cycle re-entry
A revised view of retinoblastoma
Retinoblastoma is a rare but devastating childhood cancer of the retina that has also served as a research model and has facilitated major advances in our understanding of tumorigenesis (reviewed by Burkhart and Sage, 2008; van den Heuvel and Dyson, 2008). Based on its ability to prevent excessive cell growth by inhibiting cell cycle progression until a cell is ready to divide, retinoblastoma 1 (Rb1) was the first gene to be identified as a tumor suppressor. Along with its related family members, p107 (Rbl1) and p130 (Rbl2), Rb1 regulates the transition of cells from G1 into the S phase of the cell cycle (Cobrinik et al., 1993; Ewen et al., 1991; Friend et al., 1986; Hannon et al., 1993; Li et al., 1993). It is now well established that the Rb proteins function to negatively regulate the E2F family of transcription factors, which activate genes that are essential for entry into the cell cycle (van den Heuvel and Dyson, 2008). Today, the Rb family members are generally considered to be transcriptional co-factors and have been shown to have other, non-tumor suppressor roles, such as the regulation of cellular differentiation and apoptosis (reviewed by Khidr and Chen, 2006; Skapek et al., 2006). This property of the Rb family is due to the ability to bind to numerous transcription factors to either promote or repress their activity (Macaluso et al., 2006; Morris and Dyson, 2001).
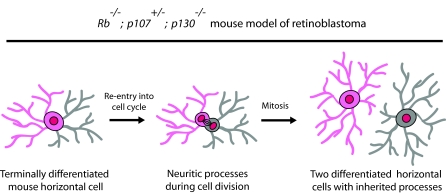
Despite the extensive literature on Rb as it relates to tumorigenesis, a retinoblastoma `cell of origin' (a cell that sustains an initiating genetic defect and goes on to give rise to a focal, clonally derived tumor) was only recently identified in a specific mouse model of retinoblastoma as being the differentiated, post-mitotic HC (Ajioka et al., 2007). These data directly challenge the widely held belief in the field of developmental biology that passage through the cell cycle is incompatible with a fully differentiated status (Skapek et al., 2001; Yang et al., 2001). The above-mentioned study was aimed at clarifying the complex functional redundancy that is exhibited by Rb family members during retinal development and tumorigenesis (Chen et al., 2004; Donovan et al., 2006; MacPherson et al., 2004; Zhang et al., 2004). Tumors from Chx10-Cre+/tg; Rbflox/flox; p130-/-; p107+/- (hereafter `p107-single') mice, which have a single functional copy of p107, were thoroughly characterized (Ajioka et al., 2007). Immunofluorescence and real-time RT-PCR analyses of the adult mutant retinae revealed that their HCs exhibit a 50-fold expansion, even though they are localized to the outer INL (Fig. 2F). Importantly, this increase in HC number did not arise from a significant increase in the number of embryonic HC precursors. Rather, such HC tumors were derived from fully mature HCs that re-entered the cell cycle. Transmission electron microscopy (TEM) on adult p107-single retinal tumors revealed that the proliferating HCs, identified by the presence of condensed chromosomes, exhibit several differentiated hallmarks of mature HCs, including the presence of synaptic triads with photoreceptors and bipolar cells. These data indicate, for the first time, that a fully differentiated neuron with dendritic and axonal processes and their associated synaptic connections can re-enter the cell cycle and divide (Fig. 5). These data were corroborated by directly imaging HC mitosis in p107-single retinal explant cultures. Additionally, single cells were injected with neurobiotin. If the injected cell is electrically coupled to neighboring cells, as is the case for mouse HCs, the neurobiotin passes into these cells and fills their processes. Indeed, the neurobiotin tracer was transported to neighboring HCs, which indicates that the mitotic (injected) cells were coupled to other nearby HCs via gap junctions. These data provide further, functional evidence for the HC identity of the mitotic cells. Finally, the p107-single mice eventually developed an extremely metastatic bi-lateral retinoblastoma.
Fig. 5.
Differentiated HCs can divide and give rise to retinoblastoma. HCs from Rb-/-; p107+/-; p130-/- mice (referred to as p107-single mice because they carry one normal p107 allele) have been shown to undergo mitosis while in a fully differentiated state whereby HC processes exhibited by the mitotic cell were inherited by the daughter cell.
The study by Ajioka and colleagues is the first to show that a fully differentiated neuron can re-enter the cell cycle while maintaining its differentiation status and that it can give rise to an aggressive, metastatic cancer (Ajioka et al., 2007). It remains to be determined whether other neuronal cell types, under certain circumstances, can undergo mitosis while in a differentiated state. What makes the finding by Ajoika and colleagues so remarkable is that decades of tumor cell biology have indicated that differentiation and proliferation are incompatible, so that when differentiated cells are forced to re-enter the cell cycle, apoptosis often results. Furthermore, when tumors acquire certain properties of differentiated cells, they tend to be less aggressive. For example, the transcription factor Gata4 was recently shown to induce breast tumor differentiation, which leads to a repression of metastasis in vivo (Kouros-Mehr et al., 2008). Studies like these have triggered the development of cancer therapies aimed at inducing tumor cell differentiation. In light of the finding that fully differentiated HCs expand clonally, treatments to induce tumor differentiation might not be appropriate for certain tumor types, such as those originating from HCs. These data also challenge the notion that tumors necessarily develop from cancer stem cells, given that no proliferating progenitors were observed.
An important question regarding HC expansion in retinoblastoma is whether these findings translate to human patients. This is not an easy question to answer, as a recapitulation of the p107-single genetic background in humans has not been observed, and the identification of the tumor cell of origin is technically very difficult. Nevertheless, the RNAi knockdown of RB1 and the inactivation of the p53 pathway in human fetal retinal explants have revealed that proliferating neurons express the HC marker calbindin (Laurie et al., 2006). Another perplexing issue is the reason why differentiated HCs are so susceptible to tumorigenesis. Several recent papers that examine HC division during normal development might offer some insight into this issue.
A revised view of neuronal layer formation
A recent in vivo imaging study within the developing zebrafish retina has revealed that HCs undergo mitosis following their arrival within the HC stratum (Godinho et al., 2007). These data contradict the idea that functional neuronal layers form by the migration of neurons to their final layer only after they have undergone terminal mitosis (reviewed by Kriegstein et al., 2006). The HC precursors in transit toward the OPL were found to express markers of both undifferentiated and differentiated HCs. Once localized adjacent to the OPL, these cells divided once to produce a daughter HC. Although the authors make no claim that the HC precursors were derived from RPCs prior to migrating to the future HC layer, this would be expected on the basis of previous data (Turner et al., 1990). Consistent with this idea is the finding of this study that the migratory HC precursors, which ultimately divide in the INL, do not maintain apical-basal contacts during migration, as would be expected if the cells were mitotic progenitor cells. Thus, it appears that during zebrafish retinal development, a subset of progenitor cells undergo mitosis once near the OLM, and are specified as HCs. Upon migrating to the prospective HC layer (potentially by way of a bi-directional migratory trajectory comparable to that described in chicks and mice, as discussed above), the immature HC precursors undergo an additional, final mitosis that produces two daughter HCs (Fig. 2C). It is not yet clear whether mouse HCs undergo a similar developmental process. The study by Huckfeldt and colleagues (Huckfeldt et al., 2009) did not detect mitosis within the HC layer. It is possible that there is a critical time window during which such mitoses take place that was not observed in this study. A second recent paper has reported data that are consistent with the existence of chick HC precursors that divide symmetrically, giving rise to pairs of HCs that are always of the same subtype (Rompani and Cepko, 2008). As mentioned above, the chick retina contains three different types of HCs: H1, H2 and H3. Rompani and Cepko found that only the H1 and H3 types appear as pairs following a final division. It is important to note that this study did not address whether immature chick HC precursors first migrate to the INL before undergoing such terminal mitoses. However, another recent study has confirmed the existence of committed chick HC precursors and has shown that, while localized on the vitreal side of the retina near the ganglion cell layer (GCL), these cells undergo cell cycle arrest in G2 phase for up to two days. Following this period, the cells undergo a final mitosis and continue migration to the prospective HC layer (Boije et al., 2009). Again, such observations are yet to be reported for the mouse retina. Nevertheless, a vertebrate developmental program that involves HC precursor G2 arrest and subsequent terminal mitosis is extremely interesting, especially in the context of HCs being the retinoblastoma cell of origin (Ajioka et al., 2007). Perhaps HCs have an intrinsic propensity for cell cycle re-entry and, in the case of retinoblastoma, this intrinsic propensity is exploited as the HCs proliferate uncontrollably. Further research is needed to address whether Rb plays a role in HC development by regulating the terminal mitoses of committed HC precursors. An HC-specific knockout of Rb (and related family members) would be most informative.
Why do zebrafish and chick have dedicated horizontal precursors that undergo mitosis at locations removed from the ventricular surface, the site where all other mitotic divisions are believed to occur within the developing retina? One possibility is that such HC mitosis promotes a more efficient laminar coverage of the retina within these species. Should it occur within the mouse retina, however, this explanation would then appear to be at odds with the fact that different strains of mice have HC populations that exhibit conspicuous variations in their total number, and that in the presence of such natural variation (Reese et al., 2005) or following genetic manipulations in mice that yield reduced densities of HCs (Poché et al., 2008), the dendritic fields of HCs modulate their field areas to maintain a constant dendritic coverage. Indeed, this plastic feature of the HC - not witnessed within what is probably the best-studied retinal amacrine cell type with extensive laterally oriented processes, the starburst (cholinergic) amacrine cell (Farajian et al., 2004; Keeley et al., 2007) - is assumed to ensure efficient laminar coverage across the retina. As mentioned above, the dendritic patterning of the HCs is also notably plastic, being sculpted by the distribution of afferents within the OPL (Raven et al., 2007). These cells are also notorious for their plasticity in maturity, sprouting lengthy subretinal processes in retinal detachment (Lewis et al., 1998) and in retinitis pigmentosa (Fariss et al., 2000). These facts do not, of course, rule out the possibility that the mouse retina will also be shown to exhibit such inner retinal divisions of HC precursors, or that the modulation of these particular divisions is primarily responsible for the aforementioned variation in total HC number within different strains of mice (Raven et al., 2005b). They simply indicate that the immense dendritic plasticity of this cell type would appear to be sufficient for ensuring uniformity in dendritic coverage across the retinal surface.
Conclusions
Recent studies of retinal HCs have uncovered several surprising characteristics that challenge long-standing ideas in the field of developmental biology. Specifically, HCs migrate in a complex bi-directional manner before arriving at their appropriate layer, rather than exhibiting unidirectional movement to a targeted destination, which is the standard mode by which most neuronal cell types are believed to migrate radially. Live mouse HC imaging has also uncovered new aspects of neuronal mosaic formation that might be applicable to other neuronal types within the CNS. Mouse HCs also illustrate the fact that terminally differentiated neurons have the ability to re-enter the cell cycle and to give rise to clonal tumors, which is contrary to prevailing views within the field of tumor biology. Finally, zebrafish HC precursors have been shown to undergo symmetrical divisions followed by rapid tangential dispersion within the presumptive HC layer, potentially increasing laminar coverage as the retina expands. This finding is somewhat analogous to what has been observed in the cortical sub-ventricular zone, in which neural progenitor cells were observed to divide symmetrically (Noctor et al., 2004), and might ultimately be shown to occur elsewhere in the CNS.
Much work remains in order to identify the specific molecular pathways and the cellular properties that confer upon the HC such extraordinary abilities and to understand their biological significance. Specifically, the genetic programs that underlie HC migration, mosaic formation and cell cycle re-entry are not well defined. Although Lim1 has been implicated in regulating the positioning of HCs within the prospective HC layer and provides a valuable molecular entry point into the mechanism of HC migration, the primary phenotype of Lim1 mutant cells and the downstream genetic alterations following Lim1 deletion remain to be determined. With respect to neuronal mosaic formation, the molecular participants that mediate the homotypic interactions that might govern cell spacing and, later on, dendritic overlap are entirely unknown. Recently, mice with a mutation in Dscam were found to exhibit abnormal cellular clustering and hyperfasciculation in some subsets of retinal amacrine cells (Fuerst et al., 2008), suggesting a role for this cell surface molecule in mediating intercellular repulsion through homotypic contacts. Perhaps a similar molecular mechanism, which might act transiently during development, is responsible for the emerging regularity in the HC mosaic during early postnatal development. Finally, the reasons why HC precursors re-enter the cell cycle late in development, and why fully mature HCs give rise to retinoblastoma, remain a mystery. To tackle these questions, HC-specific gene profiling experiments, followed by loss-of-function studies, will be necessary, allowing us to gain further insight into these unusual properties of the HCs and to determine whether similar mechanisms are employed elsewhere in the CNS.
We thank members of Mary E. Dickinson's laboratory and Richard R. Behringer for their critical reading of the manuscript. We also thank Mary A. Raven and Ying Wang for original micrographs. Supported by the National Institutes of Health. Deposited in PMC for release after 12 months.
References
- Ajioka, I., Martins, R. A., Bayazitov, I. T., Donovan, S., Johnson, D. A., Frase, S., Cicero, S. A., Boyd, K., Zakharenko, S. S. and Dyer, M. A. (2007). Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell 131, 378-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellion, A., Baudoin, J. P., Alvarez, C., Bornens, M. and Metin, C. (2005). Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J. Neurosci. 25, 5691-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau, M. J. and Cepko, C. L. (1999). Extrinsic and intrinsic factors control the genesis of amacrine and cone cells in the rat retina. Development 126, 555-566. [DOI] [PubMed] [Google Scholar]
- Blackshaw, S., Harpavat, S., Trimarchi, J., Cai, L., Huang, H., Kuo, W. P., Weber, G., Lee, K., Fraioli, R. E., Cho, S. H. et al. (2004). Genomic analysis of mouse retinal development. PLoS Biol. 2, E247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boije, H., Edqvist, P. H. and Hallbook, F. (2009). Horizontal cell progenitors arrest in G2-phase and undergo terminal mitosis on the vitreal side of the chick retina. Dev. Biol. (in press). [DOI] [PubMed]
- Burkhart, D. L. and Sage, J. (2008). Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat. Rev. Cancer 8, 671-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal, R. S. (1893). La rétine des vertébrés. La Cellule 9, 119-225. [Google Scholar]
- Cayouette, M., Poggi, L. and Harris, W. A. (2006). Lineage in the vertebrate retina. Trends Neurosci. 29, 563-570. [DOI] [PubMed] [Google Scholar]
- Cepko, C. L., Austin, C. P., Yang, X., Alexiades, M. and Ezzeddine, D. (1996). Cell fate determination in the vertebrate retina. Proc. Natl. Acad. Sci. USA 93, 589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya, B., Di Cristo, G., Higashiyama, H., Knott, G. W., Kuhlman, S. J., Welker, E. and Huang, Z. J. (2004). Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J. Neurosci. 24, 9598-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D., Livne-bar, I., Vanderluit, J. L., Slack, R. S., Agochiya, M. and Bremner, R. (2004). Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell 5, 539-551. [DOI] [PubMed] [Google Scholar]
- Cheng, C. W., Chow, R. L., Lebel, M., Sakuma, R., Cheung, H. O., Thanabalasingham, V., Zhang, X., Bruneau, B. G., Birch, D. G., Hui, C. C. et al. (2005). The Iroquois homeobox gene, Irx5, is required for retinal cone bipolar cell development. Dev. Biol. 287, 48-60. [DOI] [PubMed] [Google Scholar]
- Cobrinik, D., Whyte, P., Peeper, D. S., Jacks, T. and Weinberg, R. A. (1993). Cell cycle-specific association of E2F with the p130 E1A-binding protein. Genes Dev. 7, 2392-2404. [DOI] [PubMed] [Google Scholar]
- Cook, J. E. and Chalupa, L. M. (2000). Retinal mosaics: new insights into an old concept. Trends Neurosci. 23, 26-34. [DOI] [PubMed] [Google Scholar]
- Cook, T. (2003). Cell diversity in the retina: more than meets the eye. BioEssays 25, 921-925. [DOI] [PubMed] [Google Scholar]
- Ding, Q., Chen, H., Xie, X., Libby, R. T., Tian, N. and Gan, L. (2009). BARHL2 differentially regulates the development of retinal amacrine and ganglion neurons. J. Neurosci. 29, 3992-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan, S. L., Schweers, B., Martins, R., Johnson, D. and Dyer, M. A. (2006). Compensation by tumor suppressor genes during retinal development in mice and humans. BMC Biol. 4, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer, M. A., Livesey, F. J., Cepko, C. L. and Oliver, G. (2003). Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat. Genet. 34, 53-58. [DOI] [PubMed] [Google Scholar]
- Edqvist, P. H. and Hallbook, F. (2004). Newborn horizontal cells migrate bi-directionally across the neuroepithelium during retinal development. Development 131, 1343-1351. [DOI] [PubMed] [Google Scholar]
- Eglen, S. J., Lofgreen, D. D., Raven, M. A. and Reese, B. E. (2008). Analysis of spatial relationships in three dimensions: tools for the study of nerve cell patterning. BMC Neurosci. 9, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshatory, Y., Everhart, D., Deng, M., Xie, X., Barlow, R. B. and Gan, L. (2007). Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J. Neurosci. 27, 12707-12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen, M. E., Xing, Y. G., Lawrence, J. B. and Livingston, D. M. (1991). Molecular cloning, chromosomal mapping, and expression of the cDNA for p107, a retinoblastoma gene product-related protein. Cell 66, 1155-1164. [DOI] [PubMed] [Google Scholar]
- Farajian, R., Raven, M. A., Cusato, K. and Reese, B. E. (2004). Cellular positioning and dendritic field size of cholinergic amacrine cells are impervious to early ablation of neighboring cells in the mouse retina. Vis. Neurosci. 21, 13-22. [DOI] [PubMed] [Google Scholar]
- Fariss, R. N., Li, Z. Y. and Milam, A. H. (2000). Abnormalities in rod photoreceptors, amacrine cells, and horizontal cells in human retinas with retinitis pigmentosa. Am. J. Ophthalmol. 129, 215-223. [DOI] [PubMed] [Google Scholar]
- Fekete, D. M., Perez-Miguelsanz, J., Ryder, E. F. and Cepko, C. L. (1994). Clonal analysis in the chicken retina reveals tangential dispersion of clonally related cells. Dev. Biol. 166, 666-682. [DOI] [PubMed] [Google Scholar]
- Feng, L., Xie, X., Joshi, P. S., Yang, Z., Shibasaki, K., Chow, R. L. and Gan, L. (2006). Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development 133, 4815-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend, S. H., Bernards, R., Rogelj, S., Weinberg, R. A., Rapaport, J. M., Albert, D. M. and Dryja, T. P. (1986). A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 323, 643-646. [DOI] [PubMed] [Google Scholar]
- Fuerst, P. G., Koizumi, A., Masland, R. H. and Burgess, R. W. (2008). Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature 451, 470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitani, Y., Fujitani, S., Luo, H., Qiu, F., Burlison, J., Long, Q., Kawaguchi, Y., Edlund, H., MacDonald, R. J., Furukawa, T. et al. (2006). Ptf1a determines horizontal and amacrine cell fates during mouse retinal development. Development 133, 4439-4450. [DOI] [PubMed] [Google Scholar]
- Galli-Resta, L., Novelli, E. and Viegi, A. (2002). Dynamic microtubule-dependent interactions position homotypic neurones in regular monolayered arrays during retinal development. Development 129, 3803-3814. [DOI] [PubMed] [Google Scholar]
- Gan, W. B. and Macagno, E. R. (1995). Interactions between segmental homologs and between isoneuronal branches guide the formation of sensory terminal fields. J. Neurosci. 15, 3243-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genis-Galvez, J. M., Prada, F. and Armengol, J. A. (1979). Evidence of three types of horizontal cells in the chick retina. Japan. J. Ophthalmol. 23, 378-387. [Google Scholar]
- Godinho, L., Williams, P. R., Claassen, Y., Provost, E., Leach, S. D., Kamermans, M. and Wong, R. O. (2007). Nonapical symmetric divisions underlie horizontal cell layer formation in the developing retina in vivo. Neuron 56, 597-603. [DOI] [PubMed] [Google Scholar]
- Grueber, W. B., Ye, B., Moore, A. W., Jan, L. Y. and Jan, Y. N. (2003). Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion. Curr. Biol. 13, 618-626. [DOI] [PubMed] [Google Scholar]
- Hannon, G. J., Demetrick, D. and Beach, D. (1993). Isolation of the Rb-related p130 through its interaction with CDK2 and cyclins. Genes Dev. 7, 2378-2391. [DOI] [PubMed] [Google Scholar]
- Hatakeyama, J. and Kageyama, R. (2004). Retinal cell fate determination and bHLH factors. Semin. Cell Dev. Biol. 15, 83-89. [DOI] [PubMed] [Google Scholar]
- Hatakeyama, J., Tomita, K., Inoue, T. and Kageyama, R. (2001). Roles of homeobox and bHLH genes in specification of a retinal cell type. Development 128, 1313-1322. [DOI] [PubMed] [Google Scholar]
- Holt, C. E., Bertsch, T. W., Ellis, H. M. and Harris, W. A. (1988). Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron 1, 15-26. [DOI] [PubMed] [Google Scholar]
- Huckfeldt, R. M., Schubert, T., Morgan, J. L., Godinho, L., Di Cristo, G., Huang, Z. J. and Wong, R. O. (2009). Transient neurites of retinal horizontal cells exhibit columnar tiling via homotypic interactions. Nat. Neurosci. 12, 35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, T., Hojo, M., Bessho, Y., Tano, Y., Lee, J. E. and Kageyama, R. (2002). Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development 129, 831-842. [DOI] [PubMed] [Google Scholar]
- Jan, Y. N. and Jan, L. Y. (2003). The control of dendrite development. Neuron 40, 229-242. [DOI] [PubMed] [Google Scholar]
- Joberty, G., Perlungher, R. R., Sheffield, P. J., Kinoshita, M., Noda, M., Haystead, T. and Macara, I. G. (2001). Borg proteins control septin organization and are negatively regulated by Cdc42. Nat. Cell Biol. 3, 861-866. [DOI] [PubMed] [Google Scholar]
- Kawauchi, D., Taniguchi, H., Watanabe, H., Saito, T. and Murakami, F. (2006). Direct visualization of nucleogenesis by precerebellar neurons: involvement of ventricle-directed, radial fibre-associated migration. Development 133, 1113-1123. [DOI] [PubMed] [Google Scholar]
- Keeley, P. W., Whitney, I. E., Raven, M. A. and Reese, B. E. (2007). Dendritic spread and functional coverage of starburst amacrine cells. J. Comp. Neurol. 505, 539-546. [DOI] [PubMed] [Google Scholar]
- Khidr, L. and Chen, P. L. (2006). RB, the conductor that orchestrates life, death and differentiation. Oncogene 25, 5210-5219. [DOI] [PubMed] [Google Scholar]
- Kinoshita, M. (2008). Insight into Septin functions from mouse models. In The Septins, 1st edn (ed. P. A. Hall, S. E. H. Russel and J. Pringle). Chichester: John Wiley & Sons.
- Kouros-Mehr, H., Bechis, S. K., Slorach, E. M., Littlepage, L. E., Egeblad, M., Ewald, A. J., Pai, S. Y., Ho, I. C. and Werb, Z. (2008). GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell 13, 141-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein, A., Noctor, S. and Martinez-Cerdeno, V. (2006). Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat. Rev. Neurosci. 7, 883-890. [DOI] [PubMed] [Google Scholar]
- Laurie, N. A., Donovan, S. L., Shih, C. S., Zhang, J., Mills, N., Fuller, C., Teunisse, A., Lam, S., Ramos, Y., Mohan, A. et al. (2006). Inactivation of the p53 pathway in retinoblastoma. Nature 444, 61-66. [DOI] [PubMed] [Google Scholar]
- Lewis, G. P., Linberg, K. A. and Fisher, S. K. (1998). Neurite outgrowth from bipolar and horizontal cells after experimental retinal detachment. Invest. Ophthalmol. Vis. Sci. 39, 424-434. [PubMed] [Google Scholar]
- Li, S., Mo, Z., Yang, X., Price, S. M., Shen, M. M. and Xiang, M. (2004). Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron 43, 795-807. [DOI] [PubMed] [Google Scholar]
- Li, Y., Graham, C., Lacy, S., Duncan, A. M. and Whyte, P. (1993). The adenovirus E1A-associated 130-kD protein is encoded by a member of the retinoblastoma gene family and physically interacts with cyclins A and E. Genes Dev. 7, 2366-2377. [DOI] [PubMed] [Google Scholar]
- Liu, W., Wang, J. H. and Xiang, M. (2000). Specific expression of the LIM/homeodomain protein Lim-1 in horizontal cells during retinogenesis. Dev. Dyn. 217, 320-325. [DOI] [PubMed] [Google Scholar]
- Livesey, F. J. and Cepko, C. L. (2001). Vertebrate neural cell-fate determination: lessons from the retina. Nat. Rev. Neurosci. 2, 109-118. [DOI] [PubMed] [Google Scholar]
- Macaluso, M., Montanari, M. and Giordano, A. (2006). Rb family proteins as modulators of gene expression and new aspects regarding the interaction with chromatin remodeling enzymes. Oncogene 25, 5263-5267. [DOI] [PubMed] [Google Scholar]
- MacNeil, M. A. and Masland, R. H. (1998). Extreme diversity among amacrine cells: implications for function. Neuron 20, 971-982. [DOI] [PubMed] [Google Scholar]
- MacPherson, D., Sage, J., Kim, T., Ho, D., McLaughlin, M. E. and Jacks, T. (2004). Cell type-specific effects of Rb deletion in the murine retina. Genes Dev. 18, 1681-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt, T., Ashery-Padan, R., Andrejewski, N., Scardigli, R., Guillemot, F. and Gruss, P. (2001). Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105, 43-55. [DOI] [PubMed] [Google Scholar]
- Mo, Z., Li, S., Yang, X. and Xiang, M. (2004). Role of the Barhl2 homeobox gene in the specification of glycinergic amacrine cells. Development 131, 1607-1618. [DOI] [PubMed] [Google Scholar]
- Morgan, J. L., Dhingra, A., Vardi, N. and Wong, R. O. (2006). Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat. Neurosci. 9, 85-92. [DOI] [PubMed] [Google Scholar]
- Morris, E. J. and Dyson, N. J. (2001). Retinoblastoma protein partners. Adv. Cancer Res. 82, 1-54. [DOI] [PubMed] [Google Scholar]
- Nadarajah, B., Brunstrom, J. E., Grutzendler, J., Wong, R. O. and Pearlman, A. L. (2001). Two modes of radial migration in early development of the cerebral cortex. Nat. Neurosci. 4, 143-150. [DOI] [PubMed] [Google Scholar]
- Nadarajah, B., Alifragis, P., Wong, R. O. and Parnavelas, J. G. (2002). Ventricle-directed migration in the developing cerebral cortex. Nat. Neurosci. 5, 218-224. [DOI] [PubMed] [Google Scholar]
- Noctor, S. C., Martinez-Cerdeno, V., Ivic, L. and Kriegstein, A. R. (2004). Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136-144. [DOI] [PubMed] [Google Scholar]
- Ohsawa, R. and Kageyama, R. (2008). Regulation of retinal cell fate specification by multiple transcription factors. Brain Res. 1192, 90-98. [DOI] [PubMed] [Google Scholar]
- Peichl, L. and Gonzalez-Soriano, J. (1994). Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis. Neurosci. 11, 501-517. [DOI] [PubMed] [Google Scholar]
- Peng, X. R., Jia, Z., Zhang, Y., Ware, J. and Trimble, W. S. (2002). The septin CDCrel-1 is dispensable for normal development and neurotransmitter release. Mol. Cell. Biol. 22, 378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poché, R. A., Kwan, K. M., Raven, M. A., Furuta, Y., Reese, B. E. and Behringer, R. R. (2007). Lim1 is essential for the correct laminar positioning of retinal horizontal cells. J. Neurosci. 27, 14099-14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poché, R. A., Raven, M. A., Kwan, K. M., Furuta, Y., Behringer, R. R. and Reese, B. E. (2008). Somal positioning and dendritic growth of horizontal cells are regulated by interactions with homotypic neighbors. Eur. J. Neurosci. 27, 1607-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven, M. A., Stagg, S. B., Nassar, H. and Reese, B. E. (2005a). Developmental improvement in the regularity and packing of mouse horizontal cells: implications for mechanisms underlying mosaic pattern formation. Vis. Neurosci. 22, 569-573. [DOI] [PubMed] [Google Scholar]
- Raven, M. A., Stagg, S. B. and Reese, B. E. (2005b). Regularity and packing of the horizontal cell mosaic in different strains of mice. Vis. Neurosci. 22, 461-468. [DOI] [PubMed] [Google Scholar]
- Raven, M. A., Oh, E. C., Swaroop, A. and Reese, B. E. (2007). Afferent control of horizontal cell morphology revealed by genetic respecification of rods and cones. J. Neurosci. 27, 3540-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese, B. E. (2008a). Mosaic architecture of the mouse retina. In Eye, Retina and Visual Systems of the Mouse (ed. L. M. Chalupa and R. W. Williams), pp. 147-155. Cambridge: MIT Press.
- Reese, B. E. (2008b). Mosaics, tiling and coverage by retinal neurons. In The Senses: A Comprehensive Reference, vol. 1 (ed. A. I. Basbaum et al.), pp. 439-456. Oxford: Elsevier. [Google Scholar]
- Reese, B. E. and Galli-Resta, L. (2002). The role of tangential dispersion in retinal mosaic formation. Prog. Retin. Eye Res. 21, 153-168. [DOI] [PubMed] [Google Scholar]
- Reese, B. E., Harvey, A. R. and Tan, S. S. (1995). Radial and tangential dispersion patterns in the mouse retina are cell-class specific. Proc. Natl. Acad. Sci. USA 92, 2494-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese, B. E., Necessary, B. D., Tam, P. P., Faulkner-Jones, B. and Tan, S. S. (1999). Clonal expansion and cell dispersion in the developing mouse retina. Eur. J. Neurosci. 11, 2965-2978. [DOI] [PubMed] [Google Scholar]
- Reese, B. E., Raven, M. A. and Stagg, S. B. (2005). Afferents and homotypic neighbors regulate horizontal cell morphology, connectivity, and retinal coverage. J. Neurosci. 25, 2167-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompani, S. B. and Cepko, C. L. (2008). Retinal progenitor cells can produce restricted subsets of horizontal cells. Proc. Natl. Acad. Sci. USA 105, 192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar, B. T. and McConnell, S. K. (2005). Cytoskeletal coordination during neuronal migration. Proc. Natl. Acad. Sci. USA 102, 13652-13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor, E., Eglen, S., Harris, B. and Wong, R. (2006). Retinal Development. Cambridge, UK: Cambridge University Press.
- Sheffield, P. J., Oliver, C. J., Kremer, B. E., Sheng, S., Shao, Z. and Macara, I. G. (2003). Borg/septin interactions and the assembly of mammalian septin heterodimers, trimers, and filaments. J. Biol. Chem. 278, 3483-3488. [DOI] [PubMed] [Google Scholar]
- Skapek, S. X., Lin, S. C., Jablonski, M. M., McKeller, R. N., Tan, M., Hu, N. and Lee, E. Y. (2001). Persistent expression of cyclin D1 disrupts normal photoreceptor differentiation and retina development. Oncogene 20, 6742-6751. [DOI] [PubMed] [Google Scholar]
- Skapek, S. X., Pan, Y. R. and Lee, E. Y. (2006). Regulation of cell lineage specification by the retinoblastoma tumor suppressor. Oncogene 25, 5268-5276. [DOI] [PubMed] [Google Scholar]
- Solecki, D. J., Model, L., Gaetz, J., Kapoor, T. M. and Hatten, M. E. (2004). Par6alpha signaling controls glial-guided neuronal migration. Nat. Neurosci. 7, 1195-1203. [DOI] [PubMed] [Google Scholar]
- Spiliotis, E. T. and Nelson, W. J. (2006). Here come the septins: novel polymers that coordinate intracellular functions and organization. J. Cell Sci. 119, 4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga, A., Taira, M. and Nakagawa, S. (2009). LIM family transcription factors regulate the subtype-specific morphogenesis of retinal horizontal cells at post-migratory stages. Dev. Biol. (in press). [DOI] [PubMed]
- Turner, D. L. and Cepko, C. L. (1987). A common progenitor for neurons and glia persists in rat retina late in development. Nature 328, 131-136. [DOI] [PubMed] [Google Scholar]
- Turner, D. L., Snyder, E. Y. and Cepko, C. L. (1990). Lineage-independent determination of cell type in the embryonic mouse retina. Neuron 4, 833-845. [DOI] [PubMed] [Google Scholar]
- van den Heuvel, S. and Dyson, N. J. (2008). Conserved functions of the pRB and E2F families. Nat. Rev. Mol. Cell Biol. 9, 713-724. [DOI] [PubMed] [Google Scholar]
- Weirich, C. S., Erzberger, J. P. and Barral, Y. (2008). The septin family of GTPases: architecture and dynamics. Nat. Rev. Mol. Cell Biol. 9, 478-489. [DOI] [PubMed] [Google Scholar]
- Wetts, R. and Fraser, S. E. (1988). Multipotent precursors can give rise to all major cell types of the frog retina. Science 239, 1142-1145. [DOI] [PubMed] [Google Scholar]
- Williams, R. W. and Goldowitz, D. (1992a). Lineage versus environment in embryonic retina: a revisionist perspective. Trends Neurosci. 15, 368-373. [DOI] [PubMed] [Google Scholar]
- Williams, R. W. and Goldowitz, D. (1992b). Structure of clonal and polyclonal cell arrays in chimeric mouse retina. Proc. Natl. Acad. Sci. USA 89, 1184-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Geldmacher, D. S. and Herrup, K. (2001). DNA replication precedes neuronal cell death in Alzheimer's disease. J. Neurosci. 21, 2661-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Gray, J., Wu, L., Leone, G., Rowan, S., Cepko, C. L., Zhu, X., Craft, C. M. and Dyer, M. A. (2004). Rb regulates proliferation and rod photoreceptor development in the mouse retina. Nat. Genet. 36, 351-360. [DOI] [PubMed] [Google Scholar]
- Zhang, S. S., Xu, X., Liu, M. G., Zhao, H., Soares, M. B., Barnstable, C. J. and Fu, X. Y. (2006). A biphasic pattern of gene expression during mouse retina development. BMC Dev. Biol. 6, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]