Summary
G-protein-coupled receptor (GPCR) 48 (Gpr48; Lgr4), a newly discovered member of the glycoprotein hormone receptor subfamily of GPCRs, is an orphan GPCR of unknown function. Using a knockout mouse model, we have characterized the essential roles of Gpr48 in bone formation and remodeling. Deletion of Gpr48 in mice results in a dramatic delay in osteoblast differentiation and mineralization, but not in chondrocyte proliferation and maturation, during embryonic bone formation. Postnatal bone remodeling is also significantly affected in Gpr48-/- mice, including the kinetic indices of bone formation rate, bone mineral density and osteoid formation, whereas the activity and number of osteoclasts are increased as assessed by tartrate-resistant acid phosphatase staining. Examination of the molecular mechanism of Gpr48 action in bone formation revealed that Gpr48 can activate the cAMP-PKA-CREB signaling pathway to regulate the expression level of Atf4 in osteoblasts. Furthermore, we show that Gpr48 significantly downregulates the expression levels of Atf4 target genes/proteins, such as osteocalcin (Ocn; Bglap2), bone sialoprotein (Bsp; Ibsp) and collagen. Together, our data demonstrate that Gpr48 regulates bone formation and remodeling through the cAMP-PKA-Atf4 signaling pathway.
Keywords: Gpr48 (Lgr4), Atf4, Bone formation, Bone remodeling, Mouse
INTRODUCTION
The principal physiological processes of skeletal formation and remodeling might be summarized as pattern formation, transition from cartilage to bone, bone matrix synthesis and secretion, and bone resorption and remodeling. There are two types of bones, derived from intramembranous or endochondral bone formation (Erlebacher et al., 1995). Intramembranous bone formation, in which mesenchymal cells develop directly into osteoblasts, is involved in the formation of the flat skull bones. Endochondral bone formation, which arises from endochondral ossification, is a complex, highly regulated physiological process and accounts for the development of most other bones. The integrity of the vertebrate skeletal system requires two distinct regulatory processes: the embryonic developmental and postnatal regulatory processes. During embryonic development, the majority of bone formation is via endochondral ossification (Karsenty and Wagner, 2002). During postnatal bone regulatory processes, referred to as bone remodeling, bone is constantly regenerated through continuous formation by osteoblasts and resorption by osteoclasts (Manolagas, 2000). Both processes are strictly controlled by a complex transcriptional network, in which activating transcription factor 4 (Atf4) plays a key role (Cancedda et al., 2000; Elefteriou et al., 2005; Yang et al., 2004). Atf4 has been shown to regulate osteoblast differentiation, collagen synthesis, expression of the osteoblast-specific genes bone sialoprotein (Bsp; Ibsp - Mouse Genome Informatics) and osteocalcin (Ocn; Bglap2), and osteoblast terminal differentiation. Mice deficient in Atf4 are runted and exhibit low bone mass (Yang et al., 2004). Atf4 can directly interact with, and modulate the transcriptional activity of, Runx2, a transcription factor essential for osteoblast and hypertrophic chondrocyte differentiation and bone formation during embryogenesis and postnatal life (Xiao et al., 2005).
G-protein-coupled receptors (GPCRs or GPRs) are integral membrane proteins involved in the transmission of signals from the extracellular environment to the cytoplasm. A variety of external stimuli, including neurotransmitters, hormones, phospholipids and growth factors, can activate GPCRs. Therefore, GPCRs and their signal transduction pathways represent important specific targets for a variety of physiological functions and therapeutic approaches, ranging from the control of blood pressure, allergic response, kidney function, hormonal disorders, neurological diseases, to bone formation and remodeling (Gensure et al., 2005; Kamenetsky et al., 2006). Binding of specific agonists/ligands to GPCRs leads to the rapid activation of heterotrimeric G-proteins (binding of GTP) and the regulation of intracellular second messengers, such as cAMP and intracellular Ca2+ levels. Gpr48, which is also known as leucine-rich repeat (LRR)-containing G-protein-coupled receptor 4 (Lgr4), is a member of the family II GPCRs, with a long N-terminal LRR domain (Hsu et al., 1998; Luo and Hsueh, 2006; Weng et al., 2008). Although Gpr48 has been implicated in a variety of physiological functions (Kato et al., 2007; Mazerbourg et al., 2004; Mohri et al., 2008; Weng et al., 2008), the roles of Gpr48 in bone formation and remodeling remain unknown. The molecular mechanism of Gpr48 regulation is also unclear.
To understand the physiological function of Gpr48 in bone formation and remodeling, we disrupted the mouse Gpr48 allele and analyzed the expression and functions of the receptor in bone. Gpr48-deficient mice displayed intra-uterus growth retardation and 60% of the homozygous mice died at day 1 or 2 after birth. Deletion of Gpr48 led to delayed embryonic bone formation and decreased differentiation and mineralization of osteoblasts. Postnatal bone formation, including the bone mineral density (BMD), bone volume/tissue volume (BV/TV) and kinetic indices of bone formation and osteoid formation were all decreased, similar to the defects observed in Atf4 knockout mice (Elefteriou et al., 2005; Yang et al., 2004). As a result, the mutant mice exhibited reduced formation of primary spongiosa, reduced trabecular bone volume and a thinning of cortical bone. In situ hybridization data suggested that the number of mature osteoblasts in bone was reduced, whereas both the number and activity of osteoclasts were increased in Gpr48-deficient mice. Furthermore, deletion of Gpr48 significantly downregulated Atf4 and Atf4-mediated gene expression, including that of Ocn, Bsp and collagen, suggesting that Gpr48 regulates bone formation and remodeling through an Atf4 pathway.
MATERIALS AND METHODS
Generation of Gpr48-/- mice, genotyping and skeletal analysis
Gpr48 knockout mice were generated based on the secretory-trap approach and genotyping was performed by PCR as described previously (Weng et al., 2008). For skeletal analysis, embryos were dissected in PBS and then skinned, eviscerated and fixed in 95% ethanol. Skeletal preparations were performed as described previously (McLeod, 1980).
Cell lines, RT-PCR and Q-PCR
The MC3T3-E1 and ATDC5 cell lines were obtained from the ATCC. BAC (RP23-405K18) from the BACPAC Resources Center (BPRC) was used for Atf4 promoter PCR. cDNA synthesis was performed using the M-MLV Reverse Transcriptase Kit (Promega). Q-PCR was performed in a quantitative real-time PCR system machine (Stratagene) using the RT real-time SYBR Green PCR Master Mix Kit (SuperArray).
Histology, in situ hybridization and immunohistochemistry
Histological analysis, Hematoxylin and Eosin (HE) staining and lacZ staining were performed on paraffin-embedded tissues and histomorphometry was performed on plastic-embedded tissues using standard protocols (McLeod, 1980). The histological detection of TRAP-positive osteoclasts in paraffin-embedded sections was performed as previously described (Elefteriou et al., 2005). Digoxigenin (DIG)-labeled antisense and sense probes were produced using the DIG Nucleic Acid Detection Kit (Roche) according to the manufacturer's directions. Probes Runx2, Atf4, Col1a1, Bsp and Ocn were the kind gift of Dr G. Karsenty (Columbia University); other probes have been described previously (Akiyama et al., 2004; Stryke et al., 2003). Antibodies specific for CREB, phospho-CREB and Sox9 were from Santa Cruz Biotechnology. Monoclonal antibodies for Bsp and Ocn were a kind gift of Dr Qin Chunlin (Baylor College of Dentistry). Polyclonal antibody specific for Atf4 was from Dr G. Karsenty. Immunohistochemical staining followed the protocol from the ABC Staining System (Santa Cruz Biotechnology).
Primary osteoblast cell culture
Mouse calvarial osteoblasts were cultured from newborn mice according to standard protocols. To isolate primary osteoblasts, calvarias were digested with collagenase P (78 μg/ml) and trypsin/EDTA (0.05%; Gibco). The cells were maintained in α-MEM containing 10% FBS, 100 units/ml penicillin and 100 μg/ml streptomycin (Gibco). Differentiation of primary osteoblasts was induced with 8 mM β-glycerophosphate (Sigma) and 50 μg/ml ascorbic acid (Sigma) in medium.
EMSA and luciferase reporter assay
Proteins were isolated from primary culture cells or tissue lysed in cell lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% NP40, 1 mM PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM sodium orthovanadate). EMSA was performed as described (Mitchell et al., 2007). For the luciferase reporter assay, 48 hours after transfection of the plasmids the cells in a 24-well plate were lysed and harvested in Reporter Lysis Buffer (Promega). Extracts were also normalized by β-galactosidase activity using the Galacto-Light Plus β-Galactosidase Reporter Gene Assay System (Promega).
Statistical analysis
All experimental data are presented as the mean ± s.e.m. Statistical significance was determined by the Mann-Whitney U-test and Student's t-test. Significance was considered at P<0.05.
RESULTS
Deletion of mouse Gpr48 induces perinatal lethality and decreases in body size and bone length
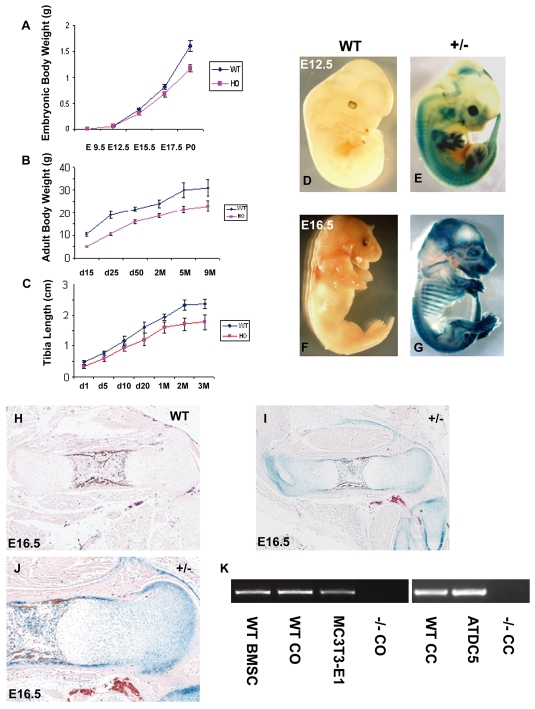
To explore the biological function of Gpr48 in vivo, we generated Gpr48 homozygous mutant mice by microinjecting gene trap-mutated Gpr48 embryonic stem (ES) cells into blastocysts of C57BL/6 mice (Weng et al., 2008). Gpr48+/- mice were phenotypically normal and fertile. However, ∼60% of the homozygous mutant mice died at day 1 or 2 after birth. Gpr48-/- mice were obtained with the expected Mendelian frequency. Gpr48-/- mice were significantly smaller than wild-type littermates during embryonic (starting from E15.5) and postnatal development (Fig. 1A-C). They exhibited prenatal (intra-uterus) and postnatal growth retardation and delay, which was characterized by a decrease in body weight and size, and the shortening of the long bones (e.g. tibia), suggesting that Gpr48 is involved in controlling the development of skeletal size.
Fig. 1.
Growth retardation of Gpr48 mutant mice and expression of Gpr48 in embryonic skeletal and bone cells. (A-C) Decrease in embryonic (A) and postnatal (B) body weight and long-bone length (C) in Gpr48-/- (HO) mice. (D-J) Whole-mount lacZ staining of E12.5 (D,E) and E16.5 (F,G) wild-type (WT) (D,F) and Gpr48+/- (E,G) mouse embryos. Tissue sections of femurs from E16.5 wild-type (H) and Gpr48+/- (I,J) mice. The wild-type bone does not show any lacZ staining (D,F,H). (K) RT-PCR analysis of Gpr48 expression in osteoblast and chondrocyte cells, showing bone marrow stromal cell (BMSC), calvarial osteoblast (CO), osteoblast cell line MC3T3-E1, primary cultured chondrocyte (CC) and chondrocyte cell line ATDC5 with Gpr48-/- calvarial osteoblast (CO) and chondrocyte (CC) as negative controls.
Expression of Gpr48 in the skeleton and bone cells
To understand the function of Gpr48 in bone formation and development, we first examined its developmental expression pattern in the skeletal tissues of Gpr48+/- mice between E12.5 and E16.5 by assessing β-galactosidase activity (lacZ expression), which derived from the correct insertion and fusion of a β-geo transcript in the gene trap vector (Fig. 1D-G). Whole-mount staining for β-galactosidase activity revealed strong lacZ staining in the cartilage, skull, ribs, spine and limbs of Gpr48+/- mice, but not in Gpr48+/+ mice (Fig. 1D,F), indicating high-level expression of Gpr48 in those tissues. Histological examination of femur tissue sections from E16.5 indicated strong expression of Gpr48 in the presumptive bone collar of the diaphyses, where vascular and osteoblast invasion was initiated, and in the primary spongiosa that constitute the precursors of eventual trabecular bone (Fig. 1I). Furthermore, expression of Gpr48 (lacZ-positive staining) could be detected in perichondrium, in resting, proliferating and hypertrophic zones during long-bone formation, as well as in the bone marrow cavity (Fig. 1J). The specificity of lacZ-positive staining was confirmed by the lack of staining in a Gpr48+/+ section (Fig. 1H). These expression patterns of Gpr48 in different tissues are in agreement with the findings of previous studies using different mouse backgrounds and insertion sites (Hoshii et al., 2007; Kato et al., 2006; Mazerbourg et al., 2004; Van Schoore et al., 2005).
To further examine the expression of Gpr48 in bone lineage cells, we performed RT-PCR analysis using Gpr48-specific primers in osteoblastic and chondrocytic lineage cells, including primary cultured bone marrow stromal cells (BMSCs), primary cultured calvarial osteoblasts (COs), the osteoblast cell line MC3T3-E1, primary cultured chondrocytes (CCs) and the chondrocyte cell line ATDC5. Our data indicate that Gpr48 is expressed in all of these cells (Fig. 1K). As a control, COs and CCs from Gpr48-/- mice exhibited no expression of Gpr48 (Fig. 1K), demonstrating the deletion of Gpr48 in osteoblasts and chondrocytes of Gpr48-/- mice.
Delayed embryonic bone formation in Gpr48-/- mice
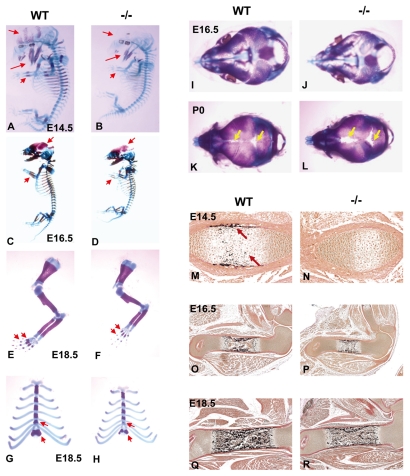
Analysis of skeletal preparations stained with Alizarin Red for mineralized tissue (bone) and Alcian Blue for cartilage revealed that at E14.5, E16.5 and E18.5, and at birth (P0), Gpr48-/- mice of either sex showed a marked reduction in the area of mineralized tissues compared with wild-type littermates (Fig. 2A-L). At E14.5, Gpr48+/+ mice already exhibited bone formation in limbs, jaw and calvarias, whereas little bone formation was observed in Gpr48-/- mice (Fig. 2A,B, red signal, arrowheads). A significant delay in bone formation was also observed in phalange and sternum at E16.5 and E18.5 in Gpr48-/- skeletal preparations (Fig. 2C-H, arrowheads). Furthermore, E16.5 Gpr48-/- mice exhibited a delay in mineralization of the skull, with frontal, parietal and interparietal bones of reduced size (Fig. 2J), resulting in a widening of cranial sutures and open fontanelles at birth (Fig. 2L, arrowheads).
Fig. 2.
Bone formation is delayed in Gpr48-/- embryos. (A-H) Whole skeletal preparation of wild-type (A,C,E,G) and Gpr48-/- mutant (B,D,F,H) mice at E14.5 (A,B), E16.5 (C,D) and E18.5 (E-H). Arrows indicate the delayed Alizarin Red staining (of bone) in the skull, jaw, sternum, clavicle, phalange and limb in Gpr48-/- embryos. (I-L) Effects of Gpr48 on intramembranous bone formation and skull ossification. Top view of the mouse skull at E16.5 (I,J) and P0 (K,L) for wild-type (I,K) and Gpr48-/- (J,L) mice after Alcian Blue/Alizarin Red staining. Arrows indicate the widening of cranial sutures and the opened fontanelles in Gpr48-/- newborn mice. (M-R) von Kossa staining of femur of wild-type (M,O,Q) and Grp48-/- (N,P,R) embryos at E14.5 (M,N) (20×), E16.5 (O,P) (5×) and E18.5 (Q,R) (5×). Arrows indicate that wild-type mice already show von Kossa signal at E14.5, but this is absent from Gpr48-/- mice.
As bone formation is related to bone mineralization, we examined the calcification status within the cartilaginous templates by von Kossa staining in wild-type and Gpr48-/- mouse embryos at E14.5 (Fig. 2M,N), E16.5 (Fig. 2O,P) and E18.5 (Fig. 2Q,R). Staining revealed significant calcification within the cartilaginous templates at E14.5 in wild-type, but not in Gpr48-/-, mice (Fig. 2M,N), indicating that Gpr48 has a significant effect on the time of initiation of mineralized osteogenesis. Together, these data suggest that Gpr48-/- mice have reduced formation of primary spongiosa (Fig. 2P,R), which may lead to bone shortening due to reduced endochondral ossification.
Gpr48 deletion has little effect on chondrocyte maturation and proliferation
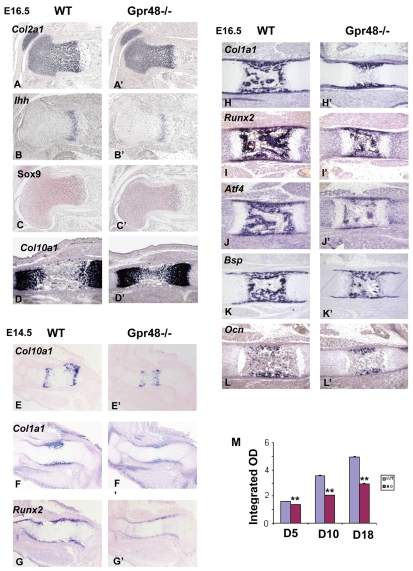
Skeletal preparations of E14.5 Gpr48-/- embryos showed delayed bone formation and mineralization, as assessed by Alizarin Red and von Kossa staining. As bone collar formation in the embryo is coupled with chondrocyte development and Gpr48 was expressed in the cartilage (Fig. 1J), we examined the status of chondrocyte maturation and proliferation in wild-type and Gpr48-/- littermates. Using in situ hybridization and immunohistochemistry, we examined the expression of four marker genes in chondrocytes at E16.5: type II collagen (Col2a1), a chondrocyte marker gene (Goldring et al., 2006); Indian hedgehog (Ihh), a gene that promotes cell cycle progression in proliferative chondrocytes and in the early stages of hypertrophic differentiation (Provot and Schipani, 2005); Sox9, a transcription factor essential for mesenchymal progenitor cell differentiation into chondrocytes (Lefebvre and Smits, 2005); and the type X collagen (Col10a1), a marker for mature hypertrophic chondrocytes. All four marker genes showed little change in Gpr48-/- as compared with wild-type embryos (Fig. 3A-D). To further examine whether Gpr48 affects chondrocyte maturation, we assessed Col10a1, Ihh and Pthrp1 (Pth1r) expression in E14.5 wild-type and Gpr48-/- embryos. Our data indicate that Gpr48 has little effect on chondrocyte maturation at E14.5 (Fig. 3E and see Fig. S1 in the supplementary material).
Fig. 3.
Deletion of Gpr48 has little effect on chondrocyte maturation, but delays osteoblast differentiation in embryonic bone. (A-D′) In situ hybridization analysis (A-B′,D,D′) and immunohistochemistry (C,C′) for chondrocyte differentiation and proliferation markers in wild-type and Gpr48-/- femur at E16.5. Probes are Col2a1 (A,A′), Ihh (B,B′), Col10a1 (D,D′) and Sox9 antibody (C,C′). (E-G′) In situ hybridization analysis of the chondrocyte and osteoblast differentiation markers Col10a1(E,E′), Col1a1 (F,F′) and Runx2 (G,G′) in E14.5 femur. (H-L′) In situ hybridization analysis of the osteoblast differentiation markers Col1a1 (H,H′), Runx2 (I,I′), Atf4 (J,J′), Bsp (K,K′) and Ocn (L,L′) in E16.5 femur. (M) Alkaline phosphatase (ALP) assays of primary cultured cavarial osteoblasts from wild-type (WT) and Gpr48-/- (HO) mice at day (D) 5, 10 and 18. **P<0.01.
To examine whether Gpr48 affects chondrocyte proliferation, we compared bromodeoxyuridine (BrdU) incorporation and proliferative cell nuclear antigen (PCNA) staining in wild-type and Gpr48-/- mice at E14.5 and E18.5. Our data indicate that there is no statistically significant difference in the number of proliferation-positive cells in Gpr48-/- versus wild-type mice (see Fig. S2 in the supplementary material). Together, our data suggest that the normal progression of chondrocyte cell proliferation and differentiation was unimpaired in Gpr48-/- mice.
Gpr48 regulates osteoblast differentiation in vivo and in vitro
To determine whether Gpr48 regulates osteoblast differentiation, we examined Col1a1 expression in the E14.5 mouse femur and observed that Col1a1-labeled osteoblast invasion of the cartilage mould was delayed in Gpr48-/- as compared with wild-type mice (Fig. 3F). Expression of Runx2, a transcription factor required for early differentiation from mesenchymal cells to osteoblasts (Kobayashi and Kronenberg, 2005), was also significantly decreased in the Gpr48-/- femur at E14.5 (Fig. 3G), suggesting that Gpr48-/- mice exhibit delayed perichondrial cell differentiation into osteoblasts. Similar results were observed with Col1a1 and Runx2 at E16.5 (Fig. 3H,I). In addition, we examined the expression levels of Atf4, Bsp and Ocn, three terminal differentiation markers of osteoblasts, at E16.5 (Yang et al., 2004). All these markers were significantly reduced in Gpr48-/- femurs (Fig. 3J-L). Since parathyroid hormone receptor (PTHR) is a known regulator of both chondrocyte differentiation and osteoblast/osteoclast function (Kronenberg, 2006; Schipani and Provot, 2003), we examined whether Gpr48 regulates the expression of Pthr1 and Pthrp (Pthlh) by RT-PCR. Our data indicate that deletion of Gpr48 has no significant effect on the expression of these two genes in primary cultured osteoblasts (see Fig. S3 in the supplementary material).
To further explore whether osteoblast differentiation capacity is impaired in Gpr48-/- mice, primary osteoblasts isolated from calvarias of wild-type and Gpr48-/- mice were cultured. Analysis of the alkaline phosphatase (ALP) activities of cultured cells differentiated for 5, 10 and 18 days showed significantly decreased ALP activity in Gpr48-/- osteoblasts during differentiation (Fig. 3M), suggesting that Gpr48 is required for osteoblast differentiation.
Gpr48 regulates bone formation and bone mass postnatally
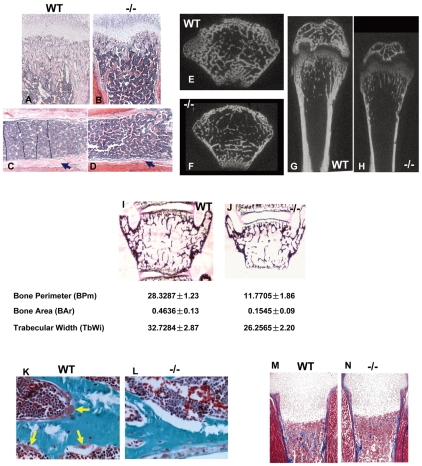
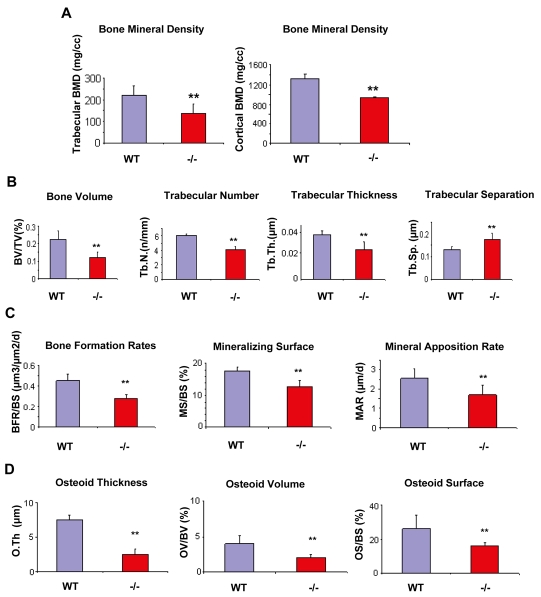
Since a significant number of Gpr48-/- mice lived beyond birth, we determined whether Gpr48 is required for bone homeostasis postnatally. Histological analysis of Gpr48-/- mice at P18 demonstrated a severe defect in bone formation, including an affect on the number and thickness of trabeculae of the trabecular bones (Fig. 4A,B). Furthermore, the cortical bone was much thinner in Gpr48-/- as compared with wild-type tibia (Fig. 4C,D, arrowheads). Quantitative micro-CT of the femur (Fig. 4E-H) and histomorphometric analyses of vertebrae (Fig. 4I,J) in 1-month-old Gpr48-/- mice demonstrated dramatic defects in the formation of trabeculae and in cortical bone volume. Specifically, the femur bone was much shorter, thinner, and had few trabecular bones, and the cortical bone was also much thinner (Fig. 4F,H). The trabecular and cortical BMD decreased by almost two-thirds in Gpr48-/- mice compared with wild-type littermates (Fig. 4E-H and Fig. 5A). From histomorphometric analyses, bone perimeter, bone area, trabecular width, BV/TV, trabecular number (Tb.N) and trabecular thickness (Tb.Th) were all significantly decreased, whereas trabecular separation (Tb.sp) was significantly increased in Gpr48-/- mice compared with the wild type (Fig. 4I,J and Fig. 5B). Kinetic indices of bone formation revealed a 50% reduction in the bone formation rate (BFR) owing to a reduction in the mineral apposition rate (MAR) (Fig. 5C). These data suggest that Gpr48 regulates both trabecular and cortical bone formation and plays important roles in the development of osteoporosis.
Fig. 4.
Gpr48 regulates bone formation postnatally. (A-D) H&E staining of tibia of wild-type (A,C) (10×) and Gpr48-/- (B,D) (10×) mice at P18. There is much less trabecular bone in Gpr48-/- than wild-type mice, and the cortical bone is much thinner in the mutant tibia (arrows). (E-H) Transverse (E,F) and frontal (G,H) microCT sections of distal and midfemoral diaphyses of 1-month-old wild-type and Gpr48-/- mice. (I,J) von Kossa staining of L3 vertebral bodies from 1-month-old wild-type and Gpr48-/- mice. There is a significant reduction in bone perimeter, bone area and trabecular width in Gpr48-/- mice. (K,L) Osteoid synthesis defect in Gpr48-/- mice demonstrated with Goldner staining (osteoid is stained red, yellow arrows). Deletion of Gpr48 significantly reduced osteoid formation (compare K with L). (M,N) Masson-Trichrome staining of osteoid (blue).
Fig. 5.
Quantitative analysis of the osteoblast defect in Gpr48-/- mice. (A) Both trabecular bone mineral density (BMD) and cortical BMD dramatically decreased in Gpr48-/- mice as assessed on microCT sections. (B) Deletion of Gpr48 leads to a reduction in bone volume/tissue volume (BV/TV), trabecular thickness, trabecular number, and increased trabecular separation as assessed by histomorphometric analysis. (C) Gpr48 regulates the kinetic indices of mineral apposition (MAR), bone formation rates (BFR/BS) and mineralizing surface (MS/BS). (D) Deletion of Gpr48 causes significant defects in osteoid characteristics, including decreased osteoid thickness (O.Th), osteoid surface (OS/BS) and osteoid volume (OV/BV). In each case, results show mean±s.d., n=3, age and sex matched; **P<0.01 in A-D.
Furthermore, in Gpr48-/- mice, the formation of osteoid was markedly reduced, as assessed by Goldner and Masson-Trichrome staining (Fig. 4K-N). Since osteoid thickness is determined by the rate of osteoid deposition by the osteoblasts and the rate of osteoid mineralization (Gal-Moscovici and Popovtzer, 2002), we quantitatively measured the osteoid thickness (O.Th), surface (OS/BS) and volume (OV/BV), and found that all were reduced in Gpr48-/- compared with wild-type control mice (Fig. 5D). The fact that Gpr48-/- mice failed to ever reach a normal bone mass indicates that the receptor is required for bone mass accrual postnatally. The bone mass defects were due in part to a failure to achieve terminal differentiation of osteoblasts, as shown by the decrease in Ocn and Bsp expression in Gpr48-deficient bones (Fig. 3K,L), as well as to a defect in osteoblast function, as shown by a 50% reduction in the rate of bone formation in adult Gpr48-deficient bones (Fig. 5C).
Gpr48 regulates osteoclast number and activity
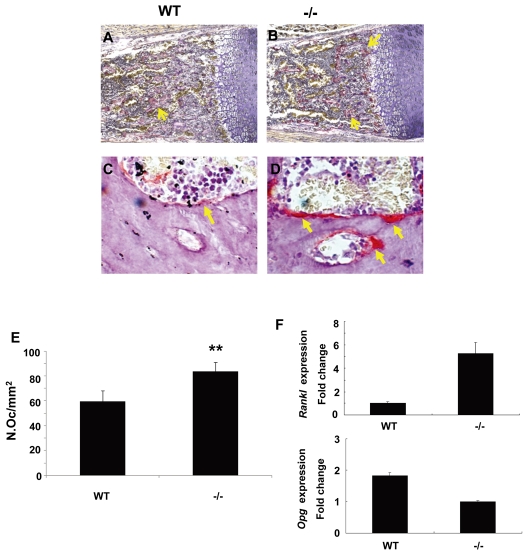
Bone remodeling is a complex physiological process involving bone formation by osteoblasts and bone resorption by osteoclasts. To understand how Gpr48 regulates bone resorption by osteoclasts, we performed tartrate-resistant acid phosphatase (TRAP) staining to examine osteoclast number and activity in wild-type and Gpr48-/- mice (Fig. 6A-F). Both the TRAP-positive cell number and activity (staining signal intensity) were increased in the proximal epiphysis of the tibia bone in E18.5 Gpr48-/- as compared with wild-type mice (Fig. 6A,B). To confirm this observation, we examined TRAP activity in the vertebrae of 1-month-old wild-type and Gpr48-/- mice. Here too, deletion of Gpr48 significantly increased TRAP-positive cell number and activity (Fig. 6C-E). These results suggest enhanced osteoclast differentiation and osteoclastic bone resorption in Gpr48-/- mice.
Fig. 6.
Activity of osteoclasts is increased in Gpr48-/- mice as assessed by TRAP staining. (A,B) TRAP staining of E18.5 tibia. TRAP-positive osteoclast cells (arrows) are in red. (C,D) TRAP staining of L3 vertebrae of 1-month-old mice. (E) The number of TRAP-positive osteoclasts per mm2 tissue area (N.Oc/mm2) is comparable in wild-type and Gpr48-/- mice. (F) Rankl and Opg expression levels in wild-type and Gpr48-/- osteoblasts as measured by Q-PCR.
To further understand the mechanism of Gpr48 action in osteoclast differentiation, we measured the expression levels of the receptor activator of nuclear factor-κB ligand (Rankl; Tnfsf11), and its decoy receptor osteoprotegerin (Opg; Tnfrsf11b), in osteoblasts. Our data indicate that Rankl expression significantly increased, whereas that of Opg decreased, in Gpr48-/- osteoblasts (Fig. 6F), suggesting that Gpr48 regulates osteoclastogenesis partially by modulating the Rankl/Opg ratio in osteoblasts.
Gpr48 activates the cAMP-PKA-CREB signaling pathway to regulate Atf4 expression in osteoblasts
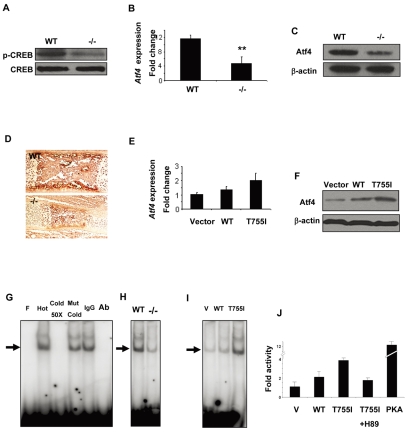
To understand the downstream signaling pathways regulated by Gpr48, we introduced various mutations in order to generate a constitutively active form of Gpr48. Among them, mutation at threonine 755 to isoleucine (T755I) of Gpr48 significantly increased the cAMP level by 3- to 4-fold (Weng et al., 2008). Increased cAMP levels can lead to the activation of cAMP-dependent protein kinase (PKA) and to the activation of the downstream transcription factor CREB (cAMP response element-binding protein) in the cell. To examine whether deletion of Gpr48 affects CREB phosphorylation and activation, we investigated the level of active phospho-CREB (phosphorylated at serine 133) in Gpr48-/- osteoblasts using a specific anti-phospho-CREB antibody in western blot analysis. Our data suggest that deletion of Gpr48 leads to an inhibition of CREB phosphorylation and activation in osteoblast cells (Fig. 7A).
Fig. 7.
Regulation of Atf4 expression by Gpr48 through the cAMP-PKA-CREB signaling pathway. (A) Phosphorylated CREB (at Ser133) protein is decreased in Gpr48-/- calvarial osteoblasts; total CREB provides a loading control. (B,C) Deletion of Gpr48 decreases the expression level of Atf4 in calvarial osteoblasts at P4 as assessed by Q-PCR (B) and western blot (C). **P<0.01. (D) Decrease in Atf4 protein in Gpr48-/- mice at E16.5 as assessed by immunohistochemistry with specific anti-Atf4 antibody. (E,F) Overexpression of Gpr48 and its constitutively active form, T755I, increased the Atf4 expression level as measured by Q-PCR (E) and western blot (F). (G-I) Gpr48 regulates the binding of CREB to the CRE site in the Atf4 promoter as assessed by EMSA. (G) Direct binding of CREB transcription factor to the CRE site in the Atf4 promoter. F, free probe; Hot, hot probe; Cold 50×, cold competitors at a 50-fold excess; Mut Cold, mutant cold competitors; Ab, CREB antibody. (H) Deletion of Gpr48 decreased CREB binding to the Atf4 promoter in osteoprogenitor cell nuclear extracts as measured by EMSA. (I) Overexpression of Gpr48 and T755I increases CREB binding to the Atf4 promoter in Gpr48-transfected cell nuclear extracts. (J) Activation of the Atf4 promoter by Gpr48 and T755I through the PKA pathway. A constitutively active PKA subunit was used as a strong activator of the promoter. A specific inhibitor of PKA, H89, abolished the Gpr48-activated promoter activity. V, vector control.
Since Gpr48-deficient mice have similar bone phenotypes to those observed in mice lacking Atf4 (Yang et al., 2004), we examined whether Gpr48 can directly or indirectly regulate Atf4, a key transcription factor in bone formation. Real-time PCR analyses demonstrated that the expression level of Atf4 mRNA was decreased by more than 50% in Gpr48-/- as compared with wild-type calvarial osteoblasts (Fig. 7B). This decrease in Atf4 mRNA was confirmed by in situ hybridization in Gpr48-/- mouse embryos (Fig. 3J). The expression of Atf4 protein was also significantly downregulated in Gpr48-/- osteoblasts, as determined by western blot analysis (Fig. 7C), and in the long bone of Gpr48-/- E16.5 embryos, as assessed by immunohistochemistry with a specific anti-Atf4 antibody (Fig. 7D). To test the idea that Gpr48 directly regulates the expression of Atf4 in vitro, we overexpressed the constitutively active form of Gpr48, Gpr48T755I, in osteoblast cell line MC3T3-E1 and then examined the expression level of Atf4. As shown in Fig. 7E,F, overexpression of wild-type Gpr48 or Gpr48T755I in MC3T3-E1 cells led to a significant increase in Atf4 expression, as measured by real-time PCR (Fig. 7E) and western blot (Fig. 7F) analyses. Together, these data demonstrate that the expression levels of both Atf4 mRNA and protein are significantly reduced in Gpr48-/- mice.
Activation of Atf4 transcription by Gpr48 through cAMP-PKA-CREB signaling
To examine how Gpr48 regulates Atf4 expression through the cAMP-CREB pathway, we analyzed the promoter region of Atf4 among different species. We found a conserved cAMP-responsive element (CRE) site (CREB binding site) at -921 CGTCA -917 in the Atf4 promoter. To determine whether Gpr48 regulates Atf4 expression through this CRE site, we performed an electrophoresis mobility-shift assay (EMSA) using specific probes from the Atf4 promoter region and osteoblast nuclear extracts. As shown in Fig. 7G, CREB was able to bind to the CRE site in Atf4. Only antibody specifically against CREB could inhibit the binding of osteoblast nuclear extracts to the CRE site (Fig. 7G), suggesting that CREB binds directly to the CRE site in the Atf4 promoter. To confirm this, we examined the binding of nuclear extracts prepared from primary culture osteoprogenitors of limb primordial cells isolated from E13.5 wild-type and Gpr48-/- mouse embryos. Our data demonstrate that deletion of Gpr48 in primary osteoprogenitor cells significantly reduced the binding of nuclear extracts to the Atf4 CRE site, as compared with nuclear extracts from wild-type mouse embryos (Fig. 7H). Also, overexpression of the constitutively active Gpr48T755I significantly increased the binding of CREB to this CRE site (Fig. 7I), supporting the idea that Gpr48 activates the cAMP-CREB signaling pathway to regulate Atf4 expression in osteoblasts.
To confirm that Gpr48 activates transcription of Atf4, we measured the promoter activity in the presence of Gpr48 using Atf4-luciferase reporter assays. Gpr48 and Gpr48T755I were able to directly activate the Atf4 promoter, which includes the CRE site. A constitutively active PKA catalytic subunit dramatically activated Atf4 promoter activity, whereas the specific PKA inhibitor, H89, abolished the Gpr48-activated promoter activity (Fig. 7J), suggesting that Gpr48 activates the Atf4 promoter through the PKA-mediated signaling pathway. Together, our data demonstrate that Gpr48 regulates Atf4 transcriptional activation and expression through the cAMP-PKA-CREB signaling pathway.
Gpr48 regulates the Atf4 target genes Ocn, Bsp and collagen
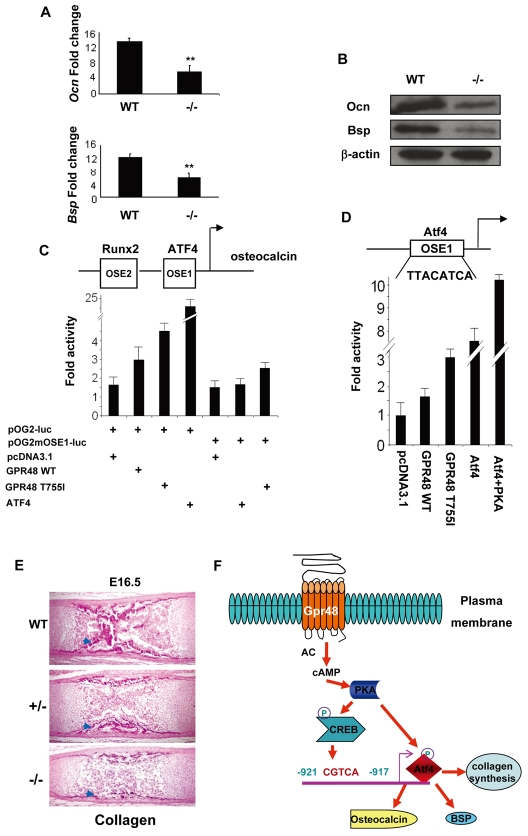
It is known that Atf4 is a crucial regulator of osteoblast differentiation and function, regulating the expression of downstream target genes such as Bsp, Ocn and collagens in mice (Yang et al., 2004). Since Gpr48 can directly regulate Atf4 expression, we examined whether Gpr48 regulates the downstream target genes that are directly modulated by Atf4. Real-time PCR array analyses indicate that the expression levels of both Ocn and Bsp decreased significantly (by 50%) in Gpr48-/- as compared with wild-type osteoblast cells (Fig. 8A). In situ hybridization analysis confirmed this finding in Gpr48-/- mouse long bones (Fig. 3K,L). Furthermore, the expression levels of the two proteins were significantly reduced in Gpr48-/- mice, as determined by western blot analyses with specific antibodies (Fig. 8B). These results suggest that Gpr48 regulates the expression of Atf4 target genes, such as Ocn and Bsp, in bone formation.
Fig. 8.
Gpr48 regulates Atf4 downstream target genes. (A,B) Downregulation of the Atf4 target genes Ocn and Bsp in Gpr48-/- osteoblasts as measured by Q-PCR (A) and western blot (B) analyses. **P<0.01. (C) Activation of the Ocn promoter by Gpr48 is abolished by a mutation at the Atf4 site (OG2mOSE1). (D) Gpr48 activation of Atf4 using p6OSE1-Luc as a reporter (100 ng). Atf4 alone, or co-expression of PKA and Atf4, strongly activated this reporter. (E) Collagen fibrils in Gpr48 wild-type, Gpr48+/- and Gpr48-/- E16.5 femur as revealed by van Gieson staining. Reduced numbers of van Gieson-stained collagen-rich trabeculae were observed in Gpr48+/- and Gpr48-/- (arrowheads). (F) Model of Gpr48-mediated signaling cascades in bone formation and remodeling. Gpr48 can activate the cAMP-PKA-CREB pathway to regulate Atf4 expression through CREB binding to the Atf4 promoter. PKA can also phosphorylate and activate Atf4 protein directly. Upregulation and activation of Atf4 lead to the expression of its downstream bone matrix target genes Ocn and Bsp and to collagen synthesis in bone formation.
To understand whether Gpr48 regulates Atf4-mediated transcriptional activation, we examined the effects of Gpr48 on the Ocn promoter, which has been extensively studied and shown to be regulated by Runx2 and Atf4 binding to OSE2 and OSE1, respectively (Fig. 8C) (Bidwell et al., 1993; Dobreva et al., 2006; Ducy and Karsenty, 1995; Ellies and Krumlauf, 2006; Yang et al., 2004). Gpr48 upregulated expression from the Ocn promoter-luciferase reporter gene containing both OSE2 and OSE1 (pOG2-luc) 3-fold in Cos7 cell transfection assays (Fig. 8C). However, a mutation in the OSE1 Atf4 binding site (pOG2mOSE1-luc) prevented upregulation by Gpr48 (Fig. 8C). This suggests that Gpr48 regulates the expression of Ocn via the transcription factor Atf4, and that the Atf4 binding site in the Ocn promoter is essential for activation by Gpr48.
To further examine whether the regulation of Ocn by Gpr48 is mediated through the Atf4 binding site in the promoter, we co-transfected wild-type Gpr48 and Gpr48T755I with the 6OSE1-luciferase construct, which contains six copies of the Atf4 binding site of the Ocn promoter fused to the luciferase reporter. As shown in Fig. 8D, co-expression of Gpr48 with the 6OSE1-luciferase reporter increased the luciferase activity by ∼3-fold in Cos7 cells. Atf4 alone, and co-expression of PKA and Atf4, were used as positive controls (Fig. 8D).
To obtain further evidence for the role of Gpr48 in Atf4-mediated bone formation, we examined the synthesis of collagen, another target protein of Atf4, in E16.5 wild-type, Gpr48+/- and Gpr48-/- femur bone. Using van Gieson reagent, which specifically stains collagen fibrils, we found a dramatic decrease in the collagen content of the Gpr48-/- femur as compared with the wild-type or heterozygous femur (Fig. 8E), suggesting that Gpr48 regulates Atf4-mediated collagen synthesis. Taken together, these data suggest that Gpr48 not only regulates the expression levels of bone matrix proteins (Ocn and Bsp), but also the synthesis of collagen fibrils through the cAMP-PKA-Atf4 signaling pathways.
DISCUSSION
GPCRs play key roles in a variety of physiological processes, from sensory systems to development. In this report, we demonstrate that a novel orphan GPCR, Gpr48, regulates bone formation and remodeling by promoting osteoblast differentiation and mineralization. More importantly, we demonstrate that Gpr48 controls the expression of the key transcription factor Atf4 and its downstream target genes and proteins (Ocn, Bsp and collagen) in bone formation via the cAMP-PKA-CREB signaling pathway (Fig. 8F). Our results are the first to suggest that this GPCR can regulate bone formation and remodeling and that it could be a potential therapeutic target for bone disorders.
Gpr48 and bone formation and remodeling
The integrity of vertebrate skeletal formation and remodeling is maintained by two distinct regulatory processes: the embryonic developmental and postnatal regulatory processes (Karsenty and Wagner, 2002). Gpr48 affects both aspects of bone formation. Gpr48 is expressed from E7.5, and Gpr48-/- mice show reduced mineralization in skull and limb at E14.5 as shown by Alizarin Red and von Kossa staining. The early differentiation marker Runx2, and the terminal differentiation markers Atf4, Ocn and Bsp, are all reduced in Gpr48-/- mice, whereas the chondrocyte proliferation and differentiation markers show no difference at E14.5, E16.5 and E18.5 between wild-type and Gpr48-/- mice. Gpr48-/- primary cultured osteoblasts also show differentiation and mineralization defects during differentiation. In postnatal Gpr48-/- mice, bone mass parameters, including the BMD and BV/TV, and kinetic indices of the bone formation rate and osteoid, are all dramatically decreased, suggesting direct involvement of Gpr48 in bone formation and remodeling through osteoblasts. Bone is constantly deposited by osteoblasts and degraded by osteoclasts, the balanced action of these two cell types being crucial for the normal homeostasis of the skeletal system in adults (Kim et al., 2009). The number and activity of osteoclasts, as assessed by TRAP staining, were increased in Gpr48-/- mice, both embryonically and postnatally, indicating that Gpr48 regulates bone remodeling through osteoclasts as well. The differentiation and activity of osteoclasts are regulated by the bone matrix-producing osteoblasts and by cytokines such as Opg and Rankl. To identify proteins produced by osteoblasts that regulate osteoclast formation, we compared the mRNA profiles of wild-type and Gpr48-/- osteoblasts, which showed that the Rankl/Opg ratio is markedly increased in the Gpr48-/- osteoblast. These results are consistent with those of the in vivo TRAP assay. However, besides the effects mediated by osteoblasts, the regulation of osteoclasts directly by Gpr48 cannot be excluded as Gpr48 is also expressed in osteoclasts (data not shown).
Our observations and those from the Nishimori group (Kato et al., 2006) have shown that Gpr48-/- mice present with renal hypoplasia and impaired kidney function, and renal phosphate and calcium transportation can affect bone mass (Bellorin-Font et al., 2003). Although we did not detect any significant change in serum calcium and phosphate in Gpr48-/- mice (see Fig. S4 in the supplementary material), we cannot rule out the possibility that Gpr48 might regulate urinary calcium and phosphate homeostasis and, thereby, bone mass remodeling in adult mice.
Regulation of cAMP-PKA-Atf4 pathways by Gpr48 in osteoblasts
We have shown that a novel orphan GPCR, Gpr48, operates in mammalian osteoblast precursors to promote embryonic and postnatal bone formation and remodeling. Activation of Gpr48 leads to an increase in cAMP levels and to the activation of PKA, which can phosphorylate and activate the transcription factor CREB. In this study, we demonstrate that CREB can bind to the promoter region (-921 CGTCA -917) of Atf4, activating the expression of Atf4 in osteoblasts. Furthermore, PKA can phosphorylate and activate Atf4 directly (Elefteriou et al., 2005). Atf4 is the key transcription factor in bone formation and remodeling. Gpr48-/- and Atf4-/- mice share numerous phenotypic and cellular abnormalities of the skeletal system (Yang et al., 2004). First, both Gpr48-/- and Atf4-/- mice have significantly decreased body weight and bone length in the embryo and adult. Second, Gpr48-/- and Atf4-/- mice have delayed bone formation from E14.5, including a lack of Alizarin Red staining in skull and limb, and a widening of the fontanelles in newborn mice. Third, Gpr48-/- and Atf4-/- mice have delayed mineralization, as assessed by von Kossa staining, and delayed expression of differentiation markers, as assessed by in situ hybridization. Fourth, the expression levels of the bone matrix genes Bsp and Ocn and the synthesis of collagen fibrils are significantly reduced in Gpr48-/- and Atf4-/- mice. Finally, the bone mass indicators and BFR are markedly decreased in both Gpr48-/- and Atf4-/- mice. The fact that Gpr48-/- and Atf4-/- mice fail to ever reach a normal bone mass indicates that both genes are required for bone mass formation. Besides the bone, other organs and systems, including the eye, hair, blood (anemia) and reproductive system, in which both proteins are expressed, may have similar phenotypes in Gpr48-/- and Atf4-/- mice (Hoshii et al., 2007; Jin et al., 2008; Masuoka and Townes, 2002; Mohri et al., 2008; Song et al., 2008; Weng et al., 2008).
Atf4 is a key transcription factor in the terminal differentiation of osteoblasts and regulates Ocn and Bsp in the terminal differentiation of osteoblasts. However, Atf4 does not affect Runx2 and osterix (Sp7), two key transcription factors required for early differentiation of mesenchymal cells into osteoblasts. In our studies, we demonstrate that Gpr48 can regulate both early and terminal differentiation markers such as Runx2, Ocn and Bsp, suggesting that other signaling pathways might be involved in Gpr48-mediated bone formation and remodeling. It is therefore possible that Gpr48 can initiate different intracellular signaling pathways at different development stages in response to their ligands and cellular environmental stimuli (George et al., 2002).
Gpr48 as a potential therapeutic target for osteoporosis
Several lines of evidence support a proposal that Gpr48-PKA-Atf4 signaling promotes osteoblast differentiation, bone formation from mesenchymal progenitors and osteoblast maturation. Thus, augmenting Gpr48-PKA-Atf4 signaling could be beneficial in treating osteoporosis. Furthermore, our data also show that both the number and activity of osteoclasts are increased in Gpr48-deficient mice, suggesting that Gpr48 has a negative effect on bone resorption. Therefore, agonists that activate Gpr48 signaling not only induce osteoblast differentiation and bone formation, but also suppress osteoclast function and bone resorption in osteoporosis patients. However, one must carefully evaluate the differential impacts of timing, cell type and strength of Gpr48 signaling on bone formation versus resorption, as activation of the Atf4 gene has been reported to be involved in cancer progression (Fels and Koumenis, 2006; Koumenis, 2006). The identification of Gpr48 agonists and antagonists in our future studies will provide new insights into the biology of this new hormone receptor and its role in human disease (Mak et al., 2008).
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/16/2747/DC1
Supplementary Material
We thank Dr G. Karsenty from the Department of Genetics and Development, College of Physicians and Surgeons, Columbia University, New York, for providing many probes and reagents used in our studies. This work is partially supported by the Research Platform of Cell Signaling Networks from the Science and Technology Commission of Shanghai Municipality (06DZ22923), the National Natural Science Foundation of China (No. 30800653) and a grant from the National Institutes of Health (1R01CA106479) to M.L. Deposited in PMC for release after 12 months.
References
- Akiyama, H., Lyons, J. P., Mori-Akiyama, Y., Yang, X., Zhang, R., Zhang, Z., Deng, J. M., Taketo, M. M., Nakamura, T., Behringer, R. R. et al. (2004). Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 18, 1072-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellorin-Font, E., Rojas, E., Carlini, R. G., Suniaga, O. and Weisinger, J. R. (2003). Bone remodeling after renal transplantation. Kidney Int. Suppl., S125-S128. [DOI] [PubMed]
- Bidwell, J. P., Van Wijnen, A. J., Fey, E. G., Dworetzky, S., Penman, S., Stein, J. L., Lian, J. B. and Stein, G. S. (1993). Osteocalcin gene promoter-binding factors are tissue-specific nuclear matrix components. Proc. Natl. Acad. Sci. USA 90, 3162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda, R., Castagnola, P., Cancedda, F. D., Dozin, B. and Quarto, R. (2000). Developmental control of chondrogenesis and osteogenesis. Int. J. Dev. Biol. 44, 707-714. [PubMed] [Google Scholar]
- Dobreva, G., Chahrour, M., Dautzenberg, M., Chirivella, L., Kanzler, B., Farinas, I., Karsenty, G. and Grosschedl, R. (2006). SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell 125, 971-986. [DOI] [PubMed] [Google Scholar]
- Ducy, P. and Karsenty, G. (1995). Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell. Biol. 15, 1858-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefteriou, F., Ahn, J. D., Takeda, S., Starbuck, M., Yang, X., Liu, X., Kondo, H., Richards, W. G., Bannon, T. W., Noda, M. et al. (2005). Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434, 514-520. [DOI] [PubMed] [Google Scholar]
- Ellies, D. L. and Krumlauf, R. (2006). Bone formation: the nuclear matrix reloaded. Cell 125, 840-842. [DOI] [PubMed] [Google Scholar]
- Erlebacher, A., Filvaroff, E. H., Gitelman, S. E. and Derynck, R. (1995). Toward a molecular understanding of skeletal development. Cell 80, 371-378. [DOI] [PubMed] [Google Scholar]
- Fels, D. R. and Koumenis, C. (2006). The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol. Ther. 5, 723-728. [DOI] [PubMed] [Google Scholar]
- Gal-Moscovici, A. and Popovtzer, M. M. (2002). Parathyroid hormone-independent osteoclastic resorptive bone disease: a new variant of adynamic bone disease in haemodialysis patients. Nephrol. Dial. Transplant. 17, 620-624. [DOI] [PubMed] [Google Scholar]
- Gensure, R. C., Gardella, T. J. and Juppner, H. (2005). Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem. Biophys. Res. Commun. 328, 666-678. [DOI] [PubMed] [Google Scholar]
- George, S. R., O'Dowd, B. F. and Lee, S. P. (2002). G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat. Rev. Drug Discov. 1, 808-820. [DOI] [PubMed] [Google Scholar]
- Goldring, M. B., Tsuchimochi, K. and Ijiri, K. (2006). The control of chondrogenesis. J. Cell. Biochem. 97, 33-44. [DOI] [PubMed] [Google Scholar]
- Hoshii, T., Takeo, T., Nakagata, N., Takeya, M., Araki, K. and Yamamura, K. (2007). LGR4 regulates the postnatal development and integrity of male reproductive tracts in mice. Biol. Reprod. 76, 303-313. [DOI] [PubMed] [Google Scholar]
- Hsu, S. Y., Liang, S. G. and Hsueh, A. J. (1998). Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol. Endocrinol. 12, 1830-1845. [DOI] [PubMed] [Google Scholar]
- Jin, C., Yin, F., Lin, M., Li, H., Wang, Z., Weng, J., Liu, M., Qu, J. and Tu, L. (2008). GPR48 regulates epithelial cell proliferation and migration by activating EGFR during eyelid development. Invest. Ophthalmol. Vis. Sci. 49, 4245-4253. [DOI] [PubMed] [Google Scholar]
- Kamenetsky, M., Middelhaufe, S., Bank, E. M., Levin, L. R., Buck, J. and Steegborn, C. (2006). Molecular details of cAMP generation in mammalian cells: a tale of two systems. J. Mol. Biol. 362, 623-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty, G. and Wagner, E. F. (2002). Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2, 389-406. [DOI] [PubMed] [Google Scholar]
- Kato, S., Matsubara, M., Matsuo, T., Mohri, Y., Kazama, I., Hatano, R., Umezawa, A. and Nishimori, K. (2006). Leucine-rich repeat-containing G protein-coupled receptor-4 (LGR4, Gpr48) is essential for renal development in mice. Nephron Exp. Nephrol. 104, e63-e75. [DOI] [PubMed] [Google Scholar]
- Kato, S., Mohri, Y., Matsuo, T., Ogawa, E., Umezawa, A., Okuyama, R. and Nishimori, K. (2007). Eye-open at birth phenotype with reduced keratinocyte motility in LGR4 null mice. FEBS Lett. 581, 4685-4690. [DOI] [PubMed] [Google Scholar]
- Kim, T., Kim, K., Lee, S. H., So, H. S., Lee, J., Kim, N. and Choi, Y. (2009). Identification of LRRc17 as a negative regulator of RANKL-induced osteoclast differentiation. J. Biol. Chem. 284, 15308-15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, T. and Kronenberg, H. (2005). Transcriptional regulation in development of bone. Endocrinology 146, 1012-1017. [DOI] [PubMed] [Google Scholar]
- Koumenis, C. (2006). ER stress, hypoxia tolerance and tumor progression. Curr. Mol. Med. 6, 55-69. [DOI] [PubMed] [Google Scholar]
- Kronenberg, H. M. (2006). PTHrP and skeletal development. Ann. New York Acad. Sci. 1068, 1-13. [DOI] [PubMed] [Google Scholar]
- Lefebvre, V. and Smits, P. (2005). Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res. C Embryo Today 75, 200-212. [DOI] [PubMed] [Google Scholar]
- Luo, C. W. and Hsueh, A. J. (2006). Genomic analyses of the evolution of LGR genes. Chang Gung Med. J. 29, 2-8. [PubMed] [Google Scholar]
- Mak, K. K., Bi, Y., Wan, C., Chuang, P. T., Clemens, T., Young, M. and Yang, Y. (2008). Hedgehog signaling in mature osteoblasts regulates bone formation and resorption by controlling PTHrP and RANKL expression. Dev. Cell 14, 674-688. [DOI] [PubMed] [Google Scholar]
- Manolagas, S. C. (2000). Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 21, 115-137. [DOI] [PubMed] [Google Scholar]
- Masuoka, H. C. and Townes, T. M. (2002). Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood 99, 736-745. [DOI] [PubMed] [Google Scholar]
- Mazerbourg, S., Bouley, D. M., Sudo, S., Klein, C. A., Zhang, J. V., Kawamura, K., Goodrich, L. V., Rayburn, H., Tessier-Lavigne, M. and Hsueh, A. J. (2004). Leucine-rich repeat-containing, G protein-coupled receptor 4 null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality. Mol. Endocrinol. 18, 2241-2254. [DOI] [PubMed] [Google Scholar]
- McLeod, M. J. (1980). Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 22, 299-301. [DOI] [PubMed] [Google Scholar]
- Mitchell, D. C., Stafford, L. J., Li, D., Bar-Eli, M. and Liu, M. (2007). Transcriptional regulation of KiSS-1 gene expression in metastatic melanoma by specificity protein-1 and its coactivator DRIP-130. Oncogene 26, 1739-1747. [DOI] [PubMed] [Google Scholar]
- Mohri, Y., Kato, S., Umezawa, A., Okuyama, R. and Nishimori, K. (2008). Impaired hair placode formation with reduced expression of hair follicle-related genes in mice lacking Lgr4. Dev. Dyn. 237, 2235-2242. [DOI] [PubMed] [Google Scholar]
- Provot, S. and Schipani, E. (2005). Molecular mechanisms of endochondral bone development. Biochem. Biophys. Res. Commun. 328, 658-665. [DOI] [PubMed] [Google Scholar]
- Schipani, E. and Provot, S. (2003). PTHrP, PTH, and the PTH/PTHrP receptor in endochondral bone development. Birth Defects Res. C Embryo Today 69, 352-362. [DOI] [PubMed] [Google Scholar]
- Song, H., Luo, J., Luo, W., Weng, J., Wang, Z., Li, B., Li, D. and Liu, M. (2008). Inactivation of G-protein coupled receptor 48 (Gpr48/Lgr4) impairs definitive erythropoiesis at midgestation through downregulation of ATF4 signaling pathway. J. Biol. Chem. 283, 36687-36697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryke, D., Kawamoto, M., Huang, C. C., Johns, S. J., King, L. A., Harper, C. A., Meng, E. C., Lee, R. E., Yee, A., L'Italien, L. et al. (2003). BayGenomics: a resource of insertional mutations in mouse embryonic stem cells. Nucleic Acids Res. 31, 278-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schoore, G., Mendive, F., Pochet, R. and Vassart, G. (2005). Expression pattern of the orphan receptor LGR4/GPR48 gene in the mouse. Histochem. Cell Biol. 124, 35-50. [DOI] [PubMed] [Google Scholar]
- Weng, J., Luo, J., Cheng, X., Jin, C., Zhou, X., Qu, J., Tu, L., Ai, D., Li, D., Wang, J. et al. (2008). Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proc. Natl. Acad. Sci. USA 105, 6081-6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, G., Jiang, D., Ge, C., Zhao, Z., Lai, Y., Boules, H., Phimphilai, M., Yang, X., Karsenty, G. and Franceschi, R. T. (2005). Cooperative interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J. Biol. Chem. 280, 30689-30696. [DOI] [PubMed] [Google Scholar]
- Yang, X., Matsuda, K., Bialek, P., Jacquot, S., Masuoka, H. C., Schinke, T., Li, L., Brancorsini, S., Sassone-Corsi, P., Townes, T. M. et al. (2004). ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell 117, 387-398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.