Abstract
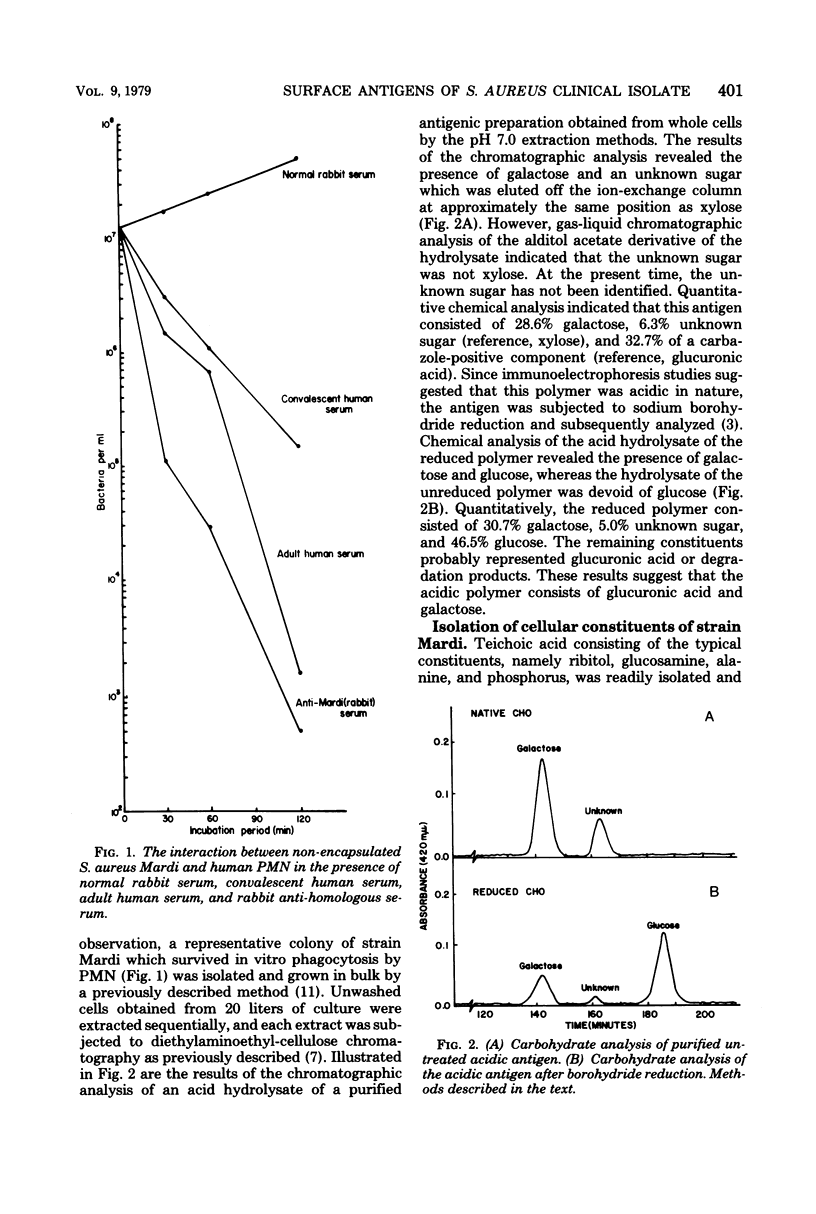
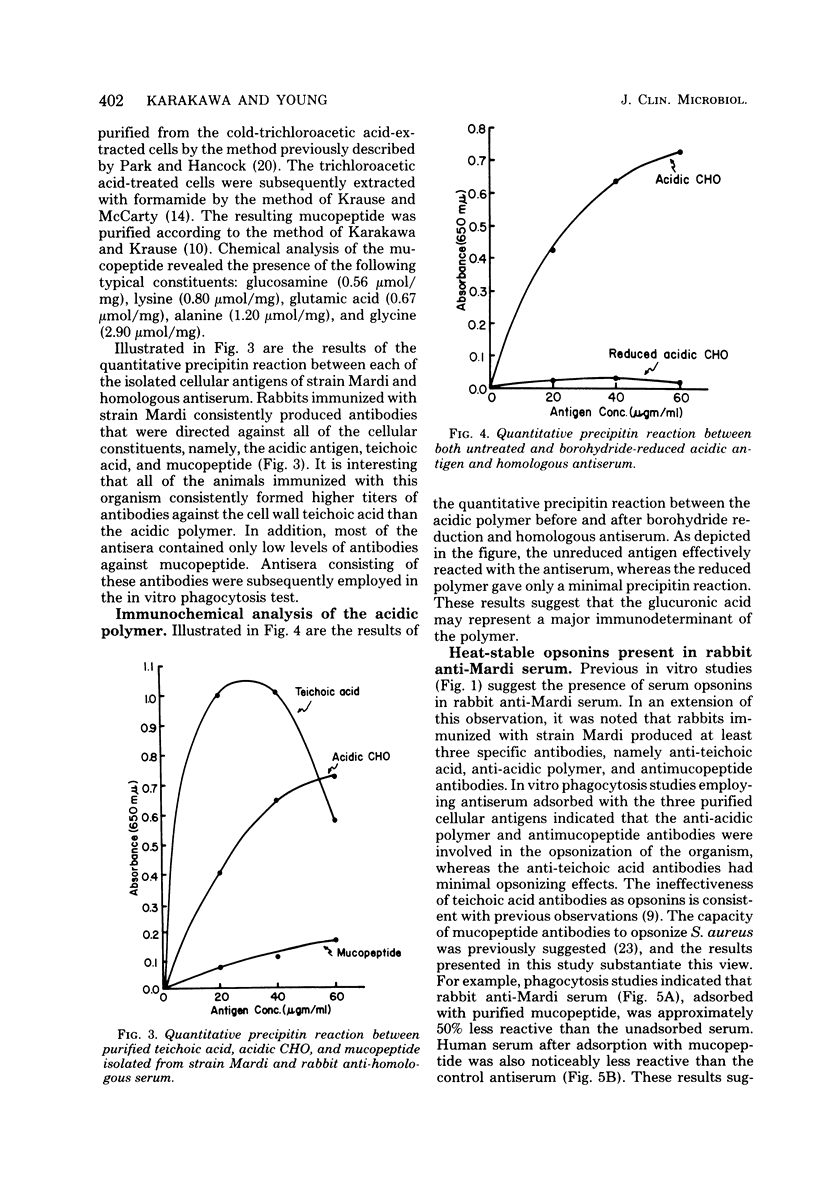
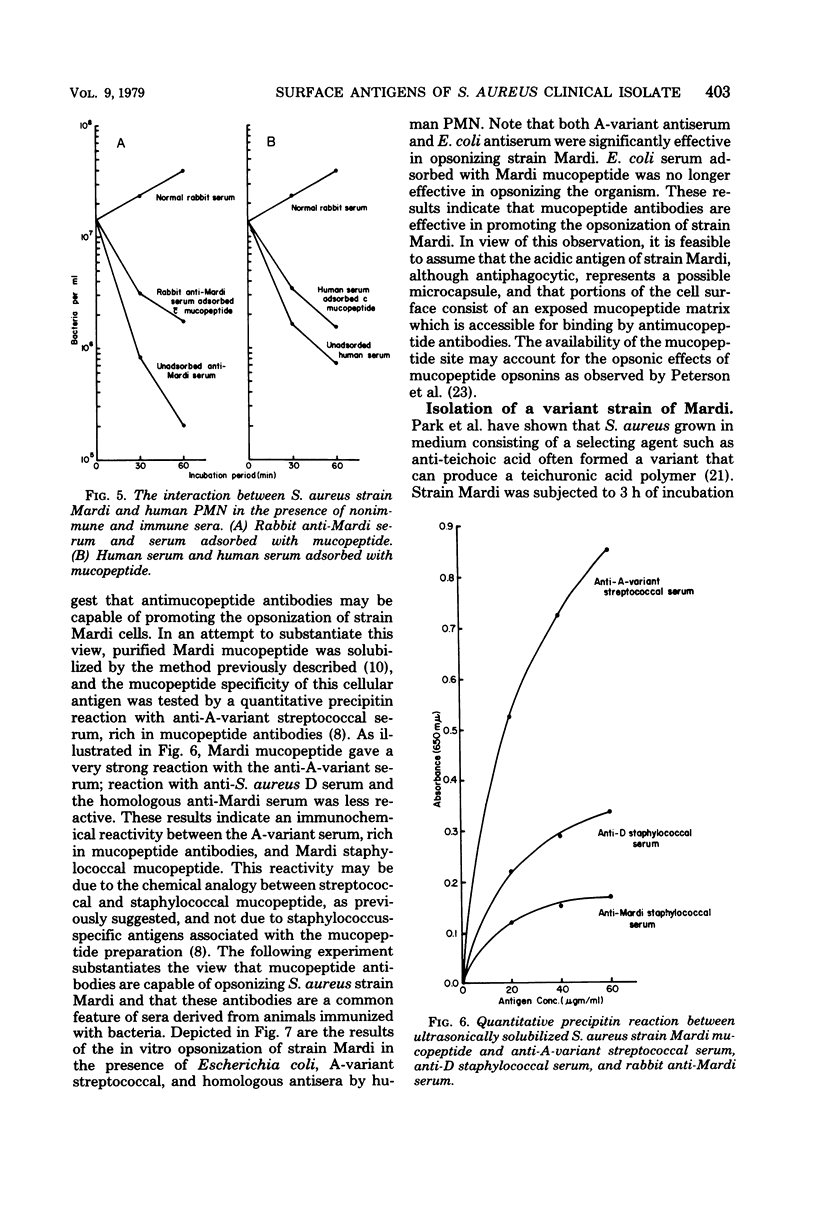
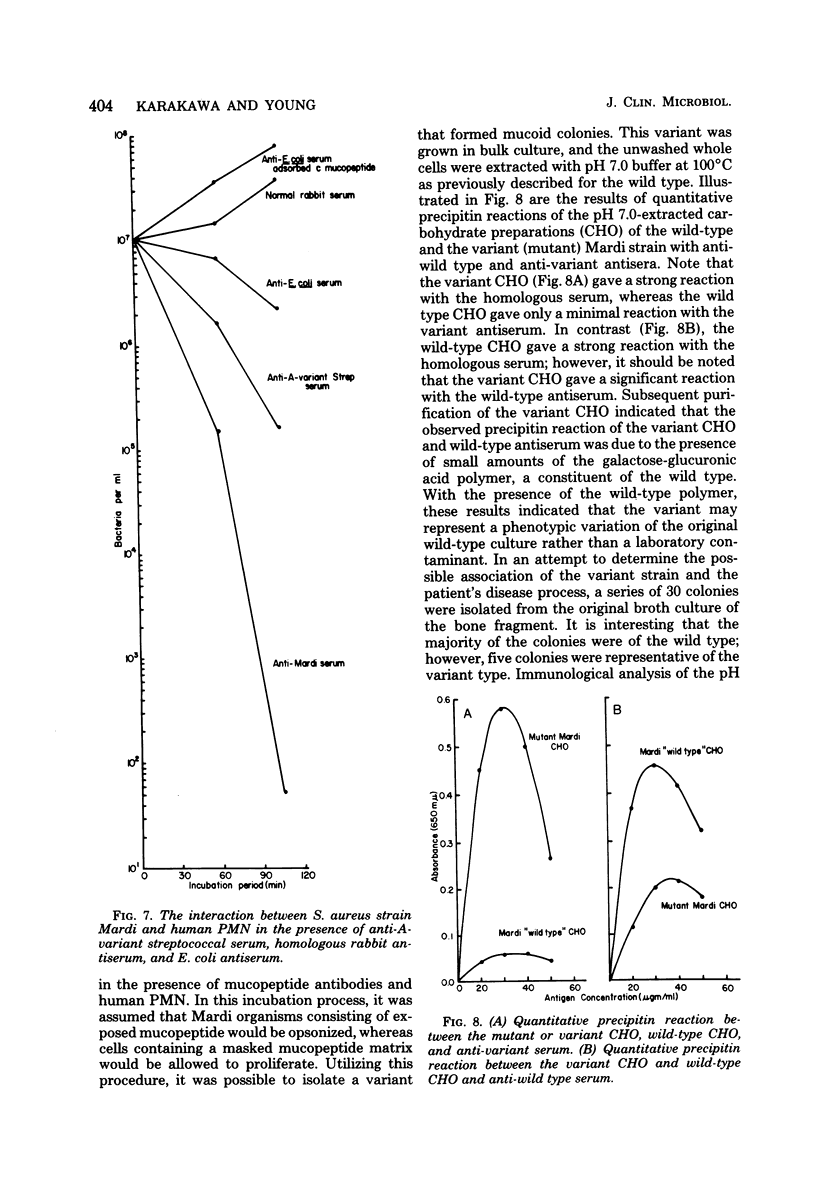
The cellular antigens of a strain of Staphylococcus aureus, isolated from a bone fragment from osteomyelitis, were analyzed immunochemically and by interaction with human phagocytic cells. When this strain was allowed to interact with human polymorphonuclear cells in the presence of antiserum, the strain was shown to have specific antiphagocytic antigens. An acidic polysaccharide consisting of galactose and glucuronic acid was isolated from the cell surface of the organism, and in vitro opsonization tests indicated that this acidic antigen impeded in vitro phagocytosis by human polymorphonuclear cells. It was also observed that antibodies directed against the mucopeptide constituents of homologous and heterologous bacterial cell walls were effective in promoting the in vitro opsonization of the organism. In the presence of antimucopeptide serum and human polymorphonuclear cells, a variant strain was isolated from the wild type, and immunochemical analysis indicated that this strain consisted of galactose and immunodominant amino-galacturonic acid residues. In vitro phagocytosis studies employing this variant strain indicated that the homologous human convalescent serum contained higher levels of opsonins against the variant strain than the original isolate, the wild type. This observation is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Easmon C. S., Glynn A. A. Comparison of subcutaneous and intraperitoneal staphylococcal infections in normal and complement-deficient mice. Infect Immun. 1976 Feb;13(2):399–406. doi: 10.1128/iai.13.2.399-406.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstedt R. D. Immune response to surface antigens of Staphylococcus aureus and their role in resistance to staphylococcal disease. Ann N Y Acad Sci. 1974 Jul 31;236(0):203–220. doi: 10.1111/j.1749-6632.1974.tb41492.x. [DOI] [PubMed] [Google Scholar]
- Fraser B. A., Mallette M. F. An improved isolation method and new composition data for Forssman hapten from sheep erythrocytes. Immunochemistry. 1973 Nov;10(11):745–753. doi: 10.1016/0019-2791(73)90176-6. [DOI] [PubMed] [Google Scholar]
- KRAUSE R. M., MCCARTY M. Studies on the chemical structure of the streptococcal cell wall. I. The identification of a mucopeptide in the cell walls of groups A and A-variant streptococci. J Exp Med. 1961 Jul 1;114:127–140. doi: 10.1084/jem.114.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. A., Karakawa W. W. Multiple polysaccharide antigens of group B streptococcus, type Ia: emphasis on a sialic acid type-specific polysaccharide. J Immunol. 1977 Jun;118(6):2155–2160. [PubMed] [Google Scholar]
- Karakawa W. W., Braun D. G., Lackland H., Krause R. M. Immunochemical studies on the cross-reactivity between streptococcal and staphylococcal mucopeptide. J Exp Med. 1968 Aug 1;128(2):325–340. doi: 10.1084/jem.128.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A. Immunochemical analysis of a Smith-like antigen isolated from two human strains of Staphylococcus aureus. J Immunol. 1975 Aug;115(2):564–568. [PubMed] [Google Scholar]
- Karakawa W. W., Krause R. M. Studies on the immunochemistry of streptococcal mucopeptide. J Exp Med. 1966 Aug 1;124(2):155–171. doi: 10.1084/jem.124.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Young D. A., Kane J. A. Structural analysis of the cellular constituents of a fresh clinical isolate of Staphylococcus aureus, and their role in the interaction between the organisms and polymorphonuclear leukocytes in the presence of serum factors. Infect Immun. 1978 Aug;21(2):496–505. doi: 10.1128/iai.21.2.496-505.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig M. G., Melly M. A. The importance of surface antigens in staphylococcal virulence. Ann N Y Acad Sci. 1965 Jul 23;128(1):231–250. doi: 10.1111/j.1749-6632.1965.tb11641.x. [DOI] [PubMed] [Google Scholar]
- Liau D. F., Hash J. H. Structural analysis of the surface polysaccharide of Staphylococcus aureus M. J Bacteriol. 1977 Jul;131(1):194–200. doi: 10.1128/jb.131.1.194-200.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCARTY M., LANCEFIELD R. C. Variation in the group-specific carbohydrate of group A streptococci. I. Immunochemical studies on the carbohydrates of variant strains. J Exp Med. 1955 Jul 1;102(1):11–28. doi: 10.1084/jem.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald H. C., Karakawa W. W. Immunochemical analysis of a uronic acid polymer of Staphylococcus epidermidis, strain 53. J Immunol. 1970 Aug;105(2):389–395. [PubMed] [Google Scholar]
- Melly M. A., Duke L. J., Liau D. F., Hash J. H. Biological properties of the encapsulated Staphylococcus aureus M. Infect Immun. 1974 Aug;10(2):389–397. doi: 10.1128/iai.10.2.389-397.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson D. S., Kazmierowski J. A., Dossett J. H., Williams R. C., Jr, Quie P. G. Studies of immune and normal opsonins during experimental staphylococcal infection in rabbits. J Immunol. 1969 May;102(5):1235–1241. [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Park J. T., Shaw D. R., Chatterjee A. N., Mirelman D., Wu T. Mutants of staphylococci with altered cell walls. Ann N Y Acad Sci. 1974 Jul 31;236(0):54–62. doi: 10.1111/j.1749-6632.1974.tb41481.x. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Sabath L. D., Quie P. G. Extracellular and bacterial factors influencing staphylococcal phagocytosis and killing by human polymorphonuclear leukocytes. Infect Immun. 1976 Aug;14(2):496–501. doi: 10.1128/iai.14.2.496-501.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Douglas S. D., Quie P. G., Verhoef J. The key role of peptidoglycan in the opsonization of Staphylococcus aureus. J Clin Invest. 1978 Mar;61(3):597–609. doi: 10.1172/JCI108971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. B. The relationship between group A and group C meningococcal polysaccharides and serum opsonins in man. J Exp Med. 1970 Mar 1;131(3):499–513. doi: 10.1084/jem.131.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. E., Melly M. A. Speculations on the immunology of staphylococcal infections. Ann N Y Acad Sci. 1965 Jul 23;128(1):274–284. doi: 10.1111/j.1749-6632.1965.tb11644.x. [DOI] [PubMed] [Google Scholar]
- Shayegani M., Hisatsune K., Mudd S. Cell Wall Component Which Affects the Ability of Serum to Promote Phagocytosis and Killing of Staphylococcus aureus. Infect Immun. 1970 Dec;2(6):750–756. doi: 10.1128/iai.2.6.750-756.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P., Winzler R. J. Determination of hexosaminitols by ion-exchange chromatography and its application to alkali-labile glycosidic linkages in glycoproteins. Arch Biochem Biophys. 1969 Feb;129(2):534–538. doi: 10.1016/0003-9861(69)90211-2. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Smith M. R., Naito Y. Biological and Immunological Properties of Encapsulated Strains of Staphylococcus aureus from Human Sources. Infect Immun. 1970 Nov;2(5):528–532. doi: 10.1128/iai.2.5.528-532.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



