Abstract
Nm23-H1significantly reduces metastasis without effects on primary tumor size and was the first discovered metastasis suppressor gene. At least three mechanisms are thought to contribute to the metastasis-suppressive effect of Nm23-H1: (a) its histidine kinase activity toward ATP-citrate lyase, aldolase C, and the kinase suppressor of ras, with the last inactivating mitogen-activated protein kinase signaling; (b) binding proteins that titer out "free" Nm23-H1 and inhibit its ability to suppress metastasis; and (c) altered gene expression downstream of Nm23-H1, particularly an inverse association with the lysophosphatidic acid receptor endothelial differentiation gene-28 (EDG2). Most metastasis suppressor genes, including Nm23-H1, affect metastatic colonization, which is the outgrowth of tumor cells in distant locations; therefore, they are of high translational interest. A phase II trial is ongoing to test the hypothesis that a compound, high-dose medroxy-progesterone acetate (MPA), used as an unconventional gluocorticoid, will stimulate breast cancer cells to reexpress Nm23-H1and limit subsequent metastatic colonization.
Background
Most cancer patients die of metastatic disease, either by direct organ compromise, paraneoplastic syndromes, or treatment complications. Despite this, few metastasis-related molecular pathways have been translated to the clinic. The development of antimetastatic therapies is conceptually challenging: by the time a high-risk patient is diagnosed and the primary tumor is treated, it is likely that tumor cells have already invaded and are sitting in distant organs as occult micrometastases. Thus, invasion and other early parts of the metastatic process have been completed and may be irrelevant for therapeutic blockade. Metastatic colonization, the growth of tumor cells in a foreign environment to form detectable and life-threatening lesions, remains incomplete in many cancer patients and may serve as an important therapeutic target (1). Metastasis suppressor genes comprise a class of genes with activity in metastatic colonization that may be amenable to therapeutic development (2).
The first discovered metastasis suppressor gene was nm23. In a screen for genes differentially expressed between tumorigenic, metastatic murine melanoma cell lines and related tumorigenic nonmetastatic lines, nm23 expression was reduced in the highly metastatic samples (3, 4). Similar trends were identified in other model systems. Low Nm23-H1 expression in human tumors often correlated with poor patient survival (5–11) although it is not considered to be an independent prognostic factor. Importantly, the transfection of nm23 into highly metastatic K-1735 melanoma cells reduced their in vivo metastatic ability by 52% to 96%, with no effect on the primary tumor size (12). No difference in tumor cell proliferation was observed in vitro, in agreement with the primary tumor size data. Similar trends were observed in transfection experiments with breast, colon, oral, and hepatocellular carcinoma and melanoma cell lines (13–19). With the use of an adeno-associated virus gene therapy vector, an nm23-H1 construct was delivered into an ovarian carcinoma model of peritoneal metastasis. It was expressed by the tumor cells and significantly extended mouse survival (20). The metastasis-suppressive effects of Nm23-H1 were confirmed when Lacombe and colleagues developed an nm23 knockout mouse. When induced to develop hepatocellular cancer, the rate of tumor formation in the liver was unchanged between the knockout and wild-type mice; however, the knockout mice developed 2-fold more pulmonary metastases (21). Although eight human nm23 homologues have been identified, only H1 and H2 have been extensively studied for metastasis-related properties (22).
His tidine Kinase Activity
The first enzymatic activity of Nm23 is as a nucleoside diphosphate kinase and results from its autophosphorylation on an internal histidine, followed by the high-energy transfer of the phosphate to a nucleoside diphosphate in a reversible manner. No correlation of nucleoside diphosphate kinase activity and metastasis suppression has been observed, which leads to the conclusion that it is not likely involved in metastasis biology.
The Nm23-H1 phosphohistidine participates in another enzymatic activity, that of a histidine protein kinase. Histidine kinases are well known in bacteria and lower eukaryotes, in which they form a major signal transduction pathway to extracellular events (two-component systems). They are poorly studied in mammalian cells (23, 24) because the acid lability of histidine phosphate renders it invisible on most gels; phosphohistidine lacks an antibody, and it has high-energy, fast kinetics.
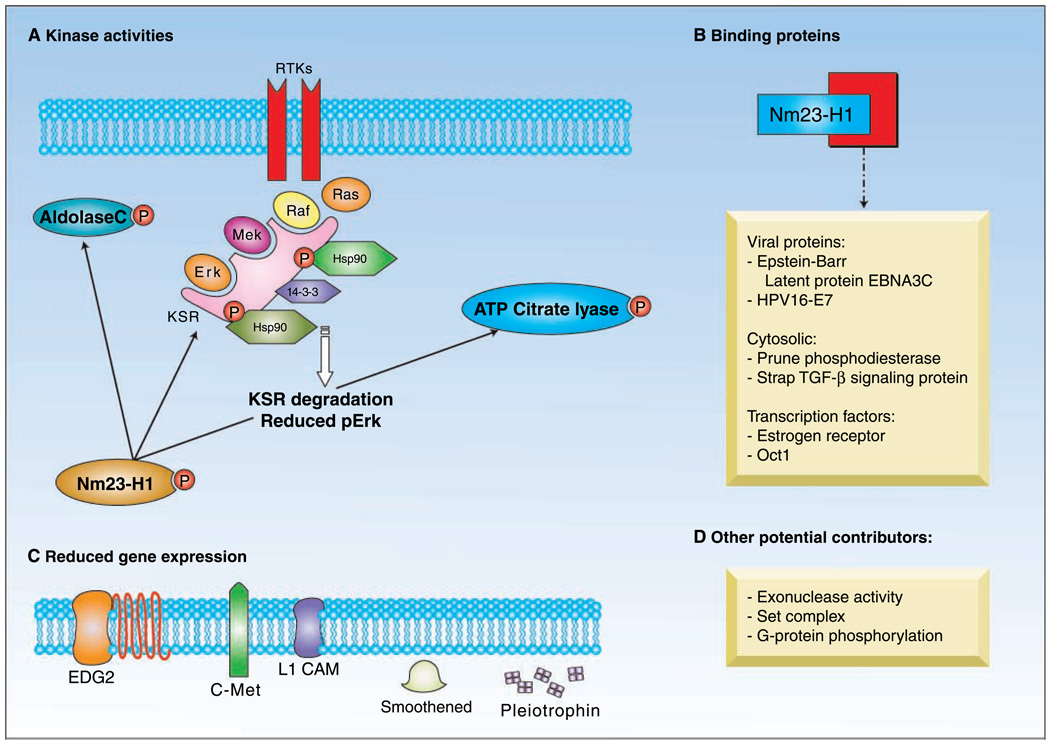
Three substrates for Nm23-H1 as a histidine kinase have been identified: ATP-citrate lyase, aldolase C, and the kinase suppressor of ras (Fig. 1A). ATP-citrate lyase (25) links lipogenesis to glucose metabolism (26). This enzyme has enjoyed a renaissance in the field of cancer energetics, with studies showing its potentiation of tumor growth (27). Nm23-H1 phosphorylates a histidine residue on ATP-citrate lyase; however, the physiologic effects of this phosphotransfer remain unknown. Aldolase C is best studied in the brain, where it protects cells from hypoxic stress (28). Aldolase C is the bestshown substrate of Nm23-H1 because it cannot autophosphorylate; Nm23-H1 phosphorylates an asparagine residue, a common substrate for histidine kinases in bacteria (29). Again, the physiologic consequences of this pathway are not worked out. The kinase suppressor of ras was identified as a potential substrate of Nm23-H1 through a homology search of two-component pathway substrates affecting the mitogen-activated protein (MAP) kinase pathway of the plant Arabidopsis. In mammalian cells, the kinase suppressor of ras is a scaffold for the extracellular signal-regulated kinase (ERK)/MAP kinase pathway; it assembles Raf, MEK, and MAP kinases, and accessory proteins into a physically tractable unit for efficient signaling. Nm23-H1 phosphorylated serines 392 and 434 of the kinase suppressor of ras, with a concomitant reduction in pErk activity in tumor cells (30). This seems to be an effect of the increased binding of heat shock protein 90 to the kinase suppressor of ras scaffold that results in the degradation of the kinase suppressor of ras (31). In agreement with these data, high Nm23-H1 expressing breast carcinoma cells were preferentially inhibited in colonization assays by the heat shock protein–90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG; ref. 31). Therefore, reduced metastatic colonization might result from low extracellular signal-regulated kinase activation due to scaffolding problems.
Fig. 1.
Nm23-H1suppression of tumor metastasis may depend on at least three pathways. A, Nm23-H1is a histidine protein kinase; its substrates include aldolase C, ATP-citrate lyase, and the Ksr, which is a scaffold for the MAP kinase pathway. Differential expression levels of Nm23-H1 result in altered binding of Hsp90 to Ksr, which leads to changes in its degradation and, consequently, ERK activation. B, binding proteins can titrate out free Nm23-H1, resulting in the loss of suppressive capacity.Viral proteins, the Pn protein, transcription factors, and TGF-β signaling intermediates bind Nm23-H1. C, a number of cell surface receptors and growth factors are up-regulated in expression as Nm23-H1is down-regulated. Of these, the receptor for serum LPA, EDG2, is capable of overcoming the Nm23-H1inhibition of both motility in vitro and metastasis in vivo. D, other activities for Nm23-H1 include a DNA exonuclease activity and a member of the set complex, which may potentially contribute to antimetastatic function. Ksr, kinase suppressor of ras; MAP, mitogen-activated protein; Hsp, heat shock protein; ERK, extracellular signal-regulated kinase; Pn, prune; TGF, transforming growth factor; LPA, lysophosphatidic acid.
It has been difficult to prove that the histidine kinase activity of Nm23-H1 is responsible for some or all of its metastasis-suppressive activity. In our laboratory, we made multiple attempts to obtain transfectants of a histidine-mutated Nm23-H1 in several tumor cell lines. We were, however, only able to obtain an occasional transfectant, thus suggesting that other mutations had also simultaneously occurred to permit viability. It would be interesting to determine if the histidine kinase inhibitors currently in development for the treatment of bacterial infections have antimetastatic activity.
Binding Proteins
High levels of Nm23-H1 protein do not completely inhibit metastasis or in vitro correlates of metastasis in any studied model system. The pathways that mediate metastasis are redundant; the inhibition of one pathway can be overcome by others that remain operative. The literature also suggests that Nm23-H1 may be present but functionally inactivated by binding proteins. The list of Nm23-H1 binding proteins is lengthy. However, the most relevant proteins are those that have been confirmed in both in vitro and in vivo experiments, in addition to those in which biological functions have been altered by the protein to protein interaction (Fig. 1B). Whereas none of the Nm23-H1 binding proteins have progressed to clinical testing, several have prompted new translational approaches to metastatic disease.
One of the best studied Nm23-H1 binding proteins is a latent protein encoded by the EBV nuclear antigen 3C. EBV is a common human virus that is associated with a number of human cancers, including Burkitt’s lymphoma, nasopharyngeal carcinoma, Hodgkin’s disease, and AIDS-associated and transplant-associated immunoblastic lymphoma. Some reports link EBV to breast (32) and gastric cancers (33) although this point is debated. Of the 90 genes encoded by the EBV genome, 9 are latently expressed. Of these, EBV nuclear antigen 1 stimulated the tumorigenicity and metastasis of a nasopharyngeal cell line (34). EBV nuclear antigen 3C, a transcription factor, interacts with Nm23-H1, which results in enhanced transactivating activity (35, 36). Both latent EBV proteins bind Nm23-H1 and inactivate its motility-suppressive effects (35). The expression of EBV nuclear antigens 1 and 3C in MDA-MB-231 human breast carcinoma cells increased ascites and lung metastases in vivo, which was reversed by Nm23-H1 overexpression (37). Similarly, the E7 oncoprotein of human papilloma virus binds Nm23-H1 and regulates tumor cell motility (38). The data suggest the very provocative hypothesis that viral proteins not only induce certain types of cancers but may be involved in their progression and highlight the potential importance of prophylactic vaccination strategies.
Another interesting Nm23-H1 binding protein is prune, which is studied in the Zollo laboratory. Homology searches revealed that prune shares domains with a family of phosphodiesterases. This activity was confirmed by the hydrolysis of the phosphodiester bonds of cyclic nucleotides by the recombinant prune protein. Prune overexpression in a breast cancer cell line stimulated its in vitro motility, which was abrogated by the simultaneous overexpression of Nm23-H1 (39). Nm23-H1 and prune were reported to bind in vitro (40), suggesting that each protein serves to titer out the "free" or bioavailable concentration of the other. Complex formation is regulated by the Nm23-H1 serine-phosphorylation status regulated by casein kinase I (41). Prune expression has been correlated with tumor aggressiveness in gastric cancer, breast cancer, and sarcoma (42–44). Dipyridamole and other inhibitors of prune phosphodiesterase activity suppress tumor motility in vitro and may have therapeutic potential (39).
Other reported Nm23-H1 binding proteins include the T-cell lymphoma invasion and metastasis 1, a guanine nucleotide exchange factor for Rac1 (45). Nm23-H1 also binds estrogen receptor α, which results in an altered pattern of estrogen-stimulated gene expression (46). Strap, a Ser-Thr kinase receptor–associated protein, binds the transforming growth factor-β receptor and inhibits its interaction with Smad7. The binding of Nm23-H1 to Strap enhanced its inhibition of transforming growth factor-β signaling (47). Proteins, such as integrin cytoplasmic domain–associated protein 1α, that tethers the β1-integrin cytoplasmic domain to control cell adhesion and motility (48) bind the H2 homologue of Nm23 that forms supercomplexes with Nm23-H1.
Because Nm23-H1 can be expressed but is biounavailable, the presence of binding proteins provides a mechanistic understanding of why Nm23-H1 expression is not of independent prognostic importance on multivariate analysis. A cautionary note: Nm23 proteins are sticky, and many nonspecific interactions have been reported with a single experimental approach. This stickiness also contributes to the difficulty in purifying Nm23 that has led to the publication of multiple activities that turned out to be contaminants.
Altered Gene Expression
A third piece of the mechanistic puzzle for metastasis suppression is changes in gene expression that result from low versus high levels of Nm23-H1 expression in tumor cells. With the use of microarrays, differential gene expression patterns were determined in control and nm23-H1-transfected breast carcinoma cell lines. The inordinate number of candidate genes was pared down with the use of gene expression data from two additional controls, breast cancer cell lines transfected with two mutations of Nm23-H1 that diminish its inhibition of tumor cell motility in vitro. Whereas few genes were up-regulated in this model system, a number of potentially important receptors and growth factors were down-regulated by wild-type, but not mutant Nm23-H1 expression, including c-Met (hepatocyte growth factor receptor), endothelial differentiation gene (EDG) 2 (lysophosphatidic acid receptor), L1CAM (cell adhesion molecule), Smoothened (part of the Hedgehog signaling pathway), Frizzled (part of the Wnt signaling pathway), and the growth factors connective-tissue growth factor and pleiotrophin. The relevance of the model system was confirmed when published breast cancer microarray databases were queried, and the mRNA expression of each protein was significantly inversely correlated with Nm23-H1 expression in human tumors. We asked if the reexpression of each protein in Nm23-H1 expressing, poorly motile breast carcinoma cells could overcome Nm23-H1 suppression and induce in vitro motility. Of these genes, only EDG2 restored motility to Nm23-H1–suppressed cells (49). To confirm and extend this observation, breast carcinoma cell lines that expressed Nm23-H1 and either a vector or EDG2 were tested for full metastatic ability in mice. EDG2 reexpression restored full metastatic ability to the Nm23-H1–expressing tumor cells (50). Again, the primary-tumor size was not significantly different. The initial steps of the metastatic colonization of the lungs were quantified by intravital microscopy on labeled tumor cells. Tumor cells that expressed Nm23-H1 and Nm23-H1/EDG2 arrived in the lungs at comparable rates after injection; however, EDG2 enhanced retention in the lungs 8-to 13-fold after several hours (50). The data establish that one mechanism of Nm23-H1 suppression of metastasis stems from its down-regulation of EDG2 expression.
EDG2 is a G-protein–coupled receptor for lysophosphatidic acid. Lysophosphatidic acid is a ubiquitous component of serum. Its levels were increased in pleural effusions of ovarian carcinoma (51) and exogenously supplied lysophosphatidic acid–enhanced metastasis in ovarian and colon carcinoma model systems (52, 53).
Other activities ascribed to Nm23-H1 include a DNA exonuclease (54) and granzyme A–activated DNase (55). The confirmation of these activities and a delineation of their mechanistic contribution to metastasis suppression are awaited.
Clinical-Translational Advances
Stimulation of Nm23-H1 expression in micrometastatic breast cancer
Suppressor-gene research has been notoriously difficult to translate to the clinic. When a panel of human breast carcinomas was interrogated for several characteristics of Nm23-H1, allelic deletion of the gene was noted; there were no mutations in the coding sequence. Only reduced Nm23-H1 expression, regardless of allelic deletion status, significantly correlated with poor patient survival (56). The data suggested that although nm23-H1 mutations are rare and that allelic deletions occur, Nm23-H1 protein levels were most important. This suggested that Nm23-H1 expression was simply "turned off" and that a compound could be identified to turn gene expression back on with potential therapeutic activity.
A number of compounds have been reported to stimulate Nm23-H1 expression in vitro. These include: (a) acetylsalicylic acid, which up-regulated Nm23 and down-regulated Bcl2 and CD44v6 expression in SW480 colon carcinoma cells, with antiproliferative and antiinvasive effects (57); (b) indomethacin, which stimulated Nm23 expression in normal mammary epithelial and MCF-7 ER+ cancer cells but not in metastatic MDA-MB-231 or MDA-MB-435 metastatic breast cancer cells (58); (c) gamma linolenic acid, which stimulated the Nm23 expression of HT-115 colon and MDA-MB-231 breast cancer cells and reduced their invasion (59); (d) all-trans-retinoic acid, which increased the Nm23-H1 expression of the 7721 hepatocellular carcinoma cell line and reduced its migration and invasion (60); (e) 1α-hydroxyvitamin D-5, which induced Nm23, intracellular adhesion molecule 1, and markers of differentiation in breast cancer cells (61); (f) trichostatin A, which up-regulated the Nm23-H1 expression of the MKN-1 and MKN-28 gastric cancer lines (62) but failed to up-regulate Nm23-H1 in metastatic breast cancer cells (63); and (g) 5-aza-deoxycytidine, which elevated the Nm23-H1 expression of two breast cancer cell lines with hypermethylated CpG islands in the nm23-H1 promoter (63). None of these compounds were expected to be specific for Nm23-H1 expression. The experience with the DNA methylation inhibitor 5-aza-deoxycytidine is illustrative of the potential problems in advancing cell line data. Whereas this agent reversed the DNA-methylation pattern of a CpG island in the nm23-H1 promoter in two metastatic breast carcinoma cell lines, the examination of multiple CpG islands in 20 human breast carcinomas found no differences in their DNA-methylation status regardless of whether the tumor cells expressed high or low Nm23-H1 protein levels (64).
The analysis of the nm23-H1 promoter revealed a 248 bp region that regulated reporter activity by 2- to 5-fold. The region contained a cassette of transcription-factor binding sites present in the mammary tumor virus–long terminal repeat, the whey-acid protein promoter, and the promoters of milk genes. The deletion of these sites reduced reporter expression and confirmed their functional involvement in regulating nm23-H1 expression (64). In the mammary tumor virus–long terminal repeat, this cassette of transcription factors is regulated by glucocorticoid response elements. In agreement with this prediction, dexamethasone elevated the Nm23-H1 expression of metastatic breast carcinoma cells when cultured in a corticosteroid-free medium; however, dexamethasone was ineffective at elevation of Nm23-H1 expression when the endogenous levels of corticosteroids in fetal bovine serum were present.
Medroxyprogesterone acetate (MPA) binds the progesterone, androgen, and glucocorticoid receptors; it binds the latter through both a conventional and a novel protein to protein interaction (65). MPA, at high doses, elevated the Nm23-H1 expression of progesterone receptor–negative (PR−) metastatic MDA-MB-231 and MDA-MB-435 breast carcinoma cell lines in vitro and inhibited their anchorage-independent colonization (66). To determine if Nm23-H1 induction or alternatively other effects were responsible for the inhibition of anchorage-independent growth by MPA, the MDA-MB-231 breast carcinoma cell line was transfected with an antisense nm23-H1 construct so that MPA could not elevate Nm23-H1 expression. Approximately 90% of the colonization-inhibitory effect of MPA was abrogated in the antisense transfectants, which indicated that the elevation of Nm23-H1 expression was a significant factor in this phenotypic effect of MPA (67). Other studies have reported that MPA can advance experimental mammary cancers. These effects are mediated through the progesterone receptor and use at least a log-higher concentration of MPA.
High-dose MPA was tested as a single agent and in combinations in advanced breast cancers as a hormonal treatment (reviewed in ref. 68). Although some responses were found, an optimal dose and schedule were never established favoring tamoxifen. Two of the older trials that used long-term MPA dosing reported 12-year and 13-year follow-up data, and a retrospective subset analysis suggested a benefit in postmenopausal patients. A total of 950 patients were randomized to chemotherapy [cyclophosphamide, methotrexate, and fluorouracil (CMF) in one trial; cyclophosphamide, doxorubicin, and fluorouracil (CAF) in the other] with or without a 6-month course of MPA, given in a 1-month induction, 5-month maintenance protocol (69, 70). In both trials, the MPA-treated postmenopausal subsets of patients had improved disease-free survival (cyclophosphamide, doxorubicin, and fluorouracil, P = 0.01; cyclophosphamide, methotrexate, and fluorouracil, P = 0.06 for node-negative and P = 0.002 for node-positive patients). Longer overall survival was noted in the postmenopausal arm (P = 0.02) for patients receiving CAF. Intriguingly, patient responses were not well correlated with progesterone receptor expression (69, 71, 72), which suggested that more recent data showing that MPA interacts with the glucocorticoid receptor (GR) may be relevant.
To test the hypothesis that high-dose MPA could stimulate the Nm23-H1 expression of breast cancer cells through the GR and inhibit metastatic colonization, estrogen-receptor–negative and progesterone-receptor–negative MDA-MB-231 breast cancer cells were injected into immunocompromised mice. Micrometastases formed in the lungs 4 weeks later, at which point mice were randomized to vehicle or MPA given on a 1-month induction and thereafter maintenance schedule. MPA reduced the number of gross pulmonary metastases by 33% to 62% as well as the incidence and size of metastases. Side effects were limited to weight gain; no difference in bone density, mammary histology, or lean to fat tissue ratio was noted (67).
Based on these data as well as the potential antiangiogenic effects of MPA, a phase II trial was initiated to test the new potential application of MPA. The primary objective is to determine the clinical benefit of MPA monotherapy and MPA+ low-dose oral cyclophosphamide and methotrexate (metronomic therapy, IdoCM) in postmenopausal patients with refractory-hormone receptor–negative metastatic breast cancer. A starting daily oral dose of 1 g MPA will be administered and increased to 1.5 g if serum concentrations are <50 ng/mL. In a second cohort, metronomic IdoCM will be administered based on its reported antiangiogenic activity (73). Preclinical studies suggested greater activity when metronomic chemotherapy is combined with a second antiangiogenic agent (74, 75).
Several pharmacodynamic measurements will estimate whether the Nm23-H1 pathway was affected in this trial. The formalin-fixed, paraffin-embedded block from the patient’s primary tumor will be stained for Nm23-H1 expression. Multiple studies indicate that metastases express Nm23-H1 levels that are either comparable or less than that of the matched primary tumor (76–80). Multiple skin biopsies will be obtained from consenting patients to determine if Nm23-H1 expression is elevated. An elevation in the Nm23-H1 expression of the basal skin layer was observed in preclinical studies.3 For the potential antiangiogenic effects of MPA, plasma thrombospondin and plasminogen activator inhibitor-1 levels will be studied.
Although MPA fell out of use in breast cancer in favor of tamoxifen, its use in endometrial cancer has continued. A recent multicenter study enrolled 45 young patients with endometrial cancer or atypical hyperplasia, with a daily oral dose of 600 mg MPA with low-dose aspirin. The study objective was to preserve fertility. Either estrogen-progestin therapy or fertility treatment was provided to responders after MPA therapy. The primary end point, pathologic complete response, was obtained in 55% of endometrial carcinoma cases and 82% of atypic hyperplasia cases. Two patients had grade 3 body weight gain, and one patient had grade 3 liver dysfunction. During a 3-year follow-up period, 12 pregnancies and 7 normal deliveries were achieved. Fourteen recurrences were found in 30 patients between 7 and 36 months (81). Similar results were reported in a second trial that used 400 mg daily (82).
Differential gene expression
Another exciting avenue of translation involves proteins whose expression is altered by low levels of Nm23-H1 expression. Data in mouse models and cohorts of human tumors indicate that low Nm23-H1– expressing breast tumors coordinately express high levels of the lysophosphatidic acid receptor EDG2 (49, 50). Lysophosphatidic acid is present at high levels in the bloodstream and could widely fuel metastatic colonization. EDG2 inhibitors may therefore represent attractive targets for breast cancer patients whose tumors express low Nm23-H1 levels. Ki16425 is an antagonist of EDG receptors, with submicromolar inhibition of EDG2 and EDG7 (83). In preclinical bone metastasis studies, animals with established MDA-BO2/green fluorescent protein bone metastases were treated daily from day 14 to 30 postinjection by 20 mg/kg Ki16425. An 81% reduction in bone lesion on X-ray and an absence of GFP signal was observed, with an 84% reduction in metastatic Ki-67 staining (84). This inhibitor and others aimed at EDG2 stand as candidates for advancement to trials.
Acknowledgments
Disclosure of Potential Conflicts of Interest
P.S. Steeg has a commercial research grant from GlaxoSmithKline.
Footnotes
Note: The deputy editor of Clinical Cancer Research is a coauthor of this paper. In keeping with the AACR’s Editorial Policy, a member of the AACR’s Publications Committee had the paper reviewed independently of the journal’s editorial process and made the decision whether to accept the paper.
P.S. Steeg, unpublished data.
References
- 1.Steeg P, Theodorescu D. Metastasis: a therapeutic target for cancer. Nature Clinical Practice Oncology. 2008;5:206–219. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steeg P. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer. 2003;3:55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- 3.Steeg PS, Bevilacqua G, Kopper L, et al. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst. 1988;80:200–204. doi: 10.1093/jnci/80.3.200. [DOI] [PubMed] [Google Scholar]
- 4.Rosengard AM, Krutzsch HC, Shearn A, et al. Reduced Nm23/Awd protein in tumor metastasis and aberrant Drosophila development. Nature. 1989;342:177–180. doi: 10.1038/342177a0. [DOI] [PubMed] [Google Scholar]
- 5.Heimann R, Ferguson D, Hellman S. The relationship between nm23, angiogenesis, and the metastatic proclivity of node-negative breast cancer. Cancer Res. 1998;58:2766–2771. [PubMed] [Google Scholar]
- 6.Hennessy C, Henry J, May FEB, Westly B, Angus B, Lennard TWJ. Expression of the antimetastatic gene nm23 in human breast cancer: an association with good prognosis. J Natl Cancer Inst. 1991;83:281–285. doi: 10.1093/jnci/83.4.281. [DOI] [PubMed] [Google Scholar]
- 7.Scambia G, Ferrandina G, Marone M, et al. Nm23 in ovarian cancer: correlation with clinical outcome and other clinico-pathological and biochemical prognostic markers. J Clin Oncol. 1996;14:334–342. doi: 10.1200/JCO.1996.14.2.334. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y-F, Chow K-C, Chang S-Y, et al. Prognostic significance of nm23-1 expression in oral squamous cell carcinoma. Br J Cancer. 2004;90:2186–2193. doi: 10.1038/sj.bjc.6601808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDermott NC, Milburn C, Curran B, Kay EW, Walsh CB, Leader MB. Immunohistochemical expression of nm23 in primary invasive malignant melanoma is predictive of survival outcome. J Pathol. 2000;190:157–162. doi: 10.1002/(SICI)1096-9896(200002)190:2<157::AID-PATH512>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 10.Pacifico MD, Grover R, Richman PI, Buffa F, Daley FM, Wilson GD. nm23 as a prognostic marker in primary cutaneous melanoma: evaluation using tissue microarray in a patient group with long-term follow-up. Melanoma Res. 2005;15:435–440. doi: 10.1097/00008390-200510000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Branca M, Giorgi C, Ciotti M, et al. Down-regulated nucleoside diphosphate kinase nm23-1 expression is unrelated to high-risk human papillomavirus but associated with progression of cervical intraepithelial neoplasia and unfavourable prognosis in cervical cancer. J Clin Pathol. 2006;59:1044–1051. doi: 10.1136/jcp.2005.033142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leone A, Flatow U, King CR, et al. Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell. 1991;65:25–35. doi: 10.1016/0092-8674(91)90404-m. [DOI] [PubMed] [Google Scholar]
- 13.Tagashira H, Hamazaki K, Tanaka N, Gao C, Namba M. Reduced metastatic potential and c-myc overexpression of colon adenocarcinoma cells (colon 26 line) transfected with nm23-2 rat nucleoside diphosphate kinase a isoform. Int J Mol Med. 1998;2:65–68. doi: 10.3892/ijmm.2.1.65. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki H, Fukuda M, Ishijima Y, et al. Overexpression of nm23-2/NDP kinase B in a human oral squamous cell carcinoma cell line results in reduced metastasis, differentiated phenotype in the metastatic site, and growth factor-independent proliferative activity in culture. Clin Cancer Res. 1999;5:4301–4307. [PubMed] [Google Scholar]
- 15.Liu F, Zhang Y, Zhang X-Y, Chen H-L. Transfection of the nm23-1 gene into human hepatocarcinoma cell line inhibits the expression of sialyl Lewis X, a1,3 fucosyltransferase VII, and metastatic potential. J Cancer Res Clin Oncol. 2002;128:189–196. doi: 10.1007/s00432-001-0314-1. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda M, Ishii A, Yasutomo Y, et al. Metastatic potential of rat mammary adenocarcinoma cells associated with decreased expression of nucleoside diphosphate kinase/nm23: reduction by transfection of NDP kinase a isoform, an nm23-2 gene homolog. Int J Cancer. 1996;65:531–537. doi: 10.1002/(SICI)1097-0215(19960208)65:4<531::AID-IJC23>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 17.Baba H, Urano T, Okada K, et al. Two isotypes of murine nm23/nucleoside diphosphate kinase, nm23-1 and nm23-2, are involved in metastatic suppression of a murine melanoma line. Cancer Res. 1995;55:1977–1981. [PubMed] [Google Scholar]
- 18.Parhar RS, Shi Y, Zou M, Farid NR, Ernst P, Al-Sedairy S. Effects of cytokine mediated modulation of Nm23 expression on the invasion and metastatic behavior of B16F10 melanoma cells. Int J Cancer. 1995;60:204–210. doi: 10.1002/ijc.2910600213. [DOI] [PubMed] [Google Scholar]
- 19.Leone A, Flatow U, VanHoutte K, Steeg PS. Transfection of human nm23-1 into the human MDA-MB-435 breast carcinoma cell line: effects on tumor metastatic potential, colonization, and enzymatic activity. Oncogene. 1993;8:2325–2333. [PubMed] [Google Scholar]
- 20.Li J, Zhou J, Chen G, et al. Inhibition of ovarian cancer metastasis by adeno-associated virus-mediated gene transfer of nm23-1in an orthotopic transplantation model. Cancer Gene Ther. 2006;13:266–270. doi: 10.1038/sj.cgt.7700899. [DOI] [PubMed] [Google Scholar]
- 21.Boissan M, Wendum D, Arnaud-Dabernat S, et al. Increased lung metastasis in transgenic NM23-Null/ SV40 mice with hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:836–845. doi: 10.1093/jnci/dji143. [DOI] [PubMed] [Google Scholar]
- 22.Lacombe M-L, Milon L, Munier A, Mehus J, Lambeth D. The human Nm23/nucleoside diphosphate kinases. J Bioenerg Biomembr. 2000;32:247–258. doi: 10.1023/a:1005584929050. [DOI] [PubMed] [Google Scholar]
- 23.Steeg P, Palmieri D, Ouatas T, Salerno M. Histidine kinases and histidine phosphorylated proteins in mammalian cell biology, signal transduction and cancer. Cancer Lett. 2003;190:1–12. doi: 10.1016/s0304-3835(02)00499-8. [DOI] [PubMed] [Google Scholar]
- 24.Besant PG, Attwood PV. Mammalian histidine kinases. Biochim Biophys Acta. 2005;1754:281–290. doi: 10.1016/j.bbapap.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Wagner P, Vu N-D. Phosphorylation of ATP-citrate lyase by nucleoside diphosphate kinase. J Biol Chem. 1995;270:21758–21764. doi: 10.1074/jbc.270.37.21758. [DOI] [PubMed] [Google Scholar]
- 26.Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9:358–365. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 27.Hatzivassiliou G, Zhao F, Bauer DE, et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Slemmer JE, Haasdijk ED, Engel DC, Plesnila N, Weber JT. Aldolase C-positive cerebellar Purkinje cells are resistant to delayed death after cerebral trauma and AMPA-mediated excitotoxicity. Eur J Neurosci. 2007;26:649–656. doi: 10.1111/j.1460-9568.2007.05708.x. [DOI] [PubMed] [Google Scholar]
- 29.Wagner P, Vu N-D. Histidine to aspartate phosphotransferase activity of nm23 protein: phosphorylation of aldolase C on Asp 319. Biochem J. 2000;346:623–630. [PMC free article] [PubMed] [Google Scholar]
- 30.Hartsough M, Morrison D, Salerno M, et al. Nm23-1 metastasis suppressor phosphorylation of kinase suppressor of ras (KSR), via a histidine protein kinase pathway. J Biol Chem. 2002;277:32389–32399. doi: 10.1074/jbc.M203115200. [DOI] [PubMed] [Google Scholar]
- 31.Salerno M, Palmieri D, Bouadis A, Halverson D, Steeg P. Nm23-1 metastasis suppressor expression level influences the binding properties, stability and function of the kinase suppressor of Ras (KSR1) Erk scaffold in breast carcinoma cells. Mol Cell Biol. 2005;25:1379–1388. doi: 10.1128/MCB.25.4.1379-1388.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins RS, Sahm K, Marando C, et al. Analysis of Epstein-Barr virus reservoirs in paired blood and breast cancer primary biopsy specimens by real time PCR. Breast Cancer Res. 2006;8:R70. doi: 10.1186/bcr1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung IM, Chung JK, Kim YA, et al. Epstein-Barr virus, β-catenin, and E-cadherin in gastric carcinomas. J Korean Med Sci. 2007;22:855–861. doi: 10.3346/jkms.2007.22.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheu LF, Chen A, Meng CL, et al. Enhanced malignant progression of nasopharyngeal carcinoma cells mediated by the expression of Epstein-Barr nuclear antigen 1 in vivo. J Pathol. 1996;180:243–248. doi: 10.1002/(SICI)1096-9896(199611)180:3<243::AID-PATH655>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 35.Subramanian C, Cotter M, Robertson E. Epstein-Barr virus nuclear protein EBNA-3C interacts with the human metastatic suppressor Nm23-1: a molecular link to cancer metastasis. Nat Med. 2001;7:350–355. doi: 10.1038/85499. [DOI] [PubMed] [Google Scholar]
- 36.Subramanian C, Robertson ES. The metastatic suppressor Nm23-1interacts with EBNA3C at sequences located between the glutamine- and proline-rich domains and can cooperate in activation of transcription. J Virol. 2002;76:8702–8709. doi: 10.1128/JVI.76.17.8702-8709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaul R, Murakami M, Choudhuri T, Robertson ES. Epstein-Barr virus latent nuclear antigens can induce metastasis in a nude mouse model. J Virol. 2007;81:10352–10361. doi: 10.1128/JVI.00886-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mileo AM, Piombino E, Severino A, Tritarelli A, Paggi MG, Lombardi D. Multiple interference of the human papillomavirus-16 E7 oncoprotein with the functional role of the metastasis suppressor Nm23-1 protein. J Bioenerg Biomembr. 2006;38:215–225. doi: 10.1007/s10863-006-9037-y. [DOI] [PubMed] [Google Scholar]
- 39.D’Angelo A, Garzia L, Andre A, et al. Prune cAMP phosphodiesterase binds nm23-1and promotes cancer metastasis. Cancer Cell. 2004;5:137–149. doi: 10.1016/s1535-6108(04)00021-2. [DOI] [PubMed] [Google Scholar]
- 40.Reymond A, Volorio S, Merla G, et al. Evidence for interaction between human Prune and nm23-1 NDPkinase. Oncogene. 1999;18:7244–7252. doi: 10.1038/sj.onc.1203140. [DOI] [PubMed] [Google Scholar]
- 41.Garzia L, D’Angelo A, Amoresano A, et al. Phosphorylation of nm23-1by CKI induces its complex formation with h-prune and promotes cell motility. Oncogene. 2008;27:1853–1864. doi: 10.1038/sj.onc.1210822. [DOI] [PubMed] [Google Scholar]
- 42.Forus A, D’Angelo A, Henriksen J, et al. Amplification and overexpression of PRUNE in human sarcomas and breast carcinomas-a possible mechanism for altering the nm23-1 activity. Oncogene. 2001;20:6881–6890. doi: 10.1038/sj.onc.1204874. [DOI] [PubMed] [Google Scholar]
- 43.Oue N, Yoshida K, Noguchi T, Sentani K, Kikuchi A, Yasui W. Increased expression of h-prune is associated with tumor progression and poor survival in gastric cancer. Cancer Sci. 2007;98:1198–1205. doi: 10.1111/j.1349-7006.2007.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zollo M, Andre A, Cossu A, et al. Overexpression of h-prune in breast cancer is correlated with advanced disease status. Clin Cancer Res. 2005;11:199–205. [PubMed] [Google Scholar]
- 45.Otsuki Y, Tanaka M, Yoshii S, Kawazoe N, Nakaya K, Sugimura H. Tumor metastasis suppressor nm23H1 regulates Rac GTPase by interaction with Tiam1. Proc Natl Acad Sci U S A. 2001;98:4385–4390. doi: 10.1073/pnas.071411598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curtis CD, Likhite VS, McLeod IX, Yates JR, Nardulli AM. Interaction of the tumor metastasis suppressor nonmetastatic protein 23 homologue H1 and estrogen receptor-α alters estrogen-responsive gene expression. Cancer Res. 2007;67:10600–10607. doi: 10.1158/0008-5472.CAN-07-0055. [DOI] [PubMed] [Google Scholar]
- 47.Seong HA, Jung H, Ha H. NM23-1 tumor suppressor physically interacts with serine-threonine kinase receptor-associated protein, a transforming growth factor-β (TGF-β) receptor-interacting protein, and negatively regulates TGF-β signaling. J Biol Chem. 2007;282:12075–12096. doi: 10.1074/jbc.M609832200. [DOI] [PubMed] [Google Scholar]
- 48.Fournier H, Dupe-Manet S, Bouvard D, et al. Integrin cytoplasmic domain-associated protein 1a (ICAP-1a) interacts directly with the metastasis suppressor nm23-2, and both proteins are targeted to newly formed cell adhesion sites upon integrin engagement. J Biol Chem. 2002;277:20895–20902. doi: 10.1074/jbc.M200200200. [DOI] [PubMed] [Google Scholar]
- 49.Horak CE, Lee JH, Elkahloun AG, et al. Nm23-1 suppresses tumor cell motility by down-regulating the lysophosphatidic acid receptor EDG2. Cancer Res. 2007;67:7238–7246. doi: 10.1158/0008-5472.CAN-07-0962. [DOI] [PubMed] [Google Scholar]
- 50.Horak CE, Mendoza A, Vega-Valle E, et al. Nm23-1 suppresses metastasis by inhibiting expression of the lysophosphatidic acid receptor EDG2. Cancer Res. 2007;67:11751–11759. doi: 10.1158/0008-5472.CAN-07-3175. [DOI] [PubMed] [Google Scholar]
- 51.Westermann AM, Havik E, Postma FR, et al. Malignant effusions contain lysophosphatidic acid (LPA)-like activity. Ann Oncol. 1998;9:437–442. doi: 10.1023/a:1008217129273. [DOI] [PubMed] [Google Scholar]
- 52.Kim KS, Sengupta S, Berk M, et al. Hypoxia enhances lysophosphatidic acid responsiveness in ovarian cancer cells and lysophosphatidic acid induces ovarian tumor metastasis in vivo. Cancer Res. 2006;66:7983–7990. doi: 10.1158/0008-5472.CAN-05-4381. [DOI] [PubMed] [Google Scholar]
- 53.Shida D, Kitayama J, Yamaguchi H, et al. Lysophosphatidic acid transactivates both c-Met and epidermal growth factor receptor, and induces cyclooxygenase-2 expression in human colon cancer LoVo cells. World J Gastroenterol. 2005;11:5638–5643. doi: 10.3748/wjg.v11.i36.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma D, McCorkle JR, Kaetzel DM. The metastasis suppressor NM23-1possesses 3’-5’ exonuclease activity. J Biol Chem. 2004;279:18073–18084. doi: 10.1074/jbc.M400185200. [DOI] [PubMed] [Google Scholar]
- 55.Fan Z, Beresford P, Oh D, Zhang D, Lieberman J. Tumor suppressor NM23-1 is a granzyme A-activated DNase during CTL-mediated apoptosis, and the nucleosome assembly protein SET is its inhibitor. Cell. 2003;112:659–672. doi: 10.1016/s0092-8674(03)00150-8. [DOI] [PubMed] [Google Scholar]
- 56.Cropp C, Lidereau R, Leone A, et al. NME1 protein expression and loss of heterozygosity mutations in primary human breast tumors. J Natl Cancer Inst. 1994;86:1167–1169. doi: 10.1093/jnci/86.15.1167. [DOI] [PubMed] [Google Scholar]
- 57.Yu H-G, Huang J-A, Yang Y-N, et al. The effects of acetylsalicylic acid on proliferation, apoptosis, and invasion of cyclooxygenase-2 negative colon cancer cells. Eur J Clin Invest. 2002;32:838–846. doi: 10.1046/j.1365-2362.2002.01080.x. [DOI] [PubMed] [Google Scholar]
- 58.Natarajan K, Mori N, Artemov D, Bhujwalla Z. Exposure of human breast cancer cells to the antiinflammatory agent indomethacin alters choline phospholipid metabolites and Nm23 expression. Neoplasia. 2002;4:409–416. doi: 10.1038/sj.neo.7900252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang W, Hiscox S, Bryce R, Horrobin D, Mansel R. The effects of n-6 polyunsaturated fatty acids on the expression of nm-23 in human cancer cells. Br J Cancer. 1998;77:731–738. doi: 10.1038/bjc.1998.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu F, Qi H-L, Chen H-L. Effects of all-trans retinoic acid and epidermal growth factor on the expression of nm23-1 in human hepatocarcinoma cells. J Cancer Res Clin Oncol. 2000;126:85–90. [PubMed] [Google Scholar]
- 61.Mehta R, Bratescu L, Graves J, Green A, Mehta R. Differentiation of human breast carcinoma cells by a novel vitamin D analog: 1 α-hydroxyvitamin D-5. Int J Oncol. 2000;16:65–73. doi: 10.3892/ijo.16.1.65. [DOI] [PubMed] [Google Scholar]
- 62.Yasui W, Oue N, Ono S, Mitani Y, Ito R, Nakayama H. Histone acetylation and gastrointestinal carcinogenesis. Ann N Y Acad Sci. 2003;983:220–231. doi: 10.1111/j.1749-6632.2003.tb05977.x. [DOI] [PubMed] [Google Scholar]
- 63.Hartsough M, Clare S, Mair M, et al. Elevation of breast carcinoma nm23-1metastasis suppressor gene expression and reduced motility by DNA methylation inhibition. Cancer Res. 2001;61:2320–2327. [PubMed] [Google Scholar]
- 64.Ouatas T, Clare S, Hartsough M, DeLaRosa A, Steeg P. MMTV-associated transcription factor binding sites increase nm23-1 metastasis suppressor gene expression in human breast carcinoma cell lines. Clin Exp Metastasis. 2002;19:35–42. doi: 10.1023/a:1013897022827. [DOI] [PubMed] [Google Scholar]
- 65.Bamberger C, Else T, Bamberger A, Beil F, Shulte H. Dissociative glucocorticoid activity of medroxyprogesterone acetate in normal human lymphocytes. J Biol Chem. 1999;84:4055–4061. doi: 10.1210/jcem.84.11.6091. [DOI] [PubMed] [Google Scholar]
- 66.Ouatas T, Halverson D, Steeg P. Dexamethasone and medroxyprogesterone acetate elevate Nm23-1 metastasis suppressor expression in metastatic human breast carcinoma cells: new uses for old compounds. Clin Cancer Res. 2003;9:3763–3772. [PubMed] [Google Scholar]
- 67.Palmieri D, Halverson D, Ouatas T, et al. Medroxyprogesterone acetate elevation of Nm23-1metastasis suppressor expression in hormone receptor-negative breast cancer. J Natl Cancer Inst. 2005;97:632–642. doi: 10.1093/jnci/dji111. [DOI] [PubMed] [Google Scholar]
- 68.Stockler M, Wilcken N, Ghersi D, Simes R. Systematic reviews of chemotherapy and endocrine therapy in metastatic breast cancer. Cancer Treat Rev. 2000;26:151–168. doi: 10.1053/ctrv.1999.0161. [DOI] [PubMed] [Google Scholar]
- 69.Focan C, Beauduin M, Salamon E, et al. Adjuvant high dose medroxyprogesterone acetate for early breast cancer: 13 years update in a multicentre randomized trial. Br J Cancer. 2001;85:1–8. doi: 10.1054/bjoc.2001.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hupperets P, Wils J, Volovics L, et al. Adjuvant chemo-hormonal therapy with cyclophosphamide, doxorubicin and 5-fluroruacil (CAF) with or without medroxyprogesterone acetate (MPA) for node-positive cancer patients, update at 12 years follow-up. The Breast. 2001;10:35–37. doi: 10.1054/brst.2000.0180. [DOI] [PubMed] [Google Scholar]
- 71.Hori T, Kodama H, Nishimura S, et al. A randomized study comparing oral and standard regimens for metastatic breast cancer. Oncol Rep. 2001;8:1067–1071. doi: 10.3892/or.8.5.1067. [DOI] [PubMed] [Google Scholar]
- 72.Byrne MJ, Gebski V, Forbes J, et al. Medroxyprogesterone acetate addition or substitution for tamoxifen in advanced tamoxifen-resistant breast cancer: a phase III randomized trial. Australian-New Zealand Breast Cancer Trials Group. J Clin Oncol. 1997;15:3141–3148. doi: 10.1200/JCO.1997.15.9.3141. [DOI] [PubMed] [Google Scholar]
- 73.Colleoni M, Rocca A, Sandri MT, et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13:73–80. doi: 10.1093/annonc/mdf013. [DOI] [PubMed] [Google Scholar]
- 74.Klement G, Baruchel S, Rak J, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Browder T, Butterfield CE, Kraling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 76.Guan-Zhen Y, Ying C, Can-Rong N, Guo-Dong W, Jian-Xin Q, Jie-Jun W. Reduced protein expression of metastasis-related genes (nm23, KISS1, KAI1 and p53) in lymph node and liver metastases of gastric cancer. Int J Exp Pathol. 2007;88:175–183. doi: 10.1111/j.1365-2613.2006.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarris M, Lee C. nm23 protein expression in colorectal carcinoma metastasis in regional lymph nodes and the liver. EJSO. 2001;27:170–174. doi: 10.1053/ejso.2000.1070. [DOI] [PubMed] [Google Scholar]
- 78.Kawakubo Y, Sato Y, Koh T, Kono H, Kareya T. Expression of nm23 protein in pulmonary adenocarcinomas: inverse correlation to tumor progression. Lung Cancer. 1997;17:103–113. doi: 10.1016/s0169-5002(97)00653-3. [DOI] [PubMed] [Google Scholar]
- 79.Kanitakis J, Euvrard S, Bourchany D, Faure M, Claudy A. Expression of the nm23 metastasis-suppressor gene product in skin tumors. J Cutan Pathol. 1997;24:151–156. doi: 10.1111/j.1600-0560.1997.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 80.Terasaki-Fukuzawa Y, Kijima H, Suto A, et al. Decreased nm23 expression, but not Ki-67 labeling index, is significantly correlated with lymph node metastasis of breast invasive ductal carcinoma. Int J Mol Med. 2002;9:25–29. [PubMed] [Google Scholar]
- 81.Ushijima K, Yahata H, Yoshikawa H, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25:2798–2803. doi: 10.1200/JCO.2006.08.8344. [DOI] [PubMed] [Google Scholar]
- 82.Yamazawa K, Hirai M, Fujito A, et al. Fertility-preserving treatment with progestin, and pathological criteria to predict responses, in young women with endometrial cancer. Hum Reprod. 2007;22:1953–1958. doi: 10.1093/humrep/dem088. [DOI] [PubMed] [Google Scholar]
- 83.Ohta H, Sato K, Murata N, et al. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- 84.Boucharaba A, Serre CM, Guglielmi J, Bordet JC, Clezardin P, Peyruchaud O. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci U S A. 2006;103:9643–9648. doi: 10.1073/pnas.0600979103. [DOI] [PMC free article] [PubMed] [Google Scholar]