Abstract
Site-specific insertion of 5-(3-aminopropyl)-2′-deoxyuridine (Z3dU) and 7-deaza-dG into the Dickerson-Drew dodecamers 5′-d(C1G2C3G4A5A6T7T8C9Z10C11G12)-3′ · 5′-d(C13G14C15G16A17A18T19T20-C21Z22C23G24)-3′ (named DDDZ10) and 5′-d(C1G2C3G4A5A6T7X8C9Z10C11G12)-3′ · 5′-d(C13G14C15G16A17A18-T19X20C21Z22C23G24)-3′ (named DDD2+Z10)(X = Z3dU; Z = 7-deaza-dG) suggests a mechanism underlying the formation of interstrand N+2 DNA cross-links by nitrogen mustards, e.g., melphalan and mechlorethamine. Analysis of the DDD2+Z10 duplex reveals that the tethered cations at base pairs A5 · X20 and X8 · A17 extend within the major groove in the 3′-direction, toward conserved Mg2+ binding sites located adjacent to N+2 base pairs C3 · Z22 and Z10 · C15. Bridging waters located between the tethered amines and either Z10 or Z22 O6 stabilize the tethered cations and allow interactions with the N + 2 base pairs without DNA bending. Incorporation of 7-deaza-dG into the DDD2+Z10 duplex weakens but does not eliminate electrostatic interactions between tethered amines and Z10 O6 and Z22 O6. The results suggest a mechanism by which tethered N7-dG aziridinium ions, the active species involved in formation of interstrand 5′-GNC-3′ cross-links by nitrogen mustards, modify the electrostatics of the major groove and position the aziridinium ions proximate to the major groove edge of the N+2 C · G base pair, facilitating interstrand cross-linking.
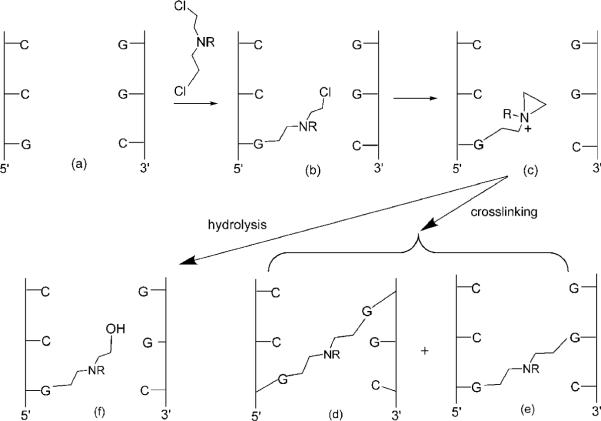
Nitrogen mustards, such as melphalan and mechlorethamine, were among the first effective clinically used anticancer compounds (1). Their biological activity is derived from DNA alkylation. Among the numerous adducts formed, N7-dG to N7-dG interstrand cross-links (ICLs)1 are a main contributor to cytotoxicity since they prevent strand separation required for DNA replication and transcription. The initial step in ICL formation is reaction with DNA (Scheme 1) to afford a reactive monofunctional N7-dG adduct, which can either react with another N7-dG site on the complementary strand, if one is available, to afford an ICL or undergo solvolysis to yield a stable monofunctional lesion. The first and second alkylation steps in the cross-linking involve aziridinium ions (Scheme 1).
Scheme 1.
Reactions of Nitrogen Mustards with DNA Giving Monofunctional and Cross-Linking Adducts
The ~7.5 Å length of the -(CH2)2N(R)(CH2)2- linkage suggests that the ICL should form between N7-dG at nearestneighbor base pairs in 5′-GC-3′ repeats (Scheme 1) (2). Molecular modeling confirms that this would be the predicted product. However, the Loechler (3, 4) and Hopkins groups (5) independently determined that the actual ICL formation occurs between N7-dG atoms at next nearest-neighbor C · G base pairs in 5′-GNC-3′ sequences, i.e., at the N + 2 base pair (Scheme 1d), with only a small amount of cross-linking at the predicted 5′-GC-3′ sequences (Scheme 1e). This result was unanticipated because formation of the 5′-GNC-3′ ICL requires a significant bending of B-DNA (6, 7); the ICL is ~1.5 Å too short to bridge the atoms in a classical B-DNA structure (8). When one considers that the second step in cross-link formation involves an aziridinium ion (Scheme 1), the disparity in the distance is actually closer to 3.0 Å. These observations lead to the conclusion that in the transition state for N + 2 ICL formation, the DNA duplex must deviate from the linear B-form, allowing the covalent linkage between the two atoms that form the second bond.
It is thought that the N + 2 ICL cross-link represents the kinetically favored reaction product (9, 10), but the mechanism of cross-link formation remains obscure. Positioning of a tethered aziridinium ion within the major groove modifies the electrostatics of the DNA duplex, which could transiently bend the duplex, thus positioning the aziridinium ion proximate to the electronegative major groove edge of the N + 2 C·G base pair that is 3′ to the point of attachment (11). The tethered 5-ω-aminopropyl-2′-deoxypyrimidine Z3-dU (Scheme 2) serves as a chemically stable model for the monofunctional N7-dG mustard adduct that is the intermediate species formed in the N + 2 cross-linking reaction. The “reach” of Z3dU in DNA as monitored by electrostatic footprinting experiments is longer than what would be predicted by the physical length of the cationic side chain (12-14). A second indication that the electrostatic interactions of the charged amino group with DNA may be important in the formation of the 5′-GNC-3′ ICL is the observation that cationic Z3dU substitutions, when appropriately phased on the same side of the helix as an intrinsically bent A-tract, reduce DNA mobility in gels (15, 16). This is suggestive of bending or anisotropic flexing of the DNA, which must be present in the transition state complex for the formation of the N + 2 ICL cross-link (9-11). The electrostatic mechanism responsible for these observations could be similar to that envisioned by Rouzina and Bloomfield for the bending of DNA by divalent cations (17).
Scheme 2.
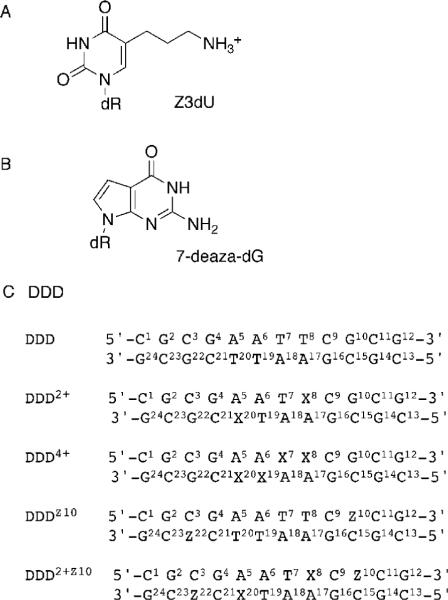
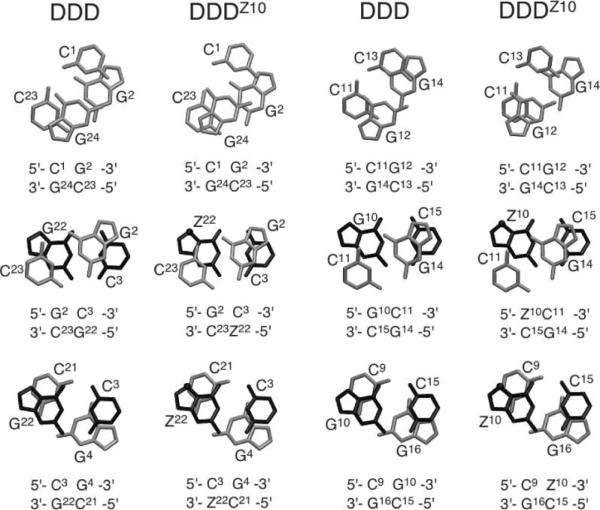
Structures of (A) Z3dU, 5-(3-Aminopropyl)-2′-deoxyuridine, (B) 7-Deaza-dG, and (C) 7-Deaza-dG and Z3dU-Modified Duplexes (X = Z3dU, and Z = 7-deaza-dG)a
a The nucleotides of one duplex strand are labeled from 1 to 12, and nucleotides of the complementary strand are labeled from 13 to 24.
To understand how localized cationic charge in the major groove affects structure, we initiated a series of studies to structurally, thermodynamically, and chemically characterize a self-complementary Dickerson-Drew dodocamer 5′-d(CGCGAATTCGCG)2-3′ in which either or both of the T residues were replaced with 5-(3-aminopropyl)-2′-deoxyuridine (Z3dU) (Scheme 2). In summary, the structure of DNA and its thermodynamic properties were dependent on where the cationic side chain was attached (14). In DDD2+, the proton NMR resonances of the Z3dU moieties were individually resolved, consistent with ordered side chain conformations in the major grove (18). At both X8 and X20, the tethered Z3dU amine exhibited a 3′-orientation with respect to the major groove. This corroborated both the chemical footprinting experiments and the predictions of molecular modeling. The NMR data suggested that the Z3dU cationic amines were transiently located in the vicinity of the electronegative N7 and O6 atoms at the N + 2 position on the floor of the major groove (18), implicating at least transient axial bending by the tethered cation. A 1.6 Å resolution crystal structure was reported in the presence of Tl+ (19). The X8- and X20-tethered amines were also oriented in the 3′-direction. In contrast, the basic amino side chains at positions X7 and X19 were directed into the major groove rather than toward the floor of the groove.
Herein, thermodynamic and crystallographic structural characterizations of the modified duplex 5′-d(C1G2C3G4A5A6T7X8C9Z10C11G12)-3′ · 5′-d(C13G14C15G16A17A18T19 X20C21Z22C23G24)-3′ (X) Z3dU, and Z) 7-deaza-dG) (DDD2+Z10) (Scheme 2) are presented. The positioning of 7-deaza-dG at positions Z10 and Z22, designed to alter the electrostatics of the major groove at base pairs C3 · Z22 and Z10·C15, facilitated crystallographic analysis of this dodecamer. The data reveal that the tethered Z3dU cations at base pairs A5 · X20 and X8 · A17 extend within the major groove in the 3′-direction, toward conserved Mg2+ binding sites located adjacent to N + 2 base pairs C3·Z22 and Z10 · C15. Bridging water molecules located between the tethered cationic amines and either Z10 or Z22 O6 stabilize the tethered Z3dU cations and allow the Z3dU amines to interact with the N + 2 base pairs without DNA bending. The distances between the cationic amines tethered to X8 or X20 and Z10 O6 or Z22 O6are ~0.5 Å longer than the distance observed between the cationic amine tethered to X8 and G10 O6 in 5′-d(C1G2C3 G4A5A6X7X8C9G10C11G12)-3′ · 5′-d(C13G14C15G16A17A18X19X20C21G22C23G24)-3′ (DDD4+), which maintains the N7 atom at nucleotides G10 and G22 (19). Incorporation of 7-deazadG into the DDD2+Z10 duplex weakens but does not eliminate electrostatic interactions between tethered cationic amines and Z10 O6 and Z22 O6. The results support a model in which the presence of tethered N7-dG aziridinium ions, which are the active species involved in formation of interstrand 5′-GNC-3′ cross-links by nitrogen mustards, modifies the electrostatics of the major groove and positions the aziridinium ions proximate to the major groove edge of the N + 2 C · G base pair, facilitating interstrand cross-linking. The thermodynamic results indicate that the 3′-orientation of the cationic Z3dU amine in the major groove is accompanied by the release of bound cations from the DDD2+ duplex, presumably located near the N7 and O6 atoms of G10 and G22. Overall, the work provides insight into how melphalanand mechlorethamine-induced ICL formation occurs between N7 G atoms at 5′-GNC-3′ sequences, i.e., at the N + 2 base pair.
MATERIALS AND METHODS
Materials
The oligodeoxynucleotides 5′-d(CGCGAATTCZCG)-3′ (DDDZ10) and 5′-d(CGCGAATXCZCG)-3′ (DDD2+Z10) were synthesized (20) and purified using reversedphase semipreparative HPLC (Phenomenex, Phenyl-Hexyl, 5 μm, 250 mm × 10.0 mm) equilibrated with 0.1 M ammonium formate (pH 7.0) and desalted using a G25 column. The samples were characterized by capillary gel electrophoresis and mass spectrometry. The oligodeoxynucleotide concentrations were determined using an extinction coefficient of 1.10 × 105 M-1 cm-1 at 260 nm (21).
Temperature-Unfolding Profiles (Melting Curves)
Heat capacities versus temperature profiles were measured with a VP-DSC differential scanning calorimeter (Microcal, Inc., Northampton, MA). The dry oligodeoxynucleotides were dissolved in 10 mM sodium phosphate buffer (pH 7) and adjusted to the desired ionic strength with NaCl for all unfolding experiments. DSC melting curves were obtained in the differential mode, by comparing a duplex solution against a buffer solution, in the temperature range of 0-105 °C. The experimental curve was normalized by the heating rate, and a buffer versus buffer scan was subtracted and normalized for the number of moles. The resulting monophasic, or biphasic, curves were then analyzed by deconvolution with Origin version 5.0 (Microcal); their integration (∫ΔCp dT) yielded the molar unfolding enthalpy (ΔHcal), which was independent of the nature of the transition (22). The molar entropy (ΔScal) was obtained similarly, using ∫(ΔCp/T) dT. The free energy change at any temperature T was then obtained with the Gibbs equation: ΔG°(T) = ΔHcal - TΔScal.
Absorption versus temperature profiles (UV melts) for each duplex were measured at 260 nm using a thermoelectrically controlled UV-vis Aviv 14DS or Lambda 40-Perkin-Elmer spectrophotometers. The temperature was scanned at heating rates of 0.75-1.00 °C/min. UV melts were measured in the salt range of 10-200 mM NaCl at pH 7, and at a constant total strand concentration of 7 μM, to determine the differential binding of counterions, ΔnNa+, which accompanied their helix-coil transitions. The TM values of biphasic transitions were determined from the differential UV melts and only using the TM of the duplex→random coil transition. This linking number was measured experimentally with the assumption that counterion binding to the helical and coil states of each oligonucleotide took place with a similar type of binding using the relationship (23) ΔnNa+ = 1.1(ΔHcal/RTM2)(∂TM/∂ ln[Na+]). The numerical factor corresponded to the conversion of ionic activities into concentrations. The first term in parentheses, (ΔHcal/RTM2), was a constant determined directly from DSC experiments, where R was the gas constant. The second term in parenthese was also determined experimentally from the dependencies of TM on salt concentration.
Crystallization
Crystals of the DDDZ10 and DDD2+Z10 DNAs were grown at 18 °C by the hanging drop vapor diffusion method, using the Nucleic Acid Miniscreen (Hampton Research) (24). Droplets containing 0.6 mM oligodeoxynucleotide, 5% 2-methyl-2,4-pentanediol (MPD), 20 mM sodium cacodylate (pH 6.0), 6 mM spermine tetrahydrochloride, 40 mM KCl, and 10 mM MgCl2 were equilibrated against a reservoir of 35% MPD. Orthorhombic crystals in space group P212121 appeared within 7 days and grew to a size of 0.1 mm × 0.1 mm × 0.3 mm over 2-3 days. A wide range of Mg2+ and spermine concentrations and pH values were assayed. It was concluded that both modified duplexes showed a propensity to crystallize at low cationic strengths.
Data Collection and Processing
Diffraction data were collected on beamline 22-ID at SER-CAT, APS (Argonne National Laboratory, Argonne, IL), using a MAR-165 CCD detector at -160 °C. Data were scaled, integrated, and reduced with HKL2000 (25).
Refinement
Both structures were determined by the molecular replacement method using EPMR (26) with the DDD structure with PDB entry BDL084 (27) as a search model. The structures of DDDZ10 and DDD2+Z10 were initially refined with simulated annealing by using CNS (28), and subsequently by geometric constraint/maximum-likelihood and isotropic temperature factor refinement for individual atoms by using REFMAC 5.0/CCP4 (29, 30). In later refinement stages of both structures, solvent water molecules were added on the basis of Fourier 2Fo - Fc sum and Fo -Fc difference electron density maps, and accepted on the basis of standard distance and B-factor criteria. X8 in the DDD2+Z10 structure and Z10 in both structures were introduced into DNA duplexes on the basis of Fourier electron density maps; corresponding geometry/topology files were adapted, and anisotropic temperature factor refinement was carried out afterward. TURBO-FRODO (31) was used to display electron density maps, manually rebuild the duplex models, and add or delete water molecules. The geometries of the DNA duplexes were analyzed with CURVES (version 5.3) (32).
RESULTS
Unfolding Thermodynamics of Dodecamer Duplexes
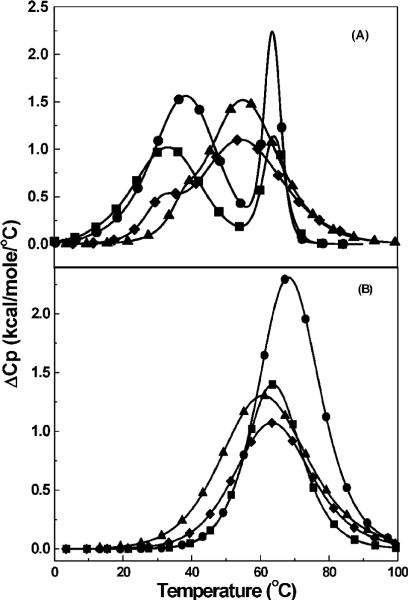
Heat capacity thermograms for the unfolding of each duplex are shown in panels A and B of Figure 1 at overall Na+concentrations of 16 and 116 mM, respectively. At the lower salt concentration, all curves exhibited biphasic transitions that shifted to monophasic transitions at higher temperatures with the increase in salt concentration. This was due to the stronger effect of salt on the first (duplex) transition, relative to the second (hairpin) transition, confirming the helix → hairpin → random coil transitions of each dodecamer duplex (33). Deconvolution analysis of differential scanning calorimetry (DSC) curves yielded the associated enthalpies; however, only the model-independent enthalpies of the duplex → random coil transitions are reported in Table 1. At low salt concentrations, enthalpies in the following order were obtained: DDD (116 kcal/mol) > DDDZ10 > DDD2+Z10 > DDD2+ (68 kcal/mol). The increase in salt concentration yielded lower enthalpy values but a similar trend: DDD (110 kcal/mol) > DDDZ10 > DDD2+Z10 > DDD2+ (60 kcal/mol). Relative to DDD, the dT → Z3dU substitution, the dG → 7-deaza-dG substitution, or both were destabilizing at low and high salt concentrations (34-36). The lowering of the enthalpies at higher salt concentrations suggested the presence of heat capacity effects. The heat capacities were in the following order: DDD (-0.8 kcal K-1 mol-1) ~ DDDZ10 < DDD2+ ~ DDD2+Z10 (-0.3 kcal K-1 mol-1). Comparison of DDD with DDD2+ and the second pair, DDDZ10 with DDD2+Z10, suggested that the decrease in heat capacity was due to weakened hydrophobic interactions from the incorporation of the charged Z3dU chain in the helical states of these oligodeoxynucleotides.
Figure 1.
DSC curves of duplexes in 10 mM sodium phosphate buffer (pH 7) and total Na+ concentrations of (A) 16 and (B) 116 mM: DDD (●), DDD2+ (■), DDDZ10 (▲), and DDD2+Z10 (◆).
Table 1.
Thermodynamic Profiles for the Folding of the Dodecamersa
| [NaCl] (mM) | TM (°C) | ΔHcal (kcal/mol) | TΔScal (kcal/mol) | ΔG°20 (kcal/mol) | ΔnNa+ (mol/mol) |
|---|---|---|---|---|---|
| DDD | |||||
| 10 | 33.3 | -116 | -109 | -6.9 | -2.3 ± 0.2 |
| 100 | 57.7 | -110 | -94.1 | -15 | -1.8 ± 0.1 |
| DDDZ10 | |||||
| 10 | 35.7 | -106 | -99.9 | -6.1 | -1.7 ± 0.1 |
| 100 | 50.0 | -87.7 | -77.0 | -11 | -1.3 ± 0.1 |
| DDD2+ | |||||
| 10 | 29.8 | -68.0 | -64.7 | -3.3 | -1.5 ± 0.1 |
| 100 | 45.3 | -60.0 | -52.1 | -7.9 | -1.2 ± 0.1 |
| DDD2+Z10 | |||||
| 10 | 31.9 | -74.3 | -70.6 | -3.7 | -1.2 ± 0.1 |
| 100 | 42.2 | -70.2 | -61.2 | -9.0 | -1.1 ± 0.1 |
All parameters were measured from UV (TM) and DSC melting curves in 10 mM sodium phosphate buffer (pH 7) and at the indicated NaCl concentrations. All TM's correspond to a strand concentration of 10 mM. The experimental uncertainties were as follows: ±0.5 °C for TM, ±5% for ΔHcal, ±7% for ΔG°20, and ±5% for TΔScal.
Thermodynamic Profiles for the Formation of Each Duplex
Table 1 lists thermodynamic profiles for the formation of each duplex at 20 °C. The magnitude of the ΔG°20 terms indicated that each oligodeoxynucleotide formed a stable duplex. The formation of each duplex resulted from the partial compensation of favorable enthalpy and unfavorable entropy contributions. Favorable enthalpy contributions included formation of base pairs and base-pair stacks, the immobilization of water around charges (electrostricted hydration), and the release of structural water around polar and nonpolar groups, while the unfavorable entropy term arose from contributions of the ordering of two strands, counterion condensation, and immobilization of water molecules. The free energy changes for duplex formation were in the following order: DDD (-6.9 kcal/mol) < DDDZ10 < DDD2+Z10 < DDD2+ (-3.3 kcal/mol) at low salt concentrations and DDD (-15.0 kcal/mol) < DDDZ10 < DDD2+Z10 < DDD2+ (-7.9 kcal/mol) at high salt concentrations. Relative to DDD, both dT → Z3dU and dG → 7-deaza-dG substitutions were destabilizing at low and high salt concentrations.
Differential Thermodynamic Binding of Counterions
Increasing the salt concentration shifted the melting curves to higher temperatures, consistent with the expectation that the duplex states had higher charge density parameters. The linear TM dependencies on salt concentration are shown in Figure 2. The slopes of these lines in conjunction with the ΔH/RTM2 terms allowed measurement of differential counterion binding. The ΔnNa+ values for the formation of each duplex (Table 1) were found in the following order: -2.3 (DDD) < -1.7 (DDDZ10) < -1.5 (DDD2+) < -1.2 (DDD2+Z10) in 10 mM NaCl. A similar trend was observed at 100 mM NaCl; however, the ΔnNa+ values (Table 1) were lower due to higher screening of the phosphates. The main effect was that the incorporation of Z3dU, 7-deaza-dG, or both into the dodecamer duplexes, assuming similar random coils at high temperatures, caused a weaker binding of counterions (35, 36).
Figure 2.
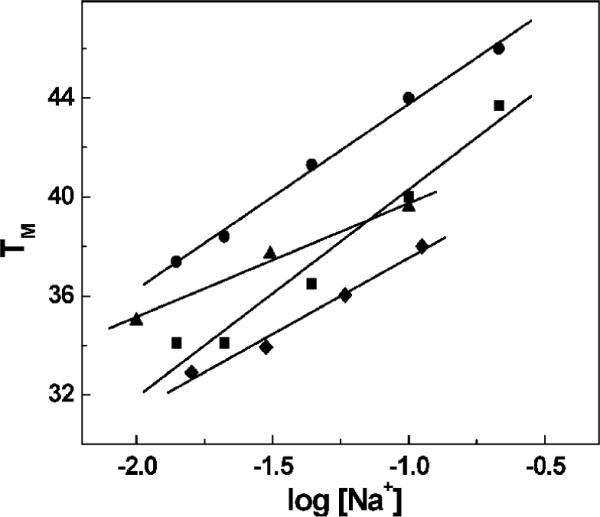
TM dependencies on salt concentration of duplexes in 10 mM sodium phosphate buffer (pH 7) and a total strand concentration of 7 μM. All TM values correspond to the duplex → random coil transition: DDD (●), DDD2+ (■), DDDZ10 (▲), and DDD2+Z10 (◆).
DNA Conformation in the Solid or Crystalline State
Crystal Packing
The crystals of the modified DDDZ10 and DDD2+Z10 dodecamers were isomorphous with those of the native DDD dodecamer. The two strands were not symmetryrelated, and the lattice interactions between the chemically modified DDDZ10 and DDD2+Z10 duplexes were preserved, as compared to those in the crystals of the native DDD dodecamer. Within the crystal, the DDD2+Z10 duplexes were related by crystallographic 21 symmetries. They were arranged in a head-to-tail fashion and formed extended columns along the crystallographic c-axis. Their ends formed 2 bp overlaps, involving direct hydrogen bonds between adjacent guanines and resulting in base triples (Figure S1 of the Supporting Information). Each G · G pair was stabilized by two N2-H ··· N3 hydrogen bonds. This packing contact is a common feature of orthorhombic P212121 crystals of Dickerson-Drew-type dodecamers (37, 38). Similar features were observed for the modified DDDZ10 duplex (Figure S2 of the Supporting Information).
Overall Structures
The structures of both DDDZ10 and DDD2+Z10 were refined to a resolution of 1.6 Å. The quality of the final electron density maps in both instances was excellent (Figure 3). The rmsd values for bond distances were 0.006 Å in both instances and for angles 1.6° in both instances (Table 2). The high resolutions achieved for the structures allowed a detailed analysis of geometric and conformational changes in the duplexes as a consequence of the introduced modifications. In addition to a single duplex per crystallographic asymmetric unit, a total of 110 and 95 water molecules were placed in the case of the DDDZ10 and DDD2+Z10 structures, respectively (Table 2). Neither Mg2+nor spermine were located in the DDDZ10 or DDD2+Z10 dodecamers.
Figure 3.
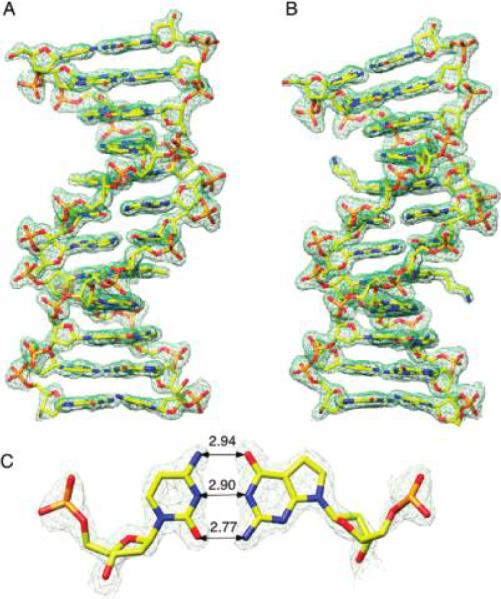
Sum electron density contoured at the 1.5σ level (green meshwork) around modified duplexes (A) DDDZ10 and (B) DDD2+Z10 and (C) the 7-deaza-dG · dC base pair with Watson-Crick geometry.
Table 2.
Selected Crystal Data and Refinement Statistics
| DDDZ10 | DDD2+Z10 | |
|---|---|---|
| (A) Crystal Data | ||
| unit cell (α, β, and γ angles are 90°) | ||
| a (Å) | 24.19 | 24.86 |
| b (Å) | 40.33 | 41.06 |
| c (Å) | 65.69 | 66.62 |
| space group | P212121 | P212121 |
| (B) Data Collection | ||
| temperature of data collection (°C) | -160.0 | -160.0 |
| total no. of reflections | 164005 | 359861 |
| no. of independent reflections | 9994 | 9574 |
| completeness (%) | 96.6 | 93.4 |
| maximum resolution (Å) | 1.42 | 1.42 |
| (C) Structure Refinement | ||
| resolution range (Å) | 34.36-1.60 | 34.94-1.60 |
| no. of reflections used in refinement | 7824 | 8368 |
| no. of reflections used in the test set | 471 | 497 |
| rmsd of bonds from ideal (Å) | 0.006 | 0.006 |
| rmsd of angles from ideal (deg) | 1.6 | 1.6 |
| no. of DNA atoms | 486 | 492 |
| no. of water molecules | 110 | 95 |
| R-factor(test set)a,b | 0.262 | 0.233 |
| R-factor(work set)b | 0.179 | 0.178 |
| R-factor(work+test)b | 0.186 | 0.180 |
Calculated using 6% of the reflection data that were not used in the refinement.
R-factor = Σhkl|F(hkl)o - F(hkl)c|/ΣhklF(hkl)o.
Comparison of the DDDZ10 and DDD Duplexes
A superimposition of the DDDZ10 and DDD (27) (PDB entry BDL084) duplexes is shown in Figure 4. Bending was evident at one end of the duplex in the case of the DDD duplex (27) compared to the DDDZ10 duplex. In the native DDD duplex structure, there was an ordered Mg2+ ion bound at the G2 · C23 → C3 · G22 base-pair step (Figure 5). The cation contacted the N7 and O6 atoms of G2 and G22 in the opposite strands via coordinated waters (39, 40). It also bridged the O2P oxygens of P6c and P7c of an adjacent molecule and stabilized the close interduplex contact between P2 and P7c (39-41). The bend at this end of the DDD duplex was associated with the coordination of a Mg2+ ion. In the modified DDDZ10 duplex, this ordered Mg2+ was not observed. The degree of the bend where the Mg2+ was located in the DDD dodecamer was reduced (Figure 4 and Figure S3 of the Supporting Information), and disorder was observed for the phosphate group between C1 and G2.
Figure 4.
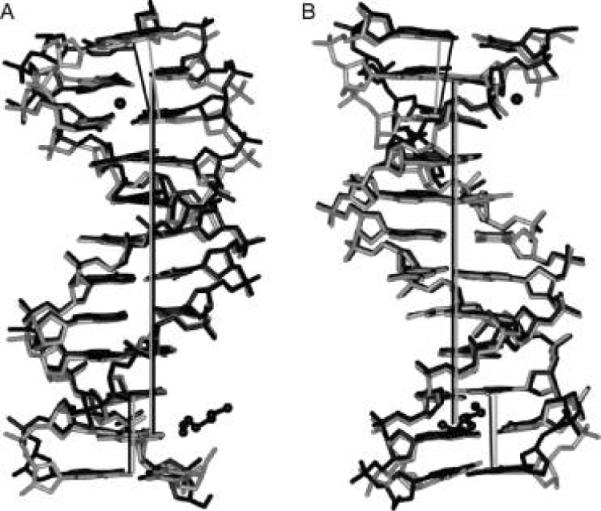
Superimposition of the DDD (PDB entry BDL084) (black) and DDDZ10 (gray) duplexes. The view is into the (A) minor groove and (B) major groove, with respect to the central base pairs. Mg2+ and a partial spermine molecule in the structure of the native DDD are shown in a ball-and-stick representation. The helix axes are based on the central hexamer duplexes and are coincident (straight gray and black lines for DDDZ10 and DDD, respectively). In both duplexes, the helix axes defined by base pairs G10 · C15, C11 · G14, and G12 · C13 at one end are parallel with those of the central hexamers. Conversely, the helix axis defined by base pairs C1 · G24, G2 · C23, and C3 · G22 at the other end is inclined more in the native DDD than in DDDZ10 relative to the respective helix axis.
Figure 5.
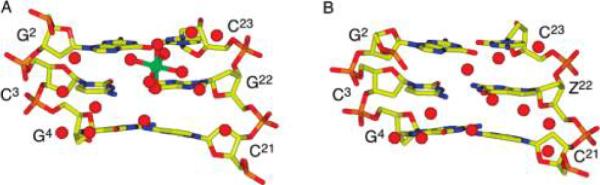
(A) Unmodified DDD duplex (PDB entry BDL084), showing the contact of Mg2+ with O6 and N7 of G2 and G22 through coordinated waters at the G2 · C23 → C3 · G22 base-pair step. (B) DDDZ10 duplex, showing the reorganization of waters and the absence of the Mg2+ ion. The Mg2+ ion is colored green in panel A, and the waters are colored red.
The substitution of 7-deaza-dG was accompanied by changes in hydration in the major groove (Figure 5). Two waters contacting G2 and G4 O6 and N7 in the DDDZ10 duplex maintained their positions as in the DDD duplex, while two waters close to Z22 O6 C7 reorganized versus the natural dodecamer. The phosphodiester linkage between C1 and G2 flipped to the major groove with the disappearance of the Mg2+ ion at the G2pC3 step (Figure 5). Changes were evident in the rise and roll and, to a smaller degree, in the twist parameters (Figure 6). In contrast, the other end of the duplex was not perturbed (Figure 4 and Figure S3 of the Supporting Information). In the unmodified DDD duplex, one of the internal nitrogen atoms of a spermine molecule was bound to G10 N7 (3.4 Å) (Figure 4) (27). The 7-deaza-dG modification at G10 in the DDDZ10 or DDD2+Z10 duplex made this H-bond impossible, and indeed, no ordered spermine was present.
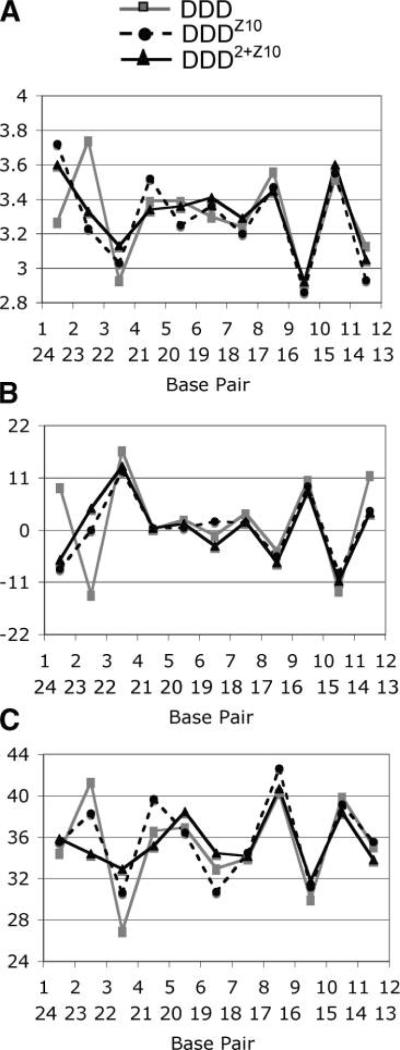
Figure 6.
Interbase pair parameters: (A) helical rise, (B) roll, and (C) twist for the DDDZ10, DDD2+Z20, and DDD (PDB entry BDL084) duplexes.
Structural Perturbations from the 7-Deaza-dG Modification
The C3 · Z22 and C15 · Z10 base pairs were maintained in the DDDZ10 or DDD2+Z10 duplexes despite dG being substituted with 7-deaza-dG (Figure 3). In the case of the C3 · Z22 base pair, the distances from N4 to O6, from N3 to N1, and from O2 to N2 were 2.9, 2.9, and 2.8 Å, respectively. For the Z10 · C15 base pair, the distances from N4 to O6, from N3 to N1, and from O2 to N2 were 2.9, 2.9, and 2.7 Å, respectively. These distances differed slightly from those observed for unmodified duplex C3 · G22 (2.8, 2.9, and 2.7 Å from N4 to O6, from N3 to N1, and from O2 to N2, respectively) and G10 · C15 (3.1, 2.9, and 2.7 Å from N4 to O6, from N3 to N1, and from O2 to N2, respectively). In terms of the base stacking interactions between the C1 · G24 and G4 · C21 pairs in the DDDZ10 duplex as compared to those in the DDD duplex (27), the base overlaps at the C3 · Z22 → G4 · C21 steps were similar in the two duplexes (Figure 8B). However, the G2 · C23 → C3 · Z22 and C1 · G24 → G2 · C23 steps were slightly perturbed. Similar features were observed for the base pairs at the other end of the duplex (Figure 7).
Figure 8.
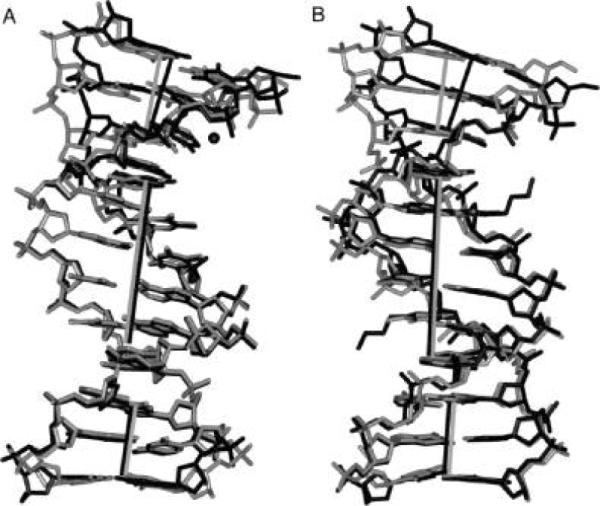
(A) Superimposition of the DDD (PDB entry BDL084, in black) and DDDZ10 (gray) duplexes. (B) Superimposition of the DDD2+Z10 (black) and DDDZ10 (gray) duplexes. The view is into the major groove. The Mg2+ ion in the structure of the native DDD duplex is shown in a ball-and-stick representation. The helix axes based on the central hexamer duplexes are coincident (black line for DDD and DDD2+Z10 and gray line for DDDZ10). For both, the helix axes defined by base pairs G10 · C15, C11 · G14, and G12 · C13 are parallel with those of the central hexamers. The helix axes defined by base pairs C1 · G24, G2 · C23, and C3 · G22 in either DDD2+Z10 or DDD are bent as compared to that the DDDZ10 duplex.
Figure 7.
Base stacking interactions in the CGCG portions at both ends of the DDD (PDB entry BDL084) and DDDZ10 duplexes. The conformations of the tetramer duplexes in the structures of the DDD and DDDZ10 duplexes indicate adjustments in the relative orientations of the modified and flanking base pairs in the latter. The modified base pair in DDDZ10 and the respective base pair in the unmodified DDD duplex are colored black, and atom C7 of 7-deaza-dG is highlighted with a black ball.
Comparison of the DDDZ10 and DDD2+Z10 Duplexes
A superimposition of the modified DDDZ10 and DDD2+Z10 duplexes is shown in Figure 8B. While neither the DDDZ10 duplex nor the DDD2+Z10 duplex exhibited a bound Mg2+, an increased axial bending of 20° was observed at one end of the duplex for DDD2+Z10, as compared to an axial bend of 10° for the DDDZ10 duplex (Figure 8B and Figure S3 of the Supporting Information). The conformational change was accompanied by alterations in the helical twist (Figure 6C). The other end of the duplex was not perturbed. In both instances, the asymmetric bending was attributed to a combination of cation binding and lattice interactions (41, 42).
Orientation and Conformation of the Tethered Cationic Side Chains
Both Z3dU modifications could be identified in sum and difference electron density maps (Figure 3). Both tethers extended toward the 3′-direction in the major groove. The two side chains at X8 and X20 adopted different conformations. Compared to that of the DDDZ10 duplex, a 20° bend was induced by the tethered cation at X20 in DDD2+Z10 at one end of the duplex. The effect of this tethered cation was similar to that of the Mg2+ ion bound in the major groove close to G22 O6 in the unmodified DDD duplex (27) (Figure 8). The conformation of the Cα -Cβ -Cγ-N+ torsion angle for the tethered cationic side chain at X20 was 177°, which was close to the ideal trans conformation. At the opposite end of the duplex, the tethered cation of X8 did not induce a conformational change. The conformation of the Cα -Cβ -Cγ-N+ torsion angle for the tethered cationic side chain at X8 was 200°, somewhat perturbed from the ideal trans conformation.
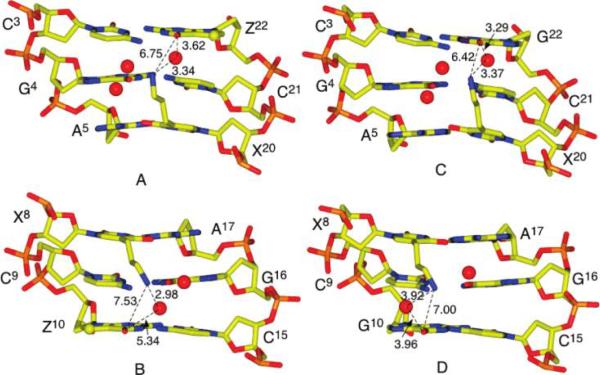
A superimposition of the DDD2+Z10 and DDD4+ (19) (PDB entry 1Z5T) duplexes is shown in Figure 9. For the 7-deazadG-containing duplex, there was a 0.5 Å increase in distance between the tethered Z3dU amine at X8 and the Z10 O6 atom. There were two waters observed at <5 Å with respect to the Z3dU amine at the bent end of the DDD2+Z10 and DDD4+ duplexes (Figure 10B,D). In the DDD4+ duplex, one water molecule mediated the interaction between the Z3dU amine and G10 O6 (Figure 10D), with distances of 4.0 Å between H2O O and G10 O6 and 3.9 Å between H2O O and Z3dU N+. The 7-deaza-dG modification in DDD2+Z10 resulted in the relocation of the bridging water molecule to a position further removed from the Z10 O6 atom (Figure 10B,D). The distance between H2O O and Z10 O6 was lengthened to 5.3 Å. This reflected a weakened interaction between the Z3dU amine and G10 O6. The other water molecule was farther from the Z3dU amine, with distances of 4.7 and 4.1 Å in the DDD2+Z10 and DDD4+ duplexes, respectively (Figure 10B,D). This water molecule mediated the interaction between the Z3dU amine and G16 O6 in the DDD2+Z10 and DDD4+ modified duplexes.
Figure 9.
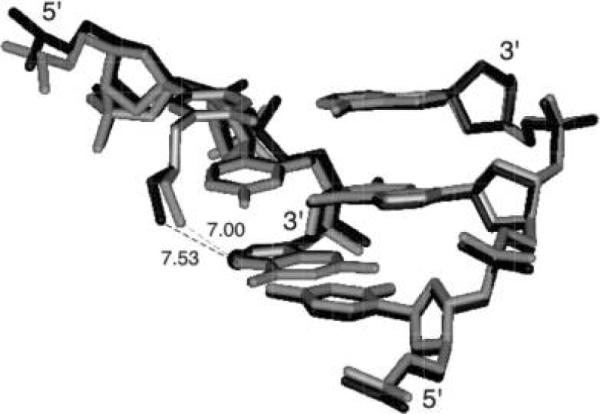
View of the superimposed DDD2+Z10 (black) and DDD4+ (gray) (PDB entry 1Z5T) duplexes. The distances between the tethered Z3dU cations at X8 and G/Z10 O6 in the two duplexes are in angstroms. Atom C7 in 7-deaza-dG is highlighted as a black sphere.
Figure 10.
Interactions among the Z3dU amine, a bridging water molecule, and G/Z O6 in the major groove in the (A and B) DDD2+Z10 and (C and D) DDD4+ (PDB entry 1Z5T) duplexes.
The positions and conformations of the tethered cationic side chains were affected by the water organization in the major groove. The Cα-Cβ-Cγ-N+ torsion angles of the Z3dU moieties were 200° and 207° for DDD2+Z10 and DDD4+, respectively. At the other end of the duplexes, the bridging water molecules were also relocated due to the 7-deaza-dG modifications at G22 (Figure 10A,C). Thus, the 3.6 Å distance between the Z3dU amine and Z22 O6 was greater than the distance of 3.3 Å between the Z3dU amine and G22 O6. The closest water molecule to the X20-tethered amine made a 6.8 Å bridge to Z22 O6, as compared to a 7.5 Å bridge from the X8-tethered amine to Z10 O6.
DISCUSSION
The formation of the DNA double helix creates an electrostatic environment that results in the sequencedependent binding of inorganic cations and reactive electrophiles in the major and minor grooves (17, 40, 43-48), in addition to the array of cations that surround the polyanionic phosphate backbone (49-53). The effect of the environment on DNA conformation is dramatic. While quantitative and qualitative changes in mobile cations can globally alter DNA structure (54-57), DNA affinity binding molecules, including proteins and organic ligands, regioselectively modulate the electrostatic environment of DNA and concomitantly cause localized changes in conformation (58-62).
A Bridging Water between the Tethered Z3dU Amine and Z10 O6
The observation of bridging water molecules between the tethered Z3dU amines and the dG O6 atoms of base pairs C3 · G22 and C3 · Z22 and base pairs G10 · C15 and Z10 · C15, in the crystallographic structures of the DDD4+ (19) and DDD2+Z10 duplexes, respectively, is significant (Figure 10). These bridging water molecules play a structural role in orienting the Z3dU moiety toward the 3′-direction in the major groove. The 7-deaza-dG modification in DDD2+Z10 results in the relocation of the bridging water molecule and a lengthening of the distance between H2O O and Z10 O6 to 5.3 Å. This is attributed to a weakened interaction between the Z3dU amine and G10 O6. Likewise, the bridging water molecule that mediates the interaction between the Z3dU amine and G16 O6 is farther from the Z3dU amine, with distances of 4.7 and 4.1 Å in the DDD2+Z10 and DDD4+duplexes, respectively (Figure 10).
Relevance to N + 2 ICL Cross-Link Formation by Nitrogen Mustards
The ability of the bridging waters to effectively lengthen the 4 Å Z3dU tether so that the combination of the Z3dU tether and the bridging water can span the distance to the next-nearest-neighbor base pairs in the 3′-direction relative to the Z3dU-modified nucleotides, without requiring significant bending of the DNA duplex, suggests that formation of N + 2 ICLs by nitrogen mustards (3-5) might involve a similar mechanism. A similar orientation of the monofunctional N7-dG-tethered aziridinium ion in the major groove at 5′-GNC-3′ sequences, involving a water bridge, might also be accommodated with minimal helical bending of the DNA duplex, thus kinetically favoring formation of the bent N + 2 transition state geometry. These data probably also explain why previous NMR solution data for tethered Z3dU amines were equivocal with respect to DNA bending (18); on average, the solution structure remains linear due to the presence of the bridging water molecules between the cationic amines and dG O6 of the next-nearestneighbor base pair. Exchange of the bridging water molecule then releases the tethered Z3dU moiety, facilitating formation of a high-energy bent structure, in which the Z3dU amine is positioned adjacent to dG N7 and O6 of the next-nearestneighbor base pair. This structure models the intermediate leading to N + 2 ICL formation by nitrogen mustards. Alternatively, the dislocation of the bridging water releases the Z3dU from the floor of the major groove.
Thermodynamic Consequences of Positioning Z3dU in the Major Groove
The differential thermodynamic profiles for the incorporation of the tethered Z3dU cation in the major groove of DDD (last four rows of Table 3) indicate that inclusion of two Z3dU chains in the DDD duplex is destabilizing (ΔΔG = 5.6 kcal/mol), resulting from the compensation of an unfavorable enthalpy contribution (ΔΔH = 48.8 kcal/mol) with a favorable entropy contribution [Δ(TΔS) = 43.2 kcal/mol (63)] and average counterion release of 0.7 mol of Na+/mol of duplex. In comparison, the incorporation of two Z3dU chains in DDDZ10 yielded lower magnitudes for each parameter: ΔΔG = 2.1 kcal/mol, ΔΔH = 24.6 kcal/mol, Δ(TΔS) = 22.5 kcal/mol, and ΔΔnNa+ = 0.35 mol of Na+/mol of duplex. The unfavorable enthalpy terms explain differences in both base stacking and hydration contributions between each pair of duplexes, while the favorable entropy terms are consistent with the release of counterions. The lower magnitudes of the latter profile are consistent with the perturbations caused by the inclusion of two 7-deaza-dG bases.
Table 3.
Differential Thermodynamic Profiles for Pairs of Dodecamer Duplexes
| [NaCl] (mM) | ΔΔHcal (kcal/mol) | Δ(TΔScal) (kcal/mol) | ΔΔG°20 (kcal/mol) | ΔΔnNa+ (mol of Na+/mol) |
|---|---|---|---|---|
| Substitution of dG with 7-Deaza-dG in DDD (DDDZ10 minus DDD) | ||||
| 10 | 10.0 | 9.2 | 0.8 | 0.6 |
| 100 | 21.8 | 17 | 4.8 | 0.5 |
| Incorporation of Z3dU into DDD (DDD2+ minus DDD) | ||||
| 10 | 48.0 | 44.4 | 3.6 | 0.8 |
| 100 | 49.5 | 41.9 | 7.6 | 0.6 |
| Incorporation of Z3dU into DDDZ10 (DDD2+Z10 minus DDDZ10) | ||||
| 10 | 31.7 | 29.3 | 2.4 | 0.5 |
| 100 | 17.5 | 15.8 | 1.7 | 0.2 |
The placement of the Z3dU side chains in the major groove of DDD or DDDZ10 reduces the level of base-pair stacking interactions and reorganizes both counterions and water molecules. These effects can be explained by the fact that the physical presence of the tethered propyl chain displaces counterions and waters in the major groove proximal to the site of attachment, while the positively charged amine indirectly neutralizes negative phosphate charges (17, 35). For instance, if it was assumed that the incorporation of two amino propyl chains effectively neutralized two negative phosphate charges of the 22 formal charges of DDD, then the average residual charge per phosphate predicted from Manning condensation theory (49) would correspond to a reduction in the level of counterion binding of 0.5 counterions for DDD2+, which is in good agreement with the experimental ΔΔnNa+ value of 0.8. A smaller release of counterions, ~0.3 mol of Na+/mol of duplex, was obtained for the DDDZ10 duplex, confirming the weaker binding of counterions by the duplex state of DDDZ10. This is consistent with the dG → 7-deaza-dG substitution into DDD at G10 eliminating this ion binding site (45). The overall thermodynamics and structural data seem to suggest that it is the electrostatic attraction of the electron dense region at the edge of G10 that propels the tethered aminopropyl chain toward the 3′-direction rather than out into solvent (18, 19, 36).
Structural and Thermodynamic Consequences of the 7-Deaza-dG Modification
The rationale for incorporation of 7-deaza-dG was to alter the electrostatics of the major groove at base pairs C3 · Z22 and Z10 · C15. This incorporation of 7-deaza-dG at two sites in DDD facilitated these crystallographic studies. Thus, the results presented here also provide the first crystallographic characterization of the 7-deaza-dG modification in duplex DNA. It results in minimal structural perturbation of the Dickerson dodecamer, with Watson-Crick pairing being maintained at base pairs C3 · Z22 and Z10 · C15. The 7-deaza-dG modification does alter the electrostatics at the site typically occupied by a Mg2+ hexahydrate ion in unmodified DDD crystal lattices (19) and removes a proton acceptor from the major groove. Neither Mg2+ nor spermine is located in the crystal structure of the DDDZ10 dodecamer, as compared to the native DDD dodecamer (Figure 5). The contact of the bound Mg2+ ion with O6 and N7 of G2 and G22 through coordinated waters at the G2 · C23 → C3 · G22 and C11 · G14 → G10 · C15 steps may stabilize these base pairs with respect to breathing. NMR studies on the DDDZ10 duplex revealed the rapid exchange of the Watson-Crick hydrogen-bonded imino protons at the 5′-neighboring base pairs relative to the 7-deaza-dG-modified bases, the G2 · C23 and C11 · G14 base pairs (36). This suggests that in solution, the 7-deaza-dG modification increases the level of breathing at the 5′-neighbor base pair, related to the role of major groove cations in stabilizing DNA structure. The reduction in stability (ΔΔG ~ 2 kcal/mol) due to the 7-deaza-dG substitution is dominated by an unfavorable ΔΔHcal term, which suggests changes in stacking and/or hydration in the 7-deaza-dG-containing duplexes. The unfavorable ΔΔH is compensated, in part, by a favorable Δ(TΔScal). This is consistent with a reduced level of release of salts and H2O from the 7-deaza-dG-substituted duplexes and chemical footprinting showing enhanced accessibility of the G2 · C23 and C11 · G14 base pairs (36).
Summary
These structural and thermodynamic studies suggest an electrostatic mechanism underlying the formation of interstrand N+2 cross-links by the nitrogen mustards, e.g., melphalan and mechlorethamine. The tethered Z3dU cations extend within the major groove in the 3′-direction, toward conserved Mg2+ binding sites located adjacent to N+2 base pairs C3 · Z22 and Z10 · C15. Bridging waters located between the tethered cationic amines and either Z10 or Z22 O6 stabilize the tethered Z3dU cations and allow the Z3dU amines to interact with the N+2 base pairs without DNA bending. On the basis of the results, we envision a model in which the presence of tethered N7-dG aziridinium ions, which are the active species involved in formation of interstrand 5′-GNC-3′ cross-links by nitrogen mustards, modifies the electrostatics of the major groove and positions the aziridinium ions proximate to the major groove edge of the N+2 C · G base pair, facilitating interstrand cross-linking.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants CA-76049 (B.G., L.A.M., and M.P.S.) and GM-55237 (M.E.), National Cancer Institute Center Grants CA-36727 (University of Pittsburgh) and CA-68485 (Vanderbilt University), and NSF Grant MCB-0315746 (L.A.M.). Crystallographic data were collected on beamline 22-ID by the Southeast Regional Collaborative Access Team (SER-CAT) at the Advanced Photon Source, Argonne National Laboratory (Argonne, IL). Supporting institutions may be found at www.ser-cat.org/members.html. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under Contract W-31-109-Eng-38.
Footnotes
Crystallographic coordinates and structure factors have been deposited in the Protein Data Bank as entries 2QEG (DDDZ10) and 2QEF (DDD2+Z10).
Abbreviations: CCD, charge-coupled device; 7-deaza-dG, 7-deaza-2¢-deoxyguanosine; DDD, Dickerson-Drew dodecamer; 5′-d (C1 G2 C3 G4 A5 A6 T7 T8 C9 G1 0 C1 1 G1 2) - 3 ′ · 5 ′ -d(C13G14C15G16A17A18T19T20C21G22C23G24)-3′; DDDZ10, Dickerson-Drew dodecamer containing 7-deaza-dG at the G10 nucleotide position; DDD2+Z10, Dickerson-Drew dodecamer containing 7-deaza-dG at the G10 nucleotide position and Z3dU at the T8 position; DDD4+, Dickerson-Drew dodecamer containing Z3dU at the T7 and T8 positions; DSC, differential scanning calorimetry; ICL, interstrand crosslink; MPD, 2-methyl-2,4-pentanediol; Z3-dU, 5-(3-aminopropyl)-2′-deoxyuridine.
SUPPORTING INFORMATION AVAILABLE Hydrogen bonding interactions mediating direct contacts between neighboring DDD duplexes in the crystal lattice for duplex DDDZ10 (Figures S1 and S2), comparison of the global axis curvature (Figure S3), and comparison of backbone and glycosyl torsion angles in the crystal structures of DDD, DDDZ10, and DDD2+Z10 duplexes (Figure S4). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Colvin M. Pharmacological Principles of Cancer Treatment. W. B. Saunders; Philadelphia: 1980. The alkylating agents; pp. 276–308. [Google Scholar]
- 2.Brookes P, Lawley PD. The reaction of mono and di-functional alkylating agents with nucleic acids. Biochem. J. 1961;80:496–503. doi: 10.1042/bj0800496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ojwang JO, Grueneberg DA, Loechler EL. Synthesis of a duplex oligonucleotide containing a nitrogen mustard interstrand DNA-DNA cross-link. Cancer Res. 1989;49:6529–6537. [PubMed] [Google Scholar]
- 4.Grueneberg DA, Ojwang JO, Benasutti M, Hartman S, Loechler EL. Construction of a human shuttle vector containing a single nitrogen mustard interstrand, DNA-DNA cross-link at a unique plasmid location. Cancer Res. 1991;51:2268–2272. [PubMed] [Google Scholar]
- 5.Millard JT, Raucher S, Hopkins PB. Mechlorethamine cross-links deoxyguanosine residues at 5′-GNC sequences in duplex DNA fragments. J. Am. Chem. Soc. 1990;112:2459–2460. [Google Scholar]
- 6.Rink SM, Solomon MS, Taylor MJ, Rajur SB, McLaughlin LW, Hopkins PB. Covalent structure of a nitrogen mustard-induced DNA interstrand cross-link. J. Am. Chem. Soc. 1993;115:2551–2557. [Google Scholar]
- 7.Rink SM, Hopkins PB. A mechlorethamine-induced DNA interstrand cross-link bends duplex DNA. Biochemistry. 1995;34:1439–1445. doi: 10.1021/bi00004a039. [DOI] [PubMed] [Google Scholar]
- 8.Arnott S, Hukins DWL. Optimised parameters for A-DNA and B-DNA. Biochem. Biophys. Res. Commun. 1972;47:1504–1509. doi: 10.1016/0006-291x(72)90243-4. [DOI] [PubMed] [Google Scholar]
- 9.Dong Q, Barsky D, Colvin ME, Melius CF, Ludeman SM, Moravek JF, Colvin OM, Bigner DD, Modrich P, Friedman HS. A structural basis for a phosphoramide mustard-induced DNA interstrand cross-link at 5′-d(GAC) Proc. Natl. Acad. Sci. U.S.A. 1995;92:12170–12174. doi: 10.1073/pnas.92.26.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer GB, Povirk LF. Specificity and kinetics of interstrand and intrastrand bifunctional alkylation by nitrogen mustards at a G-G-C sequence. Nucleic Acids Res. 1997;25:1211–1218. doi: 10.1093/nar/25.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold B. Effect of cationic charge localization on DNA structure. Biopolymers. 2002;65:173–179. doi: 10.1002/bip.10215. [DOI] [PubMed] [Google Scholar]
- 12.Liang G, Encell L, Nelson MG, Switzer C, Gold B. The role of electrostatics in the sequence selective reaction of charged alkylating agents with DNA. J. Am. Chem. Soc. 1995;117:10135–10136. [Google Scholar]
- 13.Dande P, Liang G, Chen FX, Roberts C, Nelson MG, Hashimoto H, Switzer C, Gold B. Regioselective effect of zwitterionic DNA substitutions on DNA alkylation: Evidence for a strong side chain orientational preference. Biochemistry. 1997;36:6024–6032. doi: 10.1021/bi962602u. [DOI] [PubMed] [Google Scholar]
- 14.Gold B, Marky LM, Stone MP, Williams LD. A review of the role of the sequence-dependent electrostatic landscape in DNA alkylation patterns. Chem. Res. Toxicol. 2006;19:1402–1414. doi: 10.1021/tx060127n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strauss JK, Prakash TP, Roberts C, Switzer C, Maher LJ. DNA bending by a phantom protein. Chem. Biol. 1996;3:671–678. doi: 10.1016/s1074-5521(96)90135-0. [DOI] [PubMed] [Google Scholar]
- 16.Strauss JK, Roberts C, Nelson MG, Switzer C, Maher LJ. DNA bending by hexamethylene-tethered ammonium ions. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9515–9520. doi: 10.1073/pnas.93.18.9515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rouzina I, Bloomfield VA. DNA bending by small, mobile multivalent cations. Biophys. J. 1998;74:3152–3164. doi: 10.1016/S0006-3495(98)78021-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Huang L, Dande P, Gold B, Stone MP. Structure of a tethered cationic 3-aminopropyl chain incorporated into an oligodeoxynucleotide: Evidence for 3′-orientation in the major groove accompanied by DNA bending. J. Am. Chem. Soc. 2002;124:8553–8560. doi: 10.1021/ja0201707. [DOI] [PubMed] [Google Scholar]
- 19.Moulaei T, Maehigashi T, Lountos GT, Komeda S, Watkins D, Stone MP, Marky LA, Li JS, Gold B, Williams LD. Structure of B-DNA with cations tethered in the major groove. Biochemistry. 2005;44:7458–7468. doi: 10.1021/bi050128z. [DOI] [PubMed] [Google Scholar]
- 20.Heystek LE, Zhou HQ, Dande P, Gold B. Control over the localization of positive charge in DNA: The effect on duplex DNA and RNA stability. J. Am. Chem. Soc. 1998;120:12165–12166. [Google Scholar]
- 21.Cavaluzzi MJ, Borer PN. Revised UV extinction coefficients for nucleoside-5′-monophosphates and unpaired DNA and RNA. Nucleic Acids Res. 2004;32:e13. doi: 10.1093/nar/gnh015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marky LA, Breslauer KJ. Calculating thermodynamic data for transitions of any molecularity from equilibrium melting curves. Biopolymers. 1987;26:1601–1620. doi: 10.1002/bip.360260911. [DOI] [PubMed] [Google Scholar]
- 23.Kaushik M, Suehl N, Marky LA. Calorimetric unfolding of the bimolecular and i-motif complexes of the human telomere complementary strand, d(C3TA2)4. Biophys. Chem. 2007;126:154–164. doi: 10.1016/j.bpc.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 24.Berger I, Kang CH, Sinha N, Wolters M, Rich A. A highly efficient 24-condition matrix for the crystallization of nucleic acid fragments. Acta Crystallogr. 1996;D52:465–468. doi: 10.1107/s0907444995013564. [DOI] [PubMed] [Google Scholar]
- 25.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Acta Crystallogr. 1997;A276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 26.Kissinger CR, Gehlhaar DK, Fogel DB. Rapid automated molecular replacement by evolutionary search. Acta Crystallogr. 1999;D55:484–491. doi: 10.1107/s0907444998012517. [DOI] [PubMed] [Google Scholar]
- 27.Shui X, McFail-Isom L, Hu GG, Williams LD. The B-DNA dodecamer at high resolution reveals a spine of water on sodium. Biochemistry. 1998;37:8341–8355. doi: 10.1021/bi973073c. [DOI] [PubMed] [Google Scholar]
- 28.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. 1998;D54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 29.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. 1997;D53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 30.Collaborative Computational Project Number 4 The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. 1994;D50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 31.Cambillau C, Roussel A. TURBO FRODO. version OpenGL.1 Université Aix-Marseille II; Marseille, France: 1997. [Google Scholar]
- 32.Ravishankar G, Swaminathan S, Beveridge DL, Lavery R, Sklenar H. Conformational and helicoidal analysis of 30 ps of molecular dynamics on the d(CGCGAATTCGCG) double helix: `Curves', dials, and windows. J. Biomol. Struct. Dyn. 1989;6:669–699. doi: 10.1080/07391102.1989.10507729. [DOI] [PubMed] [Google Scholar]
- 33.Marky LA, Blumenfeld KS, Kozlowski S, Breslauer KJ. Salt-dependent conformational transitions in the self-complementary deoxydodecanucleotide d(CGCAATTCGCG): Evidence for hairpin formation. Biopolymers. 1983;22:1247–1257. doi: 10.1002/bip.360220416. [DOI] [PubMed] [Google Scholar]
- 34.Soto AM, Kankia BI, Dande P, Gold B, Marky LA. Thermodynamic and hydration effects for the incorporation of a cationic 3-aminopropyl chain into DNA. Nucleic Acids Res. 2002;30:3171–3180. doi: 10.1093/nar/gkf430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shikiya R, Li JS, Gold B, Marky LA. Incorporation of cationic chains in the Dickerson-Drew dodecamer: Correlation of energetics, structure, and ion and water binding. Biochemistry. 2005;44:12582–12588. doi: 10.1021/bi050897i. [DOI] [PubMed] [Google Scholar]
- 36.Ganguly M, Wang F, Kaushik M, Stone MP, Marky LA, Gold B. A study of 7-deaza-2′-deoxyguanosine 2′-deoxycytidine base pairing in DNA. Nucleic Acids Res. 2007;35:6181–6195. doi: 10.1093/nar/gkm670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu TK, Kaczor-Grzeskowiak M, Dickerson RE. Absence of minor groove monovalent cations in the crosslinked dodecamer C-G-C-G-A-A-T-T-C-G-C-G. J. Mol. Biol. 1999;292:589–608. doi: 10.1006/jmbi.1999.3075. [DOI] [PubMed] [Google Scholar]
- 38.Tsunoda M, Kondo J, Karino N, Ueno Y, Matsuda A, Takenaka A. Water mediated Dickerson-Drew-type crystal of DNA dodecamer containing 2′-deoxy-5-formyluridine. Biophys. Chem. 2002;95:227–233. doi: 10.1016/s0301-4622(01)00259-9. [DOI] [PubMed] [Google Scholar]
- 39.Tereshko V, Minasov G, Egli M. The Dickerson-Drew B-DNA dodecamer revisited at atomic resolution. J. Am. Chem. Soc. 1999;121:470–471. [Google Scholar]
- 40.Minasov G, Tereshko V, Egli M. Atomic-resolution crystal structures of B-DNA reveal specific influences of divalent metal ions on conformation and packing. J. Mol. Biol. 1999;291:83–99. doi: 10.1006/jmbi.1999.2934. [DOI] [PubMed] [Google Scholar]
- 41.Egli M, Tereshko V. Lattice- and sequence-dependent binding of Mg2+ in the crystal structure of a B-DNA dodecamer. ACS Symp. Ser. 2004;884:87–109. [Google Scholar]
- 42.Pallan PS, Ittig D, Heroux A, Wawrzak Z, Leumann CJ, Egli M. Crystal structure of tricyclo-DNA: An unusual compensatory change of two adjacent backbone torsion angles. Chem. Commun. 2008:883–885. doi: 10.1039/b716390h. [DOI] [PubMed] [Google Scholar]
- 43.Wurdeman RL, Douskey MC, Gold B. DNA methylation by N-methyl-N-nitrosourea: Methylation pattern changes in single- and double-stranded DNA, and in DNA with mismatched or bulged guanines. Nucleic Acids Res. 1993;21:4975–4980. doi: 10.1093/nar/21.21.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiu TK, Dickerson RE. 1 Å crystal structures of B-DNA reveal sequence-specific binding and groove-specific bending of DNA by magnesium and calcium. J. Mol. Biol. 2000;301:915–945. doi: 10.1006/jmbi.2000.4012. [DOI] [PubMed] [Google Scholar]
- 45.Howerton SB, Sines CC, VanDerveer D, Williams LD. Locating monovalent cations in the grooves of B-DNA. Biochemistry. 2001;40:10023–10031. doi: 10.1021/bi010391+. [DOI] [PubMed] [Google Scholar]
- 46.Hud NV, Polak M. DNA-cation interactions: The major and minor grooves are flexible ionophores. Curr. Opin. Struct. Biol. 2001;11:293–301. doi: 10.1016/s0959-440x(00)00205-0. [DOI] [PubMed] [Google Scholar]
- 47.Egli M. DNA-cation interactions. Quo vadis? Chem. Biol. 2002;9:277–286. doi: 10.1016/s1074-5521(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 48.Howerton SB, Nagpal A, Williams LD. Surprising roles of electrostatic interactions in DNA-ligand complexes. Biopolymers. 2003;69:87–99. doi: 10.1002/bip.10319. [DOI] [PubMed] [Google Scholar]
- 49.Manning GS. Molecular theory of polyelectrolyte solutions with applications to electrostatic properties of polynucleotides. Q. Rev. Biophys. 1978;11:179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- 50.Fenley MO, Manning GS, Olson WK. Approach to the limit of counterion condensation. Biopolymers. 1990;30:1191–1203. doi: 10.1002/bip.360301305. [DOI] [PubMed] [Google Scholar]
- 51.Anderson CF, Record MT. Salt nucleic-acid interactions. Annu. Rev. Phys. Chem. 1995;46:657–700. doi: 10.1146/annurev.pc.46.100195.003301. [DOI] [PubMed] [Google Scholar]
- 52.Bloomfield VA. DNA condensation by multivalent cations. Biopolymers. 1997;44:269–282. doi: 10.1002/(SICI)1097-0282(1997)44:3<269::AID-BIP6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 53.Manning GS. Electrostatic free energy of the DNA double helix in counterion condensation theory. Biophys. Chem. 2002;101:461–473. doi: 10.1016/s0301-4622(02)00162-x. [DOI] [PubMed] [Google Scholar]
- 54.Saenger W. Principles of Nucleic Acid Structure. Springer; New York: 1984. [Google Scholar]
- 55.McConnell KJ, Beveridge DL. DNA structure: What's in charge? J. Mol. Biol. 2000;304:803–820. doi: 10.1006/jmbi.2000.4167. [DOI] [PubMed] [Google Scholar]
- 56.Manning GS. Comments on selected aspects of nucleic acid electrostatics. Biopolymers. 2003;69:137–143. doi: 10.1002/bip.10361. [DOI] [PubMed] [Google Scholar]
- 57.Manning GS. The contribution of transient counterion imbalances to DNA bending fluctuations. Biophys. J. 2006;90:3208–3215. doi: 10.1529/biophysj.105.078865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paolella DN, Liu Y, Fabian MA, Schepartz A. Electrostatic mechanism for DNA bending by bZIP proteins. Biochemistry. 1997;36:10033–10038. doi: 10.1021/bi970515b. [DOI] [PubMed] [Google Scholar]
- 59.Dickerson RE. DNA bending: The prevalence of kinkiness and the virtues of normality. Nucleic Acids Res. 1998;26:1906–1926. doi: 10.1093/nar/26.8.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams LD, Maher LJ. Electrostatic mechanisms of DNA deformation. Annu. Rev. Biophys. Biomol. Struct. 2000;29:497–521. doi: 10.1146/annurev.biophys.29.1.497. [DOI] [PubMed] [Google Scholar]
- 61.Horton NC, Perona JJ. Crystallographic snapshots along a protein-induced DNA-bending pathway. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5729–5734. doi: 10.1073/pnas.090370797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manning GS. Is a small number of charge neutralizations sufficient to bend nucleosome core DNA onto its superhelical ramp? J. Am. Chem. Soc. 2003;125:15087–15092. doi: 10.1021/ja030320t. [DOI] [PubMed] [Google Scholar]
- 63.Marky LA, Kupke DW. Enthalpy-entropy compensations in nucleic acids: Contribution of electrostriction and structural hydration. Methods Enzymol. 2000;323:419–441. doi: 10.1016/s0076-6879(00)23376-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.