Abstract
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel. The mutations G551D and G1349D, which affect the nucleotide-binding domains (NBDs) of CFTR protein, reduce channel activity. This defect can be corrected pharmacologically by small molecules called potentiators. CF mutations residing in the intracellular loops (ICLs), connecting the transmembrane segments of CFTR, may also reduce channel activity. We have investigated the extent of loss of function caused by ICL mutations and the sensitivity to pharmacological stimulation. We found that E193K and G970R (in ICL1 and ICL3, respectively) cause a severe loss of CFTR channel activity that can be rescued by the same potentiators that are effective on NBD mutations. We compared potency and efficacy of three different potentiators for E193K, G970R, and G551D. The 1,4-dihydropyridine felodipine and the phenylglycine PG-01 [2-[(2-1H-indol-3-yl-acetyl)-methylamino]-N-(4-isopropylphenyl)-2-phenylacetamide] were strongly effective on the three CFTR mutants. The efficacy of sulfonamide SF-01 [6-(ethylphenylsulfamoyl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid cycloheptylamide], another CFTR potentiator, was instead significantly lower than felodipine and PG-01 for the E193K and G970R mutations, and almost abolished for G551D. Furthermore, SF-01 modified the response of G551D and G970R to the other two potentiators, an effect that may be explained by an allosteric antagonistic effect. Our results indicate that CFTR potentiators correct the basic defect caused by CF mutations residing in different CFTR domains. However, there are differences among potentiators, with felodipine and PG-01 having a wider pharmacological activity, and SF-01 being more mutation specific. Our observations are useful in the prioritization and development of drugs targeting the CF basic defect.
The cystic fibrosis transmembrane conductance regulator (CFTR) is a plasma membrane channel permeable to Cl- and other anions (Sheppard and Welsh, 1999). Each single CFTR polypeptide is composed of (from the amino to the carboxyl terminus) a transmembrane domain, a nucleotide-binding domain (NBD-1), a regulatory R region, a second transmembrane domain, and a second nucleotide-binding domain (NBD-2). The two transmembrane domains, each one having six segments that cross completely the phospholipid bilayer, contribute to the formation of the hydrophilic channel through which anions are transported (Dawson et al., 1999). NDB-1 and NDB-2 are exposed instead to the cytosol. They possess particular sequences, called Walker A, Walker B, and LSGGQ motifs, highly conserved among ATP-binding cassette transporters (Gottesman and Ambudkar, 2001). Such motifs form together the binding sites for ATP, two binding sites per CFTR molecule (Kidd et al., 2004). Under resting conditions, the CFTR channel is closed. It is believed that phosphorylation of CFTR at the R domain by cAMP-dependent protein kinase A favors the interaction between the two NBDs (Mense et al., 2006). Two ATP-binding sites are then generated at the interface between the NBD domains. Binding of ATP to NBDs allows the opening of the CFTR channel and consequently anion transport (Ikuma and Welsh, 2000).
Mutations in the CFTR gene are the cause of the genetic disease, cystic fibrosis (CF), in which defective Cl- transport is responsible for impaired mucociliary clearance and bacterial colonization of the airways (McAuley and Elborn, 2000). There are more than 1500 CF mutations that can be grouped in five classes according to the mechanisms through which they cause CFTR loss of function (Welsh and Smith, 1993; McAuley and Elborn, 2000). In particular, class III includes mutations that strongly reduce CFTR activity even in the presence of a maximal cAMP stimulus. Such mutations cause an alteration in channel gating so that the mutant protein remains in the closed state for a much longer time compared with wild-type CFTR. The most studied class III mutations are G551D and G1349D (Gregory et al., 1991; Bompadre et al., 2007), which alter two highly conserved glycines in the LSGGQ motif of the NBD1 and NBD2, respectively. ΔF508, the most frequent CF mutation, is also localized in NBD1 and causes both a severe impairment of CFTR maturation, with entrapment of the mutant protein in the endoplasmic reticulum (Gregory et al., 1991), and a gating defect (Dalemans et al., 1991). The gating defect of ΔF508, G551D, and G1349D can be corrected pharmacologically by small molecules called CFTR potentiators (Galietta and Moran, 2004; Verkman et al., 2006). Such compounds, which include flavonoids (genistein, apigenin), fluorescein derivatives, xanthines, benzimidazolones, phenylglycines, sulfonamides, 1,4-dihydropyridines, and tetrahydrobenzothiophenes (Illek et al., 1999; Bulteau et al., 2000; Al-Nakkash et al., 2001; Cai and Sheppard, 2002; Yang et al., 2003; Pedemonte et al., 2005a,b), enhance mutant CFTR activity through a decrease in the time spent by the channel in the closed state (Pedemonte et al., 2005a,b). The mechanism of action of CFTR potentiators is unknown but it has been hypothesized that they bind CFTR at the level of NBDs, possibly at the interface between them (Zegarra-Moran et al., 2007), thus promoting NBD dimerization and favoring CFTR opening. This mechanism may counteract the effects of ΔF508, G551D, and G1349D that instead alter NBD function.
Mutations lying outside the NBDs, in particular, in the CFTR intracellular loops (ICL1–ICL4), which connect the transmembrane segments, also cause a gating defect (Seibert et al., 1996, 1997). ICLs now are considered particularly interesting because they may couple changes in NBD conformation to movements of transmembrane segments, thus allowing gating of the CFTR pore (Mendoza and Thomas, 2007; He et al., 2008; Mornon et al., 2008; Serohijos et al., 2008). In the present study, we have investigated the ability of CFTR potentiators to overcome the gating defect caused by ICL mutations. For this purpose, we have chosen felodipine, PG-01, and SF-01, representative of three different classes of potentiators: 1,4-dihydropyridines, phenylglycines, and sulfonamides, respectively (Pedemonte et al., 2005a,b). Our results demonstrate that potentiators are also effective on ICL mutants, thus indicating that their action is not restricted to NBDs but involves changes in protein conformation that also affect other CFTR regions. However, we also found differences in potency and effectiveness among potentiators with respect to CF mutations, thus suggesting the possible existence of more than one binding site and/or mechanism of action.
Materials and Methods
Cell Culture. COS-7 cells were cultured in Dulbecco's modified Eagle's medium/F12 (1:1) medium with 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. HEK-293 cells were grown in Dulbecco's modified Eagle's medium with Glutamax containing 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Fisher rat thyroid (FRT) epithelial cells were cultured in Coon's modified Ham's F12 medium supplemented with 5% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Mutagenesis. Mutations were introduced into the wild-type CFTR coding sequence by use of the QuickChange XL site-directed mutagenesis kit (Stratagene, Cambridge, UK). The CFTR coding sequence was fully sequenced to confirm the mutagenesis reaction.
YFP-Based Functional Assay. CFTR activity was determined in transiently transfected COS-7 cells with use of the halide-sensitive yellow fluorescent protein (YFP)-H148Q/I152L (Galietta et al., 2001a) as also described previously (Caci et al., 2008). Cells were plated in 96-well microplates (25,000 cells/well) in 100 μl of antibiotic-free culture medium and, after 6 h, cotransfected with plasmids carrying the coding sequence for CFTR and the halide-sensitive YFP. For each well, 0.2 μg of total plasmid DNA and 0.5 μl of Lipofectamine 2000 were premixed in 50 μl of Opti-MEM I(Invitrogen, Carlsbad, CA) to generate transfection complexes that were then added to the cells. After 24 h, the complexes were removed by replacement with fresh culture medium. The CFTR functional assay was carried out 48 h after transfection. For this purpose, the cells were washed two times with phospate-buffered saline (PBS) and incubated for 20 to 30 min with 60 μl of PBS containing forskolin (20 μM) with and without potentiators at various concentrations. After incubation, cells were transferred to an Olympus IX 50 fluorescence microscope equipped with a 20× objective, optical filters for detection of EYFP fluorescence [Chroma Technology Corp. (Brattleboro, VT)]; excitation, HQ500/20X, 500 ± 10 nm; emission, HQ535/30M, 535 ± 15 nm; dichroic, 515 nm) and a photomultiplier tube [Hamamatsu Corporation (Bridgewater, NJ)]. For each well, cell fluorescence was measured continuously before and after addition of 165 μl of a modified PBS containing 137 mM NaI instead of NaCl (final NaI concentration in the well, 100 mM). The signal from the photomultiplier tube was digitized by use of a PowerLab 2/25 acquisition system (ADInstruments Ltd., Chalgrove, Oxfordshire, UK). After background subtraction, cell fluorescence recordings were normalized for the initial average value measured before addition of I-. The signal decay caused by YFP fluorescence quenching was fitted with a double-exponential function to derive the maximal slope that corresponds to initial influx of I- into the cells (Galietta et al., 2001a,b). Maximal slopes were converted to rates of variation of intracellular I- concentration (in millimolar per second) with use of the eq. 1:
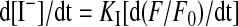 |
(1) |
where KI is the affinity constant of YFP for I- (Galietta et al., 2001a), and F/F0 is the ratio of the cell fluorescence at a given time versus initial fluorescence.
Stable Transfections. FRT cells with stable expression of wild-type and mutant CFTR were generated by transfection of the corresponding plasmid with Lipofectamine 2000 followed by selection with 0.75 mg/ml G418. Positive clones were identified by measuring transepithelial Cl- currents (see below).
Transepithelial Cl- Current Measurements. FRT cells expressing wild-type or mutant CFTR were seeded at high density on Snapwell permeable supports [Corning Inc. (Corning, NY); 500,000 cells/insert]. After 4 to 7 days from plating, when the cells had generated a monolayer with high electrical resistance, the Snapwell supports were mounted in a Ussing chamber-like system (vertical diffusion chamber). The apical chamber was filled with a low-Cl--containing solution: 65 mM NaCl, 65 mM sodium gluconate, 2.7 mM KCl, 1.5 mM KH2PO4, 2 mM CaCl2, 0.5 mM MgCl2, 10 mM glucose, 10 mM Na-HEPES, pH 7.4. The basolateral chamber was filled instead with 130 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgCl2, 10 mM glucose, 10 mM Na-HEPES, pH 7.4. Experiments were performed at 37°C with a continuous bubbling with air on both sides.
Hemichambers were connected to a DVC-1000 voltage clamp (World Precision Instruments Inc., Sarasota, FL) via Ag/AgCl electrodes and 1 M KCl agar bridges. The transepithelial electrical potential difference was clamped at zero to measure CFTR Cl- currents across the apical membrane.
CFTR Protein Analysis: Transient Transfection and Western Blot. HEK-293 cells were grown on 60-mm-diameter dishes. Subconfluent cells (60%) were transfected by lipofection with use of Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Confluent monolayers were harvested 24 h after transfection in PBS once, pelleted at 2000g at 4°C for 10 min, and then resuspended in 150 μl of cold lysis buffer (20 mM HEPES, pH 7, 150 mM NaCl, 1 mM EDTA, and 1% Igepal, all from Sigma-Aldrich, St. Louis, MO) supplemented with complete protease inhibitors (Roche Applied Sciences, Indianapolis, IN) for 30 min at 4°C. Cell lysates were spun at 20,000g for 15 min at 4°C to pellet-insoluble material. One-tenth of lysates was heated in Laemmli buffer at 37°C for 15 min and loaded on a 7.5% SDS-polyacrylamide gel electrophoresis and electroblotted from the gels to a polyvinylidene difluoride membrane (GE Healthcare, Little Chalfont, Buckinghamshire, UK). CFTR was immunodetected by use of monoclonal antibody MM13-4 (1:1000) followed by horseradish peroxidase-conjugated anti-mouse (1:50,000), and visualized by chemiluminescence with ECL Advance kit (GE Healthcare) according to the manufacturer's instructions. Direct recording of the chemiluminescence was performed with the charge-coupled device camera of the GeneGnome analyzer and quantification performed with the GeneTools software (Syngene BioImaging Systems, Synoptics Ltd., Cambridge, UK).
Data Analysis and Statistics. Dose-response relationships from each experiment were fitted with the Hill equation with use of the Igor software (WaveMetrics, Portland, OR) to calculate Kd, maximal activity, and Hill coefficient.
Data are reported as representative traces and as mean ± S.E.M. To determine the significance of differences between groups of data, we used the Student's t test by means of Statview software (SAS Institute, Cary, NC).
Results
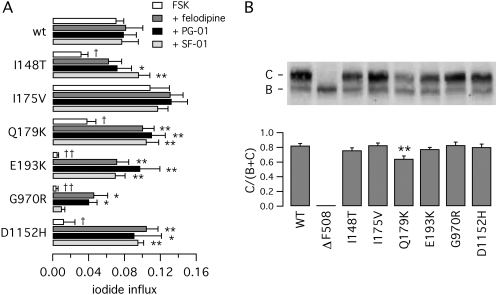
We performed an initial screening to identify those mutations in ICLs that cause a severe defect in CFTR activity. To this respect, we considered I148T, I175V, Q179K, and E193K in ICL1 (Seibert et al., 1997) and G970R in ICL3 (Seibert et al., 1996). We also considered D1152H, a mutation residing at the end of last transmembrane segment, which has a reduced activity and showed responsiveness to potentiators in primary cultures of airway epithelial cells (Vankeerberghen et al., 1998; Pedemonte et al., 2005a). We introduced all of these CF mutations, separately, in the CFTR coding sequence. The resulting mutant plasmids were expressed in COS-7, and CFTR activity was measured as iodide influx using the halide-sensitive YFP assay. Elevation of intracellular cAMP with a maximal concentration of forskolin (20 μM) elicited a ∼20-fold increase in iodide influx rate in cells transfected with wild-type CFTR (Fig. 1A). Under the same conditions, the mutants of ICLs showed different levels of activity. E193K and G970R showed the most severe defect, with more than 10-fold decreased activity relative to wild-type CFTR (Fig. 1A). D1152H was significantly more active than E193K and G970R but approximately five times less than the wild-type protein. In contrast, I148T and Q179K had a forskolin response that was 45 to 55% than that of the normal protein, a behavior that is not consistent with such amino acid changes being CF-causing mutations (Fig. 1A). This discrepancy was even more striking for I175V, which was associated with an anion transport rate greater than wild-type CFTR.
Fig. 1.
Functional and biochemical characterization of CFTR mutants. A, anion transport measured in COS-7 cells with the fluorescence YFP assay. Cells were transfected with wild-type or mutant CFTR as indicated. Before the assay, cells were stimulated with forskolin (FSK, 20 μM) with and without 5 μM felodipine, PG-01, or SF-01. Bars represent the average ± S.E.M. of 4 to 8 experiments. *, p < 0.05; **, < 0.01 versus forskolin alone of the same mutant. †, p < 0.05; ††, p < 0.01 versus forskolin alone of wild-type CFTR. B, analysis of CFTR maturation by Western blot experiments. The top shows a representative experiment. The positions of mature (band C) and immature (band B) forms of CFTR protein are indicated. The bottom summarizes the results of Western blot experiments as band C intensity normalized for total CFTR protein (mean ± S.E.M., n = 5–11). **, p < 0.01 versus wild-type CFTR.
Anion transport for wild-type and mutant CFTR was also measured in the presence of potentiators, namely felodipine, PG-01, or SF-01, in combination with maximal forskolin. In general, the three potentiators stimulated CFTR mutants so that anion transport rates became comparable with those of the normal protein (Fig. 1A). The effect was obviously more dramatic for the mutants having low forskolin response like E193K, G970R, and D1152H. For example, potentiators increased anion transport of E193K by more than 15-fold relative to forskolin alone (Fig. 1A). However, a significant exception was represented by G970R, which was less responsive to potentiators and, in particular, to SF-01. As expected, wild-type CFTR showed very little response to potentiators because its activity can be stimulated to maximal levels by the physiological cAMP pathway (Fig. 1A). The activity of I175V was also not significantly affected by potentiators, a behavior that further indicates that this protein is already totally activated with maximal forskolin and therefore behaves essentially as the normal protein.
We evaluated the maturation efficiency of wild-type and mutant CFTR by determining the electrophoretic mobility in Western blot experiments (Fig. 1B). As expected, the wild-type CFTR protein showed the typical pattern consisting of band C, the fully glycosylated mature form of the protein, plus a less intense band B, the core-glycosylated protein that is still in the endoplasmic reticulum. As also expected, ΔF508 showed only the immature (band B) form of CFTR (Denning et al., 1992). In contrast, all other mutants, with the exception of Q179K, had a normal pattern of CFTR mobility, with a ratio band C/(band B + band C), close to that of wild-type CFTR (Fig. 1B). The Q179K mutant displayed a ratio of 0.64 ± 0.04 (n = 11) that was significantly lower than that of the wild-type protein (0.82 ± 0.03, n = 8, p < 0.01). This finding may indicate a partial maturation defect (Fig. 1B).
To confirm the results obtained with the functional assay, and to determine precisely the potency and maximal effect for each potentiator, we generated stable transfectants for E193K and G970R, the two ICL mutants having the most severe deficit in cAMP response. FRT cells with stable expression of E193K- and G970R-CFTR were used to measure transepithelial Cl- currents in parallel with FRT cells expressing G551D-CFTR, a classical mutant causing a severe channel-gating defect.
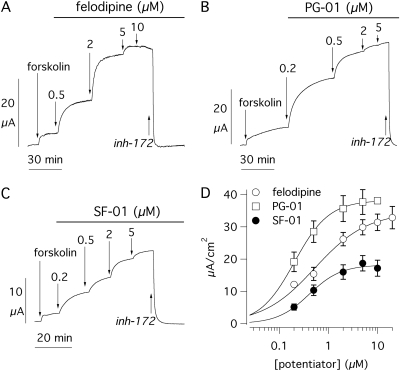
Figure 2 shows data obtained from the E193K. Stimulation with forskolin elicited a short-circuit current (Isc) increase of 4.20 ± 0.34 μA/cm2 (n = 24). This mutant displayed marked responses to PG-01 and felodipine with a 7- to 9-fold increase of the forskolin-activated current at maximal potentiator concentration (Fig. 2, A–C), whereas SF-01 had a more reduced efficacy (∼60% of the other two potentiators; Fig. 2D). The apparent Kd for the three potentiators was 0.22 ± 0.03 (n = 6), 0.74 ± 0.19 (n = 9), and 0.67 ± 0.14 μM (n = 9), respectively (Table 1). At the end of each experiment (Fig. 2, A–C), we added CFTRinh-172 (10 μM), a selective CFTR inhibitor (Caci et al., 2008). This compound returned transepithelial current to values close to zero, thus indicating that forskolin- and potentiator-activated currents depended on CFTR activity.
Fig. 2.
Pharmacological stimulation of the E193K mutant. A to C, representative short-circuit current recordings from transfected FRT cells showing response of the E193K-CFTR mutant to different concentrations of felodipine, PG-01, and SF-01. Before addition of potentiators, cells were stimulated with maximal forskolin (20 μM). At the end of the experiments, CFTR-dependent currents were blocked with CFTRinh-172 (10 μM). D, dose-response relationships for the three potentiators. Each point is the average ± S.E.M. of six to nine experiments. Data were fitted with a Hill equation. inh-172, CFTRinh-172.
TABLE 1.
Sensitivity of CFTR mutants to potentiators Mean data ± S.E.M. for Kd, maximal current (Imax), and Hill coefficient values obtained by fitting dose-response relationships of CFTR potentiators in Ussing chamber experiments.
| Kd | Imax | nH | n | |
|---|---|---|---|---|
| μM | μA/cm2 | |||
| E193K | ||||
| PG-01 | 0.22 ± 0.03 | 37.4 ± 3.6 | 1.5 ± 0.2 | 6 |
| SF-01 | 0.74 ± 0.19 | 20.6 ± 2.7 | 1.3 ± 0.1 | 9 |
| Felodipine | 0.67 ± 0.14 | 32.7 ± 2.4 | 1.4 ± 0.1 | 9 |
| G970R | ||||
| PG-01 | 0.45 ± 0.07** | 60.2 ± 8.1 | 1.4 ± 0.2 | 10 |
| SF-01 | 0.45 ± 0.07ns | 17.6 ± 2.7 | 1.6 ± 0.2 | 10 |
| Felodipine | 2.03 ± 0.39** | 75.7 ± 7.4 | 1.2 ± 0.1 | 9 |
| G551D | ||||
| PG-01 | 1.94 ± 0.54*† | 21.5 ± 4.4 | 1.4 ± 0.2 | 8 |
| SF-01 | 1.10 ± 0.12ns | 5.9 ± 0.7 | 1.8 ± 0.3 | 15 |
| Felodipine | 10.22 ± 1.12**†† | 68.4 ± 5.4 | 2.2 ± 0.4 | 9 |
ns, nonsignificant
P < 0.05
P < 0.01; vs. the same potentiator in E193K
P < 0.05
P < 0.01; vs. the same potentiator in G970R
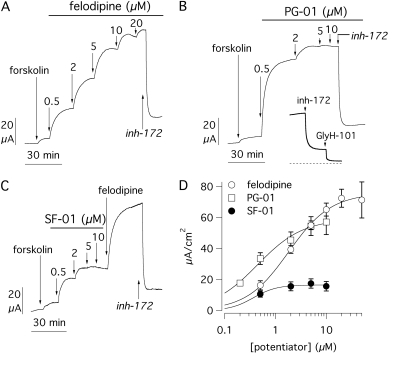
Similar experiments were performed on FRT cells expressing the G970R mutant (Fig. 3). The absolute effect of forskolin was 5.70 ± 0.63 μA/cm2 (n = 29). Subsequent stimulation with potentiators elicited a dose-dependent increase of transepithelial currents (Fig. 3, A–C), thus confirming that the defective activity of this CFTR mutant is also pharmacologically correctable. At maximal concentrations, PG-01 and felodipine caused a 12- and 20-fold increase of the forskolin-activated current, respectively. In contrast, SF-01 efficacy was only 20 to 30% that of felodipine and PG-01 (Fig. 3D). The addition of felodipine after SF-01 caused a further increase of the Cl- current (Fig. 3C). This result indicates that SF-01 is a partial agonist for this particular CFTR mutant. Regarding potency, Kd was 0.45 ± 0.07 μM for PG-01 (n = 10), 2.03 ± 0.39 μM for felodipine (n = 9), and 0.45 ± 0.07 μM for SF-01 (n = 10) (Table 1). The former two values were approximately 2-fold higher than those measured for E193K. On the contrary, SF-01 potency was not significantly different between E193K and G970R. The G970R mutant showed another interesting characteristic consisting in a noncomplete inhibition by CFTRinh-172 at 10 μM (compare data in Fig. 3 with data in Figs. 2, 4, and 5). In all experiments, CFTRinh-172 reduced the G970R current by only 79 ± 2%, whereas for the other mutants and for wild-type CFTR the inhibition was greater than 95% (98 ± 1% for E193K, p < 0.01). This partial effect was confirmed by the lack of inhibition after the increase of CFTRinh-172 concentration to 20 μM (not shown). The residual current remaining after CFTRinh-172 could be fully blocked with another CFTR inhibitor, GlyH-101 (Fig. 3B, inset) (Muanprasat et al., 2004).
Fig. 3.
Pharmacological stimulation of the G970R mutant. A to C, short-circuit current recordings from transfected FRT cells showing response of the G970R-CFTR mutant to potentiators as in Fig. 2. C, a single concentration of felodipine (10 μM) was added after the SF-01 dose response. B, inset shows that the current activated by PG-01 and partially inhibited by CFTRinh-172 (10 μM) can be further inhibited close to zero (dotted line) by GlyH-101 (50 μM). D, dose-response relationships for the three potentiators. Each point is the average ± S.E.M. of 9 to 10 experiments. inh-172, CFTRinh-172.
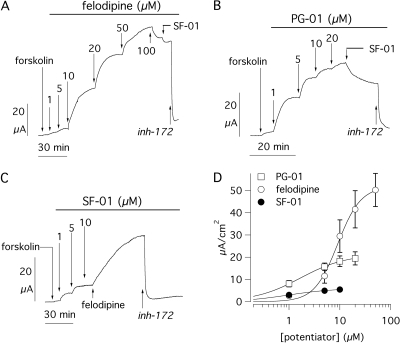
Fig. 4.
Pharmacological stimulation of the G551D mutant. A to C, short-circuit current recordings from transfected FRT cells showing response of G551D-CFTR to felodipine, PG-01, and SF-01. Forskolin concentration, added before potentiators, was 20 μM as in Figs. 2 and 3. In A and B, SF-01 (10 μM) was added after the dose response to felodipine and PG-01, respectively. In C, felodipine (10 μM) was added after SF-01. All currents were blocked with CFTRinh-172 (10 μM). D, dose-response relationships for the three potentiators. Each point is the average ± S.E.M. of 8 to 16 experiments. inh-172, CFTRinh-172.
Fig. 5.
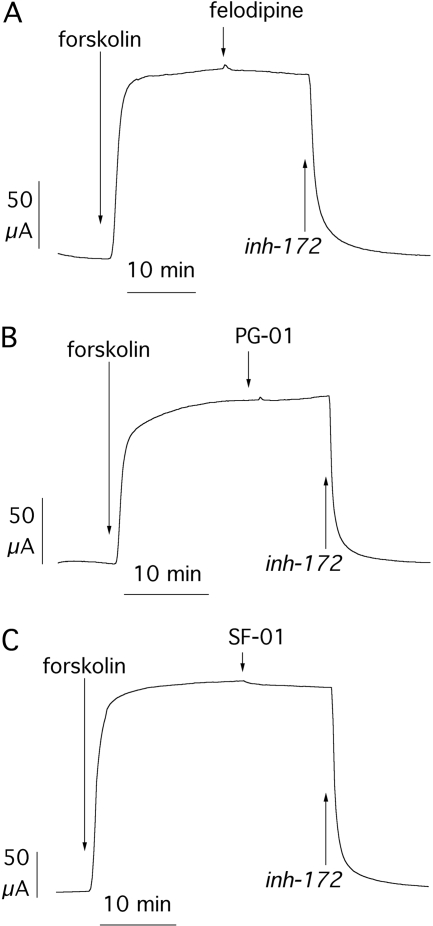
Potentiators on wild-type CFTR. A to C, short-circuit current recordings from FRT cells expressing wild-type CFTR. Cells were maximally stimulated with forskolin (20 μM) followed by a single concentration (10 μM) of felodipine, PG-01, and SF-01, as indicated. CFTRinh-172 (10 μM) was added at the end to fully block CFTR-dependent Cl- currents. inh-172, CFTRinh-172.
The results obtained from ICL mutants were compared with those obtained from G551D (Fig. 4). The response to forskolin was almost undetectable (1.89 ± 0.21 μA/cm2, n = 33), as reported previously (Zegarra-Moran et al., 2002). Previous studies have also shown that potentiators display a significantly reduced potency toward this NBD1 mutant (Zegarra-Moran et al., 2002; Pedemonte et al., 2005a,b). In agreement with such reports, we found that PG-01 and felodipine Kd values were indeed increased by ∼10-fold in G551D compared with E193K (Fig. 4, A–C). Despite the decrease in potency, these two potentiators were able to activate large Cl- currents, although felodipine was consistently more effective than PG-01 (Table 1). On the contrary, SF-01 behaved in a completely different manner. First, its efficacy was dramatically smaller, the maximal current being only 9 and 24% that of felodipine and PG-01, respectively (Fig. 4D). Second, its potency, although difficult to measure because of the small size of the currents, was not different from that measured for E193K and G970R (Table 1). Addition of felodipine to cells treated with SF-01 elicited a strong effect, thus demonstrating that the sulfonamide agent had caused only a partial activation of G551D-CFTR (Fig. 4C).
To investigate the mechanism for SF-01-reduced efficacy, we added this compound at 10 μM after a full dose response of PG-01 or felodipine on G970R and G551D cells. Under these conditions, we found that SF-01 had an inhibitory effect (Fig. 4, A and B). No negative interaction was found between PG-01 and felodipine (not shown).
The inhibition caused by SF-01 can be explained in different ways. One possibility is that SF-01 acts also on an inhibitory site on CFTR protein. Accordingly, the poor effectiveness of SF-01 on G551D or G970R could be explained by the prevalence of inhibitory over stimulatory activities. Alternatively, it may be possible that SF-01 antagonizes the effect of other potentiators. To elucidate this issue, we performed two types of experiments.
In the first set of experiments, we applied potentiators to cells expressing wild-type CFTR after maximal stimulation with forskolin (Fig. 5). Under these conditions, CFTR is fully activated and potentiators are expected to cause little further stimulation. We had expected a significant inhibition of CFTR currents by SF-01 if this compound had a separate inhibitory binding site. In contrast, we found no inhibition by SF-01 under these conditions (Fig. 5). In general, we observed very few current changes by PG-01, SF-01, and felodipine on wild-type CFTR currents after maximal cAMP stimulation.
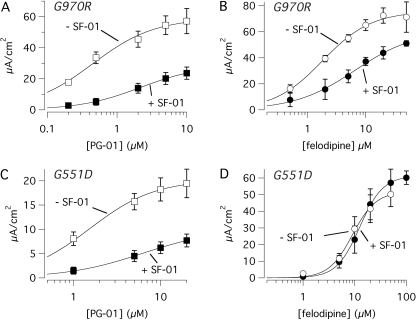
In the second set of experiments, we compared PG-01 or felodipine dose responses obtained in the presence and the absence of SF-01 at 10 μM (Fig. 6). We found that, in G970R cells, the addition of SF-01 caused a significant reduction of the currents elicited by the subsequent application of PG-01 with a shift of the dose-response relationship. The apparent Kd for PG-01 increased from 0.45 ± 0.07 (n = 10) to 2.73 ± 0.85 μM (n = 7) in the absence and in the presence of SF-01, respectively (p < 0.01; Fig. 6A). The dose response of felodipine was also altered in G970R cells by the presence of SF-01 (Fig. 6B). The apparent Kd of felodipine was increased from 2.03 ± 0.39 (n = 9) to 4.76 ± 0.98 μM (n = 5) by SF-01 (p < 0.01). Although not clearly evident from the average data in Fig. 6, the dose-response relationships of individual experiments showed that the maximal current elicited by PG-01 and felodipine was significantly reduced by SF-01 (by 53 and 35%, respectively). In G551D cells, this type of experiment produced results that were in part different from those found in G970R cells. The activity of PG-01 was altered by SF-01 as in G970R; the apparent Kd was increased by more than 3-fold (from 1.94 ± 0.54 to 7.42 ± 2.15 μM, n = 7–8, p < 0.05), and the maximal response was reduced by 50% (Fig. 6C). In contrast, felodipine potency and efficacy in G551D cells were not affected by SF-01 (Fig. 6D). Very large currents were evoked by felodipine even in the presence of SF-01 and the apparent Kd for felodipine was not modified by previous application of the sulfonamide compound (10.22 ± 1.51 versus 13.74 ± 2.47 μM, n = 5–6).
Fig. 6.
Interaction between potentiators. A to D, dose responses for felodipine and PG-01 in the presence/absence of SF-01 (10 μM). The graphs report short-circuit current data from FRT cells expressing G970R (A and B) or G551D (C and D). Each point is the average ± S.E.M. of 5 to 10 experiments.
Discussion
CFTR potentiators are an interesting group of small molecules potentially useful as drugs to treat the basic defect in CF (Verkman et al., 2006; Verkman and Galietta, 2009). CFTR potentiators are typically effective on CF mutations localized in the NBDs, like G551D, G1349D, and ΔF508. Actually, many potentiators have been found by high-throughput screening using ΔF508-expressing cells (Pedemonte et al., 2005a,b). After detection in the primary screening, many of the active compounds have later been found to be active also on G551D and G1349D. To be effective on ΔF508-CFTR, cells must first be incubated at low temperature or treated with pharmacological chaperones to rescue the mutant protein from the endoplasmic reticulum (Denning et al., 1992; Pedemonte et al., 2005c). Therefore, potentiators are not expected to be effective in vivo on ΔF508 patients unless they are also treated with a corrector of ΔF508 mistrafficking. Accordingly, clinical trials of potentiators like VX-770 are restricted to G551D patients who represent a very small fraction of all CF-affected individuals.
The aim of our study was to assess the effectiveness of CFTR potentiators on CF mutations lying outside the NBDs. This issue has a therapeutic relevance because it demonstrates that potentiators may be generally effective and beneficial for a wider number of CF patients. Furthermore, the effect of potentiators on various CFTR mutants also has mechanistic implications that may help to understand their effect on CFTR protein.
To test the potentiators, we evaluated a series of mutations localized in CFTR ICLs. We took into consideration various mutations in ICL1, G970R in ICL3, and D1152H, which lies between the last transmembrane segment and NBD2. Our results show that E193K, G970R, and, to a lesser extent, D1152H cause a marked decrease in CFTR activity. However, I148T and Q179K activity was ∼50% that of wild-type CFTR. This behavior is inconsistent with the latter mutations being causative of CF and suggests that they are instead benign CFTR variants. In fact, it has been shown that I148T is in linkage disequilibrium with a deletion of two amino acids (3199del6) localized more distantly toward the carboxyl terminus (Rohlfs et al., 2002; Monaghan et al., 2004) and that this latter sequence change is the one causing CFTR loss of function. I175V may be another benign variant because its activity was comparable with or even higher than that of the native protein. However, we must consider that CFTR may also have other functions in addition to Cl- transport. Such functions include regulation of other channels involved in cystic fibrosis pathogenesis such as the epithelial sodium channel, ENaC. Therefore, it is possible that some mutations such as I175V and I148T have a more profound effect on CFTR as a regulator of other proteins (Schreiber et al., 1999).
We focused our study on E193K and G970R, the two ICL mutants having the most severe loss in CFTR activity, and, for comparison, on G551D. We found a complex pattern of pharmacological sensitivity. First, PG-01, SF-01, and felodipine were effective on E193K and the apparent affinity for this CFTR mutant was close to that reported previously for ΔF508 under similar conditions of stimulation with forskolin (Pedemonte et al., 2005a,b). PG-01 and felodipine were also effective on G970R although with a significant decrease in potency relative to E193K and ΔF508. A further decrease in potency was observed when the two potentiators were tested on G551D, in agreement with previous studies (Zegarra-Moran et al., 2002; Pedemonte et al., 2005a). However, SF-01 efficacy was nearly halved in E193K, severely reduced in G970R, and almost abolished in G551D, whereas potency, at least in G970R, was not significantly different from that measured in E193K.
Another characteristic of SF-01 was the inhibitory effect on currents that had been previously activated with the other two potentiators. We considered various hypotheses to explain this behavior. The first hypothesis postulates that SF-01 binds to two different sites: 1) an activating site that is shared with other potentiators and 2) an inhibitory site. It may be possible that the efficacy of SF-01 as a potentiator is reduced in G970R and G551D so that the inhibitory effect becomes prevalent. This possibility would explain why SF-01 appears as a partial agonist and why this compound has an antagonistic activity on other potentiators. However, we found no inhibition of wild-type CFTR currents by SF-01 after maximal stimulation with cAMP. If an inhibitory site for SF-01 existed, we would have expected a reduction of the currents.
The second hypothesis is that SF-01, PG-01, and felodipine have a unique binding site as potentiators but the efficacy of SF-01 is reduced relative to the other two potentiators, in particular, for G970R and G551D mutants. Accordingly, SF-01 would behave as a partial agonist and therefore as a competitive antagonist of full agonists like PG-01 and felodipine.
The third hypothesis postulates that SF-01 antagonizes the other potentiators allosterically. Interaction of SF-01 with a second site would cause a modification of the orthosteric binding site and thus a decrease in potency and efficacy for the other potentiators.
Our data seem to favor the third type of mechanism. We found that the presence of SF-01 caused an alteration in the dose-response relationships for PG-01 and felodipine in G970R, and for PG-01 alone in G551D. These findings are difficult to reconcile with a mechanism based on competition for the same binding site. In such a case, for G551D-CFTR, we would have expected SF-01 to also have an inhibitory effect on felodipine. Indeed, the effect of orthosteric antagonists is generally agonist-independent (Kenakin, 2004). In other words, all agonists for the same site are equally affected by a given antagonist. Therefore, our findings are more suggestive of an allosteric type of antagonism. This mechanism is often agonist-dependent. For example, the order of potency of an agonists series for the same orthosteric site may be altered dramatically in the presence of an allosteric antagonist (Kenakin, 2004). Under these conditions, the potency of some agonists is decreased, whereas that of others may remain unaltered.
In summary, our data suggest that PG-01 and felodipine share a common binding site separate from that of SF-01. This seems supported by the fact that the Kd for PG-01 and felodipine is increased by the same extent in G970R (∼2-fold) and in G551D (∼10-fold) with respect to E193K, whereas the apparent potency of SF-01 does not change. Mutations like G551D may cause a decrease in the affinity for ligands on one site and, conversely, a decrease in efficacy on the other site.
Another interesting observation regarding the G970R mutant is the noncomplete inhibition produced by CFTRinh-172. After a maximal concentration of this compound, there was a residual current that could be blocked by a different inhibitor, GlyH-101, which acts by blocking the CFTR pore (Muanprasat et al., 2004). It is possible that the G970R mutation alters the CFTR activity so that it becomes partially refractory to CFTRinh-172. In this respect, it is important to remember that CFTRinh-172, different from GlyH-101, acts by altering CFTR gating with an allosteric mechanism (Caci et al., 2008).
In conclusion, we found that CFTR potentiators have a more general ability than previously expected to correct CFTR channels with gating mutations. This finding has practical implications because it implies that potentiators developed for the ΔF508 mutation may also be effective on other CF patients having class III mutations within and outside the NBDs. Although each one of these mutations has a very low frequency, their total number may account for a significant fraction of CF patients. For example, at the moment, the potentiator VX-770 is being tested on CF patients with the G551D mutation. Initial results are promising, with a significant improvement in CFTR function as measured by sweat chloride assay and nasal potential measurements. Our results suggest that a compound active on G551D is probably effective also on other gating mutations, thus extending its field of therapeutic application. This has important implications in the prioritization of potentiators for drug development. In fact, some classes of potentiators, although being very potent on ΔF508, such as sulfonamides and tetrahydrobenzothiophenes (Yang et al., 2003; Pedemonte et al., 2005a), have limited efficacy on other mutants.
Our study has also mechanistic implications revealing complex interactions between CFTR and potentiators that may indicate the presence of more than one binding site and mechanisms of action. Further studies are needed to elucidate the presence and location of separate binding sites for the potentiators.
This work was supported in part by Cystic Fibrosis Foundation Therapeutics; the Telethon Foundation [Grant GGP05103]; the National Institutes of Health [Grant P30-DK072517]; and Fondazione Italiana per la Ricerca sulla Fibrosi Cistica [Grant FFC 3/2006]. This work was also supported by public grants from Institut National de la Santé et de la Recherche Médicale.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.154146.
ABBREVIATIONS: CFTR, cystic fibrosis transmembrane conductance regulator; CF, cystic fibrosis; YFP, yellow fluorescent protein; NBD, nucleotide-binding domain; ICL, intracellular loop; FRT, Fisher rat thyroid; PBS, phosphate-buffered saline; PG-01, 2-[(2-1H-indol-3-yl-acetyl)-methylamino]-N-(4-isopropylphenyl)-2-phenylacetamide; SF-01, 6-(ethylphenylsulfamoyl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid cycloheptylamide; CFTRinh-172, 3-[(3-trifluoromethyl)phenyl]-5-[(4-carboxyphenyl)methylene]-2-thioxo-4-thiazolidinone; GlyH-101, N-(2-naphthalenyl)-[(3,5-dibromo-2,4-dihydroxyphenyl)methylene]glycine hydrazide; VX-770, N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide.
References
- Al-Nakkash L, Hu S, Li M, and Hwang TC (2001) A common mechanism for cystic fibrosis transmembrane conductance regulator protein activation by genistein and benzimidazolone analogs. J Pharmacol Exp Ther 296 464-472. [PubMed] [Google Scholar]
- Bompadre SG, Sohma Y, Li M, and Hwang TC (2007) G551D and G1349D, two CF-associated mutations in the signature sequences of CFTR, exhibit distinct gating defects. J Gen Physiol 129 285-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulteau L, Dérand R, Mettey Y, Métayé T, Morris MR, McNeilly CM, Folli C, Galietta LJ, Zegarra-Moran O, Pereira MM, et al. (2000) Properties of CFTR activated by the xanthine derivative X-33 in human airway Calu-3 cells. Am J Physiol Cell Physiol 279 C1925-C1937. [DOI] [PubMed] [Google Scholar]
- Caci E, Caputo A, Hinzpeter A, Arous N, Fanen P, Sonawane N, Verkman AS, Ravazzolo R, Zegarra-Moran O, and Galietta LJ (2008) Evidence for direct CFTR inhibition by CFTRinh-172 based on Arg347 mutagenesis. Biochem J 413 135-142. [DOI] [PubMed] [Google Scholar]
- Cai Z and Sheppard DN (2002) Phloxine B interacts with the cystic fibrosis transmembrane conductance regulator at multiple sites to modulate channel activity. J Biol Chem 277 19546-19553. [DOI] [PubMed] [Google Scholar]
- Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, Crystal RG, Pavirani A, Lecocq JP, and Lazdunski M (1991) Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature 354 526-528. [DOI] [PubMed] [Google Scholar]
- Dawson DC, Smith SS, and Mansoura MK (1999) CFTR: mechanism of anion conduction. Physiol Rev 79 S47-S75. [DOI] [PubMed] [Google Scholar]
- Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, and Welsh MJ (1992) Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 358 761-764. [DOI] [PubMed] [Google Scholar]
- Galietta LJ and Moran O (2004) Identification of CFTR activators and inhibitors: chance or design? Curr Opin Pharmacol 4 497-503. [DOI] [PubMed] [Google Scholar]
- Galietta LJ, Haggie PM, and Verkman AS (2001a) Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett 499 220-224. [DOI] [PubMed] [Google Scholar]
- Galietta LJ, Springsteel MF, Eda M, Niedzinski EJ, By K, Haddadin MJ, Kurth MJ, Nantz MH, and Verkman AS (2001b) Novel CFTR chloride channel activators identified by screening of combinatorial libraries based on flavone and benzoquinolizinium lead compounds. J Biol Chem 276 19723-19728. [DOI] [PubMed] [Google Scholar]
- Gottesman MM and Ambudkar SV (2001) Overview: ABC transporters and human disease. J Bioenerg Biomembr 33 453-458. [DOI] [PubMed] [Google Scholar]
- Gregory RJ, Rich DP, Cheng SH, Souza DW, Paul S, Manavalan P, Anderson MP, Welsh MJ, and Smith AE (1991) Maturation and function of cystic fibrosis transmembrane conductance regulator variants bearing mutations in putative nucleotide-binding domains 1 and 2. Mol Cell Biol 11 3886-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Aleksandrov AA, Serohijos AW, Hegedus T, Aleksandrov LA, Cui L, Dokholyan NV, and Riordan JR (2008) Multiple membrane-cytoplasmic domain contacts in the cystic fibrosis transmembrane conductance regulator (CFTR) mediate regulation of channel gating. J Biol Chem 283 26383-26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma M and Welsh MJ (2000) Regulation of CFTR Cl- channel gating by ATP binding and hydrolysis. Proc Natl Acad Sci U S A 97 8675-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illek B, Zhang L, Lewis NC, Moss RB, Dong JY, and Fischer H (1999) Defective function of the cystic fibrosis-causing missense mutation G551D is recovered by genistein. Am J Physiol Cell Physiol 277 C833-C839. [DOI] [PubMed] [Google Scholar]
- Kenakin T (2004) Allosteric modulators: the new generation of receptor antagonist. Mol Interv 4 222-229. [DOI] [PubMed] [Google Scholar]
- Kidd JF, Ramjeesingh M, Stratford F, Huan LJ, and Bear CE (2004) A heteromeric complex of the two nucleotide binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) mediates ATPase activity. J Biol Chem 279 41664-41669. [DOI] [PubMed] [Google Scholar]
- McAuley DF and Elborn JS (2000) Cystic fibrosis: basic science. Paediatr Respir Rev 1 93-100. [DOI] [PubMed] [Google Scholar]
- Mendoza JL and Thomas PJ (2007) Building an understanding of cystic fibrosis on the foundation of ABC transporter structures. J Bioenerg Biomembr 39 499-505. [DOI] [PubMed] [Google Scholar]
- Mense M, Vergani P, White DM, Altberg G, Nairn AC, and Gadsby DC (2006) In vivo phosphorylation of CFTR promotes formation of a nucleotide-binding domain heterodimer. EMBO J 25 4728-4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan KG, Highsmith WE, Amos J, Pratt VM, Roa B, Friez M, Pike-Buchanan LL, Buyse IM, Redman JB, Strom CM, et al. (2004) Genotype-phenotype correlation and frequency of the 3199del6 cystic fibrosis mutation among I148T carriers: results from a collaborative study. Genet Med 6 421-425. [DOI] [PubMed] [Google Scholar]
- Mornon JP, Lehn P, and Callebaut I (2008) Atomic model of human cystic fibrosis transmembrane conductance regulator: membrane-spanning domains and coupling interfaces. Cell Mol Life Sci 65 2594-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, and Verkman AS (2004) Discovery of glycine hydrazide pore-occluding CFTR Inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol 124 125-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedemonte N, Sonawane ND, Taddei A, Hu J, Zegarra-Moran O, Suen YF, Robins LI, Dicus CW, Willenbring D, Nantz MH, et al. (2005a) Phenylglycine and sulfonamide correctors of defective deltaF508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol Pharmacol 67 1797-1807. [DOI] [PubMed] [Google Scholar]
- Pedemonte N, Diena T, Caci E, Nieddu E, Mazzei M, Ravazzolo R, Zegarra-Moran O, and Galietta LJ (2005b) Antihypertensive 1,4-dihydropyridines as correctors of the cystic fibrosis transmembrane conductance regulator channel gating defect caused by cystic fibrosis mutations. Mol Pharmacol 68 1736-1746. [DOI] [PubMed] [Google Scholar]
- Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJ, and Verkman AS (2005c) Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest 115 2564-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfs EM, Zhou Z, Sugarman EA, Heim RA, Pace RG, Knowles MR, Silverman LM, and Allitto BA (2002) The I148T CFTR allele occurs on multiple haplotypes: a complex allele is associated with cystic fibrosis. Genet Med 4 319-323. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Hopf A, Mall M, Greger R, and Kunzelmann K (1999) The first-nucleotide binding domain of the cystic fibrosis transmembrane conductance regulators is important for inhibition of the epithelial Na+ channel. Proc Natl Acad Sci U S A 96 5310-5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert FS, Linsdell P, Loo TW, Hanrahan JW, Riordan JR, and Clarke DM (1996) Cytoplasmic loop three of cystic fibrosis transmembrane conductance regulator contributes to regulation of chloride channel activity. J Biol Chem 271 27493-27499. [DOI] [PubMed] [Google Scholar]
- Seibert FS, Jia Y, Mathews CJ, Hanrahan JW, Riordan JR, Loo TW, and Clarke DM (1997) Disease-associated mutations in cytoplasmic loops 1 and 2 of cystic fibrosis transmembrane conductance regulator impede processing or opening of the channel. Biochemistry 36 11966-11974. [DOI] [PubMed] [Google Scholar]
- Sheppard DN and Welsh MJ (1999) Structure and function of the CFTR chloride channel. Physiol Rev 79 S23-S45. [DOI] [PubMed] [Google Scholar]
- Serohijos AW, Hegedus T, Aleksandrov AA, He L, Cui L, Dokholyan NV, and Riordan JR (2008) Phenylalanine-508 mediates a cytoplasmic-membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc Natl Acad Sci U S A 105 3256-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankeerberghen A, Wei L, Teng H, Jaspers M, Cassiman JJ, Nilius B, and Cuppens H (1998) Characterization of mutations located in exon 18 of the CFTR gene. FEBS Lett 437 1-4. [DOI] [PubMed] [Google Scholar]
- Verkman AS and Galietta LJ (2009) Chloride channels as drug targets. Nat Rev Drug Discov 8 153-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS, Lukacs GL, and Galietta LJ (2006) CFTR chloride channel drug discovery—inhibitors as antidiarrheals and activators for therapy of cystic fibrosis. Curr Pharm Des 12 2235-2247. [DOI] [PubMed] [Google Scholar]
- Welsh MJ and Smith AE (1993) Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 73 1251-1254. [DOI] [PubMed] [Google Scholar]
- Yang H, Shelat AA, Guy RK, Gopinath VS, Ma T, Du K, Lukacs GL, Taddei A, Folli C, Pedemonte N, et al. (2003) Nanomolar affinity small molecule correctors of defective Delta F508-CFTR chloride channel gating. J Biol Chem 278 35079-35085. [DOI] [PubMed] [Google Scholar]
- Zegarra-Moran O, Monteverde M, Galietta LJ, and Moran O (2007) Functional analysis of mutations in the putative binding site for cystic fibrosis transmembrane conductance regulator potentiators. Interaction between activation and inhibition. J Biol Chem 282 9098-9104. [DOI] [PubMed] [Google Scholar]
- Zegarra-Moran O, Romio L, Folli C, Caci E, Becq F, Vierfond JM, Mettey Y, Cabrini G, Fanen P, and Galietta LJ (2002) Correction of G551D-CFTR transport defect in epithelial monolayers by genistein but not by CPX or MPB-07. Br J Pharmacol 137 504-512. [DOI] [PMC free article] [PubMed] [Google Scholar]