Abstract
Peptides with broad-spectrum antimicrobial activity are found in the mucosal surfaces at many sites in the body, including the airway, the oral cavity, and the digestive tract. Based on their in vitro antimicrobial and other immunomodulatory activities, these host defense peptides have been proposed to play an important role in the innate defense against pathogenic microbial colonization. The genes that encode these peptides are up-regulated by pathogens, further supporting their role in innate immune defense. However, the differences in the local microbial environments between the generally sterile airway and the highly colonized oral cavity suggest a more complex role for these peptides in innate immunity. For example, β-defensin genes are induced in the airway by all bacteria and Toll-like receptor (TLR) agonists primarily through an NF-κB-mediated pathway. In contrast, the same genes are induced in the gingival epithelium by only a subset of bacteria and TLR ligands, via different pathways. Furthermore, the environments into which the peptides are secreted—specifically saliva, gingival crevicular fluid, and airway surface fluid—differ greatly and can effect their respective activities in host defense. In this review, we examine the differences and similarities between host defense peptides in the oral cavity and the airway, to gain a better understanding of their contributions to immunity.
Keywords: defensin, cathelicidin, innate immunity, antimicrobial peptide, host-pathogen interaction
Introduction
The human airway and the oral cavity both represent mucosal surfaces open to the environment. This allows for constant exposure to the micro-organisms that could colonize and lead to disease. Primary among the mechanisms to prevent such pathogenic colonization is the innate immune system, which provides a non-specific, rapid defense against invading pathogens. However, the airway is predominantly a sterile environment, while the oral cavity is host to hundreds of species of micro-organisms, many of which are commensal. An examination of the similarities and differences between these two epithelial surfaces will help us understand the roles played by innate immune mediators. These include several classes of molecules initially identified as antimicrobial peptides (AMP). With the increasing discovery of their multiple activities, they will here be referred to as host defense peptides (HDPs).
The cells which comprise the innate immune response are primarily phagocytes, including neutrophils and macrophages, and the cells that line the epithelial mucosa. It had been originally thought that the epithelium provided defense primarily as a barrier against microbial invasion. However, it has been more recently recognized that these cells play an important active role in the recognition of microbes, eliciting a defensive response similar to that found in cells of the myeloid lineage. In general, the innate immune response is driven by the recognition of microbe-associated molecular patterns, such as lipopolysaccharide (LPS) or bacterial DNA, or by Toll-like receptors (TLRs), which are specific for these patterns. Other receptors, such as Dectin-1, a glucan receptor, or mannose-binding lectin (Takahashi et al., 2006), also participate in this recognition. Recognition of the patterns by the receptors, often with co-receptors such as CD14 or MD2, usually results in the activation of a signal transduction pathway, which ultimately activates transcription factors, often including NF-κB, and in turn the induction of innate immune gene expression. These genes include pro-inflammatory cytokines such as IL-1β and IL-8, and host defense peptides such as defensins, cathelicidins, and histatins.
Host Defense Peptides
Defensins
Initially identified in phagocytic cells, defensins have been predicted to play a major role in innate antimicrobial host defense. These peptides are 3-5 kDa in size, highly cationic, and have a characteristic 6-cysteine motif, which results in a three-disulfide-bonded secondary structure. There are two main subgroups, the α- and β-defensins, which differ in their cysteine motifs, but share a similar secondary structure and are both rich in cationic residues (Fig. 1). Peptides in both groups are encoded by unique genes, which are primarily localized to a region of human chromosome 8p. The peptides are expressed as larger precursors, with a putative signal sequence at the N-terminus of the precursor. α-defensins have an acidic pro-region which undergoes a secondary cleavage to release the mature peptide, while mature β-defensins are predicted to mature by signal peptidase (Beckloff and Diamond, 2008).
Figure 1.

ClustalW alignment of human α- and β-defensins. The mature peptide sequences of α- and β-defensins are aligned with the number of amino acids listed on the right. Amino acids are annotated based on the ClustalW scheme for physical characteristics: uppercase for small and hydrophobic, italic for acidic, boldface for basic, and underlined for hydroxyl, amide, and basic. Highly conserved residues in each sequence are denoted by an asterisk.
The α-defensins are 29-35 residues in length and exhibit broad-spectrum activity against Gram-positive and Gram-negative bacteria (Ganz and Lehrer, 1995), fungi (Lehrer et al., 1986), viruses (Klotman and Chang, 2006), and mycobacteria (Kisich et al., 2001). In humans, they are found predominantly in the neutrophils, where the 4 α-defensins [known as human neutrophil peptides (HNP) 1, 2, 3, and 4] make up approximately 30% of the total protein in the azurophilic granule (Liu et al., 1997). The presence of neutrophils during airway inflammation can result in the introduction of high concentrations of HNP into inflamed tissue. Increases in HNP levels in broncho-alveolar lavage fluid has been observed in lung inflammatory diseases (Ashitani et al., 2007a,b; Mukae et al., 2007). Similarly, an increase in HNP levels in the saliva has been observed in oral diseases (Mizukawa et al., 1999, 2000). However, variability in levels of HNP in gingival crevicular fluid in both healthy persons and those with periodontitis (Lundy et al., 2004) suggests that they may be regulated by pathogens that affect neutrophil migration and function, such as Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans.
β-defensins were initially discovered in a study to characterize the role of antimicrobial peptides in the innate immune response in the airway (Diamond et al., 1991). Human β-defensins (hBD)1-4, the 4 human β-defensin peptides characterized to date, are all expressed in epithelial cells as well as certain cell types of the myeloid lineage (reviewed in Diamond et al., 2004). These peptides are slightly larger than the α-defensins, at 36-42 amino acid residues in length. Both classes of peptide exhibit broad-spectrum activity against Gram-positive and Gram-negative bacteria, mycobacteria, and fungi (reviewed in Lehrer and Ganz, 2002). Since they are expressed by epithelial cells, the peptides have been found in the airway surface fluid that lines the respiratory tract (Laube et al., 2006), and in saliva (Mathews et al., 1999; Sahasrabudhe et al., 2000). Thus, they have been proposed to play a role in either the prevention of bacterial colonization in the airway, or in the maintenance of steady-state levels of flora in the oral cavity (Weinberg et al., 1998).
Cathelicidins
In contrast to defensins, which are characterized by a specific sequence motif in the mature peptide, cathelicidins are defined by a conserved sequence of about 100 residues in the N-terminus of the precursor, named the cathelin domain (Zanetti et al., 1995). After proteolytic cleavage, an active C-terminal domain is released. This active peptide is highly heterogeneous between species (reviewed in Zanetti, 2004). Each mammalian species examined has cathelicidins, but each contains a different set of related genes. The sole human cathelicidin, LL-37, is a 37-residue peptide found at the C-terminus of the human cationic antimicrobial protein 18 (hCAP-18) encoded by the cathelicidin-related antimicrobial peptide gene (Larrick et al., 1995; Gudmundsson et al., 1996). LL-37 peptide was initially found in the specific granules of neutrophils at approximately 1 μM (Sorensen et al., 1997). The peptide was subsequently identified in monocytes, T-cells (Agerberth et al., 2000), the surface epithelia of conducting airways (Bals et al., 1998b), and in broncho-alveolar lavage fluid (Agerberth et al., 1999).
LL-37 has broad-spectrum activity similar to that of defensins, against both Gram-positive and Gram-negative bacteria, as well as Candida albicans (Larrick et al., 1995). Furthermore, the N-terminal cathelin domain was also found to have distinct antimicrobial activity after the proteolytic maturation step (Zaiou et al., 2003). This cathelin segment is active against bacterial strains that are resistant to LL-37, including methicillin-resistant Staphylococcus aureus, suggesting a complementary host defense mechanism by a single gene.
Histatins
A component of saliva from humans and higher primates, histatins are a class of related α-helical HDPs with potent activity primarily against fungi, including both forms of C. albicans (reviewed in Edgerton and Koshlukova, 2000). These peptides bind to a surface protein on Candida, and translocate into the cytoplasam, where they interact with cellular processes, ultimately killing the cell (Edgerton et al., 1998; Koshlukova et al., 1999). Since they are not found in the airway, they represent a unique peptide-based antifungal defense of the oral cavity.
Defense Against Microbial Infections
Antibacterial Activity
Most HDPs generally carry an overall structural cationic charge that allows them to bind to negatively charged prokaryotic cellular membranes. Direct correlations between cationicity and antimicrobial activity have been demonstrated, suggesting a mechanism for the selectivity of microbes, as opposed to the host cell membranes, which are composed of more neutral phospholipids (Matsuzaki et al., 1995). Once bound to the microbial surface, the peptides are predicted to lead to membrane disruption by insertion, but may also translocate into the microbe and kill by intracellular mechanisms (reviewed in Brogden, 2005).
In general, the peptides exhibit broad-spectrum antimicrobial activity. Each peptide, however, has differential activity against different micro-organisms, as measured by in vitro assays. Predominant bacterial pathogens in human lung infections include Pseudomonas aeruginosa, Haemophilus influenzae, Klebsiella pneumoniae, Burkhoderia cepacia, Bordetella pertussis, and Mycobacterium tuberculosis. Minimal inhibitory concentrations (MICs) for defensins and LL-37 against these pathogens can range from less than 10 μg/mL to greater than 250 μg/mL (Miyakawa et al., 1996; Saiman et al., 2001; Starner et al., 2005) (Table 1). However, the susceptibility of a given species is often strain-specific and dependent on conditions, including salt concentration and interaction with other antimicrobials (Bals et al., 1998a; Garcia et al., 2001b; Starner et al., 2002; Fattorini et al., 2004).
Table 1.
Susceptibilities of Oral and Airway Pathogens to HDPs
| Species | Strain | Minimal Inhibitory Concentration, μg/mL | Reference | ||||
|---|---|---|---|---|---|---|---|
| HNP1-3 | hBD1 | hBD2 | hBD3 | LL-37 | |||
| S. sanguinis | MPC-1 | 31.3 | 62.5 | Ji et al., 2007a | |||
| 66×49 | 62.5 | 37.2 | Ji et al., 2007a | ||||
| A. actinomycetemcomitans | Y4 | >250 | 47.9 | Joly et al., 2004 | |||
| 49.6 | 37.8 | Ji et al., 2007a | |||||
| 50 | 200 | 200 | Ouhara et al., 2005 | ||||
| >500 | Miyasaki and Lehrer, 1998 | ||||||
| P. gingivalis | ATCC33277 | 34.6 | 5.7 | Joly et al., 2004 | |||
| 31.3 | >125 | Ji et al., 2007a | |||||
| W50 | >250 | >250 | Joly et al., 2004 | ||||
| 50 | 200 | 100 | Ouhara et al., 2005 | ||||
| FDC381 | nd | Miyasaki and Lehrer, 1998 | |||||
| T. denticola | ATCC33521 | 15.7 | 39.4 | Ji et al., 2007a | |||
| >100 | >100 | Brissette and Lukehart, 2002 | |||||
| F. nucleatum | ATCC10953 | 7.8 | 4.9 | Ji et al., 2007a | |||
| 1908 | >250 | 13.2 | Joly et al., 2004 | ||||
| ATCC49256 | 10.3 | 4.5 | Joly et al., 2004 | ||||
| ATCC25586 | >100 | Miyasaki and Lehrer, 1998 | |||||
| 21 | 20 | 12.5 | 12.5 | Ouhara et al., 2005 | |||
| P. aeruginosa | NCTC 6750 | 1 | Varkey and Nagaraj, 2005 | ||||
| MR3007 | >250 | 4.7 | Turner et al., 1998 | ||||
| (not specified) | 10 | Harder et al., 1997 | |||||
| clinical | 64 | Saiman et al., 2001 | |||||
| PA01 | 26.5 | Garcia et al., 2001a | |||||
| ATCC27853 | 100 | 75 | 25 | Huang et al., 2007 | |||
| 20 mM NaCl | 16 | Bals et al., 1998b | |||||
| 155 mM NaCl | 250 | Bals et al., 1998b | |||||
| 300 mM NaCl | 300 | Bals et al., 1998b | |||||
| S. aureus | 29213 | 9.5 | 210 | 20 | Kisich et al., 2007 | ||
| COL | >50 | 10 | 5 | 5 | Midorikawa et al., 2003 | ||
| NCTC8530 | 0.8 | Varkey and Nagaraj, 2005 | |||||
| (not specified) | 100 | Harder et al., 1997 | |||||
| 67395 | 7.9 | 2.9 | Turner et al., 1998 | ||||
| B. cepacia | ATCC25416 | >250 | 79.1 | Turner et al., 1998 | |||
| ATCC17770 | 6.6 | Garcia et al., 2001a | |||||
Similarly, in the oral cavity, activity of peptides against bacterial species is highly variable. The α-defensins are generally inactive against most oral bacteria tested (Miyasaki et al., 1990). Human β-defensins, however, are active, but demonstrate strain-specific variability, with greater activity in general against aerobic species compared with anaerobic species (Joly et al., 2004). Furthermore, a study has suggested that periodontopathogenic bacteria were more resistant to both LL-37 and hBD3 than were non-pathogenic bacteria (Ji et al., 2007a). Results from the same strain, however, vary between laboratories (Table 1).
While HDPs were initially identified as peptides with potent antimicrobial activity in vitro, most of these antimicrobial assays were carried out in low-salt conditions, and in the absence of host fluids such as serum or saliva. Subsequent investigations have determined that the activity of many HDPs is inhibited by high levels of salt and serum proteins, which inhibit the potential for the initial electrostatic interactions necessary for activity (Dorschner et al., 2006). For example, saliva reduces the activity of hBD1-3 and LL-37 by 20-50% (Mineshiba et al., 2003; Ouhara et al., 2005), compared with an 80% reduction by serum (Mineshiba et al., 2003). Cationic antimicrobial peptides are apparently active in airway fluid (Cole et al., 2002b), although the effect of airway fluid on activity is unknown for most peptides. It has been observed that LL-37 can bind to airway mucins, which can inhibit its activity (Felgentreff et al., 2006). A full examination of the species-specificity and activity in respective fluids (e.g., oral pathogens in saliva, or airway pathogens in airway fluid) remains to be carried out.
Defense against Viral Infections
While initially examined as antibacterial peptides, more recently defensin activity against viral infections has been studied. Interestingly, initial experiments demonstrating the role of defensins as direct antiviral agents suggested a selectivity against enveloped viruses, but analysis of recent data has shown that defensins and other antimicrobial peptides have even broader activity against both enveloped and non-enveloped viruses and include DNA, RNA, and retroviruses. The mechanisms of viral inactivation also vary and include not only direct binding of the virus to the peptide, but also indirect methods of inactivation via intracellular modulation of the viral replication, modulation of signaling pathways necessary for antiviral effects, and recruitment of immune cells that contribute to antiviral activity in vivo. Thus, both oral and respiratory epithelial cells are capable of influencing antiviral immunity via the induction of antimicrobial peptides by either bacteria or viruses.
Early studies showed that a human neutrophil α-defensin, HNP-1, exhibited in vitro inactivation of the enveloped viruses, Herpes Simplex virus (HSV)-1, HSV-2, influenza virus, cytomegalovirus, vesticular stomatitis virus, and Sendai virus, whereas the non-enveloped viruses, echovirus type 11 and reovirus type 3, were not inactivated (Daher et al., 1986). In this study, HSV-1, which was most susceptible, bound directly to HNP-1 in a temperature-dependent manner. To support the selectivity against enveloped viruses, HNP-1, hBD-1, and hBD-2 did not inhibit viral replication of vaccinia virus, a non-enveloped virus, despite strong activity against E. coli (Howell et al., 2004). In contrast to defensins, the human cathelicidin LL-37 effectively kills vaccinia virus, a non-enveloped virus, in vitro and in vivo (Howell et al., 2004). However, subsequent studies showed that interaction with the viral envelope is only one mechanism of defensin antiviral activity, and that antimicrobial peptide activity against viruses depends on the type of virus and the type of peptide involved.
Various mechanisms of defensin activity have been demonstrated by studies on an enveloped RNA virus that infects respiratory epithelium, influenza virus. In addition to the direct inhibition of viral plaques mentioned above by HNP-1, retrocyclin 2, hBD-3, and another innate immune molecule, mannan-binding lectin, all inhibited influenza virus hemagglutinin fusion with the cellular membrane of respiratory epithelial cells (Leikina et al., 2005) at concentrations similar to those reported for hBD-3 inhibition of HIV-1 infection–from 10 to 20 μg/mL (Quinones-Mateu et al., 2003). Other indirect defensin mechanisms against influenza virus also appear to be at work, suggesting that inhibiting membrane fusion with the virus is only one method of antiviral activity. Recently, it was demonstrated that HNP-1 inhibited the replication and protein synthesis of influenza virus by affecting host cell signal transduction pathways, inhibiting protein kinase C activation in several target cells, including A549, a human lung alveolar type II cell line derived from an adenocarcinoma (Salvatore et al., 2007). In addition, HNP-1 and hBD-2 can induce aggregation of influenza virus particles and increase their uptake by neutrophils. In addition, these peptides can also interact with surfactant protein D, a collectin present in alveolar epithelium that also binds influenza virus and increases respiratory burst in neutrophils (Hartshorn et al., 2006; Tecle et al., 2007; White et al., 2007).
Defense against HSV-1, an enveloped DNA virus that infects oral epithelium, may also involve mechanisms other than the direct binding of the virus to the peptide. HSV-1 infection of CaSki cells, a cell line derived from human cervical epithelial cells, is blocked by two α-defensins, HD-5 and HNP-1, at the stage of viral gene expression 4 hrs after viral entry. Coupled with the observation that these α-defensins accumulate intracellularly inside the epithelial cells (the source being from other cells such as neutrophils) and the fact that they are cationic, this result suggested that some of the post-entry anti-HSV-1 activity may be mediated by interactions of defensins with viral DNA (Hazrati et al., 2006). Both synthetic and rhesus-monkey-derived retrocyclins, which are theta-defensins, also have effects against both HSV-1 and HSV-2 at the point of viral entry and inhibition of HSV-2 VP-16 protein translocation. However, not all retrocyclins are effective against both HSV-1 and HSV-2, and each retrocyclin can have different mechanisms of inhibition of HSV-2 (Yasin et al., 2004), suggesting that mechanisms of defensin inhibition of viral neutralization in the Herpes family can vary. The role of defensins in HSV-1 infection of oral epithelium has yet to be studied, and the variable mechanisms among the different defensins against HSV-2 show that these specific mechanisms cannot be extrapolated from these studies to infer mechanisms of HSV-1 in oral epithelium.
The mechanism of defensin activity against HIV-1 infection is more complex and also involves different mechanisms. Defensins, both natural (Nakashima et al., 1993; Zhang et al., 2002, 2004; Chang et al., 2003; Mackewicz et al., 2003; Tanabe et al., 2004) and synthetic retrocyclin, a theta-defensin (Cole et al., 2002a), exhibit direct in vitro activity against human immunodeficiency virus (HIV). LL-37 has also been demonstrated to inhibit HIV replication in numerous isolates, although the mechanism has not been elucidated (Bergman et al., 2007). In addition to the direct interaction with the HIV envelope, defensins can participate in antiviral defense using indirect mechanisms by modulating either the CXCR4 (β-defensins) (Quinones-Mateu et al., 2003; Feng et al., 2006) or the CD4 (α-defensins) (Furci et al., 2007) HIV co-receptors on peripheral blood mononuclear cells. In the oral epithelium, HIV-1 can induce the gene expression of hBD-2 and hBD-3, which then can modulate HIV entry into T-lymphocytes (Quinones-Mateu et al., 2003).
Other mechanisms, not involving the interaction between defensins and the envelope, are also apparent. Defensins are also active against non-enveloped viruses. Human α-defensins HNP 1-3 and HD-5 are active against human papillomavirus by blocking viral escape from endocytotic vesicles, but not by viral binding and internalization (Buck et al., 2006). Overexpression of defensins in cell cultures also provided the cultures with increased resistance to infections with both bacteria and adenovirus (Gropp et al., 1999). Both α- and β-defensins inhibit the infectivity of adenovirus (Bastian and Schafer, 2001) and adeno-associated virus in vitro (Virella-Lowell et al., 2000). Unlike with enveloped viruses, the mechanism for neutralization of multiple human adenovirus serotypes by the human α-defensins HNP-1 and HD-5 is not restriction of virus-binding to cells via its primary receptor, CD 46. Instead, HD-5 and HNP-1 use a later step in viral entry—the direct interaction with the virus capsid, blocking the dissociation of the capsid vertex region (containing pVI) in the endosome and preventing pVI-mediated endosomalysis. This interaction prevents viral escape from the endosome, leading to prolonged residency in the endosome, with subsequent accumulation in the lysosomes, thus resulting in failure of the virus to reach the nuclear membrane (Smith and Nemerow, 2008).
In addition to the antiviral activity of defensins, the attraction of cells important to viral immunity to the site of infection by human β-defensins further supports the hypothesis that β-defensins also play a role in the innate immune response to viruses. For example, cells producing hBD-1 or hBD-2 could influence chemotaxis, since both defensins have been shown to bind to HEK293 cells expressing the chemokine receptor CCR6, and hBD-2 attracted immature monocyte-derived dendritic cells and memory T-cells via CCR6 in vitro (Yang et al., 1999). There are a few clinical examples where the presence or infection of epithelial cells with virus leads to the induction of β-defensins, which then could function by attracting immune cells to the local area of infection. For example, a high level of gene expression of hBD-1, -2, and -3 was detected in papillomavirus-induced epithelial lesions compared with normal oral epithelial cells (Chong et al., 2006). Another example shows the correlation with increased levels of hBD-2 mRNA and peptide by airway epithelium infected with rhinovirus-16 in healthy human volunteers (Proud et al., 2004). Decreased viral titers correlated with the increased β-defensin levels detected following infection. The mechanisms of β-defensin antiviral activity are not well-understood.
One cell that has recently become recognized as being very important in innate immunity against viruses is the plasmacytoid dendritic cell (PDC) (Siegal et al., 1999, reviewed in Fitzgerald-Bocarsly and Feng, 2007; Fitzgerald-Bocarsly et al., 2008). Once known as “natural interferon producing cell”, “plasmacytoid T-cells”, or “plasmacytoid monocytes”, these cells can produce extraordinary amounts of IFN-α—as much as 1-2 IU or 3-10 pg of IFN-α per cell—upon stimulation with enveloped viruses (Cederblad and Alm, 1990; Howell et al., 1994). Enveloped viruses that stimulate PDC to produce IFN-α include HSV, influenza virus, HIV, and Sendai virus. PDCs are found both in the lung (Lommatzsch et al., 2007; Ryan et al., unpublished observations) and in the oral cavity (Santoro et al., 2005; Kajita et al., 2007) in numerous pathological conditions. These cells express both hBD-1 mRNA and peptide (Ryan et al., 2003), which is induced upon stimulation with HSV-1, influenza, and Sendai virus (Ryan et al., submitted). Since hBD-1 is not reported to exhibit antiviral activity in vitro (Quinones-Mateu et al., 2003; Sun et al., 2005; Hazrati et al., 2006), possibly due to its unstable nature outside the cell, its role may be chemotactic for the recruitment of other antiviral cells, or it has intracellular antiviral effects. In our laboratory, HSV-1 plaque formation on Vero cells was inhibited by hBD-1 (at concentrations similar to the direct antiviral activity reported in other studies), but this antiviral activity was observed only with freshly reconstituted recombinant hBD-1 incubated with virus in serum-free medium prior to addition of the virus to the target cell line (Ryan et al., unpublished observations). The importance of the native peptide state for direct inactivation of HSV-1 has been shown with HNP-1 (Daher et al., 1986), and the relative inactivity of the β-defensins may simply reflect the loss of the active peptide state.
Other Roles of HDPs in Innate Immune Defenses
Besides their ability to permeate membranes, several other properties of HDPs have been discovered. The amphiphilic cationic design facilitates maximum interactions in biological systems. Once inside the bacterial cell, HDPs have been shown to exhibit microbicidal behaviors, inhibiting protein, cell wall, and nucleic acid synthesis (Zasloff, 2002). Along with the bovine peptide Indolicidin, they accumulate in the cytoplasm, binding both DNA and RNA (Hsu et al., 2005; Jenssen et al., 2006). Many classes of HDPs can bind the anionic-binding pocket of aminoglycoside-modifying enzymes, thus inhibiting their activity (Jenssen et al., 2006).
In addition to their direct antimicrobial activity, defensins, both α- and β-, exhibit numerous other biological activities (reviewed in Yang et al., 2004). Primary among these is selective chemotactic activity for a variety of host defense cells. Specifically, HNP1-3 and hBD1-3 are chemotactic for immature dendritic cells (Yang et al., 1999, 2000b); hBD2 exhibits chemotactic activity for mast cells (Niyonsaba et al., 2002); and hBD2 and HNPs for selective T-lymphocytes (Yang et al., 1999, 2000b). Together, the defensins can provide a potent inflammatory response, in that products of mast cell degranulation [which can be induced by both α- and β-defensins (Yamashita and Saito, 1989; Befus et al., 1999; Niyonsaba et al., 2001) and CXCL8 (which is induced on bronchial epithelial cells by HNPs (Van Wetering et al., 1997)] are neutrophil chemotactic agents (Echtenacher et al., 1996; Malaviya et al., 1996; Baggiolini, 1998). Other pro-inflammatory cytokines, such as TNF-α and IL-1β, are induced by HNPs as well (Chaly et al., 2000).
While these and other studies demonstrate a role for defensins as a link between innate and adaptive immunity, there are also examples of defensin effects directly on the adaptive immune response. HNPs can enhance the antigen-specific immune response to ovalbumin when introduced intranasally, increasing the production of ovalbumin-specific IgG and CD4+ T-cells (Lillard et al., 1999). β-defensins were also shown to enhance adaptive immunity, using a fusion vector containing a sequence encoding a mouse β-defensin fused to that of a B-cell lymphoma-specific epitope. The resultant DNA vaccine provided enhanced antitumor activity (Biragyn et al., 1999, 2002).
Most recently, a β-defensin was identified as a regulator of coat color in dogs (Candille et al., 2007), primarily due to its binding to a melanocortin receptor. This suggests that they may participate in other functions in the cells as well.
While initially isolated as an antimicrobial peptide, LL-37 has been discovered to exhibit numerous other activities. Since its expression in epithelium is induced in response to infection and inflammation (Dorschner et al., 2001), it was proposed to play additional roles in inflammation besides antimicrobial. Indeed, LL-37 demonstrates chemotactic activity for neutrophils, monocytes, and some T-cells (Yang et al., 2000a), and induces IL-8 secretion from epithelial cell lines (Tjabringa et al., 2003). Furthermore, LL-37 affects dendritic cell (DC) maturation, and can act synergistically with the DC-maturation cytokine GM-CSF to activate signal transduction pathways in monocytes (Scott et al., 2002). Together, these multiple activities of LL-37 (as reviewed in Bowdish et al., 2005) suggest that it plays an important, multifunctional role in host defense.
Expression of HDPs in Mucosal Epithelia
HDP Expression in the Airway Epithelium
The respiratory tract is continuously exposed to a variety of micro-organisms that can adhere and potentially cause infection. Epithelial cells lining the mammalian trachea release numerous antimicrobial factors, including β-defensins and LL-37, forming a crucial site in the host defense against airborne microbial pathogens (reviewed in Diamond et al., 2000b). Deficiencies in these defenses, or conditions where they are inactive, may result in recurrent airway infections such as those seen in cystic fibrosis (CF) (Smith et al., 1996).
In several systems, expression of β-defensin genes responds to the presence of pathogens (reviewed in Kaiser and Diamond, 2000). For example, in primary cultures of bovine and human tracheal epithelial cells (TEC), significant increases in the steady-state mRNA levels of the bovine β-defensin tracheal antimicrobial peptide (TAP) and its human homologue β-defensin-2 (hBD-2) were noted upon incubation with Pseudomonas aeruginosa and Escherichia coli lipopolysaccharides (LPS) (Diamond et al., 1996; Becker et al., 2000). P. aeruginosa can also induce hBD-3 and -4 in human lung A549 cells (Harder et al., 2000; Garcia et al., 2001b). A list of peptides and their regulation can be found in Table 2.
Table 2.
Expression of HDP Genes in Oral and Airway Epithelium
| Oral Cavity | |||
|---|---|---|---|
| Peptide | Site of Expression | Regulation | Stimulant |
| hBD-1 | Gingival epithelium | Inducible | P. gingivalis |
| hBD-2 | Salivary glands, epithelium | Inducible | F. nucleatum, A. actinomycetemcomitans, IL-1β |
| hBD-3 | Keratinocytes | Inducible |
F. nucleatum, A. actinomycetemcomitans |
| LL-37 | Gingival Epithelium | Inducible | Vitamin D |
| Neutrophils | Constitutive | - | |
| Histatins | Salivary glands | Constitutive | - |
| Airway | |||
| Peptide | Site of Expression | Regulation | Stimulant |
| hBD-1 | Ciliated epithelium | Constitutive | - |
| Plasmacytoid DCs | Inducible | Enveloped viruses | |
| hBD-2 | Ciliated epithelium | Inducible | Bacteria, TLR agonists, cytokines |
| hBD-3 | Ciliated epithelium | Inducible | Bacteria, TLR agonists, cytokines |
| LL-37 | Ciliated epithelium | Inducible | Vitamin D |
| Neutrophils | Constitutive | - | |
In vivo studies showing the induction of β-defensin gene expression further support the hypothesis that defensins play a role in antimicrobial defense. Intratracheal instillation of Mannheimia (Pasteurella) haemolytica into a single lobe of a cow lung caused an increase in β-defensin expression in the airway epithelium localized to the site of infection (Stolzenberg et al., 1997). Similarly, in the mouse, intratracheal instillation of P. aeruginosa caused an increased expression of mouse β-defensin-3 (mBD-3), the murine homologue for hBD-2, in the tracheal epithelium (Bals et al., 1999).
LL-37 expression is observed in the respiratory epithelium, and its mRNA and protein can be induced in vitro by physiological concentrations of Vitamin D (Yim et al., 2007) and in vivo by injury (Dorschner et al., 2001). Thus, both classes of peptides appear to be regulated as part of an innate immune response in the airway.
HDP Expression in the Oral Mucosa
In contrast to the predominantly sterile airway, the oral cavity is host to a magnitude of microbial organisms, which necessitates a protective mechanism to prevent the onset of disease, yet without a sensitive response to the commensal organisms. HDPs are found both in saliva (Edgerton and Koshlukova, 2000; reviewed in Abiko and Saitoh, 2007) and in crevicular fluid (Diamond et al., 2001; Dale et al., 2006), suggesting that they play a role in the maintenance of microbial homeostasis. In human gingival tissue, hBD-1 and hBD-2 are both expressed in normal, uninflamed tissue, at the highest levels at the gingival margin near the site of plaque formation, and within the sulcular epithelium during states of inflammation (Dale et al., 2001). In contrast, hBD3 expression was observed primarily in the basal layer, as well as in Merkel and Langerhans cells in healthy tissue. However, the expression changed in persons with periodontitis, with hBD3 expression extending to the superficial spinous layers (Lu et al., 2005). Another study demonstrated that hBD2 mRNA levels were increased in tissue biopsies from persons with gingivitis, and both hBD2 and hBD3 mRNA levels were increased in biopsies from periodontitis tissue (Dommisch et al., 2005). Furthermore, immunohistochemical analysis demonstrated the expression of hBD2 in the buccal epithelium in tissue from persons infected with C. albicans, but not from uninfected individuals (Sawaki et al., 2002).
In vitro studies have demonstrated that P. gingivalis induces the expression of hBD1 (Vankeerberghen et al., 2005) and hBD2 (Taguchi and Imai, 2006) in cultured human gingival epithelial cells (HGEC), while Fusobacterium nucleatum and A. actinomycetemcomitans stimulate the production of hBD-2 and -3 (Feucht et al., 2003; Vankeerberghen et al., 2005). The response to A. actinomycetemcomitans is both strain-specific and variable between individuals (Feucht et al., 2003; Vankeerberghen et al., 2005), with a reduced response observed in cells from a person with Localized Aggressive Periodontitis (Laube et al., 2008). In all cases, however, the induction was not mediated by LPS, suggesting that regulation of the induction of these mediators of innate immunity in the oral mucosa is controlled by more complex mechanisms of innate immunity. In the case of P. gingivalis, it was demonstrated that one of its secreted proteases, RgpB, could stimulate hBD2 expression via a protease-activated receptor (Chung et al., 2004; Dommisch et al., 2007). With A. actinomycetemcomitans, the induction can be attributed at least in part to the outer membrane protein OMP100 (Ouhara et al., 2006).
The variability in the responses of oral epithelial cells to different bacteria, and the variable sensitivity of oral flora to the peptides (Ji et al., 2007a), led to the hypothesis that commensal bacteria with increased resistance to killing by the peptides would induce the expression of peptides to kill the more sensitive pathogens (Weinberg et al., 1998; Dale and Fredericks, 2005). Early studies, which demonstrated that F. nucleatum induced hBD2 expression in gingival epithelial cells, while P. gingivalis did not (Krisanaprakornkit et al., 2000; Vankeerberghen et al., 2005), seemed to support this. A more recent study comparing the responses of bacteria associated with periodontal disease with those of other oral bacteria not associated with periodontal disease demonstrated that there was a wide range of responses in cultured HOK-16B cells (Ji et al., 2007b). Specifically, Prevotella intermedia induced all peptide genes (hBD1, 2, and 3, as well as LL-37); F. nucleatum induced hBD2 and 3; and P. gingivalis induced hBD2. Other species associated with periodontal disease, such as Tannerella forsythia and Treponema denticola, either did not induce expression, or led to a reduction in steady-state mRNA levels. LL-37 expression in an oral epithelial cell line was similarly complex, with P. gingivalis demonstrating no induction, while A. actinomycetemcomitans and F. nucleatum induced significant amounts of both mRNA and peptide (Hosokawa et al., 2006). Finally, a study using biofilm cultures showed that early stages of biofilm formation could induce β-defensin expression, but later, more mature, biofilms did not (Eberhard et al., 2008). These results suggest that the response may be more complicated than previously hypothesized, and that it will be difficult to develop an accurate model of the innate immune response based on in vitro studies.
Few studies have been carried out in animal models. Dixon et al. examined several innate immune mediators in gingival tissue from conventionally reared mice in comparison with germ-free mice. Their results indicated significant differences in IL-1β levels (higher protein in conventionally reared mice, yet lower mRNA levels) (Dixon et al., 2004), suggesting that commensal micro-organisms may influence innate immune gene expression. Our recently published results demonstrated that introduction of both pathogenic bacteria and commensal bacteria can transiently induce β-defensin gene expression in rat gingival tissue (Kurland et al., 2006).
In the oral cavity, LL-37 was initially identified in infiltrating neutrophils (Dale et al., 2001), but was subsequently observed in salivary glands as well, in both human (Woo et al., 2003) and murine oral tissue (Murakami et al., 2002). Furthermore, the LL-37 gene was inducible in gingival epithelial cells by bacteria, including A. actinomycetemcomitans (Hosokawa et al., 2006), suggesting a role for LL-37 in the natural defense against colonization by periodontal pathogens, such as A. actinomycetemcomitans.
Regulation of HDP Gene Expression
In response to microbes, microbial components, or pro-inflammatory cytokines, a typical general result is gene induction mediated by the activation of transcription factors. In the airway, LPS induces both TAP and hBD-2 gene expression through its binding to epithelial-cell-expressed CD14. This stimulates a signal transduction pathway, which results in the activation of NF-κB, which binds to an NF-κB consensus sequence upstream from the TAP and hBD-2 genes (Becker et al., 2000; Diamond et al., 2000a). Other microbe-associated molecular patterns known to activate the NF-κB pathway also stimulate β-defensin gene expression in airway epithelial cells, including Pam3CSK4 (Klein-Patel et al., 2006), flagellin (Takahashi et al., 2001), poly IC (Duits et al., 2003), and CpG DNA (Platz et al., 2004). In gingival epithelial cells, however, the mechanism is somewhat different. We and others have shown that gingival epithelial cells appear to be insensitive to LPS (Krisanaprakornkit et al., 2002; Laube et al., 2008). However, they are extraordinarily sensitive to cell wall extract from the commensal bacterium F. nucleatum, which induces hBD2 expression (Krisanaprakornkit et al., 2000). Similarly, live pathogenic bacteria, both Gram-positive and -negative, can induce this response in oral epithelial cells (Chung and Dale, 2004; Laube et al., 2008). However, there are apparently two different pathways leading to increased hBD2 levels. Cell wall extract from F. nucleatum induces expression primarily through activation of p38 and JNK pathways (Krisanaprakornkit et al., 2002), while induction by pathogens proceeds through the NF-κB pathway (Chung and Dale, 2004). A more recent study further defines this response, demonstrating that pathogens such as P. gingivalis and A. actinomycetemcomitans utilize an alternative pathway leading to NF-κB activation, via the IKKα/TRAF3 pathway (Chung and Dale, 2008). When gingival epithelial cells were stimulated with F. nucleatum, however, the response induced hBD2, but not through any NF-κB-stimulating pathway. This suggests that there is a unique response of gingival cells leading to the regulation of β-defensin gene expression, in contrast to that of airway cells. A model showing known microbe-associated molecular patterns and their respective pathways that stimulate HDP gene expression in the two tissues is shown in Fig. 2.
Figure 2.
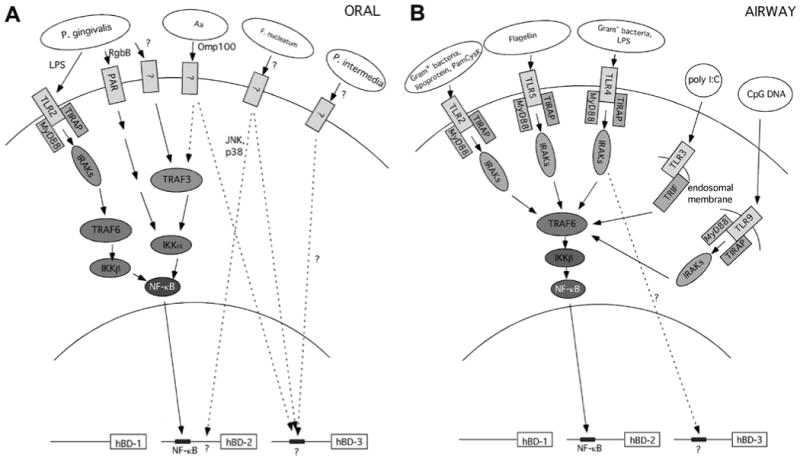
Pathways stimulated by microbes and their associated molecular patterns in oral (A) and airway (B) epithelial cells leading to β-defensin expression. These models are based solely on published studies demonstrating the role of the respective receptors, adapter molecules, and transcription factors. In some cases, e.g., stimulation of hBD-2 by P. intermedia, specific stimulatory molecule, receptor, pathway, and/or transcription factors have not been identified, and are shown as a question mark.
Diseases Associated with HDPs
Since HDPs are gene-encoded, it follows that mutations in these genes should lead to increased susceptibility to infection. Such phenotypes associated with HDP mutations have been rarely observed, possibly due to the redundancy of peptide-based antimicrobial defense systems, with numerous peptides expressed in the same tissues or cells. Supporting this hypothesis was the observation that a deletion of the entire region encoding both α- and β-defensins (8p- syndrome) results in recurrent airway infections (Ostergaard and Tommerup, 1989). In the airway, it was first observed that the airway surface fluid from persons with CF exhibited characteristics different from that of non-CF individuals. In a report by Smith et al. (Smith et al., 1996), it was determined that the salt concentration was elevated in CF airway surface fluid, and β-defensin activity was inhibited at such a high salt concentration. Subsequently, other investigators have identified HDPs in the airway with both salt-dependent (Bals et al., 1998a; Garcia et al., 2001b) and salt-independent activities (Turner et al., 1998; Bals et al., 2001; Garcia et al., 2001a), and further hypothesized that the difference between CF and non-CF airway surface fluid lies in characteristics besides salt concentration (Matsui et al., 1998; Bals et al., 2001). Nonetheless, it appears that numerous differences in the ability of HDPs to kill airway pathogens appear in CF airways compared with normal airways.
In the oral cavity, associations between polymorphisms in some defensin genes and infections has been observed. Jurevic et al. demonstrated that individuals, both diabetic and non-diabetic, with a SNP in the 5′ UTR of the hBD1 sequence are more likely to exhibit lower levels of Candida carriage (Jurevic et al., 2003). Furthermore, Tao et al. (2005) observed an association between lower levels of salivary α-defensins HNP1-3 and the presence of dental caries.
A rare disorder, infantile congenital agranulocytosis, also known as morbus Kostmann (MK), is associated with a complete absence of LL-37, and is characterized by, among other symptoms, chronic periodontitis and overgrowth with A. actinomycetemcomitans (Putsep et al., 2002; Carlsson et al., 2006). Individuals with another genetic disorder, Papillon-Lefèvre Syndrome, demonstrate a deficiency in LL-37 and exhibit severe periodontitis. This may be due to a deficiency in the serine proteinases that process hCAP-18 to the mature, active LL-37 peptide (de Haar et al., 2006). Together, these studies suggest a role for LL-37 in the natural defense against colonization by periodontal pathogens. Surprisingly, neither of these conditions apparently exhibits increased airway infections, suggesting that LL-37 plays a different role in the oral cavity than in the airway. Since MK is a disorder characterized by a lack of mature neutrophils, it can be treated with the hematopoietic growth factor G-CSF, which leads to normal neutrophil counts. However, even with these normal neutrophil counts, persons with MK still exhibit a deficiency in LL-37 and have severe periodontal disease (Defraia and Marinelli, 2001). This suggests that this particular peptide plays an important role in defense against colonization by A. actinomycetemcomitans. This supports a more crucial role of the neutrophil in the containment of periodontal infections compared with lung infections. Variability in the inducibility of β-defensins in oral epithelial cells by oral pathogens can also lead to reduced levels of these peptides (Krisanaprakornkit et al., 2000; Vankeerberghen et al., 2005; Ji et al., 2007b; Laube et al., 2008).
In addition to inherent deficiencies in HDP activity leading to increased infections, exogenous factors that inhibit their activity have been proposed to reduce their defensive capacity. Bacterial virulence factors can suppress the innate immune response that naturally leads to the up-regulation of HDP expression. A type III secretion factor from the airway pathogen, Bordetella bronchiseptica, inhibits the activation of NF-κB in airway epithelial cells, leading to a reduced ability to induce β-defensin gene expression (Legarda et al., 2005). Similarly, a penicillin-binding protein from Group B streptococcus provides protection against killing in rat lungs (Jones et al., 2007). Environmental factors, such as components of inhaled air pollutant particulates, can also inhibit this response, predisposing individuals in highly polluted areas to bacterial infections (Klein-Patel et al., 2006). In the oral cavity, bacterial virulence factors can have similar activities. Arginine- and lysine-specific proteases secreted by P. gingivalis can cleave to and inactivate the cationic HDPs (Devine et al., 1999), and the A. actinomycetemcomitans leukotoxin can suppress the phagocytic capability of neutrophils, resulting in an indirect inhibition of the host defense activity of the α-defensins found in such high concentrations in those cells.
Therapeutic Potential of HDPs
Use of Peptides and Peptide Genes
Since HDPs exhibit broad-spectrum antimicrobial activity, and demonstrate a decreased ability to develop resistance (Zasloff, 2002), they represent an ideal potential therapeutic agent in any tissue or system that is a site for microbial infection, including the lung and oral cavity. After their initial discovery, numerous HDPs were examined for such therapeutic use. Unfortunately, several issues stood in the way of their development (reviewed in Marr et al., 2006). These include: difficulty and expense of manufacturing; short half-lives due to proteolytic degradation; and inhibition by host molecules, such as those found in serum as well as physiological salt concentrations in some cases.
Since it was observed that certain HDPs, including β-defensins and cathelicidin, are regulated at the transcriptional level, it has been suggested that, rather than direct application of the peptide, exogenous modifiers of HDP expression could be used (Laube et al., 2006). Unfortunately, the earliest discovered modifiers of HDP expression included microbial factors such as LPS, and inflammatory mediators, such as TNF-α and IL-1β. The inflammatory response that would be induced by these factors would outweigh the potential therapeutic value of increasing HDP levels. Recently, however, it was discovered that expression of the gene encoding LL-37 could be induced by a less toxic agent, the biologically active form of vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3]. This was initially observed in a variety of cell lines (Wang et al., 2004; Gombart et al., 2005), but it has also been found in primary cultures of airway epithelial cells (Yim et al., 2007) as well as gingival epithelial cells (Diamond et al., unpublished observations). This suggests a potential for the therapeutic modulation of host defense peptide genes to address infections in both sites.
Peptide Mimetics
One recently developed method to address the problems associated with HDP therapeutics is the use of non-peptidic analogues of AMPs. These molecules have many advantages over peptides because of their small size, which increases stability and enhances tissue distribution, and their ability to fine-tune their physical properties for optimization of potency and safety. As a first step in this direction, various laboratories have designed antimicrobial peptides by idealizing the amphiphilic alpha-helical arrangement of the side-chains observed in the natural structures, leading to a large number of potent and selective antimicrobial compounds (reviewed in Zasloff, 2002). More recently, peptidomimetic approaches that mimic the peptide primary structure have been pursued with amide bond isosteres or modifications of the peptide backbone by chain extension or hetero-atom incorporation (Merrifield et al., 1994). Several groups have designed β-peptides, peptides with an additional methylene group in their backbone, which maintained this amphiphilic architecture (DeGrado et al., 1982; Barron and Zuckermann, 1999). A systematic analysis of β-peptide structure and activity revealed that facial amphiphilicity as well as a precise ratio of charged to hydrophobic residues was essential for potent and selective activity, similar to what has been described for the antimicrobial β-peptides (Barron and Zuckermann, 1999). Helical, cationic, facially amphiphilic peptoid mimics (poly-N-substituted glycine) of magainin-2 have also been produced that show selective and potent antibacterial activity against Gram-positive and Gram-negative bacteria. Activity is length-dependent (12-17 mers) and, for several of the peptoids, is comparable with the antibacterial and hemolytic activities of synthetic magainin analogues and the antibacterial β-peptides (Stigers et al., 1999). Significantly, the β-peptides and peptoids are resistant to proteases, providing important advantages over alpha-peptides. However, β-peptides and peptoids are relatively difficult and expensive to synthesize in large quantities. Therefore, the de novo design of inexpensive oligomers and polymers that adopt amphiphilic secondary structures and exhibit potent and selective antimicrobial activity was pursued (DeGrado et al., 1982).
A third approach to the discovery of synthetic antimicrobial compounds sought to transfer the physiochemical compounds of AMP to arylamide backbones. These molecules, containing amide bonds similar to those of AMP, mimic the essential architecture for amphipathicity without the helical structure (Tew et al., 2006). Composed of a hydrocarbon polyphenylethylene backbone, these biologically active molecules can be enhanced, through the addition of side-chains, tuning their molecular weight (MW) (Ilker et al., 2004). These non-peptidic analogues have many advantages over peptides, including small size, increased stability, and enhancement of physical properties for optimization of potency and safety (Rennie et al., 2005). Several variations have been produced using similar hydrocarbon backbone (Ilker et al., 2004; Tew et al., 2006).
One such compound, named mPE, has shown improved activity and selectivity over its first-generation counterparts (Tew et al., 2006). It displays a high level of selectivity against erythrocytes (88 μg/mL), while maintaining excellent activity against many clinical isolates, including antibiotic-resistant bacteria, and better than the magainin derivative oligomers mentioned previously (Rennie et al., 2005). Vibrational spectroscopic evidence by Tew et al. showed that the ability of mPE to disrupt cellular membranes with great efficiency is the result of its ability to insert itself perpendicularly into a bacterial membrane (Chen et al., 2006). Analysis of the data also showed no development of resistance carried through 17 passages, as compared with two other fluoroquinolone antibiotics (ciprofloxacin and norfloxacin), whose resistance levels quadrupled against S. aureus (Rennie et al., 2005). Using in vitro antimicrobial assays, we have demonstrated the potent activity of mPE against oral pathogens, both Gram-positive and -negative bacteria, as well as several Candida species (Beckloff et al., 2007). This molecule is active against both planktonic and biofilm cultures, suggesting that it can be developed as an antimicrobial therapeutic agent for use in the oral cavity.
Conclusions
Antimicrobial peptides and the genes that encode them have been studied extensively in both the oral cavity and the airway. While they exhibit potent, broad-spectrum antimicrobial activity in vitro, the evidence that their most important role in either of these locations is directly antimicrobial is still circumstantial. Regardless, gene expression studies demonstrate that the predominantly sterile airway exhibits a sensitive, but broad, response to microbial challenge. In contrast, the oral epithelium exhibits a complex regulation of AMP genes, suggesting that they participate in the maintenance of the homeostasis of the oral flora. More recent studies on their immunomodulatory activities suggest that their development as therapeutic antibiotics may pose problems in either site, with the complexity of the oral flora, which includes both commensal and pathogenic species, being particularly problematic. Further examination of their role in the host defense of these mucosal epithelia, especially the factors that control AMP gene expression, is essential if their potential is to be understood.
References
- Abiko Y, Saitoh M. Salivary defensins and their importance in oral health and disease. Curr Pharm Des. 2007;13:3065–3072. doi: 10.2174/138161207782110417. [DOI] [PubMed] [Google Scholar]
- Agerberth B, Grunewald J, Castanos-Velez E, Olsson B, Jörnvall H, Wigzell H, et al. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med. 1999;160:283–290. doi: 10.1164/ajrccm.160.1.9807041. [DOI] [PubMed] [Google Scholar]
- Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- Ashitani J, Matsumoto N, Nakazato M. Elevated alpha-defensin levels in plasma of patients with pulmonary sarcoidosis. Respirology. 2007a;12:339–345. doi: 10.1111/j.1440-1843.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- Ashitani J, Matsumoto N, Nakazato M. Elevated levels of antimicrobial peptides in bronchoalveolar lavage fluid in patients with chronic eosinophilic pneumonia. Respiration. 2007b;74:69–75. doi: 10.1159/000090199. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, et al. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest. 1998a;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998b;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R, Wang X, Meegalla RL, Wattler S, Weiner DJ, Nehls MC, et al. Mouse beta-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect Immun. 1999;67:3542–3547. doi: 10.1128/iai.67.7.3542-3547.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R, Weiner DJ, Meegalla RL, Accurso F, Wilson JM. Salt-independent abnormality of antimicrobial activity in cystic fibrosis airway surface fluid. Am J Respir Cell Mol Biol. 2001;25:21–25. doi: 10.1165/ajrcmb.25.1.4436. [DOI] [PubMed] [Google Scholar]
- Barron AE, Zuckermann RN. Bioinspired polymeric materials: in-between proteins and plastics. Curr Opin Chem Biol. 1999;3:681–687. doi: 10.1016/s1367-5931(99)00026-5. [DOI] [PubMed] [Google Scholar]
- Bastian A, Schafer H. Human alpha-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul Pept. 2001;101:157–161. doi: 10.1016/s0167-0115(01)00282-8. [DOI] [PubMed] [Google Scholar]
- Becker MN, Diamond G, Verghese MW, Randell SH. CD14-dependent lipopolysaccharide-induced beta-defensin-2 expression in human tracheobronchial epithelium. J Biol Chem. 2000;275:29731–29736. doi: 10.1074/jbc.M000184200. [DOI] [PubMed] [Google Scholar]
- Beckloff N, Diamond G. Computational analysis suggests beta-defensins are processed to mature peptides by signal peptidase. Protein Peptide Lett. 2008;15:536–540. doi: 10.2174/092986608784567618. [DOI] [PubMed] [Google Scholar]
- Beckloff N, Laube D, Castro T, Furgang D, Park S, Perlin D, et al. Activity of an antimicrobial peptide mimetic against planktonic and biofilm cultures of oral pathogens. Antimicrob Agents Chemother. 2007;51:4125–4132. doi: 10.1128/AAC.00208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Befus AD, Mowat C, Gilchrist M, Hu J, Solomon S, Bateman A. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J Immunol. 1999;163:947–953. [PubMed] [Google Scholar]
- Bergman P, Walter-Jallow L, Broliden K, Agerberth B, Soderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res. 2007;5:410–415. doi: 10.2174/157016207781023947. [DOI] [PubMed] [Google Scholar]
- Biragyn A, Tani K, Grimm MC, Weeks S, Kwak LW. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat Biotechnol. 1999;17:253–258. doi: 10.1038/6995. [DOI] [PubMed] [Google Scholar]
- Biragyn A, Belyakov IM, Chow YH, Dimitrov DS, Berzofsky JA, Kwak LW. DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood. 2002;100:1153–1159. doi: 10.1182/blood-2002-01-0086. [DOI] [PubMed] [Google Scholar]
- Bowdish DM, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock RE. Impact of LL-37 on anti-infective immunity. J Leukoc Biol. 2005;77:451–459. doi: 10.1189/jlb.0704380. [DOI] [PubMed] [Google Scholar]
- Brissette CA, Lukehart SA. Treponema denticola is resistant to human beta-defensins. Infect Immun. 2002;70:3982–3984. doi: 10.1128/IAI.70.7.3982-3984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Buck CB, Day PM, Thompson CD, Lubkowski J, Lu W, Lowy DR, et al. Human alpha-defensins block papillomavirus infection. Proc Natl Acad Sci USA. 2006;103:1516–1521. doi: 10.1073/pnas.0508033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candille SI, Kaelin CB, Cattanach BM, Yu B, Thompson DA, Nix MA, et al. A beta-defensin mutation causes black coat color in domestic dogs. Science. 2007;318:1418–1423. doi: 10.1126/science.1147880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson G, Wahlin YB, Johansson A, Olsson A, Eriksson T, Claesson R, et al. Periodontal disease in patients from the original Kostmann family with severe congenital neutropenia. J Periodontol. 2006;77:744–751. doi: 10.1902/jop.2006.050191. [DOI] [PubMed] [Google Scholar]
- Cederblad B, Alm G. Infrequent but efficient interferon-α-producing human mononuclear leukocytes induced by herpes simplex virus in vitro studies by immunoplaque and limiting dilution assays. J Interferon Res. 1990;10:65–73. doi: 10.1089/jir.1990.10.65. [DOI] [PubMed] [Google Scholar]
- Chaly YV, Paleolog EM, Kolesnikova TS, Tikhonov II, Petratchenko EV, Voitenok NN. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur Cytokine Netw. 2000;11:257–266. [PubMed] [Google Scholar]
- Chang TL, Francois F, Mosoian A, Klotman ME. CAF-mediated human immunodeficiency virus (HIV) type 1 transcriptional inhibition is distinct from alpha-defensin-1 HIV inhibition. J Virol. 2003;77:6777–6784. doi: 10.1128/JVI.77.12.6777-6784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Tang H, Even MA, Wang J, Tew GN, Chen Z. Observing a molecular knife at work. J Am Chem Soc. 2006;128:2711–2714. doi: 10.1021/ja057029t. [DOI] [PubMed] [Google Scholar]
- Chong KT, Xiang L, Wang X, Jun EL, Xi LF, Schweinfurth JM. High level expression of human epithelial beta-defensins (hBD-1, 2 and 3) in papillomavirus induced lesions. Virol J. 2006;3:75. doi: 10.1186/1743-422X-3-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WO, Dale BA. Innate immune response of oral and foreskin keratinocytes: utilization of different signaling pathways by various bacterial species. Infect Immun. 2004;72:352–358. doi: 10.1128/IAI.72.1.352-358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WO, Dale BA. Differential utilization of nuclear factor-kappaB signaling pathways for gingival epithelial cell responses to oral commensal and pathogenic bacteria. Oral Microbiol Immunol. 2008;23:119–126. doi: 10.1111/j.1399-302X.2007.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WO, Hansen SR, Rao D, Dale BA. Protease-activated receptor signaling increases epithelial antimicrobial peptide expression. J Immunol. 2004;173:5165–5170. doi: 10.4049/jimmunol.173.8.5165. [DOI] [PubMed] [Google Scholar]
- Cole AM, Hong T, Boo LM, Nguyen T, Zhao C, Bristol G, et al. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc Natl Acad Sci USA. 2002a;99:1813–1818. doi: 10.1073/pnas.052706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AM, Liao HI, Stuchlik O, Tilan J, Pohl J, Ganz T. Cationic polypeptides are required for antibacterial activity of human airway fluid. J Immunol. 2002b;169:6985–6991. doi: 10.4049/jimmunol.169.12.6985. [DOI] [PubMed] [Google Scholar]
- Daher KA, Selsted ME, Lehrer RI. Direct inactivation of viruses by human granulocyte defensins. J Virol. 1986;60:1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale BA, Fredericks LP. Antimicrobial peptides in the oral environment: expression and function in health and disease. Curr Issues Mol Biol. 2005;7:119–133. doi: 10.1093/jac/dki103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale BA, Kimball JR, Krisanaprakornkit S, Roberts F, Robinovitch M, O'Neal R, et al. Localized antimicrobial peptide expression in human gingiva. J Periodontal Res. 2001;36:285–294. doi: 10.1034/j.1600-0765.2001.360503.x. [DOI] [PubMed] [Google Scholar]
- Dale BA, Tao R, Kimball JR, Jurevic RJ. Oral antimicrobial peptides and biological control of caries. BMC Oral Health. 2006;6(Suppl 1):13. doi: 10.1186/1472-6831-6-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haar SF, Hiemstra PS, van Steenbergen MT, Everts V, Beertsen W. Role of polymorphonuclear leukocyte-derived serine proteinases in defense against Actinobacillus actinomycetemcomitans. Infect Immun. 2006;74:5284–5291. doi: 10.1128/IAI.02016-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defraia E, Marinelli A. Oral manifestations of congenital neutropenia or Kostmann syndrome. J Clin Pediatr Dent. 2001;26:99–102. doi: 10.17796/jcpd.26.1.n1vhq267271378l1. [DOI] [PubMed] [Google Scholar]
- DeGrado WF, Musso GF, Lieber M, Kaiser ET, Kezdy FJ. Kinetics and mechanism of hemolysis induced by melittin and by a synthetic melittin analogue. Biophys J. 1982;37:329–338. doi: 10.1016/S0006-3495(82)84681-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine DA, Marsh PD, Percival RS, Rangarajan M, Curtis MA. Modulation of antibacterial peptide activity by products of Porphyromonas gingivalis and Prevotella spp. Microbiology. 1999;145(Pt 4):965–971. doi: 10.1099/13500872-145-4-965. [DOI] [PubMed] [Google Scholar]
- Diamond G, Zasloff M, Eck H, Brasseur M, Maloy WL, Bevins CL. Tracheal antimicrobial peptide, a novel cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci (USA) 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G, Russell JP, Bevins CL. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc Natl Acad Sci USA. 1996;93:5156–5160. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G, Kaiser V, Rhodes J, Russell JP, Bevins CL. Transcriptional regulation of β-defensin gene expression in tracheal epithelial cells. Infect Immun. 2000a;68:113–119. doi: 10.1128/iai.68.1.113-119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000b;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- Diamond DL, Kimball JR, Krisanaprakornkit S, Ganz T, Dale BA. Detection of beta-defensins secreted by human oral epithelial cells. J Immunol Methods. 2001;256:65–76. doi: 10.1016/s0022-1759(01)00442-2. [DOI] [PubMed] [Google Scholar]
- Diamond G, Laube D, Klein-Patel ME. Mammalian β-defensins in mucosal defences. In: Hancock REW, Devine DA, editors. Mammalian host defence peptides. Cambridge, UK: Cambridge University Press; 2004. pp. 111–138. [Google Scholar]
- Dixon DR, Reife RA, Cebra JJ, Darveau RP. Commensal bacteria influence innate status within gingival tissues: a pilot study. J Periodontol. 2004;75:1486–1492. doi: 10.1902/jop.2004.75.11.1486. [DOI] [PubMed] [Google Scholar]
- Dommisch H, Acil Y, Dunsche A, Winter J, Jepsen S. Differential gene expression of human beta-defensins (hBD-1, -2, -3) in inflammatory gingival diseases. Oral Microbiol Immunol. 2005;20:186–190. doi: 10.1111/j.1399-302X.2005.00211.x. [DOI] [PubMed] [Google Scholar]
- Dommisch H, Chung WO, Rohani MG, Williams D, Rangarajan M, Curtis MA, et al. Protease-activated receptor 2 mediates human beta-defensin 2 and CC chemokine ligand 20 mRNA expression in response to proteases secreted by Porphyromonas gingivalis. Infect Immun. 2007;75:4326–4333. doi: 10.1128/IAI.00455-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A streptococcus. J Invest Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- Dorschner RA, Lopez-Garcia B, Peschel A, Kraus D, Morikawa K, Nizet V, et al. The mammalian ionic environment dictates microbial susceptibility to antimicrobial defense peptides. FASEB J. 2006;20:35–42. doi: 10.1096/fj.05-4406com. [DOI] [PubMed] [Google Scholar]
- Duits LA, Nibbering PH, van Strijen E, Vos JB, Mannesse-Lazeroms SP, van Sterkenburg MA, et al. Rhinovirus increases human beta-defensin-2 and -3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol Med Microbiol. 2003;38:59–64. doi: 10.1016/S0928-8244(03)00106-8. [DOI] [PubMed] [Google Scholar]
- Eberhard J, Menzel N, Dommisch H, Winter J, Jepsen S, Mutters R. The stage of native biofilm formation determines the gene expression of human beta-defensin-2, psoriasin, ribonuclease 7 and inflammatory mediators: a novel approach for stimulation of keratinocytes with in situ formed biofilms. Oral Microbiol Immunol. 2008;23:21–28. doi: 10.1111/j.1399-302X.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- Edgerton M, Koshlukova SE. Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv Dent Res. 2000;14:16–21. doi: 10.1177/08959374000140010201. [DOI] [PubMed] [Google Scholar]
- Edgerton M, Koshlukova SE, Lo TE, Chrzan BG, Straubinger RM, Raj PA. Candidacidal activity of salivary histatins. Identification of a histatin 5-binding protein on Candida albicans. J Biol Chem. 1998;273:20438–20447. doi: 10.1074/jbc.273.32.20438. [DOI] [PubMed] [Google Scholar]
- Fattorini L, Gennaro R, Zanetti M, Tan D, Brunori L, Giannoni F, et al. In vitro activity of protegrin-1 and beta-defensin-1, alone and in combination with isoniazid, against Mycobacterium tuberculosis. Peptides. 2004;25:1075–1077. doi: 10.1016/j.peptides.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Felgentreff K, Beisswenger C, Griese M, Gulder T, Bringmann G, Bals R. The antimicrobial peptide cathelicidin interacts with airway mucus. Peptides. 2006;27:3100–3106. doi: 10.1016/j.peptides.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Feng Z, Dubyak GR, Lederman MM, Weinberg A. Cutting edge: human beta defensin 3—a novel antagonist of the HIV-1 coreceptor CXCR4. J Immunol. 2006;177:782–786. doi: 10.4049/jimmunol.177.2.782. [DOI] [PubMed] [Google Scholar]
- Feucht EC, DeSanti CL, Weinberg A. Selective induction of human beta-defensin mRNAs by Actinobacillus actinomycetemcomitans in primary and immortalized oral epithelial cells. Oral Microbiol Immunol. 2003;18:359–363. doi: 10.1046/j.0902-0055.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P, Feng D. The role of type I interferon production by dendritic cells in host defense. Biochimie. 2007;89:843–855. doi: 10.1016/j.biochi.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P, Dai J, Singh S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev. 2008;19:3–19. doi: 10.1016/j.cytogfr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furci L, Sironi F, Tolazzi M, Vassena L, Lusso P. Alpha-defensins block the early steps of HIV-1 infection: interference with the binding of gp120 to CD4. Blood. 2007;109:2928–2935. doi: 10.1182/blood-2006-05-024489. [DOI] [PubMed] [Google Scholar]
- Ganz T, Lehrer RI. Defensins. Pharmacol Ther. 1995;66:191–205. doi: 10.1016/0163-7258(94)00076-f. [DOI] [PubMed] [Google Scholar]
- Garcia JR, Jaumann F, Schulz S, Krause A, Rodriguez-Jimenez J, Forssmann U, et al. Identification of a novel, multifunctional beta-defensin (human beta- defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 2001a;306:257–264. doi: 10.1007/s004410100433. [DOI] [PubMed] [Google Scholar]
- Garcia JR, Krause A, Schulz S, Rodrigues-Jimenez FJ, Kluver E, Adermann K, et al. Human beta-defensin 4: a novel inducible peptide with a salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001b;15:1819–1821. [PubMed] [Google Scholar]
- Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- Gropp R, Frye M, Wagner TO, Bargon J. Epithelial defensins impair adenoviral infection: implication for adenovirus-mediated gene therapy. Hum Gene Ther. 1999;10:957–964. doi: 10.1089/10430349950018355. [DOI] [PubMed] [Google Scholar]
- Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL 39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996;238:325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, Schröder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- Harder J, Meyer-Hoffert U, Teran LM, Schwichtenberg L, Bartels J, Maune S, et al. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol. 2000;22:714–721. doi: 10.1165/ajrcmb.22.6.4023. [DOI] [PubMed] [Google Scholar]
- Hartshorn KL, White MR, Tecle T, Holmskov U, Crouch EC. Innate defense against influenza A virus: activity of human neutrophil defensins and interactions of defensins with surfactant protein D. J Immunol. 2006;176:6962–6972. doi: 10.4049/jimmunol.176.11.6962. [DOI] [PubMed] [Google Scholar]
- Hazrati E, Galen B, Lu W, Wang W, Ouyang Y, Keller MJ, et al. Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. J Immunol. 2006;177:8658–8666. doi: 10.4049/jimmunol.177.12.8658. [DOI] [PubMed] [Google Scholar]
- Hosokawa I, Hosokawa Y, Komatsuzawa H, Goncalves RB, Karimbux N, Napimoga MH, et al. Innate immune peptide LL-37 displays distinct expression pattern from beta-defensins in inflamed gingival tissue. Clin Exp Immunol. 2006;146:218–225. doi: 10.1111/j.1365-2249.2006.03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DM, Feldman SB, Kloser P, Fitzgerald-Bocarsly PA. Decreased frequency of functional natural interferon-producing cells in peripheral blood of patients with the aquired immune deficiency syndrome. Clin Immunol Immunopathol. 1994;71:223–230. doi: 10.1006/clin.1994.1076. [DOI] [PubMed] [Google Scholar]
- Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol. 2004;172:1763–1767. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- Hsu CH, Chen C, Jou ML, Lee AY, Lin YC, Yu YP, et al. Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: evidence for multiple conformations involved in binding to membranes and DNA. Nucleic Acids Res. 2005;33:4053–4064. doi: 10.1093/nar/gki725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LC, Jean D, Proske RJ, Reins RY, McDermott AM. Ocular surface expression and in vitro activity of antimicrobial peptides. Curr Eye Res. 2007;32:595–609. doi: 10.1080/02713680701446653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilker MF, Nusslein K, Tew GN, Coughlin EB. Tuning the hemolytic and antibacterial activities of amphiphilic polynorbornene derivatives. J Am Chem Soc. 2004;126:15870–15875. doi: 10.1021/ja045664d. [DOI] [PubMed] [Google Scholar]
- Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S, Hyun J, Park E, Lee BL, Kim KK, Choi Y. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J Periodontal Res. 2007a;42:410–419. doi: 10.1111/j.1600-0765.2006.00962.x. erratum in J Periodontal Res 43:126, 2008. [DOI] [PubMed] [Google Scholar]
- Ji S, Kim Y, Min BM, Han SH, Choi Y. Innate immune responses of gingival epithelial cells to nonperiodontopathic and periodontopathic bacteria. J Periodontal Res. 2007b;42:503–510. doi: 10.1111/j.1600-0765.2007.00974.x. [DOI] [PubMed] [Google Scholar]
- Joly S, Maze C, McCray PB, Jr, Guthmiller JM. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42:1024–1029. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AL, Mertz RH, Carl DJ, Rubens CE. A streptococcal penicillin-binding protein is critical for resisting innate airway defenses in the neonatal lung. J Immunol. 2007;179:3196–3202. doi: 10.4049/jimmunol.179.5.3196. [DOI] [PubMed] [Google Scholar]
- Jurevic RJ, Bai M, Chadwick RB, White TC, Dale BA. Single-nucleotide polymorphisms (SNPs) in human beta-defensin 1: high-throughput SNP assays and association with candida carriage in type I diabetics and nondiabetic controls. J Clin Microbiol. 2003;41:90–96. doi: 10.1128/JCM.41.1.90-96.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser V, Diamond G. Expression of mammalian defensin genes. J Leukoc Biol. 2000;68:779–784. [PubMed] [Google Scholar]
- Kajita K, Honda T, Amanuma R, Domon H, Okui T, Ito H, et al. Quantitative messenger RNA expression of Toll-like receptors and interferon-alpha1 in gingivitis and periodontitis. Oral Microbiol Immunol. 2007;22:398–402. doi: 10.1111/j.1399-302X.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- Kisich KO, Heifets L, Higgins M, Diamond G. Antimycobacterial agent based on mRNA encoding human beta-defensin 2 enables primary macrophages to restrict growth of Mycobacterium tuberculosis. Infect Immun. 2001;69:2692–2699. doi: 10.1128/IAI.69.4.2692-2699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisich KO, Howell MD, Boguniewicz M, Heizer HR, Watson NU, Leung DY. The constitutive capacity of human keratinocytes to kill Staphylococcus aureus is dependent on beta-defensin 3. J Invest Dermatol. 2007;127:2368–2380. doi: 10.1038/sj.jid.5700861. [DOI] [PubMed] [Google Scholar]
- Klein-Patel ME, Diamond G, Boniotto M, Saad S, Ryan LK. Inhibition of β-defensin gene expression in airway epithelial cells by low doses of residual oil fly ash is mediated by vanadium. Toxicol Sci. 2006;92:115–125. doi: 10.1093/toxsci/kfj214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6:447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- Koshlukova SE, Lloyd TL, Araujo MW, Edgerton M. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J Biol Chem. 1999;274:18872–18879. doi: 10.1074/jbc.274.27.18872. [DOI] [PubMed] [Google Scholar]
- Krisanaprakornkit S, Kimball JR, Weinberg A, Darveau RP, Bainbridge BW, Dale BA. Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect Immun. 2000;68:2907–2915. doi: 10.1128/iai.68.5.2907-2915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisanaprakornkit S, Kimball JR, Dale BA. Regulation of human beta-defensin-2 in gingival epithelial cells: the involvement of mitogen-activated protein kinase pathways, but not the NF-kappaB transcription factor family. J Immunol. 2002;168:316–324. doi: 10.4049/jimmunol.168.1.316. [DOI] [PubMed] [Google Scholar]
- Kurland AR, Schreiner H, Diamond G. In vivo β-defensin gene expression in rat gingival epithelium in response to Actinobacillus actinomycetemcomitans infection. J Periodontal Res. 2006;41:567–572. doi: 10.1111/j.1600-0765.2006.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube DM, Yim S, Ryan LK, Kisich KO, Diamond G. Antimicrobial peptides in the airway. Curr Top Microbiol Immunol. 2006;306:153–182. doi: 10.1007/3-540-29916-5_6. [DOI] [PubMed] [Google Scholar]
- Laube D, Dongari-Bagtzoglou A, Kashleva H, Eskdale J, Gallagher G, Diamond G. Differential regulation of innate immune response genes in gingival epithelial cells stimulated with Aggregatibacter actinomycetemcomitans. J Periodontal Res. 2008;43:116–123. doi: 10.1111/j.1600-0765.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- Legarda D, Klein-Patel M, Yim S, Yuk MH, Diamond G. Suppression of NF-κB-mediated beta-defensin gene expression in the mammalian airway by the Bordetella type III secretion system. Cell Microbiol. 2005;7:489–497. doi: 10.1111/j.1462-5822.2004.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer RI, Ganz T. Defensins of vertebrate animals. Curr Opin Immunol. 2002;14:96–102. doi: 10.1016/s0952-7915(01)00303-x. [DOI] [PubMed] [Google Scholar]
- Lehrer RI, Szklarek D, Ganz T, Selsted ME. Synergistic activity of rabbit granulocyte peptides against Candida albicans. Infect Immun. 1986;52:902–904. doi: 10.1128/iai.52.3.902-904.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikina E, Delanoe-Ayari H, Melikov K, Cho MS, Chen A, Waring AJ, et al. Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat Immunol. 2005;6:995–1001. doi: 10.1038/ni1248. [DOI] [PubMed] [Google Scholar]
- Lillard JW, Jr, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci USA. 1999;96:651–656. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhao C, Heng HHQ, Ganz T. The human β-defensin-1 and α-defensins are encoded by adjacent genes: two peptide families with differing disulfide topology share a common ancestry. Genomics. 1997;43:316–320. doi: 10.1006/geno.1997.4801. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Bratke K, Bier A, Julius P, Kuepper M, Luttmann W, et al. Airway dendritic cell phenotypes in inflammatory diseases of the human lung. Eur Respir J. 2007;30:878–886. doi: 10.1183/09031936.00036307. erratum, Eur Respir J 31:226, 2008. [DOI] [PubMed] [Google Scholar]
- Lu Q, Samaranayake LP, Darveau RP, Jin L. Expression of human beta-defensin-3 in gingival epithelia. J Periodontal Res. 2005;40:474–481. doi: 10.1111/j.1600-0765.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- Lundy FT, Orr DF, Gallagher JR, Maxwell P, Shaw C, Napier SS, et al. Identification and overexpression of human neutrophil alpha-defensins (human neutrophil peptides 1, 2 and 3) in squamous cell carcinomas of the human tongue. Oral Oncol. 2004;40:139–144. doi: 10.1016/s1368-8375(03)00142-8. [DOI] [PubMed] [Google Scholar]
- Mackewicz CE, Yuan J, Tran P, Diaz L, Mack E, Selsted ME, et al. Alpha-defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. AIDS. 2003;17:23–32. doi: 10.1097/00002030-200309260-00001. erratum, AIDS 17:31, 2003. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- Marr AK, Gooderham WJ, Hancock RE. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol. 2006;6:468–472. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Mathews M, Jia HP, Guthmiller JM, Losh G, Graham S, Johnson GK, et al. Production of beta-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect Immun. 1999;67:2740–2745. doi: 10.1128/iai.67.6.2740-2745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K, Sugishita K, Fujii N, Miyajima K. Molecular basis for membrane selectivity of an antimicrobial peptide, magainin 2. Biochemistry. 1995;34:3423–3429. doi: 10.1021/bi00010a034. [DOI] [PubMed] [Google Scholar]
- Merrifield RB, Merrifield EL, Juvvadi P, Andreu D, Boman HG. Design and synthesis of antimicrobial peptides. Ciba Found Symp. 1994;186:5–20. [PubMed] [Google Scholar]
- Midorikawa K, Ouhara K, Komatsuzawa H, Kawai T, Yamada S, Fujiwara T, et al. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect Immun. 2003;71:3730–3739. doi: 10.1128/IAI.71.7.3730-3739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineshiba F, Takashiba S, Mineshiba J, Matsuura K, Kokeguchi S, Murayama Y. Antibacterial activity of synthetic human β defensin-2 against periodontal bacteria. J Int Acad Periodontol. 2003;5(2):35–40. [PubMed] [Google Scholar]
- Miyakawa Y, Ratnakar P, Rao AG, Costello ML, Mathieu-Costello O, Lehrer RI, et al. In vitro activity of the antimicrobial peptides human and rabbit defensins and porcine leukocyte protegrin against Mycobacterium tuberculosis. Infect Immun. 1996;64:926–932. doi: 10.1128/iai.64.3.926-932.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaki KT, Lehrer RI. Beta-sheet antibiotic peptides as potential dental therapeutics. Int J Antimicrob Agents. 1998;9:269–280. doi: 10.1016/s0924-8579(98)00006-5. [DOI] [PubMed] [Google Scholar]
- Miyasaki KT, Bodeau AL, Ganz T, Selsted ME, Lehrer RI. In vitro sensitivity of oral, Gram-negative, facultative bacteria to the bactericidal activity of human neutrophil defensins. Infect Immun. 1990;58:3934–3940. doi: 10.1128/iai.58.12.3934-3940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukawa N, Sugiyama K, Ueno T, Mishima K, Takagi S, Sugahara T. Levels of human defensin-1, an antimicrobial peptide, in saliva of patients with oral inflammation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:539–543. doi: 10.1016/s1079-2104(99)70130-7. [DOI] [PubMed] [Google Scholar]
- Mizukawa N, Sugiyama K, Ueno T, Mishima K, Takagi S, Sugahara T. Detection of human alpha-defensin-1, an antimicrobial peptide, in the fluid of jaw cysts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:78–81. doi: 10.1067/moe.2000.107055. [DOI] [PubMed] [Google Scholar]
- Mukae H, Ishimoto H, Yanagi S, Ishii H, Nakayama S, Ashitani J, et al. Elevated BALF concentrations of alpha- and beta-defensins in patients with pulmonary alveolar proteinosis. Respir Med. 2007;101:715–721. doi: 10.1016/j.rmed.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Murakami M, Ohtake T, Dorschner RA, Gallo RL. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J Dent Res. 2002;81:845–850. doi: 10.1177/154405910208101210. [DOI] [PubMed] [Google Scholar]
- Nakashima H, Yamamoto N, Masuda M, Fujii N. Defensins inhibit HIV replication in vitro. AIDS. 1993;7:1129. doi: 10.1097/00002030-199308000-00019. [DOI] [PubMed] [Google Scholar]
- Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol. 2001;31:1066–1075. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Niyonsaba F, Iwabuchi K, Matsuda H, Ogawa H, Nagaoka I. Epithelial cell-derived human beta-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int Immunol. 2002;14:421–426. doi: 10.1093/intimm/14.4.421. [DOI] [PubMed] [Google Scholar]
- Ostergaard GZ, Tommerup N. The 8p- syndrome. Ann Genet. 1989;32:87–91. [PubMed] [Google Scholar]
- Ouhara K, Komatsuzawa H, Yamada S, Shiba H, Fujiwara T, Ohara M, et al. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, β-defensins and LL37, produced by human epithelial cells. J Antimicrob Chemother. 2005;55:888–896. doi: 10.1093/jac/dki103. [DOI] [PubMed] [Google Scholar]
- Ouhara K, Komatsuzawa H, Shiba H, Uchida Y, Kawai T, Sayama K, et al. Actinobacillus actinomycetemcomitans outer membrane protein 100 triggers innate immunity and production of beta-defensin and the 18-kilodalton cationic antimicrobial protein through the fibronectin-integrin pathway in human gingival epithelial cells. Infect Immun. 2006;74:5211–5220. doi: 10.1128/IAI.00056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz J, Beisswenger C, Dalpke A, Koczulla R, Pinkenburg O, Vogelmeier C, et al. Microbial DNA induces a host defense reaction of human respiratory epithelial cells. J Immunol. 2004;173:1219–1223. doi: 10.4049/jimmunol.173.2.1219. [DOI] [PubMed] [Google Scholar]
- Proud D, Sanders SP, Wiehler S. Human rhinovirus infection induces airway epithelial cell production of human beta-defensin 2 both in vitro and in vivo. J Immunol. 2004;172:4637–4645. doi: 10.4049/jimmunol.172.7.4637. [DOI] [PubMed] [Google Scholar]
- Putsep K, Carlsson G, Boman HG, Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360:1144–1149. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- Quinones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:39–48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- Rennie J, Arnt L, Tang H, Nusslein K, Tew GN. Simple oligomers as antimicrobial peptide mimics. J Ind Microbiol Biotechnol. 2005;32:296–300. doi: 10.1007/s10295-005-0219-0. [DOI] [PubMed] [Google Scholar]
- Ryan LK, Diamond G, Amrute S, Feng Z, Weinberg A, Fitzgerald-Bocarsly P. Detection of HBD1 peptide in peripheral blood mononuclear cell subpopulations by intracellular flow cytometry. Peptides. 2003;24:1785–1794. doi: 10.1016/j.peptides.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Sahasrabudhe KS, Kimball JR, Morton TH, Weinberg A, Dale BA. Expression of the antimicrobial peptide, human beta-defensin 1, in duct cells of minor salivary glands and detection in saliva. J Dent Res. 2000;79:1669–1674. doi: 10.1177/00220345000790090601. [DOI] [PubMed] [Google Scholar]
- Saiman L, Tabibi S, Starner TD, San Gabriel P, Winokur PL, Jia HP, et al. Cathelicidin peptides inhibit multiply antibiotic-resistant pathogens from patients with cystic fibrosis. Antimicrob Agents Chemother. 2001;45:2838–2844. doi: 10.1128/AAC.45.10.2838-2844.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore M, Garcia-Sastre A, Ruchala P, Lehrer RI, Chang T, Klotman ME. Alpha-defensin inhibits influenza virus replication by cell-mediated mechanism(s) J Infect Dis. 2007;196:835–843. doi: 10.1086/521027. [DOI] [PubMed] [Google Scholar]
- Santoro A, Majorana A, Roversi L, Gentili F, Marrelli S, Vermi W, et al. Recruitment of dendritic cells in oral lichen planus. J Pathol. 2005;205:426–434. doi: 10.1002/path.1699. [DOI] [PubMed] [Google Scholar]
- Sawaki K, Mizukawa N, Yamaai T, Fukunaga J, Sugahara T. Immunohistochemical study on expression of alpha-defensin and beta-defensin-2 in human buccal epithelia with candidiasis. Oral Dis. 2002;8:37–41. doi: 10.1034/j.1601-0825.2002.1o770.x. [DOI] [PubMed] [Google Scholar]
- Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Smith JG, Nemerow GR. Mechanism of adenovirus neutralization by human alpha-defensins. Cell Host Microbe. 2008;3:11–19. doi: 10.1016/j.chom.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- Starner TD, Swords WE, Apicella MA, McCray PB., Jr Susceptibility of nontypeable Haemophilus influenzae to human beta-defensins is influenced by lipooligosaccharide acylation. Infect Immun. 2002;70:5287–5289. doi: 10.1128/IAI.70.9.5287-5289.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starner TD, Agerberth B, Gudmundsson GH, McCray PB., Jr Expression and activity of beta-defensins and LL-37 in the developing human lung. J Immunol. 2005;174:1608–1615. doi: 10.4049/jimmunol.174.3.1608. [DOI] [PubMed] [Google Scholar]
- Stigers KD, Soth MJ, Nowick JS. Designed molecules that fold to mimic protein secondary structures. Curr Opin Chem Biol. 1999;3:714–723. doi: 10.1016/s1367-5931(99)00030-7. [DOI] [PubMed] [Google Scholar]
- Stolzenberg ED, Anderson GM, Ackermann MR, Whitlock RH, Zasloff M. Epithelial antibiotic induced in states of disease. Proc Natl Acad Sci USA. 1997;94:8686–8690. doi: 10.1073/pnas.94.16.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Finnegan CM, Kish-Catalone T, Blumenthal R, Garzino-Demo P, La Terra Maggiore GM, et al. Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J Virol. 2005;79:14318–14329. doi: 10.1128/JVI.79.22.14318-14329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi Y, Imai H. Expression of beta-defensin-2 in human gingival epithelial cells in response to challenge with Porphyromonas gingivalis in vitro. J Periodontal Res. 2006;41:334–339. doi: 10.1111/j.1600-0765.2006.00879.x. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Wada A, Ogushi K, Maeda K, Kawahara T, Mawatari K, et al. Production of beta-defensin-2 by human colonic epithelial cells induced by Salmonella enteritidis flagella filament structural protein. FEBS Lett. 2001;508:484–488. doi: 10.1016/s0014-5793(01)03088-5. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Ip WE, Michelow IC, Ezekowitz RA. The mannose-binding lectin: a prototypic pattern recognition molecule. Curr Opin Immunol. 2006;18:16–23. doi: 10.1016/j.coi.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe H, Ouellette AJ, Cocco MJ, Robinson WE., Jr Differential effects on human immunodeficiency virus type 1 replication by alpha-defensins with comparable bactericidal activities. J Virol. 2004;78:11622–11631. doi: 10.1128/JVI.78.21.11622-11631.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Jurevic RJ, Coulton KK, Tsutsui MT, Roberts MC, Kimball JR, et al. Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob Agents Chemother. 2005;49:3883–3888. doi: 10.1128/AAC.49.9.3883-3888.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecle T, White MR, Gantz D, Crouch EC, Hartshorn KL. Human neutrophil defensins increase neutrophil uptake of influenza A virus and bacteria and modify virus-induced respiratory burst responses. J Immunol. 2007;178:8046–8052. doi: 10.4049/jimmunol.178.12.8046. [DOI] [PubMed] [Google Scholar]
- Tew GN, Clements D, Tang H, Arnt L, Scott RW. Antimicrobial activity of an abiotic host defense peptide mimic. Biochim Biophys Acta. 2006;1758:1387–1392. doi: 10.1016/j.bbamem.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, et al. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6696. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wetering S, Mannesse-Lazeroms SP, Van Sterkenburg MA, Daha MR, Dijkman JH, Hiemstra PS. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol. 1997;272:888–896. doi: 10.1152/ajplung.1997.272.5.L888. [DOI] [PubMed] [Google Scholar]
- Vankeerberghen A, Nuytten H, Dierickx K, Quirynen M, Cassiman JJ, Cuppens H. Differential induction of human beta-defensin expression by periodontal commensals and pathogens in periodontal pocket epithelial cells. J Periodontol. 2005;76:1293–1303. doi: 10.1902/jop.2005.76.8.1293. [DOI] [PubMed] [Google Scholar]
- Varkey J, Nagaraj R. Antibacterial activity of human neutrophil defensin HNP-1 analogs without cysteines. Antimicrob Agents Chemother. 2005;49:4561–4566. doi: 10.1128/AAC.49.11.4561-4566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virella-Lowell I, Poirier A, Chesnut KA, Brantly M, Flotte TR. Inhibition of recombinant adeno-associated virus (rAAV) transduction by bronchial secretions from cystic fibrosis patients. Gene Ther. 2000;7:1783–1789. doi: 10.1038/sj.gt.3301268. [DOI] [PubMed] [Google Scholar]
- Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. erratum, J Immunol 173:6489, 2004. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Krisanaprakornkit S, Dale BA. Epithelial antimicrobial peptides: review and significance for oral applications. Crit Rev Oral Biol Med. 1998;9:399–414. doi: 10.1177/10454411980090040201. [DOI] [PubMed] [Google Scholar]
- White MR, Tecle T, Crouch EC, Hartshorn KL. Impact of neutrophils on antiviral activity of human bronchoalveolar lavage fluid. Am J Physiol Lung Cell Mol Physiol. 2007;293:1293–1299. doi: 10.1152/ajplung.00266.2007. [DOI] [PubMed] [Google Scholar]
- Woo JS, Jeong JY, Hwang YJ, Chae SW, Hwang SJ, Lee HM. Expression of cathelicidin in human salivary glands. Arch Otolaryngol Head Neck Surg. 2003;129:211–214. doi: 10.1001/archotol.129.2.211. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Saito K. Purification, primary structure, and biological activity of guinea pig neutrophil cationic peptides. Infect Immun. 1989;57:2405–2409. doi: 10.1128/iai.57.8.2405-2409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000a;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol. 2000b;68:9–14. [PubMed] [Google Scholar]
- Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- Yasin B, Wang W, Pang M, Cheshenko N, Hong T, Waring AJ, et al. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J Virol. 2004;78:5147–5156. doi: 10.1128/JVI.78.10.5147-5156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D3. J Cystic Fibrosis. 2007;6:403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiou M, Nizet V, Gallo RL. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J Invest Dermatol. 2003;120:810–816. doi: 10.1046/j.1523-1747.2003.12132.x. [DOI] [PubMed] [Google Scholar]
- Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yu W, He T, Yu J, Caffrey RE, Dalmasso EA, et al. Contribution of human alpha-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science. 2002;298:995–1000. doi: 10.1126/science.1076185. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lopez P, He T, Yu W, Ho DD. Retraction of an interpretation. Science. 2004;303:467. doi: 10.1126/science.303.5657.467b. [DOI] [PubMed] [Google Scholar]


