Abstract
The responsiveness of the rat anterior substantia nigra pars reticulata (SNR) GABAergic neurons to GABAAergic drugs changes with age and gender, altering its role in seizure control. To determine whether maturational and gender-specific differences in the properties of spontaneous GABAARs-mediated inhibitory postsynaptic currents (sIPSCs) underlie these events, we studied sIPSCs at baseline and after application of the α1 GABAARs subunit selective agonist zolpidem, at postnatal days (PN) 5-9, PN12-15, and PN28-32. Results were correlated with the α1 and α3 GABAARs subunit immunoreactivity (-ir) at PN5, PN15, and PN30, using immunochemistry. The mean frequency, amplitude and charge transfer increased whereas the 10–90% rise time and decay time accelerated with age in both genderes. The faster sIPSC kinetics in older rats were paralleled by increased α1-ir and decreased α3-ir. At PN5-9, males had more robust sIPSCs (frequency, amplitude, charge carried per event and charge transfer) than females. At PN28-32, males exhibited higher amplitudes and faster kinetics than females. The zolpidem-induced increase of decay times, amplitude and charge transfer and α1-ir expression were the lowest in PN5-9 males but increased with age, in both genders. Our findings demonstrate that alterations in GABAARs subunit expression partially underlie age- and gender-specific sIPSC changes in SNR neurons. However, the observation of gender differences in sIPSC kinetics that cannot be attributed to changes in perisomatic α1 expression suggests the existence of additional gender-specific factors that control the sIPSC kinetics in rat SNR.
Keywords: patch clamp, seizures, development, synaptic inhibition, immunohistochemistry, zolpidem
The substantia nigra reticulata (SNR) is a midbrain structure involved in the regulation of movement and seizure control (Iadarola and Gale, 1982, Moshe and Albala, 1984, Gale, 1985, Deransart et al., 1998, Veliskova and Moshe, 2006). The majority of cells in the SNR are fast spiking GABAergic neurons (Richards et al., 1997), which receive inhibitory input via postsynaptic GABAARs from the striatum but also from the globus pallidus (Smith and Bolam, 1989, Smith and Bolam, 1991, Bolam et al., 2000, Misgeld, 2004). GABAARs are heteropentamers composed of homologous subunits (α1-6, β1-3, γ1-3, δ, ε, π, θ) (Levitan et al., 1988, Olsen et al., 1991, Barnard et al., 1998, Benarroch, 2007). Many studies have shown that in particular the α subunit subtypes undergo significant changes in expression during brain development. In most studied brain regions, including the SNR, the α2 and α3 subunits are highly expressed in the early stages of postnatal maturation and their levels gradually decrease with age while the α1 subunit mRNA and protein levels rise (Laurie et al., 1992, Fritschy et al., 1994, Veliskova et al., 1998). The type of α subunit in GABAARs changes their pharmacological and kinetic properties. The decay time of the inhibitory postsynaptic currents (IPSCs) is faster when the α1 subunit is present, whereas it slows down if GABAARs contain α2 or α3 subunits instead (Verdoorn, 1994, Gingrich et al., 1995, Lavoie et al., 1997). Our previous studies on the α1 mRNA expression in the SNR showed higher levels in PN30 than in PN15 rats (Moshe et al., 1994) and in female rats than age-matched male rats (Veliskova et al., 1998, Ravizza et al., 2003).
In this study, we seek to determine whether there are age- and gender-related changes in GABAARs-mediated sIPSCs and zolpidem sensitivity in the GABAergic SNR neurons of PN5-32 rats.
Experimental procedures
We used Sprague-Dawley rats of both genders divided into 3 different age groups PN5-9, PN12-15 and PN28-32, with the date of birth taken as PN0 (Taconic Farms, New York, USA). Rats were kept at constant temperature (21 – 23°C), relative humidity (40 – 60%) and a 12 h dark/12 h light cycle (lights on at 7:00am) with food and water ad libitum in our animal facility accredited by the American Association for the Accreditation of Laboratory Animal Care. Rats younger than 21 days were kept with a dam. All procedures and experiments were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Slices containing SNR were prepared from rats of either gender at PN5-9, 12-15 and 28-32. Rats were deeply anesthetized with isoflurane and decapitated. The brain was quickly removed and placed in oxygenated (95% O2/5% CO2) ice-cold sucrose slicing solution containing (in mM): 187 sucrose, 3 KCl, 2 CaCl2, 1.9 MgCl2, 1.2 NaH2PO4, 26 NaHCO3 and 20 D-glucose, pH 7.4, 300–310 mOsm. 300 μM thick sagittal slices were cut using a vibratome (Leica, VT1000S). Slices were transferred into oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM): 124 NaCl; 2.5 KCl; 1 NaH2PO4; 26 NaHCO3; 2 CaCl2; 1.3 MgSO4 and 20 glucose, pH 7.3-7.4, 290–300 mOsm, and allowed to recover at room temperature for at least 1 hour before recording.
Cells were visualized with an upright Eclipse E600-FN microscope (Nikon) in the anterior part of the SNR (Veliskova and Moshe, 2001). Whole-cell patch clamp recordings were made from electrophysiologically identified GABAergic neurons using an Axopatch 200B amplifier (Molecular Devices, Union City, CA). Patch pipettes were pulled using Flaming/Brown micropipette puller (Sutter Instruments Co, Novato, CA) from thin-wall borosilicate glass tubing (1.5 mm OD; World Precision Instruments, Sarasota, FL) and had open tip resistance 2–3 MΩ when filled with an intracellular solution containing (in mM): 140 CsCl, 4 NaCl, 1 MgCl2, 10 HEPES, 10 EGTA, 2 Mg-ATP, 290 mOsm, pH 7.3 adjusted with CsOH. No correction was made for the liquid junction potential of +4.3 mV. Slices were continuously perfused at a rate of 4 ml/min with oxygenated aCSF solution. All recordings were performed at room temperature. Neurons were voltage-clamped at a holding potential of −70 mV and broken in to establish whole cell configuration recordings. We waited 3–5 minutes after breaking-in until the holding current stabilized.
The SNR consists predominantly of GABAergic neurons but it also contains a small portion of dopaminergic cells. The two populations can be distinguished by their electrophysiological responses to hyperpolarizing current. To determine whether neurons were GABAergic, they were stepwise hyperpolarized in current clamp configuration by injection of negative current (from −70 mV to −130 mV) and the decisive parameter for accepting a cell as GABAergic was lack of hyperpolarization-induced inward rectification or sag (Richards et al., 1997, Radnikow and Misgeld, 1998). After determining the cell phenotype, we switched back to the voltage clamp mode and held the cell at −70 mV. All GABAergic events were thus observed as inward currents. Series resistance was estimated by measuring the transient current in response to 1- to 5-mV 200 ms-long hyperpolarizing voltage steps. Cells were accepted for further analysis provided that the series resistance after 40–60% compensation did not exceed 15 MΩ and/or did not change by more than 15% during data acquisition. The input resistance could not be exactly measured due to the high intracellular Cs+ concentration, which blocks K+ channels (Shao and Dudek, 2005). Synaptic currents were recorded in the presence of glutamate antagonists D-(−)-2-Amino-5-phosphonopentanoic acid (D-AP5, 50 μM) and 6-cyano-2,3-dihydroxy-7-nitro-quinoxaline (CNQX, 10 μM) to block excitatory amino acid-mediated transmission.
Bicuculline methobromide (BIM) and D-AP5 were dissolved in distilled water whereas CNQX and zolpidem were dissolved in dimethyl sulfoxide (DMSO, final dilution 0.001%). All drugs were bath applied. BIM and zolpidem were purchased from Sigma-Aldrich, St. Louis, MO; D-AP5 and CNQX from Tocris Bioscience, Ellisville, MO. Sprague –Dawley male and female rats were transcardially perfused with saline and then formalin at PN5, PN15 and PN30. Their brains were collected, fixed overnight in formalin, immersed in 30% sucrose and when they sank they were frozen and kept at −80°C till use. Sagittal 40μm sections were cut in a MICROM cryostat (MICROM International, Walldorf, Germany) and were stained with rabbit antibodies specific for the α1 or α3 GABAARs subunits (Millipore, Billerica MA). The anti-α1 antibody recognizes the aminoacid sequence 1–16 of the rat α1 subunit protein, whereas the anti-α3 antibody recognizes the aminoacid sequence 1–15 of the rat α3 subunit protein. Both were used at a dilution of 1:800. Immunochemistries were done in free-floating sections. The steps included incubation with 1% H2O2 in Tris based saline (TBS) for 30 minutes at room temperature (RT); blocking in TBS with 10% normal goat serum (NGS) and 0.4% Triton-X-100 (90 minutes, RT); incubation with the primary antibody in TBS with Triton X100 0.4% and 3% NGS (2–3 days, 4°C with shaking); incubation with secondary biotinylated anti-rabbit antibody (Vector Labs, Burlingame, CA) (1:200 dilution) in TBS with 0.4% Triton-X-100 and 3% NGS, RT; and further peroxidase based staining as per manufacturer’s protocols (Vector Labs; ABC Elite kit and 3,3′-diaminobenzidine/nickel substrate kit). In every assay a representative brain from all groups was included to minimize inter-assay variability. Brains were coded to permit blinded assessment of the values.
Because the SNR is sparsely populated, densitometric analysis of cellular perisomatic immunostaining was done by sampling representative stained cells from 4–5 anterior SNR sections per rat, using the Image J software (Wayne Rasband, Research Services Branch, NIMH, Bethesda Maryland, USA). The densitometry values of these cells were averaged for each brain and results were used in the statistical analysis. Details of this densitometric analysis are as described in (Galanopoulou, 2006). Similar densitometric analysis of immunochemically stained sections has been used extensively to provide semi-quantitative assessment of the level of protein expression in the stained cells (Rieux et al., 2002, Galanopoulou, 2006, Galanopoulou, 2008).
Synaptic currents were filtered at 2 kHz (low-pass Bessel filter), sampled at 10 kHz and recorded with pClamp 8 analysis software (Molecular Devices Co, Sunnyvale, CA) through a Digidata 1322A digitizer (Molecular Devices Co, Sunnyvale, CA). Spontaneous inhibitory postsynaptic currents (sIPSCs) were analyzed offline using Mini Analysis Program (Synaptosoft, Decatur, GA). Individual events were automatically selected if their amplitude and area under curve were 5-fold higher than the set threshold detection parameters. All recordings were subsequently visually checked to remove artifacts. Both single and multiple peaked events were included into the analysis. A minimum of 50 accepted events per cell was analyzed (on average 450 events) and averaged to obtain mean values. The amplitude was measured from the baseline to the peak of the synaptic current. We further analyzed the 10–90% rise time, the 37 % decay time (measured as a time required for the current to decay to 37% of its peak amplitude) and the charge transferred by a single sIPSC (calculated by the software as an integrated area under curve). The charge transfer was calculated as a product of the mean sIPSC frequency and charge transferred by averaged sIPSC (q = fmean × qaveraged sIPSC). Baseline sIPSCs were analyzed before zolpidem 0.5 μM was applied and compared with events when the drug was present in the slice for at least 5 minutes. Two-way ANOVA followed by post hoc t-test (Tukey) was used to compare age and gender differences in sIPSCs properties. Because the sensitivity of the two-way ANOVA and Tukey post hoc decreases as the number of inter-group comparisons increases, we utilized unpaired t-test to explore whether significant differences in the studied variables existed in specific same age groups that demonstrated visible gender-related differences. The paired t-test was used to assess zolpidem effects. The Kolmogorov-Smirnov (K-S) two-sample, two-tailed test was used to compare cumulative amplitude, 10–90% rise time and decay time distributions. Statistics on the densitometry measurements were carried on with repeated measures multiple factor ANOVA and Tukey post hoc comparisons, using Statview and JMP softwares (SAS Institute, Cary, NC, USA). All values are expressed as least square mean value ± standard error (SE).
Results
Baseline sIPSCs
Spontaneous IPSCs were recorded in the presence of CNQX 10 μM and D-AP5 50 μM. Under these conditions all sIPSCs were blocked by BIM, which confirms that they were GABAARs-mediated (Fig. 1A,B,C). The sIPSCs could be detected at all studied ages in both genders. The events reversed close to 0 mV, the theoretical equilibrium potential for Cl− ions (Fig. 2A,B). All numeric values are summarized in Table 1.
Fig. 1.
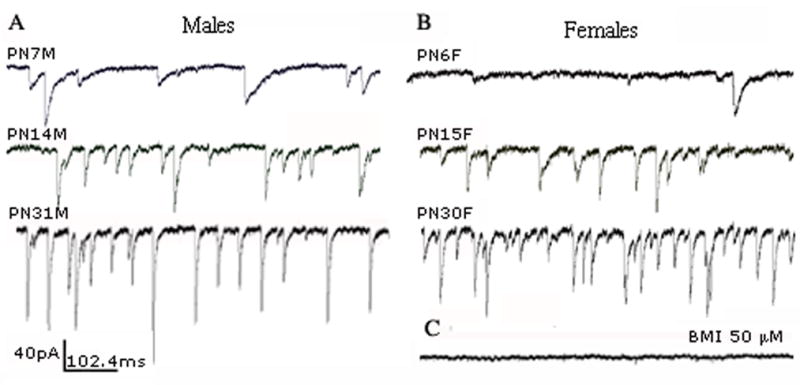
Raw traces of spontaneous GABAARs-mediated inhibitory postsynaptic currents (sIPSCs) recorded in voltage-clamp configuration from GABAergic SNR neurons. Whole cell patch clamp recordings were made at a holding potential of −70 mV in the presence of glutamate antagonists CNQX 10 μM and D-AP5 50 μM in males (A) and females (B) of different ages. All sIPSCs were invariably blocked by GABAARs antagonist bicuculline methobromide (BIM) 50 μM in all groups (C).
Fig. 2.
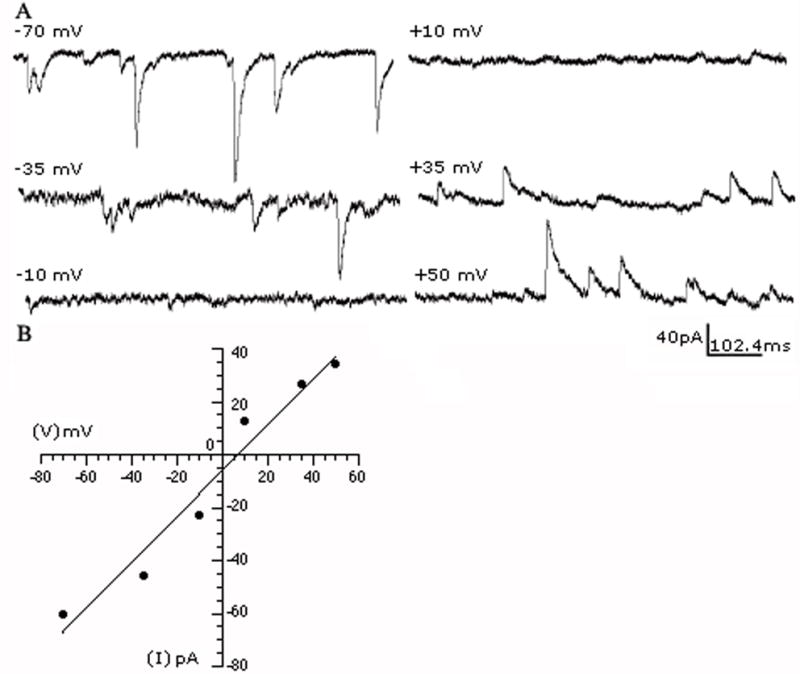
GABAARs-mediated sIPSC reversal potential. (A) The recorded sIPSCs were inward at negative holding potentials and outward at positive holding potentials. (B) A current-voltage plot of the average sIPSC amplitude vs. holding potential from the cell in (A) shows that the events reversed close to the theoretical equilibrium potential for Cl−, as expected with symmetrical intra- and extracellular chloride concentrations, confirming that they were mediated by GABAARs. The plot was best fitted with a linear regression.
Table 1.
Baseline properties of sIPSCs
| Age group/sex | n (cells) | Frequency (Hz) | Amplitude (pA) | 10–90% rise time (ms) | Decay time (ms) | Charge per averaged event (fC) | Charge transfer (fC/s) |
|---|---|---|---|---|---|---|---|
| PN5-9 M | 21 | 6.4±1.9# | 65.5±7.1# | 2.61±0.1 | 11.37±0.5 | 706±51.9# | 5326±1553# |
| PN12-15 M | 28 | 7.0±1.7 | 73.9±6.1 | 2.03±0.1 | 7.57±0.4 | 506±44.9 | 4400±1345 |
| PN28-32 M | 16 | 16.4±2.2 | 119.2±8.1# | 1.4±0.2# | 5.1±0.6# | 566±59.4 | 10010±1779 |
| PN5-9 F | 18 | 3.7±2.1 | 48.6±7.6 | 2.67±0.1 | 9.86±0.5 | 457±56.0 | 2061±1678 |
| PN12-15 F | 23 | 8.8±1.8 | 67.9±6.8 | 1.94±0.1 | 7.52±0.5 | 467±49.6 | 5025±1484 |
| PN28-32 F | 14 | 18.5±2.4 | 90.6±8.7 | 1.72±0.2 | 6.19±0.6 | 528±63.4 | 10894±1902 |
P<0.05 value different from animals of opposite sex in the same age group, unpaired t-test. F=female, M=male.
The results of the two-way ANOVA for the studied sIPSC parameters and inter-group comparisons are presented in Figure 3. Specifically, the mean sIPSCs frequency significantly rises with age in both genders. Although overall gender differences were not found using the two-way ANOVA, separate comparisons of the average sIPSCs frequencies by the unpaired t-test in the individual age groups revealed that PN5-9 males have greater frequency than PN5-9 females (Fig. 3A, Table 1). The mean sIPSCs amplitude also significantly increased with age in both genders. Furthermore, male SNR neurons had higher sIPSC amplitudes than female ones (Fig. 3B). Although, post hoc comparisons with Tukey test did not show any significant gender differences between same age groups, possibly due to the high number of comparisons, the unpaired t-test revealed significantly higher sIPSC amplitudes in males than in females in the PN5-9 and PN28-32 groups (Figs. 3B, 4Aa,b, Table 1). Changes in the sIPSCs kinetics seen in our experiments were similar to those found in other studies (Dunning et al., 1999, Okada et al., 2000). Both the 10–90% rise time and decay time progressively accelerated with age (Figs. 3C,D, 4A,B, Table 1). The unpaired t-test showed that both these parameters are significantly faster in PN28-32 males than females. In the face of significant changes in the amplitude and kinetic parameters, the charge transferred by averaged sIPSC remained quite stable during development. The only exceptions were events in PN5-9 males, which transferred significantly bigger charge than PN5-9 females (Fig. 3E, Table 1, Tukey post hoc and unpaired t-test). The total charge transfer carried by synaptic currents gradually grew with maturation in both males and females. PN5-9 males demonstrated higher increase than same age females (Fig. 3F and Table 1, unpaired t-test only).
Fig. 3.
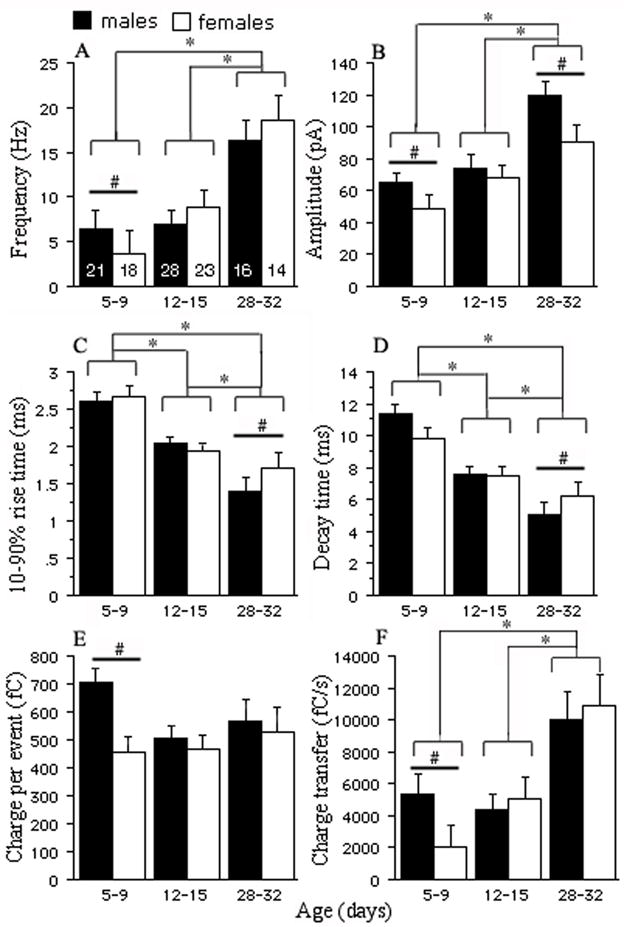
Baseline properties of the sIPSCs during development. (A) The mean sIPSCs frequency in PN28-32 groups is significantly higher than in the two remaining groups in both genders (P<0.05, two-way ANOVA, see Table 1 for numerical values). An additional inter-group analysis using the unpaired t-test (see Methods) showed that the mean sIPSCs frequency is higher in PN5-9 males than in females (P<0.05, unpaired t-test). (B) The mean sIPSCs amplitude is greatest in the oldest group compared with PN5-9 and PN12-15 animals and males have higher amplitudes than females (P<0.05, two-way ANOVA). The unpaired t-test comparisons demonstrate that PN5-9 and PN28-32 males exhibit higher amplitudes than their female counterparts (P<0.05). The mean 10–90% rise time (C) and decay time (D) shorten with age in both genders and significant differences were observed between each age group (P<0.05, two-way ANOVA). The only sex differences were noted in PN28-32 animals, males having faster decay and 10–90% rise time than females (P<0.05, unpaired t-test). (E) The mean charge transferred by averaged sIPSC remains constant during development in both males and females. PN5-9 males have significantly greater charge than PN5-9 females (P<0.05, unpaired t-test). (F) The importance of synaptic GABAARs-mediated inhibition rises with age as the synaptic charge transfer considerably grows in both genders (P<0.05, two-way ANOVA). No differences were found between PN5-9 and 12–15 animals. The unpaired t-test revealed that the PN5-9 males have bigger charge transfer than PN5-9 females (P<0.05). (*P<0.05 two-way ANOVA; #P<0.05, unpaired t-test; numbers in graph A represent the numbers of cells).
Fig. 4.
Age-related changes in the averaged GABAARs-mediated sIPSC traces of male and female GABAergic SNR neurons. (Aa) and (Ab) show averaged representative sIPSC traces derived from 232–469 events recorded from PN7, PN14, PN31 male and PN6F, PN15, PN30 female GABAergic SNR neurons. The overlapped traces demonstrate acceleration of decay time with age in both genders. The mean amplitude is highest in both PN28-32 groups. (Ba) and (Bb) figures in expanded time scale illustrate shortening of the 10–90% rise time during development.
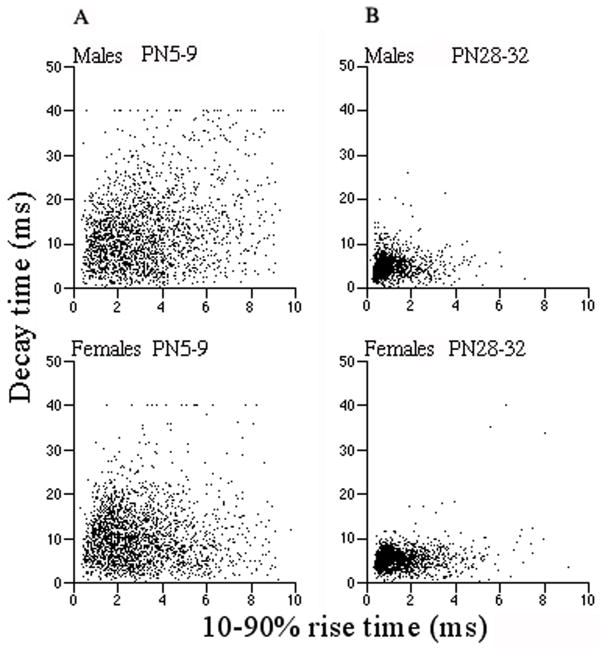
The occurrence of slow sIPSC kinetics in younger groups and the opposite pattern observed in older animals (Figs. 3,4) may result from the known maturational subunit changes in GABAAergic synapses. We performed an additional analysis of the sIPSC 10–90% rise times and decay times in a subset of PN5-9, PN12-15 and PN28-32 male and female cells with almost similar series resistances (Rs ranged from 10.8 to 11.7 MΩ, P>0.05) to assess the datapoints distribution. Only single events emerging from the baseline were included to avoid distortion of the kinetic parameters and amplitudes by overlapping events. We analyzed 2223–2309 events from each group and plotted decay times of individual events against their 10–90% rise times. The widespread distribution of events in PN5-9 groups most likely reflected greater subunit heterogeneity of GABAARs. (Fig. 5A). In contrast, the scattergrams of PN28-32 groups demonstrated that the occurrence of mainly fast events was confined to a very small area of the plot (Fig. 5B) suggesting that the composition of GABAARs was more homogeneous (Moshe et al., 1994).
Fig. 5.
The sIPSCs decay times of single events of SNR GABAergic neurons were plotted against 10–90% rise times in PN5-9 and PN28-32 animals of both genders. The broad dispersion of datapoints in the PN5-9 group plots (column A) reflects heterogeneous GABAARs subunit composition. The majority of events have slow activation and deactivation times. The situation is different in PN28-32 groups (column B), where the decay vs. rise time plots show that most events are confined to one limited area of the graph representing fast values. This finding suggests that GABAARs subunit composition is more homogenous.
Zolpidem
The age- and gender-dependent changes in sIPSCs amplitude and kinetics and different α1 mRNA subunit expression during maturation led us to investigate whether pharmacological responses to zolpidem would also alter with age and gender.
Zolpidem 0.5 μM, an α1 subunit-selective positive modulator of type I benzodiazepine receptors (Pritchett et al., 1989), was bath applied and changes in the mean sIPSCs decay time and amplitude were analyzed after at least a 5-minute exposure to the drug.
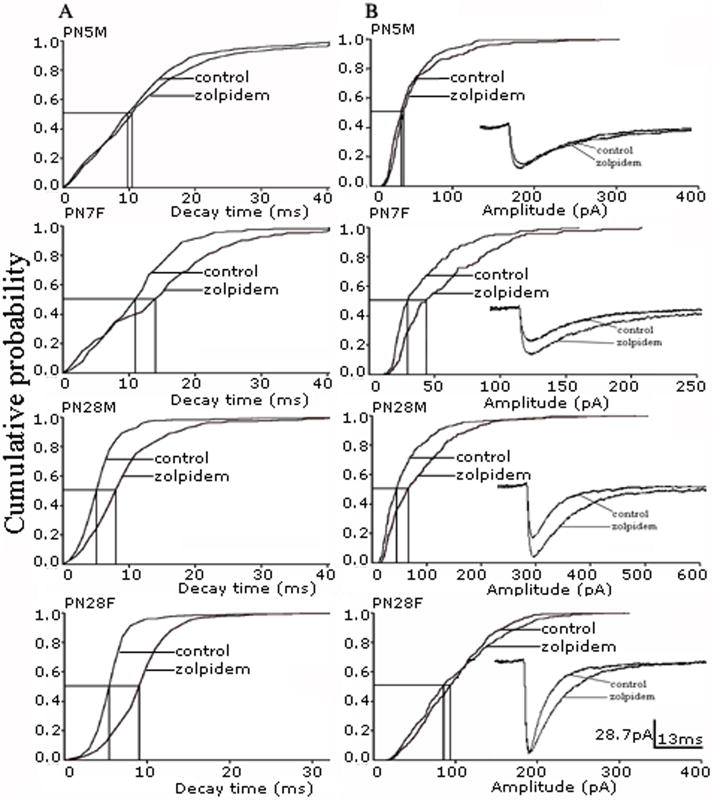
Zolpidem significantly prolonged the decay time and increased the amplitude and charge per event compared with baseline sIPSCs in most groups (P<0.05, K-S test, Fig. 6 P<0.05, paired t-test, Fig. 7). The only exception was PN5-9 males where there was no significant increase in any of these variables in response to zolpidem. This may mean that GABAA receptors in PN5-9 male SNR neurons have less α1 subunits than the other groups. In PN28-32 females, zolpidem significantly increased the decay time and charge per event but not the sIPSC amplitude (Fig. 6 and 7). As PN28-32 females had the largest baseline amplitudes compared to the other groups, this may reflect a ceiling effect (baseline amplitude 82.5±12.5 pA, zolpidem amplitude 89.8±11.5 pA, n=6, p>0.05, mean±SE, paired t-test).
Fig. 6.
Decay time (column A) and amplitude (column B) cumulative probability plots were constructed from representative cells in PN5-9 (male cell 450 events, female 164) and 28–32 groups (male cell 373 events, female 450). Zolpidem 0.5 μM significantly changed cumulative amplitude and decay distribution in all cells shown (P<0.05, K-S test) except in the PN5M cell (no change in decay time and amplitude) and PN28F cell (no change in amplitude) (P>0.05 K-S test). While the lack of zolpidem effect both on decay time and amplitude in the PN5M cell can be explained by low α1 subunit expression, the near-to-complete saturation of GABAARs may underlie the failure of zolpidem to augment the average sIPSCs amplitude in the PN28F cell. Please note that PN12-15 male and female SNR neurons were also responsive to zolpidem (data not shown). The insets in graphs in the column B show overlapped baseline and zolpidem averaged events from the same cells.
Fig. 7.
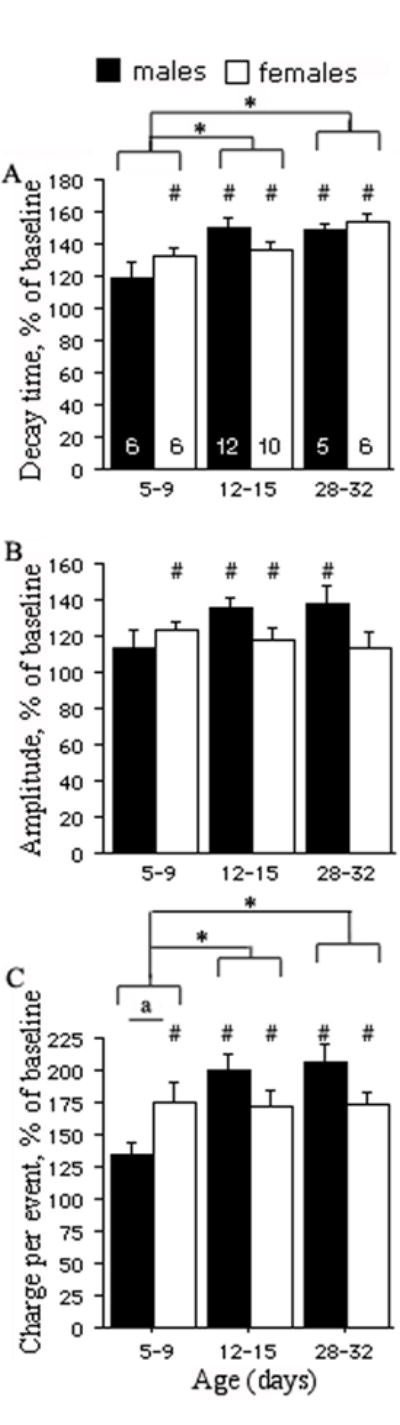
The percentage increase relative to the pre-drug baseline of decay time (panel A), amplitude (panel B), and charge per event (panel C) was used to compare age- and sex-related differences in response to zolpidem 0.5 μM. In most groups, zolpidem increased decay time, amplitude and charge per event compared to their baseline values, as indicated by the pound key (#) marks (P<0.05, paired t-test, data no shown). However, the only group that showed no response to zolpidem was the PN5-9 males. In PN28-32 females, zolpidem increased decay time and charge per event but not the amplitude of sIPSCs. This probably reflects a ceiling effect, as the baseline amplitudes in this group were already the highest (baseline amplitude 82.5±12.5 pA, zolpidem amplitude 89.8±11.5 pA, n=6, p>0.05, mean±SE, paired t-test).
(A) The graph shows a significantly greater prolongation of decay time in PN12-15 and PN28-32 groups compared with PN5-9 groups. No sex-related differences were detected (unpaired t-test). (B) In contrast, the increase of sIPSCs amplitude by zolpidem does not differ significantly across ages and genders. (C) The overall increase of charge transferred per event is greater in PN12-15 and PN28-32 animals compared with PN5-9 groups. There is a significant effect of the age*sex interaction, which indicates sex-specific patterns in the developmental changes in zolpidem sensitivity. Specifically, PN5-9 females show significantly bigger enhancement in zolpidem compared with PN5-9 males (“a” indicates P≤0.05, unpaired t-test), and this zolpidem sensitivity remains stable till PN28-32. In contrast, there is a significant increase in zolpidem sensitivity in males between PN5-9 and PN12-15. The pound keys (#) indicate groups in which zolpidem induced significant changes compared to baseline levels (P<0.05, paired t-test, data no shown). The asterisks (*) indicate significant differences from the respective PN5-9 group (P<0.05, two-way ANOVA, numbers in the plot A indicate the number of cells).
The overall age- and gender-related differences in sensitivity to zolpidem 0.5 μM were determined using the two-way ANOVA by comparing ratios of drug-induced responses to the pre-drug values. The zolpidem-induced percentage increases of the mean decay time and charge per event were greater in older animals than in younger ones (P<0.05) but no gender differences were observed (Fig. 7). No significant overall age and gender specific differences in zolpidem-induced percentage increase of the mean sIPSCs amplitude were observed. (Fig. 7).
These results suggest that SNR neurons are sensitive to zolpidem at all studied ages, with single exception the PN5-9 males, and that responsiveness to zolpidem increases with age.
Age- and gender-related differences in the expression of α1 and α3 GABAARs subunits in the anterior SNR
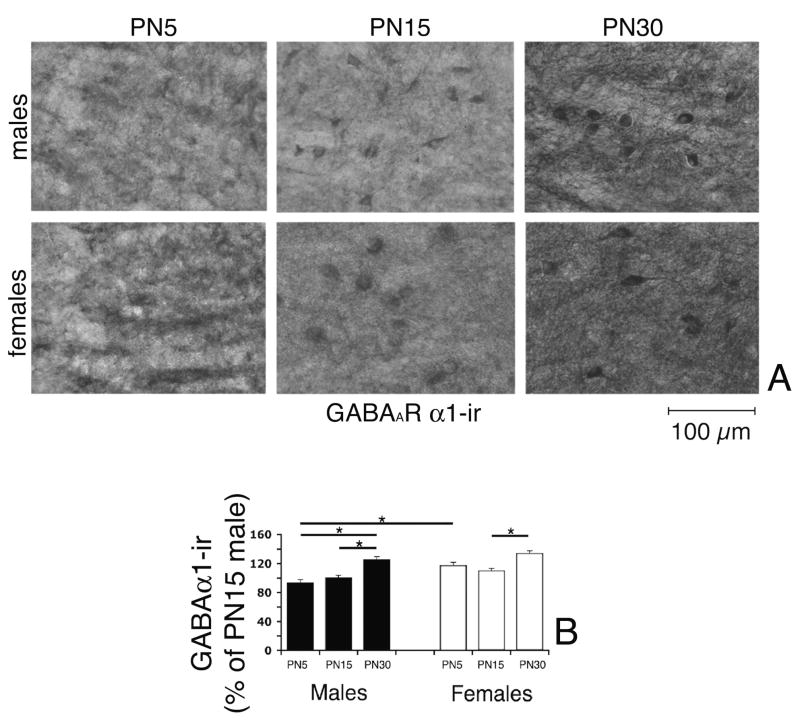
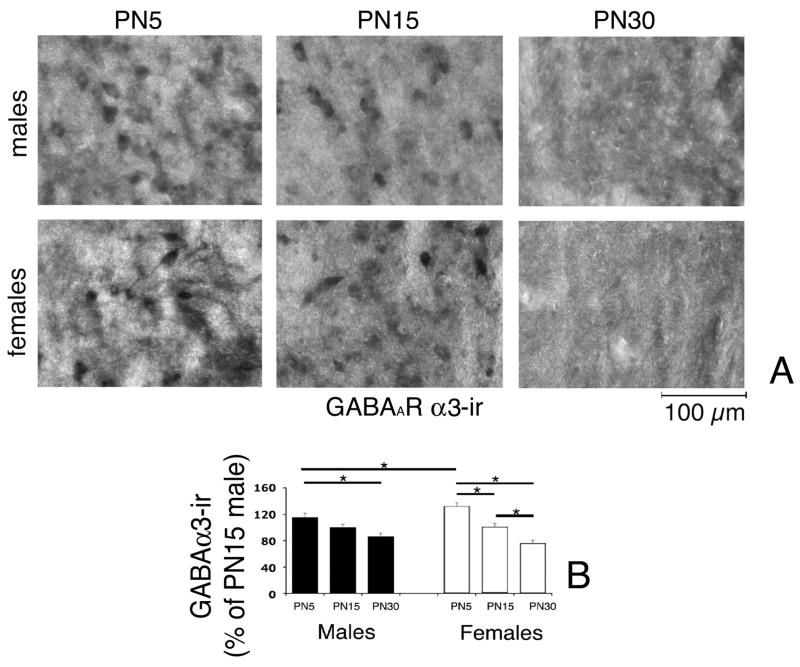
To correlate the described age- and gender-specific changes in the electrophysiological properties of sIPSCs and zolpidem effects, we compared the expression of the α1 and α3 GABAARs subunits in the anterior SNR of PN5, PN15, and PN30 male and female rats, using specific immunochemistries. Representative photos and the results of the statistical comparisons of the densitometric comparison of perisomatic α1-immunoreactivity (-ir) and α3-ir are presented in Table 2 and Figs. 8 and 9. The GABAARs α1-ir increased between PN15 and PN30 in both genders (Table 2, Fig. 8). Gender had a significant overall effect, with females expressing more GABAARs α1-ir than males (Table 2, Fig. 8). However, in inter-group comparisons of same age groups, only the PN5 females had statistically higher expression of GABAARs α1-ir than males (Table 2, Fig. 8). In contrast, the GABAARs α3-ir decreased between PN5 and PN30 in both genders, without any significant gender differences (Table 2, Fig. 9). The decrease was steeper in females, with significant statistical differences among all 3 age groups. In males, significant differences were observed only between PN5 and PN30 rats (Table 2, Fig. 9). Once again, gender differences were only observed at PN5, when females expressed more GABAARs α3-ir than males (Table 2, Fig. 9).
Table 2.
Age and sex specific effects on the expression of α1 and α3 GABAAR-ir in rat anterior SNR.
| A. Main effects, two-way ANOVA, repeated measures. | ||||
|---|---|---|---|---|
| Groups | n (rats/group) | Age (F-value) | Sex (F-value) | Age*Sex (F-value) |
| α1 GABAAR-ir | 5 | 8.4* | 6.5* | 0.6 |
| α3 GABAAR-ir | 4 | 12.2* | 0.2 | 1.8 |
| B. Least squares means tables. | ||||
|---|---|---|---|---|
| Groups | α1 GABAAR-ir somatic expression | α3 GABAAR-ir somatic expression | ||
| n (rats) | Least Squares Means (% of PN15 male group) | n (rats) | Least Squares Means (% of PN15 male group) | |
| PN5 M | 5 | 93.1±4.7# | 4 | 115.4±6.1# |
| PN15 M | 5 | 100±4.3# | 4 | 100±5.5 |
| PN30 M | 5 | 124.8±4.5 | 4 | 86±5.5 |
| PN5 F | 5 | 116.7±4.8§ | 4 | 131.9±6# |
| PN15 F | 5 | 109.4±4.3# | 4 | 100.9±5.6 # ¶ |
| PN30 F | 5 | 133.2±4.6 | 4 | 75.56±5.5 |
P<0.05
P<0.05 vs PN30 same sex group;
P<0.05 vs PN5 males;
P<0.05 vs PN5 females; Tukey post hoc comparisons.
Fig. 8.
Expression of GABAARs α1-ir in the anterior SNR of PN5, PN15, and PN30 male and female rats.
Panel A: Representative photographs of anterior SNR neurons stained with anti-GABAARs α1 specific antibody. The α1-ir increases with age in both genders, in both the somata and the dendritic processes. The scale bar indicates 100μm distance.
Panels B: Densitometric comparisons of perisomatic α1-ir confirmed the developmental increase in GABAARs α1-ir in both male and female rats between PN5 and PN30. As the SNR is sparsely populated, densitometry was done on individual α1-ir anterior SNR cells and was averaged for each brain. At PN5, females expressed more GABAARs α1-ir than PN5 males. The asterisks indicate statistically significant differences (P<0.05) between the groups linked with bars.
Fig. 9.
Expression of GABAAR α3-ir in the anterior SNR of PN5, PN15, and PN30 male and female rats.
Panel A: Representative photographs of anterior SNR neurons stained with anti-GABAARs α3 specific antibody. The α3-ir decreases with age. The scale bar indicates 100μm distance.
Panels B: Densitometric comparisons of perisomatic α3-ir confirmed the developmental decrease in GABAARs α3-ir in both male and female rats between PN5 and PN30. Densitometry was done on individual α3-ir anterior SNR cells and was averaged for each brain. At PN5, females expressed more GABAARs α3-ir than males. The asterisks indicate statistically significant differences (P<0.05) between the groups linked with bars.
Discussion
The current study describes age- and gender-related differences in the properties of sIPSCs in GABAergic neurons of the anterior SNR, a region that undergoes developmental changes in terms of its ability to modify seizure thresholds (Veliskova and Moshe, 2001). Our data show that the mean frequency, amplitude and charge transfer increase and 10–90% rise time and decay time accelerate in both genders with age. The developmental increase in GABAARs α1-ir and parallel decrease in GABAARs α3-ir may partially explain some of these changes. The amount of charge transferred by averaged sIPSC remains practically constant during maturation in both genders except in PN5-9 males. Gender differences are detected in some of the studied parameters in PN5-9 and PN28-32 groups. The potency of zolpidem 0.5 μM to prolong the decay time is age-dependent in both genders. However, the responsiveness to zolpidem appears earlier in females (PN5-9 group), and this may be due to the higher expression of GABAARs α1-ir in female than in male PN5-9 SNR.
Increased mean sIPSCs frequency and higher amplitude in PN28-32 GABAergic rat SNR neurons in both genders along with marked acceleration of the sIPSCs kinetics can have several explanations including a) an increased number of GABAAergic synaptic terminals, b) higher density of postsynaptic GABAARs containing mainly α1 subunit and c) decreased expression of postsynaptic GABAARs containing α3 subunits.
The higher frequency of sIPSCs in PN28-32 animals can be due to a greater number of synapses formed on nigral GABAergic neurons with age (Phelps and Adinolfi, 1982) (Kraszewski and Grantyn, 1992, Swanwick et al., 2006). However, age-related increases in firing rates of presynaptic striatal and pallidal neurons can also contribute to higher sIPSCs frequency as we measured both action potential-dependent and -independent events.
The sIPSCs amplitude is determined by the number of open synaptic GABAARs and the amount of released GABA (Frerking et al., 1995). The role of an α subunit type in affecting the amplitude of sIPSCs may be limited because, despite the increase in α1 mRNA with age, the mean amplitude decreases in cerebellar granule cells (Brickley et al., 1996) or does not change in dentate gyrus granule cells (Hollrigel and Soltesz, 1997). Furthermore, the reduction of amplitude in α10/0 knockouts most likely relates to a decreased number of GABAARs at synapses rather than to a different subunit composition (Vicini et al., 2001, Goldstein et al., 2002). Therefore the increase in the amplitude of sIPSCs after PN15, in both genders, indicates an increased availability of postsynaptic GABAARs in GABAergic SNR neurons.
The acceleration of the decay and rise times in PN28-32 reflects age-related changes in a variety of pre- and post-synaptic factors that control the kinetics of sIPSCs. An important variable is the type of expressed α subunit (Verdoorn, 1994). Currents produced by recombinant α1 subunit-containing GABAARs deactivate faster than those mediated by GABAARs composed of α2 or α3 subunits and, as a result, such receptors close more rapidly and their decay time is reduced (Verdoorn, 1994, Gingrich et al., 1995, Lavoie et al., 1997). To determine whether the observed changes in the kinetics of sIPSCs can be attributed to changes in α subunit composition, we utilized more α1 subunit-specific functional and expression assays. We studied the responsiveness of SNR neurons to the α1-selective agonist zolpidem as well as compared the perisomatic expression of α1-ir across the different groups, using semi-quantitative densitometric immunochemical analysis. Indeed, the zolpidem-induced percentage increase of the decay time in PN28-32 animals was significantly higher than in PN5-9 group in both genders. This finding is in agreement with the developmental increase in α1 mRNA expression (Moshe et al., 1994, Veliskova et al., 1998) and α1-ir (Fig. 8) in the anterior SNR, as well as with the age-related increase in high-affinity binding sites for the α1 GABAARs agonist muscimol in rat SNR (Wurpel et al., 1988).
The zolpidem effect on the sIPSCs amplitude is mediated by increasing the affinity of closed GABAARs for GABA rather than by increasing the probability of channel opening or enhancing conductance (Perrais and Ropert, 1999, Hajos et al., 2000, Goldstein et al., 2002). Zolpidem increased the amplitude and charge per event in most age groups, although without any overall age- and gender-related differences. The only group that did not respond to zolpidem was the PN5-9 male rats, which can be explained by the lower expression of α1-ir in the anterior SNR of PN5-9 males compared to same age females. The little change of the mean sIPSCs amplitude and great enhancement of the decay observed in PN28-32 females reflects most likely near-to-complete saturation of GABAARs containing the α1 subunit.
Although the age-related changes in decay time correlated well with the α1-specific assays (zolpidem responses and α1-ir), additional gender-specific factors, not necessarily related to α1 subunit, seem to interfere with the shaping of sIPSC kinetics in PN5-9 and PN28-32 males and females. The absence of significant gender differences in decay time at PN5-9, despite the enhanced α1-ir expression and zolpidem sensitivity in PN5-9 females, may be partially explained by the higher expression of α3-ir in PN5-9 females. The increased number of α3-containing GABAARs in PN5-9 females may therefore create a subpopulation of sIPSCs with slower kinetics, blunting the differences in decay times between males and females.
In the absence of gender differences in zolpidem sensitivity and perisomatic α1-ir or α3-ir, the slower decay and 10–90% rise times of sIPSCs, along with the lower amplitude, in PN28-32 females may suggest a more distant site of origin of their sIPSCs compared to same age males. The significantly slower rise times combined with lower amplitudes in PN28-32 females thus support the hypothesis that their synaptic events may be more subjected to dendritic filtering than those in PN28-32 males. There are no studies to date describing sexual dimorphism in the organization and size of the dendritic tree of GABAergic neurons and GABAAergic synapses in the SNR of PN28-32 animals but gender differences have been reported in other brain structures, e.g. in the anterior cingulate cortex (Markham and Juraska, 2002), accessory olfactory bulb (Caminero et al., 1991) and subiculum (Andrade et al., 2000).
Based on our in situ hybridization data showing higher α1 mRNA expression in PN15 and PN30 females (Ravizza et al., 2003), we expected that the baseline decay time would be faster and zolpidem-induced prolongation of the decay time significantly greater in females than in males. Our current electrophysiological data did not, however, show gender differences between PN12-15 groups and the only significant difference was found between PN28-32 males and females, males having quicker decay time (Fig. 3D, Table 1). The discrepancy between in situ α1 mRNA hybridization, somatic α1-ir and expected kinetic data in PN12-15 and PN28-32 rats may have the following explanations: First, despite the higher α1 mRNA in PN15 females, the synaptic α1 protein levels may be similar in males and females as reflected by the same decay time and somatic α1-ir (Fig. 8). This thought is further supported by the same zolpidem-induced prolongation of the decay time. PN12-15 females, however, may have an increased α1 subunit expression in the extrasynaptic compartment. Such α1-containing GABAARs do not participate in shaping the decay time. This hypothesis is currently being a subject of our next experiments as some studies have already suggested that α1-containing GABAARs may indeed be present extrasynaptically and play an important role in modulating tonic GABAARs-mediated currents by benzodiazepines such as zolpidem or lorazepam (Liang et al., 2004, Shen et al., 2005). Second, as discussed earlier, it is possible that the previously reported increased α1 mRNA expression in PN30 females may reflect α1 subunit that is ultimately targeted to GABAARs located at distal dendritic synaptic sites. Additional variable that may influence the sIPSC shape is the vesicular transmitter release. The vesicular GABA release may be more asynchronous in PN5-9 animals than in PN28-32, yielding sIPSCs with slower decay and rise times in the younger age group (Vautrin and Barker, 1995, Williams et al., 1998). Another factor that may contribute to the gender differences in GABAA receptor function in the SNR are naturally occurring neurosteroids as their site of action was recently identified in the α1 (Ueno et al., 2004, Rahman et al., 2008) and other α subunit subtypes (Hosie et al., 2009). Neurosteroids or their metabolites may also regulate the expression of specific GABAAR subunit (Maguire and Mody, 2007, Peden et al., 2007). However, more studies will be necessary to confirm whether they could underlie observed age and gender sIPSCs differences in the SNR. The amount of charge transferred by averaged sIPSC is conserved during maturation. The amount of charge is determined to a large extent by the amplitude and decay time of sIPSC. Therefore, older animals, despite shorter decay time, develop equal charge transfer owing to the higher amplitude. The importance of phasic inhibition during development increases nonetheless as the charge transfer is significantly augmented in PN28-32 males and females. The main reason is increased frequency of sIPSCs due most likely to higher firing rate of presynaptic neurons and/or increased number of active GABAAergic synapses.
The above findings support that during development, PN5-32 GABAergic anterior SNR neurons acquire more active GABAAergic synapses per neuron, more activated synaptic GABAARs per sIPSC, their sIPSCs have faster kinetics and increased zolpidem-sensitivity, which is partially attributed to the increase in α1-ir and decrease in α3-ir. This developmental transformation may contribute to the late appearance of the GABAA-sensitive anticonvulsant region of the anterior SNR, which has been linked to an increase in high affinity binding sites for muscimol, an agonist for α1-containing GABAARs (Wurpel et al., 1988).
Acknowledgments
The authors thank Drs. Jana Veliskova, Libor Velisek and James G. Heida for thoughtful comments and critiques. We would like to acknowledge the excellent technical assistance of Mrs. Qianyun Li.
This project was supported by NIH NINDS grants NS20253, NS045243, NS58303, NS62947, and grants from the International Rett Syndrome Foundation, PACE, and Heffer Family Foundation. SLM is a recipient of a Martin A and Emily L Fisher Fellowship in Neurology and Pediatrics.
List of abbreviations
- SNR
substantia nigra pars reticulata
- sIPSCs
spontaneous inhibitory postsynaptic currents
- PN
postnatal days
- -ir
immunoreactivity
- GABAARs
GABAA receptors
- aCSF
artificial cerebrospinal fluid
- D-AP5
D-(−)-2-Amino-5-phosphonopentanoic acid
- CNQX
6-cyano-2,3-dihydroxy-7-nitro-quinoxaline
- BIM
bicuculline methobromide
- DMSO
dimethyl sulfoxide
- TBS
Tris based saline
- RT
room temperature
- NGS
normal goat serum
- K-S test
Kolmogorov-Smirnov test
- SE
standard error
- Rs
series resistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrade JP, Madeira MD, Paula-Barbosa MM. Sexual dimorphism in the subiculum of the rat hippocampal formation. Brain Res. 2000;875:125–137. doi: 10.1016/s0006-8993(00)02605-6. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Benarroch EE. GABAA receptor heterogeneity, function, and implications for epilepsy. Neurology. 2007;68:612–614. doi: 10.1212/01.wnl.0000255669.83468.dd. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196(Pt 4):527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497(Pt 3):753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminero AA, Segovia S, Guillamon A. Sexual dimorphism in accessory olfactory bulb mitral cells: a quantitative Golgi study. Neuroscience. 1991;45:663–670. doi: 10.1016/0306-4522(91)90279-w. [DOI] [PubMed] [Google Scholar]
- Deransart C, Vercueil L, Marescaux C, Depaulis A. The role of basal ganglia in the control of generalized absence seizures. Epilepsy Res. 1998;32:213–223. doi: 10.1016/s0920-1211(98)00053-9. [DOI] [PubMed] [Google Scholar]
- Dunning DD, Hoover CL, Soltesz I, Smith MA, O’Dowd DK. GABA(A) receptor-mediated miniature postsynaptic currents and alpha-subunit expression in developing cortical neurons. J Neurophysiol. 1999;82:3286–3297. doi: 10.1152/jn.1999.82.6.3286. [DOI] [PubMed] [Google Scholar]
- Frerking M, Borges S, Wilson M. Variation in GABA mini amplitude is the consequence of variation in transmitter concentration. Neuron. 1995;15:885–895. doi: 10.1016/0896-6273(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. Sex- and cell-type-specific patterns of GABAA receptor and estradiol-mediated signaling in the immature rat substantia nigra. Eur J Neurosci. 2006;23:2423–2430. doi: 10.1111/j.1460-9568.2006.04778.x. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABA(A) receptors. J Neurosci. 2008;28:1557–1567. doi: 10.1523/JNEUROSCI.5180-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale K. Mechanisms of seizure control mediated by gamma-aminobutyric acid: role of the substantia nigra. Fed Proc. 1985;44:2414–2424. [PubMed] [Google Scholar]
- Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995;489(Pt 2):529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein PA, Elsen FP, Ying SW, Ferguson C, Homanics GE, Harrison NL. Prolongation of hippocampal miniature inhibitory postsynaptic currents in mice lacking the GABA(A) receptor alpha1 subunit. J Neurophysiol. 2002;88:3208–3217. doi: 10.1152/jn.00885.2001. [DOI] [PubMed] [Google Scholar]
- Hajos N, Nusser Z, Rancz EA, Freund TF, Mody I. Cell type- and synapse-specific variability in synaptic GABAA receptor occupancy. Eur J Neurosci. 2000;12:810–818. doi: 10.1046/j.1460-9568.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- Hollrigel GS, Soltesz I. Slow kinetics of miniature IPSCs during early postnatal development in granule cells of the dentate gyrus. J Neurosci. 1997;17:5119–5128. doi: 10.1523/JNEUROSCI.17-13-05119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABA A receptors. Neuropharmacology. 2009;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Gale K. Substantia nigra: site of anticonvulsant activity mediated by gamma-aminobutyric acid. Science. 1982;218:1237–1240. doi: 10.1126/science.7146907. [DOI] [PubMed] [Google Scholar]
- Kraszewski K, Grantyn R. Development of GABAergic connections in vitro: increasing efficacy of synaptic transmission is not accompanied by changes in miniature currents. J Neurobiol. 1992;23:766–781. doi: 10.1002/neu.480230613. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PHMS. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABA(A) receptor channels are dependent on alpha-subunit isoform. Biophys J. 1997;73:2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan ES, Schofield PR, Burt DR, Rhee LM, Wisden W, Kohler M, Fujita N, Rodriguez HF, Stephenson A, Darlison MG, et al. Structural and functional basis for GABAA receptor heterogeneity. Nature. 1988;335:76–79. doi: 10.1038/335076a0. [DOI] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther. 2004;310:1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Juraska JM. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol Aging. 2002;23:579–588. doi: 10.1016/s0197-4580(02)00004-0. [DOI] [PubMed] [Google Scholar]
- Misgeld U. Innervation of the substantia nigra. Cell Tissue Res. 2004;318:107–114. doi: 10.1007/s00441-004-0918-2. [DOI] [PubMed] [Google Scholar]
- Moshe SL, Albala BJ. Nigral muscimol infusions facilitate the development of seizures in immature rats. Brain Res. 1984;315:305–308. doi: 10.1016/0165-3806(84)90165-2. [DOI] [PubMed] [Google Scholar]
- Moshe SL, Brown LL, Kubova H, Veliskova J, Zukin RS, Sperber EF. Maturation and segregation of brain networks that modify seizures. Brain Res. 1994;665:141–146. doi: 10.1016/0006-8993(94)91164-9. [DOI] [PubMed] [Google Scholar]
- Okada M, Onodera K, Van Renterghem C, Sieghart W, Takahashi T. Functional correlation of GABA(A) receptor alpha subunits expression with the properties of IPSCs in the developing thalamus. J Neurosci. 2000;20:2202–2208. doi: 10.1523/JNEUROSCI.20-06-02202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Bureau MH, Endo S, Smith G. The GABAA receptor family in the mammalian brain. Neurochem Res. 1991;16:317–325. doi: 10.1007/BF00966095. [DOI] [PubMed] [Google Scholar]
- Peden DR, Petitjean CM, Herd MB, Durakoglugil M, Rosahl TW, Wafford K, Homanics GE, Belelli D, Fritschy JM, Lambert JJ. Developmental maturation of synaptic and extrasynaptic GABAA receptors in mouse thalamic ventrobasal neurones. J Physiol. 2007 doi: 10.1113/jphysiol.2007.145375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D, Ropert N. Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses. J Neurosci. 1999;19:578–588. doi: 10.1523/JNEUROSCI.19-02-00578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps PE, Adinolfi AM. The postnatal development of the substantia nigra: a light and electron microscopy study. J Comp Neurol. 1982;209:123–138. doi: 10.1002/cne.902090203. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Luddens H, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Radnikow G, Misgeld U. Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. J Neurosci. 1998;18:2009–2016. doi: 10.1523/JNEUROSCI.18-06-02009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Borra VB, Isaksson M, Johansson IM, Ragagnin G, Backstrom T, Wang MD. A comparison of the pharmacological properties of recombinant human and rat alpha(1)beta(2)gamma(2L) GABA(A) receptors in Xenopus oocytes. Clin Exp Pharmacol Physiol. 2008;35:1002–1011. doi: 10.1111/j.1440-1681.2008.04946.x. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Friedman LK, Moshe SL, Veliskova J. Sex differences in GABA(A)ergic system in rat substantia nigra pars reticulata. Int J Dev Neurosci. 2003;21:245–254. doi: 10.1016/s0736-5748(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience. 1997;80:545–557. doi: 10.1016/s0306-4522(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Rieux C, Carney R, Lupi D, Dkhissi-Benyahya O, Jansen K, Chounlamountri N, Foster RG, Cooper HM. Analysis of immunohistochemical label of Fos protein in the suprachiasmatic nucleus: comparison of different methods of quantification. J Biol Rhythms. 2002;17:121–136. doi: 10.1177/074873002129002410. [DOI] [PubMed] [Google Scholar]
- Shao LR, Dudek FE. Changes in mIPSCs and sIPSCs after kainate treatment: evidence for loss of inhibitory input to dentate granule cells and possible compensatory responses. J Neurophysiol. 2005;94:952–960. doi: 10.1152/jn.01342.2004. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. Neurons of the substantia nigra reticulata receive a dense GABA-containing input from the globus pallidus in the rat. Brain Res. 1989;493:160–167. doi: 10.1016/0006-8993(89)91011-1. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. Convergence of synaptic inputs from the striatum and the globus pallidus onto identified nigrocollicular cells in the rat: a double anterograde labelling study. Neuroscience. 1991;44:45–73. doi: 10.1016/0306-4522(91)90250-r. [DOI] [PubMed] [Google Scholar]
- Swanwick CC, Murthy NR, Mtchedlishvili Z, Sieghart W, Kapur J. Development of gamma-aminobutyric acidergic synapses in cultured hippocampal neurons. J Comp Neurol. 2006;495:497–510. doi: 10.1002/cne.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Tsutsui M, Toyohira Y, Minami K, Yanagihara N. Sites of positive allosteric modulation by neurosteroids on ionotropic gamma-aminobutyric acid receptor subunits. FEBS Lett. 2004;566:213–217. doi: 10.1016/j.febslet.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Vautrin J, Barker JL. How can exocytosis account for the actual properties of miniature synaptic signals? Synapse. 1995;19:144–149. doi: 10.1002/syn.890190210. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Kubova H, Friedman LK, Wu R, Sperber EF, Zukin RS, Moshe SL. The expression of GABA(A) receptor subunits in the substantia nigra is developmentally regulated and region-specific. Ital J Neurol Sci. 1998;19:205–210. doi: 10.1007/BF02427602. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Moshe SL. Sexual dimorphism and developmental regulation of substantia nigra function. Ann Neurol. 2001;50:596–601. doi: 10.1002/ana.1248. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Moshe SL. Update on the role of substantia nigra pars reticulata in the regulation of seizures. Epilepsy Curr. 2006;6:83–87. doi: 10.1111/j.1535-7511.2006.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn TA. Formation of heteromeric gamma-aminobutyric acid type A receptors containing two different alpha subunits. Mol Pharmacol. 1994;45:475–480. [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Buhl EH, Mody I. The dynamics of synchronized neurotransmitter release determined from compound spontaneous IPSCs in rat dentate granule neurones in vitro. J Physiol. 1998;510(Pt 2):477–497. doi: 10.1111/j.1469-7793.1998.477bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurpel JN, Tempel A, Sperber EF, Moshe SL. Age-related changes of muscimol binding in the substantia nigra. Brain Res. 1988;471:305–308. doi: 10.1016/0165-3806(88)90108-3. [DOI] [PubMed] [Google Scholar]