Abstract
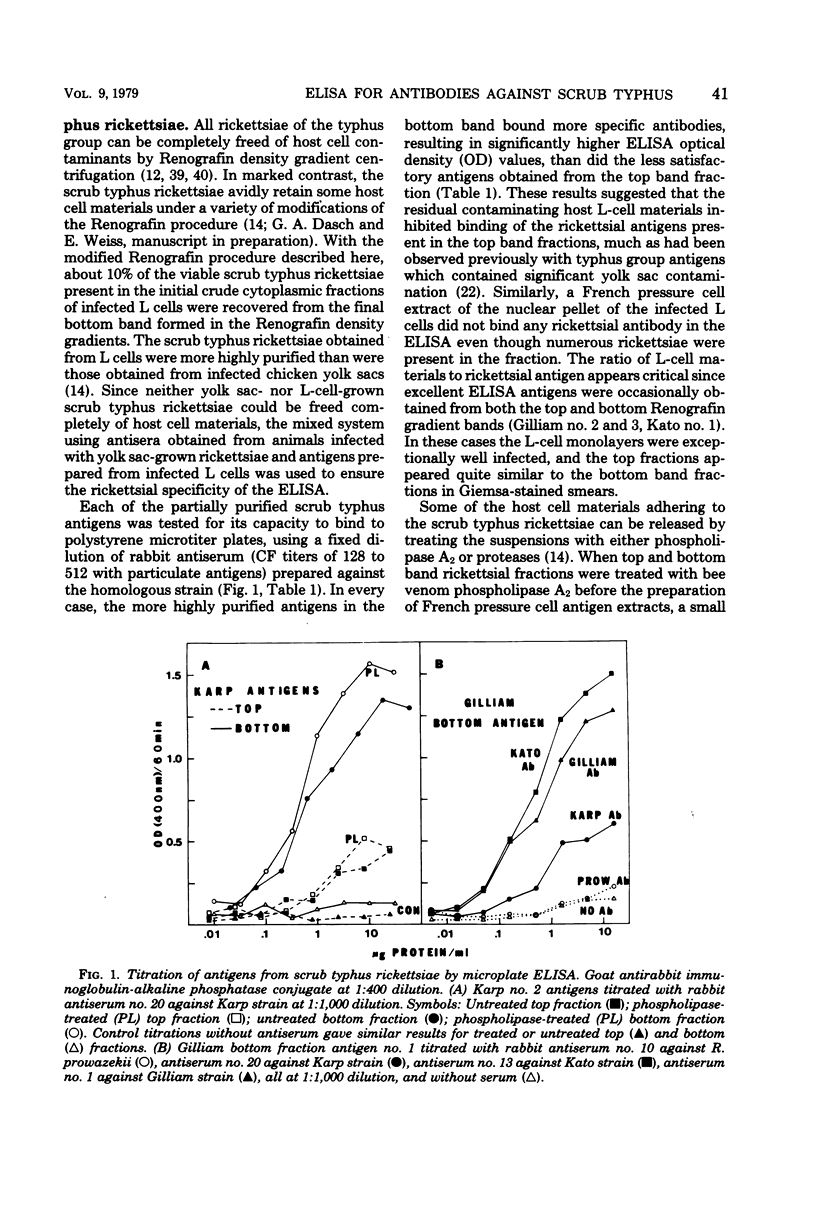
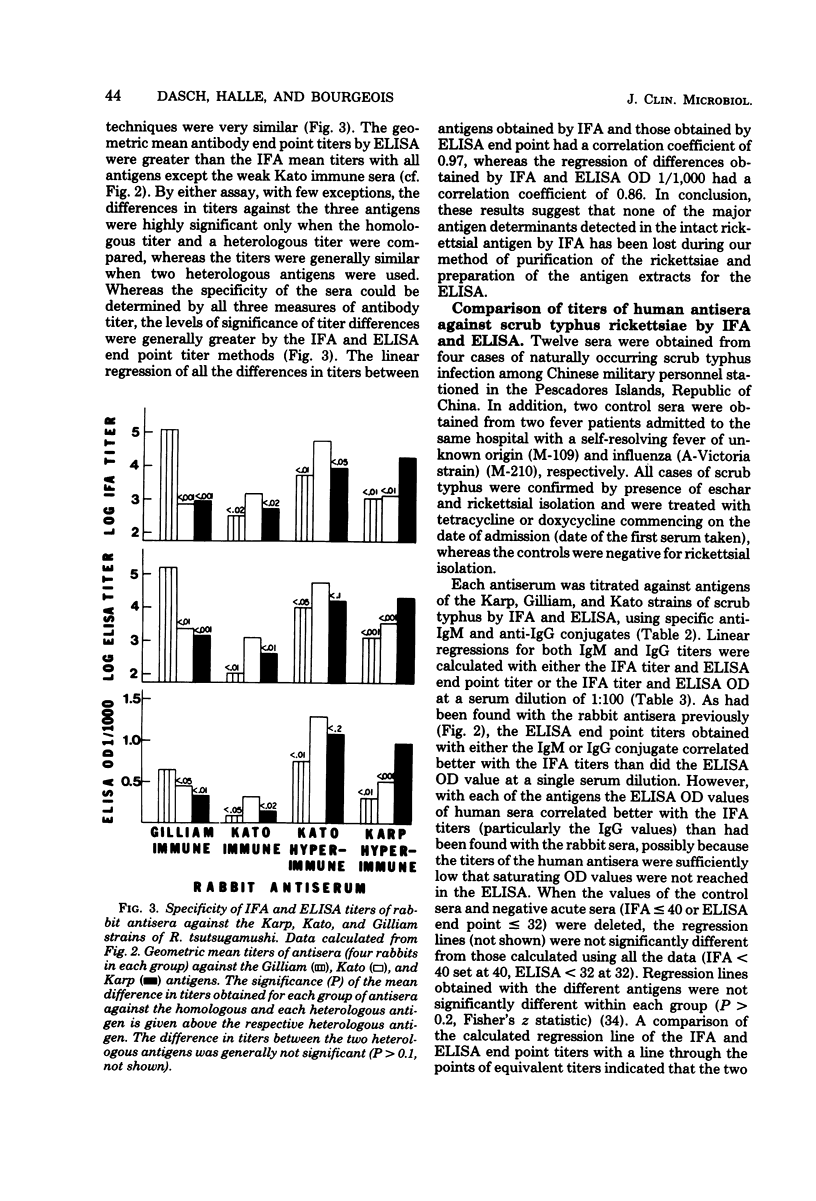
A microtiter enzyme-linked immunosorbent assay (ELISA) has been developed for the titration of antibodies against scrub typhus in human and animal sera. Scrub typhus rickettsiae were grown in monolayers of irradiated mouse LM3 cells and separated from host cell materials by differential centrifugation, filtration through a glass filter (AP-20, Millipore Corp.), and isopycnic banding in Renografin density gradients. The scrub typhus ELISA antigens were obtained from the purified viable rickettsiae by French pressure cell disruption and addition of 0.2% Formalin to the soluble extract. Antisera prepared in rabbits against the prototype Karp, the Kato, and the Gilliam strains of scrub typhus were used to standardize the ELISA and to compare its sensitivity and specificity to that of the indirect fluorescent antibody test (IFA). ELISA titers were measured as the greatest serum dilution showing an optical density 0.25 above controls or by the optical density achieved at a fixed serum dilution. The IFA and ELISA end point titers were quite similar, and all three measures of titer had comparable specificity for the strains of scrub typhus. No cross-reactions between the typhus and scrub typhus wera were observed by ELISA. Both the immunoglobulin M (IgM) and IgG antibody titers of 12 sequential sera from four patients with scrub typhus were obtained by IFA and ELISA. The IFA and ELISA end point titers for IgM and IgG had correlation coefficients of 0.91 and 0.97, respectively, whereas the ELISA optical density values at a serum dilution of 1:100 had slightly lower correlations with IFA titers (0.80 and 0.94). Early rising IgM titers followed by rising IgG titers were demonstrated by ELISA in three patients with primary scrub typhus infections, whereas the IgG response predominated in a patient with a reinfection. It is concluded that the ELISA for scrub typhus is a very satisfactory alternative to the IFA test.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOZEMAN F. M., ELISBERG B. L. Serological diagnosis of scrub typhus by indirect immunofluorescence. Proc Soc Exp Biol Med. 1963 Mar;112:568–573. doi: 10.3181/00379727-112-28107. [DOI] [PubMed] [Google Scholar]
- Bekker B. V., Dinger J. E., Wolff H. L. Scrubtyphus, an epidemiological study. Acta Leiden. 1968;36:1–8. [PubMed] [Google Scholar]
- Berman S. J., Kundin W. D. Scrub typhus in South Vietnam. A study of 87 cases. Ann Intern Med. 1973 Jul;79(1):26–30. doi: 10.7326/0003-4819-79-1-26. [DOI] [PubMed] [Google Scholar]
- Beutner E. H., Sepulveda M. R., Barnett E. V. Quantitative studies of immunofluorescent staining. Relationships of characteristics of unabsorbed antihuman IgG conjugates to their specific and non-specific staining properties in an indirect test for antinuclear factors. Bull World Health Organ. 1968;39(4):587–606. [PMC free article] [PubMed] [Google Scholar]
- Bourgeois A. L., Olson J. G., Ho C. M., Fang R. C., Van Peenen P. F. Epidemiological and serological study of scrub typhus among Chinese military in the Pescadores islands of Taiwan. Trans R Soc Trop Med Hyg. 1977;71(4):338–342. doi: 10.1016/0035-9203(77)90115-8. [DOI] [PubMed] [Google Scholar]
- Bozeman F. M., Elisberg B. L. Studies of the antibody response in scrub typhus employing indirect immunofluorescence. Acta Med Biol (Niigata) 1967 Dec;15:105–111. [PubMed] [Google Scholar]
- Brown G. W., Robinson D. M., Huxsoll D. L., Ng T. S., Lim K. J. Scrub typhus: a common cause of illness in indigenous populations. Trans R Soc Trop Med Hyg. 1976;70(5-6):444–448. doi: 10.1016/0035-9203(76)90127-9. [DOI] [PubMed] [Google Scholar]
- CARLEY J. G., DOHERTY R. L., DERRICK E. H., POPE J. H., EMANUEL M. L., ROSS C. J. The investigation of fevers in North Queensland by mouse inoculation, with particular reference to scrub typhus. Australas Ann Med. 1955 May;4(2):91–99. doi: 10.1111/imj.1955.4.2.91. [DOI] [PubMed] [Google Scholar]
- DOHERTY R. L. A clinical study of scrub typhus in North Queensland. Med J Aust. 1956 Aug 11;43(6):212–220. doi: 10.5694/j.1326-5377.1956.tb56591.x. [DOI] [PubMed] [Google Scholar]
- Dasch G. A., Samms J. R., Weiss E. Biochemical characteristics of typhus group rickettsiae with special attention to the Rickettsia prowazekii strains isolated from flying squirrels. Infect Immun. 1978 Feb;19(2):676–685. doi: 10.1128/iai.19.2.676-685.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasch G. A., Weiss E. Characterization of the Madrid E strain of Rickettsia prowazekii purified by renografin density gradient centrifugation. Infect Immun. 1977 Jan;15(1):280–286. doi: 10.1128/iai.15.1.280-286.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisberg B. L., Campbell J. M., Bozeman F. M. Antigenic diversity of rickettsia tsutsugamushi: epidemiologic and ecologic significance. J Hyg Epidemiol Microbiol Immunol. 1968;12(1):18–25. [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A., Silberman R., Peacock M., Spielman S. H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969 Jan;13(1):60–66. [PubMed] [Google Scholar]
- Gillis T. P., Thompson J. J. Quantitative fluorescent immunoassay of antibodies to, and surface antigens of, Actinomyces viscosus. J Clin Microbiol. 1978 Feb;7(2):202–208. doi: 10.1128/jcm.7.2.202-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle S., Dasch G. A., Weiss E. Sensitive enzyme-linked immunosorbent assay for detection of antibodies against typhus rickettsiae, Rickettsia prowazekii and Rickettsia typhi. J Clin Microbiol. 1977 Aug;6(2):101–110. doi: 10.1128/jcm.6.2.101-110.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaoka M., Tanaka Y. Rickettsial toxin and its specificity in 3 prototype strains, Karp, Gilliam and Kato, of Rickettsia orientalis. Acta Virol. 1973 Sep;17(5):426–434. [PubMed] [Google Scholar]
- Kobayashi Y., Nagai K., Tachibana N. Purification of complement-fixing antigens of Rickettsia orientalis by ether extraction. Am J Trop Med Hyg. 1969 Nov;18(6):942–952. doi: 10.4269/ajtmh.1969.18.942. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Ormsbee R. A., Peacock M. G., Burgdorfer W. Microimmunofluorescence test for the serological study of rocky mountain spotted fever and typhus. J Clin Microbiol. 1976 Jan;3(1):51–61. doi: 10.1128/jcm.3.1.51-61.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Weekly Reports for DECEMBER 14, 1945. Public Health Rep. 1945 Dec 14;60(50):1483–1518. [PMC free article] [PubMed] [Google Scholar]
- Robinson D. M., Brown G., Gan E., Huxsoll D. L. Adaptation of a microimmunofluorescence test to the study of human Rickettsia tsutsugamuskh antibody. Am J Trop Med Hyg. 1976 Nov;25(6):900–905. doi: 10.4269/ajtmh.1976.25.900. [DOI] [PubMed] [Google Scholar]
- SHISHIDO A. STRAIN VARIATION OF RICKETTSIA ORIENTALIS IN THE COMPLEMENT FIXATION TEST. Jpn J Med Sci Biol. 1964 Jun;17:59–72. doi: 10.7883/yoken1952.17.59. [DOI] [PubMed] [Google Scholar]
- SMADEL J. E., LEY H. L., Jr, DIERCKS R. H., CAMERON J. A. P. Persistence of Rickettsia tsutsugamushi in tissues of patients recovered from scrub typhus. Am J Hyg. 1952 Nov;56(3):294–302. doi: 10.1093/oxfordjournals.aje.a119553. [DOI] [PubMed] [Google Scholar]
- Schuurs A. H., Van Weemen B. K. Enzyme-immunoassay. Clin Chim Acta. 1977 Nov 15;81(1):1–40. doi: 10.1016/0009-8981(77)90410-7. [DOI] [PubMed] [Google Scholar]
- Shishido A., Hikita M., Sato T., Kohno S. Particulate and soluble antigens of Rickettsia tsutsugamushi in the complement fixation test. J Immunol. 1969 Sep;103(3):480–490. [PubMed] [Google Scholar]
- Van Peenen P. F., Ho C. M., Bourgeois A. L. Indirect immunofluorescence antibodies in natural and acquired Rickettsia tsutsugamushi infections of Philippine rodents. Infect Immun. 1977 Mar;15(3):813–816. doi: 10.1128/iai.15.3.813-816.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. S., Gan E., Chan Teik Chye, Muul I. Involvement of small mammals in the transmission of scrub typhus in Malaysia: isolation and serological evidence. Trans R Soc Trop Med Hyg. 1973;67(6):838–845. doi: 10.1016/0035-9203(73)90012-6. [DOI] [PubMed] [Google Scholar]
- Walls K. W., Barnhart E. R. Titration of human serum antibodies to Toxoplasma gondii with a simple fluorometric assay. J Clin Microbiol. 1978 Feb;7(2):234–235. doi: 10.1128/jcm.7.2.234-235.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman D. R., Weiss E., Dasch G. A., Bozeman F. M. Biological properties of Rickettsia prowazekii strains isolated from flying squirrels. Infect Immun. 1977 Jun;16(3):853–860. doi: 10.1128/iai.16.3.853-860.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


