Abstract
Previous studies have indicated the chemoprevention potential of citrus limonoids due to the induction of phase II detoxifying enzymes. In the present study, three citrus limonoids were purified and identified from sour orange seeds as limonin, limonin glucoside (LG), deacetylnomilinic acid glucoside (DNAG). In addition, limonin was modified to defuran limonin and limonin 7-methoxime. The structures of these compounds were confirmed by NMR studies. These five compounds were used to investigate the influence of Phase II enzymes in female A/J mice. Our results indicated that the highest induction of Glutathione S-Transferase (GST) activity against 1-chloro-2, 4-dinitrobenzene (CDNB) by DNAG (67%) in lung homogenates followed by limonin-7-methoxime (32%) in treated liver homogenates. Interestingly, the limonin-7-methoxime showed the highest GST activity (270%) in liver against 4-nitroquinoline 1-oxide (4NQO), while the same compound in stomach induced GST by 51% compared to the control. DNAG treated group induced 55% in stomach homogenates. Another Phase II enzyme, quinone reductase (QR), was significantly induced by limonin-7-methoxime by 65 and 32% in liver and lung homogenates, respectively. Defuran limonin, induced QR in lung homogenates by 45%. Our results indicated that modification of the limonin have differential induction of phase II enzymes. These findings are indicative of a possible mechanism for the prevention of cancer by aiding in detoxification of xenobiotics.
Keywords: Citrus, mice, chemoprevention, bioactive compounds, xenobiotics
1. INTRODUCTION
Health maintaining properties of citrus fruits have recently been promoted due to their potential benefits on cancer prevention based on cell culture and animal studies (1, 2). Current research has transitioned from the study of classical vitamin deficiency related diseases such as scurvy to the study of thousands of bioactive compounds that may have important role in prevention of several diseases such as cancer, heart and Alzheimer’s. Initial research on citrus limonoids was initiated to ameliorate the bitterness problem in citrus juice due to the bitter limonoid aglycones, limonin and nomilin (3).
Limonoids are a group of structurally related triterpene derivatives found in plant families such as Rutaceae and Meliaceae (4). Citrus limonoids are composed of two main nucleus structures. The structure of limonin exemplifies the first general nucleus which consists of five rings designated as A, A’, B, C, and D. The second limonoid structure, nomilin, consists of four rings designated as A, B, C, and D.
Role of limonoids on human health and their biological activities have been demonstrated in our lab (2, 5-7) and elsewhere (8-10). Citrus limonoids have been attributed to lowering cholesterol levels by reducing the production of medium apo B in cultured human liver cells HepG2 (9). Furthermore, a recent report provided evidence that limonin and nomilin have the ability of inhibiting HIV-1 replication (10). During the last decade, the chemopreventive properties of citrus limonoids have been explored using animal models. In two Japanese studies, it was reported that orange juice and citrus limonoids, obacunone and limonin, played an important role in the inhibition of azoxymethane-induced colon cancer (8). Our recent study provided supporting evidence that bioactive compounds found in grapefruit, one of them being limonin, protect against azoxymethane induced aberrant crypt foci (11). Inhibition of 7, 12-dimethylbenz[a]anthracene-induced oral tumors by citrus limonoids has also been reported (3, 12). Additionally, benzo[a]pyrene induced forestomach neoplasia in mice was inhibited by citrus limonoids (13).
In the present study, we have analyzed the induction of phase II detoxification enzymes, GST and QR by three limonoids purified from Citrus aurantium. In addition, limonin was modified to defuran limonin and limonin 7-methoxime and used for structural-activity relationship studies of phase II enzymatic activity.
2. MATERIALS AND METHODS
2.1. Materials
β-nicotanamide adenine dinucleotide phosphate reduced tetrasodium salt, 2,6-dichlorophenol-indophenol, 1-chloro-2,4-dinitrobenzene, 4-nitroquinoline 1-oxide, glutathione reduced, flavin adenine dinucleotide disodium salt, and all solvents were purchased from Sigma-Aldrich company (St. Louis, MO). Bovine Serum Albumin was obtained from Intergen (Purchase, NY). 1H and 13C Nuclear Magnetic Resonance spectra were recorded at 400 and 100 MHz, respectively, on a JEOL NMR instrument (JEOL USA, Inc., MA, USA). Tetramethylsilane (TMS) was used as internal standard.
2.2. Purification of limonoids
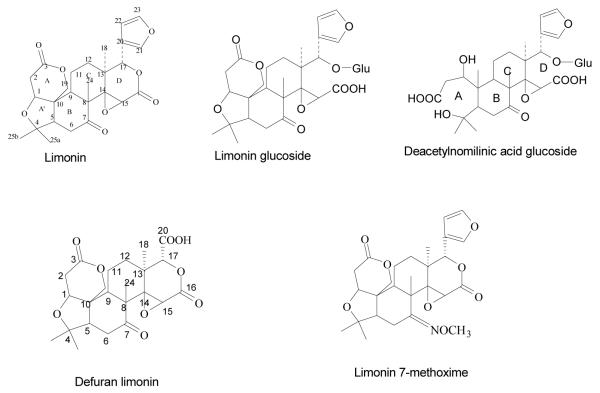
Citrus seeds (Citrus aurantium L.) were collected from the Texas A&M University-Kingsville Citrus Center Orchard, Weslaco, TX. Dried seeds were powdered (2.2 Kg) and extracted with hexane (4 L) for the removal of fatty acids. Then, the defatted powder was extracted with ethyl acetate (EtOAc) (4 L) and methanol (4 L) for 8 h separately. The extract was filtered and concentrated under vacuum to obtain a viscous liquid. Dried EtOAc extract was impregnated with silica gel and loaded to silica gel column chromatography. The silica column was eluted with different concentrations of mobile phase (chloroform and acetone) to obtain limonin. MeOH extract was fractionated on amberlite XAD-2 column and elution was carried out with increasing concentration of MeOH in water to obtain LG and DNAG (Figure 1) as per our earlier publication (14).
Figure 1.
Structure of purified limonoids from citrus and modified compounds
2.3. Preparation of limonin-7-methoxime
Two grams of limonin was dissolved in pyridine (42 mL), and methylhydoxylamine hydrochloride (2.45g) was dissolved in absolute ethanol (42 mL). Both solutions were then transferred to the round bottom flask and the reaction mixture was refluxed for 6 h at 75°C-80°C and cooled to room temperature. The completion of the reaction was determined by the disappearance of the limonin spot on TLC using chloroform:acetone (9:1) as mobile phase. Saturated solution of sodium chloride was added to the cold reaction mixture until the complete precipitation of pyridinium chloride, which was removed by filtration. The filtrate was extracted using ethyl acetate (4 × 50 mL). The pooled ethyl acetate layer was washed with 2N HCl (4 × 50 mL), followed by a washing with sodium bicarbonate (2 × 50 mL), and finally with water. The organic phase was concentrated under vacuum to obtain precipitate. Further purification was performed by silica gel column chromatography. The column was eluted with hexane and ethyl acetate as mobile phase. Limonin-7-methoxime was eluted at 30% ethyl acetate in hexane.
2.4. Preparation of defuran limonin
Mix limonin (1.5 g) acetonitrile (23 mL), carbon tetrachloride (23 mL) and water (33 mL) in a 100 mL round bottom flask and the reaction mixture was kept for stirring at room temperature for 10 min. Then, sodium periodate (9 g) and ruthenium trichloride (220 mg) were added and stirring was continued until the completion of the reaction (∼40 h). The reaction was monitored by TLC. The reaction mixture was extracted with ethyl acetate in a separating funnel, decolorized using activated charcoal, filtered and precipitated using hexane to obtain defuran limonin.
2.5. HPLC analysis
The purity of the isolated compounds were analyzed by high performance liquid chromatography using Agilent 1200 Series (Foster City, CA, USA) consists of degasser, quaternary pump, auto sampler, column oven and diode array detector as per our published method (15). The compounds were eluted using C18 Phenomenex Gemini series column (Torrence, CA, USA), 5 μm particle size, (250 × 4.6 mm) with a flow rate of 1.0 mL/min. The column temperature was set at 27 °C. The elution was carried out with gradient mobile phase (A). 3mM phosphoric acid and (B). acetonitrile, starting at 85 % of solvent A, reduced to 77 % in 5 min, 74 % after 25 min, which was further reduced to 60 % at 30 min and completing the gradient at 54 % at the end of 60 min. The column was equilibrated for 5 min with 85% solvent A and 15% solvent B before next run. The purity of the compounds were calculated using calibration curves as per our method (15).
2.6. Identification
The structures of the purified limonoids and modified compounds were elucidated by 1H, 13C NMR and DEPT studies using JEOL ECS-400 spectrometer at 298K using 5 mm broadband probe equipped with shielded z- gradient and Delta software version 4.3.6 using TMS as an internal reference. One-dimensional 1H and 13C spectra were obtained using one pulse sequence. One-dimensional 13C spectra using Distortionless Enhancement of Polarization Transfer (DEPT-135) using a 135 degree decoupler pulse was also performed to aid the structure. Mass spectrometric analyses were performed using ThermoFinnigan LCQ-DECA instrument (Thermo, San Jose, CA, USA).
2.7. Animal Studies
The animal studies model described by Lam et al. (13) was adapted to investigate the Phase II enzyme induction in mice. Female A/JOlaHsd 8-9 weeks old mice were purchased from Harlan Sprague-Dawley Laboratory (Indianapolis, IN). The mice were kept on AIN-76 semi-purified custom diet without vitamin E obtained from MPBiomedical (Solon, OH) and tap water ad libitum. Vitamin E was excluded from diet due to its ability to induce phase II enzymatic activity. The mice were housed in plastic cages in an environmentally controlled room on a 12 h light/12 h dark cycle.
The mice were divided (n=4) into five experimental groups and one control group. The treatments consisted of limonin, LG, DNAG, limonin-7-methoxime, and defuran limonin. Each compound (20 mg) was suspended in dimethyl sufoxide (DMSO): corn oil (1:1) (v/v) and administered by oral gavages for every two days. A total of five treatments were administered in 10 days. The control group was given the equivalent amount of DMSO: corn oil (1:1) without compounds. After 48h of the last treatment the mice were sacrificed by cervical dislocation. Lung, intestine, stomach, and liver were harvested and washed with cold phosphate buffer solution (PBS). A portion of the tissue was stored for future use, while the remaining portion was weighed and homogenized using a Pro200 homogenizer with PBS (10mM, pH 7.0), containing β-mercaptoethanol (1.4mM) to obtain 10% (w/v) homogenate. The homogenate was centrifuged at 22,000 × g for 45 min in a Beckman Avanti 30 centrifuge at 4°C, the supernatant was carefully removed and stored in -20°C until further use. Each organ within a group is homogenized under same conditions and the homogenates were used for bioassays.
2.8. Determination of enzyme activities and protein concentration
GST activity against CDNB was determined as described by Habig et al., (16) with slight modifications. One mL enzyme assay consisted of 100 mM phosphate assay buffer, (pH 6.5), 20 mM CDNB and 10 mM GSH and 50 μl of tissue homogenate sample. The absorbance was measured at 340 nm using Beckman DU 640 UV/Visible spectrophotometer against blank. Blank consisting of assay buffer, GSH and CDNB, without tissue homogenate in a total volume of 1mL .
GST activity against 4NQO was performed using a modified method developed by Stanley et al (17). In this reaction the glutathione replaces the nitro group of 4NQO. The 1mL enzyme assay consisted of 100 mM phosphate assay buffer pH 6.5; 5 mM 4NQO and 10 mM GSH and 20 μl of tissue homogenate. The absorbance was measured at 350 nm in a Beckman DU 640 UV/ Visible spectrophotometer against an appropriate blank consisting of assay buffer, 4NQO and GSH without tissue homogenate in a total volume of 1mL .
The QR activity was determined by slight modification of the method reported by Wang et al (18). The 1mL enzyme assay system consisted of 25 mM Tris/ HCl pH 7.5; 0.18 mg/mL BSA; 5 μM FAD; 0.2 mM NAD(P)H; 40 μM DCPIP and 20 μl of tissue homogenate. The blank contained all the above except the tissue homogenate and 0.2 mM NAD(P)H, while the control contained all except the enzyme sample. The absorbance was measured at 600 nm.
The spectrophotometer was equipped with enzyme kinetic software and programmed to calculate enzyme units. The amount of enzyme used was 1 μmole of substrate per min at 25 °C is equivalent to one unit of enzyme activity. The protein content of the samples were quantified by Bradford’s method (19). The absorbance was read at 595 nm. Bovine serum albumin was used as a standard.
2.9. Statistical Analysis
Each treatment group consisted of four mice from which lungs, intestine, stomach and liver were extracted. Each organ homogenate from each mouse represented one sample; all assays were performed in triplicates. The results were expressed as average of three replications Student’s t-test was used to asses the significance of the data.
3. RESULTS
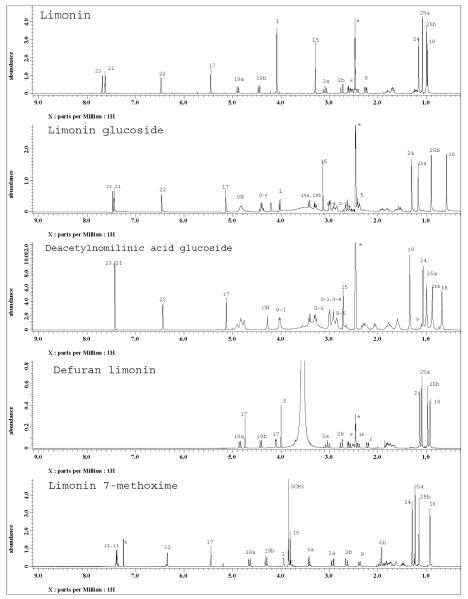
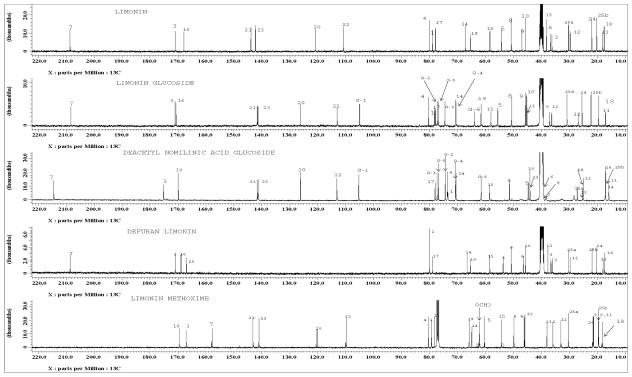
Ethyl acetate extract of Citrus aurantium L. provided three pure compounds. The purity of the isolated limonoids were analyzed by HPLC and chromatograms are presented in Figure S1. Along with three natural limonoids, limonin was modified to defuran limonin and limonin methoxime study the GST activity. The purity of the modified compounds were analyzed by HPLC using calibration graphs similar to our published paper (15). The resulting chromatograms are presented in Figure S2. The structures of the purified limonoids and modified compounds have been elucidated by MS, 1H, 13C NMR and DEPT studies. The proton and 13C spectra of five compounds with assignments of various signals are shown in Figure 2 and 3 respectively. On the basis of NMR results, the structures of the isolated compounds have been identified as limonin, limonin glucoside and deacetyl nomilinic acid glucoside. In addition, mass spectra of the modified limonoids reported the molecular ion peak at m/z 506.2294 [M + Li]+ and 447.1145 [M-H]- for limonin-7-methoxime and defuran limonin respectively. Modified compounds also confirmed as defuran limonin and limonin methoxime by 1H and 13C NMR spectra (Figure 2 and 3). Moreover, chemical shifts of isolated compounds were compared with reported values (20, 21).
Figure 2.
1H NMR spectra of purified compounds from Citrus aurantium and modified compounds recorded on JEOL ECS-400 MHz spectrometer. Assignments of various protons are made on the respective spectrum for each compound, G-1 to G-6 indicates the glucose signals. Peaks from DMSO-d6 and CDCl3 are marked with asterisks.
Figure 3.
13C NMR spectra of purified compounds from Citrus aurantium and modified compounds recorded on JEOL ECS spectrometer at 100 MHz. Peaks from DMSO-d6 and CDCl3 are marked with asterisks. Assignments of various carbons are made on the respective spectrum for each compound, G-1 to G-6 indicates the glucose signals.
Three purified citrus limonoids and two modified limonoids (Figure 1) were tested for induction of phase II enzymatic activity. The GST activities were assayed using CDNB and 4NQO as substrates. Induction of QR was also evaluated. Mice were treated with the five limonoids in order to evaluate the induction of GST activity against CDNB. In lung, DNAG was the only limonoid showed significant induction (67%) of GST activity compared to the control. Interestingly, in liver, modified methoxylated limonin-7-methoxime showed significant induction of GST activity, while LG and DNAG showed a decrease in activity (Table 1).
Table 1.
GST activity against 1-chloro-2, 4-dinitrobenzene†
| Sample | Stomach | Intestine | Liver | Lung |
|---|---|---|---|---|
| Control | 0.88 ± 0.07 | 0.86 ±0.09 | 1.78 ± 0.02 | 0.36 ± 0.08 |
| Limonin | 0.83 ± 0.13 | 0.63 ± 0.01 | 1.63 ± 0.27 | 0.34 ± 0.05 |
| Limonin-7- methoxime |
0.89 ± 0.18 | 1.34 ± 0.47 | 2.36 ±0.05** | 0.36 ± 0.03 |
| Defuran limonin | 0.86 ± 0.12 | 0.79 ± 0.09 | 1.45± 0.54 | 0.34 ± 0.08 |
| LG | 0.86 ± 0.13 | 0.75 ± 0.06 | 1.41 ± 0.08* | 0.44± 0.09 |
| DNAG | 1.15 ± 0.38 | 0.70 ± 0.13 | 1.35 ± 0.20* | 0.60 ± 0.12** |
Results are means ±SD (n=4)
specific activity (units/mg protein)
indicates statistically significant (p<0.05) decrease of activity using student’s t-test.
indicates statistically significant (p<0.05) induction of activity using student’s t-test.
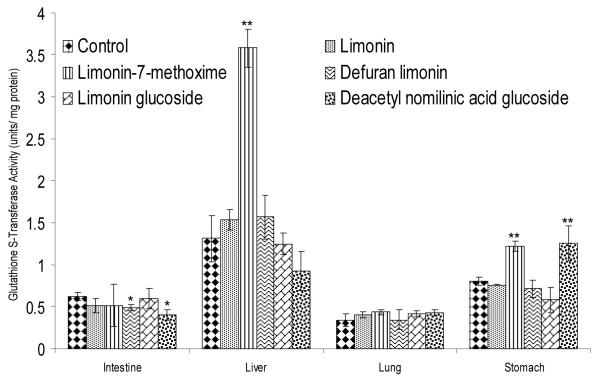
GST activity was also measured against 4NQO. In stomach homogenates, DNAG (55%) showed the highest induction of GST activity, followed by limonin-7-methoxime (51%). In liver homogenates, limonin-7-methoxime induced GST activity was three times higher (270%) than that of the control. In intestine homogenates, defuran limonin and DNAG decreased GST activity, while no activity change was observed in lung homogenates (Figure 4).
Figure 4.
GST activity in different organ homogenates against 4-Nitroquinoline 1-oxide, a potent xenobiotic tumorigenic to lung, esophagus, forestomach, glandular stomach, skin and other organs. Bars indicate mean ± S.D. (n=4). * indicate statistically significant (p<0.05) decrease in activity. ** indicate statistically significant (p<0.05) induction using student’s t-test.
Quinone reductase activity was measured in lung, intestine, liver and stomach homogenates. In liver and lung, limonin-7-methoxime showed significant induction of QR 65% and 32%, respectively compared to control. In intestine and stomach homogenates, limonin-7-methoxime increased QR activity, but induction was not statistically significant. In lung homogenates, defuran limonin showed the highest induction (45%) activity followed by limonin methoxime. Interestingly, DNAG decreased QR activity in lung. No significant change in activity was observed in stomach homogenates (Table 2).
Table 2.
Quinone reductase activity†
| Sample | Stomach | Intestine | Liver | Lung |
|---|---|---|---|---|
| Control | 8.30 ± 1.73 | 2.33 ± 0.27 | 0.52 ± 0.04 | 0.26 ± 0.05 |
| Limonin | 8.56 ± 1.58 | 2.47 ± 0.72 | 0.38 ± 0.10 | 0.29 ± 0.04 |
| Limonin-7- methoxime |
10.45 ± 1.12 | 4.01 ± 1.15 | 0.85 ± 0.11** | 0.34 ± 0.01** |
| Defuran limonin |
8.98 ± 0.79 | 2.00 ± 0.24 | 0.41 ± 0.02 | 0.38 ± 0.05** |
| LG | 8.12 ± 2.38 | 2.45 ± 0.14 | 0.47 ± 0.06 | 0.31 ± 0.05 |
| DNAG | 9.58 ± 4.07 | 2.29 ± 0.50 | 0.23 ± 0.02* | 0.28 ± 0.01 |
Results are means ±SD (n=4)
specific activity (units/mg protein)
indicates statistically significant (p<0.05) decrease of activity using student’s t-test.
indicates statistically significant (p<0.05) induction of activity using student’s t-test.
4. DISCUSSION
Some of the reported compounds in the present study are not available commercially. Hence, we have isolated and identified by spectroscopic studies as per our published papers (14) Moreover, we have quantified limonoids concentration using HPLC method (15) and the purity of the compounds were found to be more than 95%. Limonoid glucosides are tasteless water soluble compounds formed in fruits during maturation from their corresponding aglycones. On the other hand certain aglycones, are bitter compounds with low water solubility and principally found in seeds (22). Naturally occurring citrus limonoids contain a furan ring attached to the D ring at the 3-position. The overall focus of the study was on the ‘furan ring’ and the changing the ketone group at 7th position to methoxime. Interestingly, modified compounds showed very significant GST activity in liver against 4NQO. Defuran limonin is similar in structure to limonin except the absence of the furan ring. Limonin glucoside differs from limonin only by the presence of a glucose moiety at C-17 position. Deacetyl nomilinic acid glucoside differs from limonin glucoside in the A ring.
The presence of the furan moiety is thought to be responsible for the induction of the phase II detoxifying enzyme glutathione S-transferase (GST) activity. In addition to GST, another important phase II enzyme which protects against toxic and neoplastic effects of xenobiotics is NAD(P)H:quinone reductase (QR). QR protects against cytotoxicity and the increased levels are positively correlated with chemoprevention (23). Recent findings suggest that the consumption of citrus fruits, specifically grapefruit and oroblanco, modulate both phase I and phase II metabolizing enzymes in rats (24).
A study conducted in the Netherlands, reported that the habitual consumption of fruits and vegetables was positively correlated with human rectal GST activity (25). Recent research on citrus limonoids shows that both limonin and nomilin could inhibit certain chemically induced carcinogenesis in different animal models (13, 26). Additionally, Lam et al. (13) also evaluated different concentrations of limonoid treatment in mice. In a two stage model for skin carcinogenesis, it was shown that nomilin was more effective as an inhibitor during the initiation stage of carcinogenesis, while limonin was more active during the promotional phase of carcinogenesis (27). Structurally, limonin has an A and A’ ring while nomilin has only a seven membered A ring. Furthermore, it was suggested that there is a possible induction of GST activity in mice by citrus limonoids (13).
The differential induction potential of certain citrus limonoids to induce GST activity was further attributed to different structural components of the limonoid nucleus. It was suggested that an intact A ring is required for anti-neoplastic effects, such as present in nomilin (28). It is possible that modification to the B ring of the limonoid nucleus may also alter the induction of GST activity.
The D-ring of the limonoid nucleus has a furan ring attached to its third position. Several studies have been conducted on the importance of the furan ring in the induction of GST activity. Kahweol and cafestrol, furan containing diterpenes, are reported inducers of GST activity (28). All of the naturally occurring citrus limonoids contain furan moiety. Furthermore, all the citrus limonoids tested in this study have the furan moiety present except the modified, defuran limonin. Previous studies have shown that the furan moiety plays a role in the induction of GST activity (13). In the current study, defuran limonin exhibited no induction in any of the GST assays. Interestingly, some induction of QR activity due to defuran limonin was observed from lung homogenates. It seems that the furan moiety may be important for induction of phase II enzymes but not essential to chemopreventive activity.
In order to understand structural differences of the A and A’ rings of limonoids and their influence on biological activity, the induction potential of citrus limonoids with an open A ring (DNAG), and intact A and A’ rings (limonin, defuran limonin, LG and limonin-7-methoxime) was analyzed. Previous work has indicated that modifications to the A and A’ rings produce significant differences in the ability to induce GST activity (13). Authors demonstrated that citrus limonoids with intact A and A’ rings (limonin, limonol, and deoxylimonin) are not active GST inducers, while ichangin, with an open A’ ring, showed induction of GST. It is clear from the previous study that citrus limonoids with only an intact A ring were responsible for a most of the induction activity. Interestingly, our current results showed that limonin-7-methoxime, with intact A and A’ rings, had significant GST induction in liver against CDNB and in liver and stomach against 4NQO. In QR assays limonin-7-methoxime showed induction in lung and liver homogenates and defuran limonin showed induction in lung only. DNAG, with an open A ring also showed induction of GST activity in lung against CDNB and in stomach against 4NQO.
Considering modifications to the B-ring, all of the tested limonoids, except for limonin-7-methoxime, contain a ketone at the B ring. In limonin-7-methoxime, the ketone was substituted by a methoxime functional group; the rest of the limonoid structure was identical to limonin. While limonin did not show any induction activity in any of the enzymatic assays performed, limonin-7-methoxime showed induction of phase II enzymes in several organ homogenates assayed. Furthermore, limonin-7-methoxime showed induction of GST activity in liver homogenate against CDNB. In GST against 4NQO, induction was seen in liver and stomach homogenates. Additionally, limonin-7-methoxime showed QR induction in liver and lung homogenates. Our results indicate that modification to the B ring with a methoxy group plays a very important role in the induction of phase II detoxifying enzymes, as compared to the inactive limonin. LG did not show any induction of activity in any of the assays performed.
Previous findings have reported the induction of phase II enzymes by a few citrus limonoids (13, 29-31). Dosage studies by Lam (13) and Kelley (30) have used 1 - 20 mg of limonoids for the animal studies. The concentration of limonoids in the citrus fruit depends upon the variety of fruit. According to literature, limonin glucoside and deacetyl nomilin glucoside are present to the extent of 500 and 200 ppm respectively (15, 32). Hence, we have used 20 mg treatments for our studies.
In these studies, GST activity was tested against 1-chloro-2,4-dinitrobenzene (CDNB), which is a commonly used substrate for a variety of GST isozymes (33). One study suggests that the μ GST isozyme optimally conjugates 4-nitroquinoline 1-oxide (4NQO) (34). Most of the GST isozymes found in mammalian tissues are grouped under four major classes namely α, μ, π and θ (35, 36). Due to the variability of these enzymes in different mammalian tissues, it is important to explore how citrus limonoids affect the activity of GST enzymes.
The induction of phase II enzymes by citrus limonoids can potentially inhibit carcinogenesis by conjugating harmful substances into more water soluble forms. The increase in polarity of these electrophilic substances facilitates their excretion from the body. Hence, if by enhancing GST activity, more of the potentially carcinogenic xenobiotics can be expelled for the body through urine. Furthermore, it has been reported that plant-derived phase II enzyme inducers may be potentially important in the incidence of age-related macular degeneration (37). Phase II enzymes are important in the prevention of age-related degenerative conditions. Induction of phase II enzymes helps in the elimination of reactive oxygen species, which accumulate with age. Therefore, citrus limonoids could be considered as a potential anti-aging agent by inducing the activity GST and QR. Even though many studies have been performed on the health benefits of citrus limonoids, few studies have focused on the bioavailability of these valuable compounds in human (38). Further studies are needed to determine the fate of limonoid metabolite to elucidate their precise role in human health.
In conclusion, citrus limonoids with different structural characteristic were evaluated to understand their detoxification potential. To the best of our knowledge, this is the first study to report on the induction of GST using 4NQO as a substrate and QR. Additionally defuran limonin and limonin methoxime were also examined for the induction of the phase II enzymes GST and QR. The ability of these compounds to induce the activity of detoxifying phase II enzymes makes them valuable bioactive compounds in the quest to prevent cancer, anti-aging and oxidative related diseases, and deserves more in-depth research in order to improve human health.
Supplementary Material
ACKNOWLEDGEMENTS
This project is based upon the work supported by the USDA CSREES IFAFS #2001-52102-02294, NIH CA108765 and USDA-CSREES # 2005-34402-14401 “Designing Foods for Health” through the Vegetable & Fruit Improvement Center. We also thank Dr. Bimal Banik, University of Texas Pan American, for his technical suggestions during the study.
ABBREVIATIONS USED
- BSA
bovine serum albumin
- CDNB
1-chloro-2, 4-dinitrobenzene
- DCPIP
2,6-dichlorophenol-indophenol
- DMSO
dimethyl sufoxide
- DNAG
deacetylnomilinic acid glucoside
- EtOAc
ethyl acetate
- FAD
flavin adenine dinucleotide
- GSH
glutathione reduced
- GST
glutathione S-transferase
- HPLC
high performance liquid chromatography
- LG
limonin glucoside
- 4NQO
4-nitroquinoline 1-oxide
- PBS
phosphate buffer solution
- QR
quinone reductase
- NADPH
nicotanamide adenine dinucleotide phosphate
- NMR
nuclear magnetic resonance
- MeOH
methanol
- TLC
thin layer chromatography
- TMS
tetramethylsilane.
REFERENCES
- 1.Ejaz S, Ejaz A, Matsuda K, Lim CW. Review: Limonoids as cancer chemopreventive agents. J Sci Food Agric. 2006;86:339–345. [Google Scholar]
- 2.Jayaprakasha GK, Mandadi KK, Poulose SM, Jadegoud Y, Gowda G. A. Nagana, Patil BS. Novel triterpenoid from Citrus aurantium L. possesses chemopreventive properties against human colon cancer cells. Bioorganic & Medicinal Chemistry. 2008;16(11):5939–5951. doi: 10.1016/j.bmc.2008.04.063. [DOI] [PubMed] [Google Scholar]
- 3.Miller EG, Porter JL, Binnie WH, Guo IY, Hasegawa S. Further Studies on the Anticancer Activity of Citrus Limonoids. J. Agric. Food Chem. 2004;52(15):4908–4912. doi: 10.1021/jf049698g. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa S, Bennett RD, Verdon CP. Limonoids in citrus seeds: origin and relative concentration. J. Agric. Food Chem. 1980;28(5):922–925. [Google Scholar]
- 5.Jayaprakasha GK, Mandadi KK, Poulose SM, Jadegoud Y, Gowda GAN, Patil BS. Inhibition of colon cancer cell growth and antioxidant activity of bioactive compounds from Poncirus trifoliata (L.) Raf. Bioorganic & Medicinal Chemistry. 2007;15(14):4923–4932. doi: 10.1016/j.bmc.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 6.Poulose SM, Harris ED, Patil BS. Antiproliferative Effects of Citrus Limonoids Against Human Neuroblastoma and Colonic Adenocarcinoma Cells. Nutrition and Cancer. 2006;56(1):103–112. doi: 10.1207/s15327914nc5601_14. [DOI] [PubMed] [Google Scholar]
- 7.Jayaprakasha GK, Girennavar B, Patil BS. Radical scavenging activities of Rio Red grapefruits and Sour orange fruit extracts in different in vitro model systems. Bioresource Technology. 2008;99(10):4484–4494. doi: 10.1016/j.biortech.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka T, Kohnu H, Kawabata K, Honjo S, Miyake M, Wada K. Citrus limonoids obacunone and limonin inhibit the development of a precursor lesion, aberrant crypt foci, for colon cancer in rats. In: Berhow M, Hasegawa S, Manners G, editors. Citrus limonoids functional chemicals in agriculture and foods. American chemical society; Washington DC: 2000. pp. 145–163. [Google Scholar]
- 9.Kurowska EM,HS, Manners GD. Citrus Limonoids: functional chemicals in agriculture and foods. In: Berhow EW, Hasegawa S, Manners GD, editors. Regulation of apo B production in HepG2 cells by citrus limonoids. American Chemical Society; Washington, DC: 2000. pp. 175–184. [Google Scholar]
- 10.Battinelli L, Mengoni F, Lichtner M, Mazzanti G, Saija A, Mastroianni CM, Vullo V. Effect of limonin and nomilin on HIV-1 replication on infected human mononuclear cells. Planta Medica. 2003;69:910–913. doi: 10.1055/s-2003-45099. [DOI] [PubMed] [Google Scholar]
- 11.Vanamala J, Leonardi T, Patil BS, Taddeo SS, Murphy ME, Pike LM, Chapkin RS, Lupton JR, Turner ND. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis. 2006;27(6):1257–1265. doi: 10.1093/carcin/bgi318. [DOI] [PubMed] [Google Scholar]
- 12.Miller EG, Fanous R, Rivera-Hidalgo F, Binnie WH, Hasegawa S, Lam LKT. The effects of citrus limonoids on hamster buccal pouch carcinogenesis. Carcinogenesis. 1989;10(8):1535–1537. doi: 10.1093/carcin/10.8.1535. [DOI] [PubMed] [Google Scholar]
- 13.Lam LKT, Li Y, Hasegawa S. Effects of citrus limonoids on glutathione S-transferase activity in mice. J. Agric. Food Chem. 1989;37(4):878–880. [Google Scholar]
- 14.Jayaprakasha GK, Bhat N, Patil BS. Process for isolation of limonoid glucosides from citrus. 2007.
- 15.Vikram A, Jayaprakasha GK, Patil BS. Simultaneous determination of citrus limonoid aglycones and glucosides by high performance liquid chromatography. Analytica Chimica Acta. 2007;590(2):180–186. doi: 10.1016/j.aca.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Habig WH, Pabstm MJ, Jakoby WB. Glutathione S-Transferase. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 17.Stanley JS, Benson AM. The conjugation of 4-nitroquinoline 1-oxide, a potent carcinogen, by mammalian glutathione transferases. Biochemical Journal. 1988;256:303–306. doi: 10.1042/bj2560303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Liu LQ, Higuchi CM, Chen H. Induction of NADPH:quinone reductase by dietary phytoestrogens in colonic Colo205 cells. Biochemistry and Pharmocology. 1998;56:189–195. doi: 10.1016/s0006-2952(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantization of microgram quantities of proteins utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Poulose SM, Jayaprakasha GK, Mayer RT, Girennavar B, Patil BS. Purification of citrus limonoids and their differential inhibitory effects on human cytochrome P450 enzymes. J. Science Food & Agriculture. 2007;87:1699–1709. [Google Scholar]
- 21.Mandadi KK, Jayaprakasha GK, Bhat NG, Patil BS. Red Mexican grapefruit: A novel source for bioactive limonoids and their antioxidant activity. Zeitschrift Fur Naturforschung C-a Journal of Biosciences. 2007;62(34):179–188. doi: 10.1515/znc-2007-3-405. [DOI] [PubMed] [Google Scholar]
- 22.Jayaprakasha GK, Brodbelt JS, Bhat NG, Patil BS. Rapid methods for the separation of bioactive compounds from citrus. Abstracts of Papers of the American Chemical Society. 2004;228:U69–U69. [Google Scholar]
- 23.De Long MJ, Proshaska HJ, Talalay P. Induction of NAD(P)H:quinone reductase in murine hepatoma cells by phenolic antioxidants, azo dyes, and other chemoprotectors: a model system for the study of anticarcinogens. Proc Natl Acad Sci. 1986;83:787–791. doi: 10.1073/pnas.83.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn-Obercyger M, Stark AH, Z M. Grapefruit and Oroblanco enhance hepatic detoxification enzymes in rats: Possible role in protection against chemical carcinogenesis. J Agric Food Chem. 2005;53:1828–1832. doi: 10.1021/jf048547a. [DOI] [PubMed] [Google Scholar]
- 25.Wark PA, Grubben MJAL, Peters WHM, Nagengast FM, Kampman E, Kok FJ, van’t Veer P. Habitual consumption of fruits and vegetables: associations with human rectal glutathione S-transferase. Carcinogenesis. 2004;25:2135–2142. doi: 10.1093/carcin/bgh238. [DOI] [PubMed] [Google Scholar]
- 26.Lam LKT, Zhang J, Hasegawa S. Citrus limonoid reduction of chemically induced tumorigenesis. Food Technology. 1994;48:104–108. [Google Scholar]
- 27.Lam LKT, Hasegawa S, Bergstrom C, Lam SH, Kenney P. Limonin and nomilin inhibitory effects on chemical-induced tumorigenesis. In: Berhow MA, Hasegawa S, Manners GD, editors. Citrus Limonoids: functional chemicals in agriculture and foods. American Chemical Society; Washington, DC: 2000. pp. 185–200. [Google Scholar]
- 28.Lam LKT, Sparnins VL, Wattenburg LWI. Isolation and identification of kahweol palmitate and cafestol palmitate as active constituents of green coffee beans that enhance glutathione S-transferase activity. Cancer Research. 1982;42:1193–1198. [PubMed] [Google Scholar]
- 29.Tian Q, Miller EG, Ahmad H, Tang L, Patil BS. Differential inhibition of human cancer cell proliferation by citrus limonoids. Nutrition and Cancer. 2001;40:180. doi: 10.1207/S15327914NC402_15. [DOI] [PubMed] [Google Scholar]
- 30.Kelly C, Jewell C, O’Brien NM. The effect of dietary supplementation with the citrus limonoids, limonin and nomilin on xenobiotic-metabolizing enzymes in the liver and small intestine of the rat. Nutrition Research. 2003;23(5):681–690. [Google Scholar]
- 31.Ahmad HL,J, Polson M, Mackie K, Quiroga W, Patil BS. Citrus limonoids and flavanoids: Enhancement of Phase II detoxification enzymes and their potential in chemoprevention. In: Patil BS, Turner ND, Miller ED, Brodbelt JS, editors. Potential Health Benefits of Citrus. Vol. 936. American Chemical Society; Washington, DC: 2006. pp. 130–143. [Google Scholar]
- 32.Ozaki Y, Ayano S, Inaba N, Miyake M, Berhow MA, Hasegawa S. Limonoid Glucosides in Fruit, Juice and Processing by-products of Satsuma Mandarin (Chus unshiu Marcov.) Journal of Food Science. 1995;60(1):186–189. [Google Scholar]
- 33.Ahmad H, Tijerina MT, Tobola AS. Preferential expression of a class Mu glutathione S-transferase subunit in mouse liver by myristicin. Biochem Biophys Res Commun. 1997;236:825–828. doi: 10.1006/bbrc.1997.7053. [DOI] [PubMed] [Google Scholar]
- 34.Aceto A, Llio CD, Lo Bello M, Bucciarelli T, Angelucci S, G F. Differential activity of human, rat, mouse and bacteria glutathione transferase isoenzyme towards 4-nitroquinoline 1-oxide. Carcinogenesis. 1990;11:2267–2269. doi: 10.1093/carcin/11.12.2267. [DOI] [PubMed] [Google Scholar]
- 35.Mannervik B, Danielson HU. Glutathione transferase-structure and catalytic activity. CRC Crit Rev in Biochem. 1988;23:283–336. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- 36.Meyer DJ, Coles B, Pemble SE, Gilmore KS, Fraser GM, Ketterer B. Theta, a new class of glutathione transferases purified from rat and man. Biochemical Journal. 1991;274:409–414. doi: 10.1042/bj2740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Gao X, Cai B, Sparrow JR. Indirect antioxidant protection against photooxidative process initiated in retinal pigment epithelial cells by a lipofuscin pigment. Rejuv Res. 2006;9:256–263. doi: 10.1089/rej.2006.9.256. [DOI] [PubMed] [Google Scholar]
- 38.Manners GD, Jacob RA, Breksa, Schoch TK, Hasegawa S. Bioavailability of Citrus Limonoids in Humans. J. Agric. Food Chem. 2003;51(14):4156–4161. doi: 10.1021/jf0300691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.