Abstract
Since the late 1970s a number of laboratories have studied the role of vasoactive intestinal peptide (VIP) in inflammation and immunity. These studies have highlighted the dramatic effect of VIP on immune cell activation and function, and studies using animal models of disease have indicated that VIP has significant therapeutic and prophylactic potential. This review will focus on the effects of VIP on innate immune cell function and discuss the therapeutic potential for VIP in inflammatory diseases of humans.
Keywords: cytokine, innate immunity, TLR, VIP
Introduction
In 1969 Sami Said and Viktor Mutt first reported the vasodilatory effect of an octapeptide isolated from porcine lung tissue [1]; this peptide was later named vasoactive intestinal peptide (VIP) [2], heralding almost 40 years of publications by many laboratories with very disparate biological interests. The data generated by these studies show that the biochemical structure of VIP is highly conserved across many different animal phyla, suggesting an important function for this peptide throughout phylogenic evolution. VIP has pleitropic biological effects, detected throughout both the central and peripheral nervous systems [3] in tissues such as lung, heart and urinary tract [4], and in the gastrointestinal tract where it is the dominant inhibitory neurotransmitter [5,6].
Structure and phylogenic homology of VIPs
VIP is a 28 amino acid peptide which is a member of the secretin superfamily of peptides which include secretin, growth hormone releasing peptide (GHRP) and pituitary adenylate cyclase activating peptide (PACAP) [7]. VIP is synthesized as part of a larger pro-peptide which also contains peptide histidine isoleucine (PHI) in most mammals [8] or its equivalent peptide histidine methionine (PHM) in humans [9]. The amino acid structure of VIP has been remarkably well conserved during the evolutionary radiation of terrestrial animals (Table 1), and probably reflects the importance of this peptide in animal biology. The primary amino acid structure of VIP is identical in all mammals studied to date, with the exception of the guinea pig, which has four amino acid substitutions (86% homology) [10]. Mammalian VIP is also homologous to VIP from other terrestrial vertebrates such as the frog [11], alligator [12] and chicken [13], which are identical to each other and differ from the common mammalian VIP structure by four amino acids. The homologous nature of VIP is also reflected in physiological studies. For example, VIP from the cod and pig have an equipotent effect on the release of amylase from guinea pig pancreatic acini cells [14]. The conserved nature of VIP suggests that any given mammalian VIP may affect a number of different mammalian species, which may present an enormous advantage for the potential use of VIP in disease.
Table 1.
Homology between amino acid residues of vasoactive intestinal peptide (VIP) in some vertebrates.
| Species | VIP amino acid residues | |||||
|---|---|---|---|---|---|---|
| Human, pig, mouse cow rat, horse dog cat | HSDAV | FTDNY | TRLRK | QMAVK | KYLNS | ILN |
| Guinea pig | HSDAL | FTDTY | TRLRK | QMAMK | KYLNS | VLN |
| Chicken | HSDAV | FTDNY | SRFRK | QMAVK | KYLNS | VLT |
| Alligator | HSDAV | FTDNY | SRFRK | QMAVK | KYLNS | VLT |
| Frog | HSDAV | FTDNY | SRFRK | QMAVK | KYLNS | VLT |
| Cod | HSDAV | FTDNY | SRFRK | QMAAK | KYLNS | VLT |
Amino acids which have diverged from the common mammalian structure are underlined in bold type.
VIP receptors on innate immune cells
Ishihara et al.[15] were the first to identify a VIP-specific receptor in rat lung tissue (VIP1), and this was followed by the identification of a homologous (VIP2) receptor from a rat olfactory bulb cDNA library [16]. These studies were confirmed when both receptors were identified in a cDNA library from human jejunal epithelial cells and expressed subsequently in COS-7 cells [17]. The nomenclature of these receptors was then changed to VPAC1 (VIP1) and VPAC2 (VIP2) [18], and both receptors have now been identified in a wide range of tissues in different animal phyla [19]. VPAC1 and VPAC2 belong to the class II family of G-coupled protein receptors (GPCR). VIP shares 68% homology with PACAP, another secretin family member peptide [20,21], and both VPAC1 and VPAC2 bind VIP and PACAP with equivalent affinities [22]. The initial effect of VPAC1 or VPAC2 ligation by VIP is to increase significantly cyclic adenosine 5′-phosphate (cAMP) [23], adenylate cylase [24] and phospholipase C [25], which can cause different downstream effects on a variety of transcription factors. VPAC receptors are also internalized once VIP has bound to them and the receptor is then recycled to the cell membrane for further use [26], thus suggesting that a lag phase in the cellular response to VIP may occur at times when VPAC receptors are saturated. However, the biological significance of this remains unknown.
Both VPAC1 and VPAC2 are expressed by some innate immune cell types, while others express one receptor or the other. Relative levels of VPAC1 or VPAC2 expression may alter as a result of stimulation and different VPAC repertoires may be expressed by different cells. For example, human mast cells express only VPAC2 [27], whereas human neutrophils [28] and resting human peripheral blood monocytes (PBMs) express only VPAC1 [29]. Human VPAC1 is not up-regulated when PBMs are cultured with lipopolysaccharide (LPS) [30], in contrast to murine monocytes which express both VPAC1 and VPAC2 [31]. VPAC1 is also expressed constitutively by murine macrophages and VPAC2 is expressed following stimulation by LPS [32]. In the case of human and murine dendritic cells (DCs), temporal expression of VPAC1 and VPAC2 occurs during the differentiation pathway. The first studies to report VPAC1 and VPAC2 expression in DCs were performed in human monocyte-derived DCs (MDDC) [33], and this was followed by studies which showed that VPAC1 and VPAC2 were expressed in murine bone marrow-derived DC (BMDC) [34]. In both studies, VPAC1 was found to be expressed early in the differentiation pathway and after about 6 days this was followed by VPAC2 expression.
It is clear that a great deal of study remains to be conducted to determine comprehensively the function of VPAC1 and VPAC2 and their interaction under physiological and pathological conditions. Studies which dissect the role of these receptors during disease may have a significant impact on the development of future therapeutics, as will be discussed later.
The effect of VIP on innate immune cell function
Mast cells
Mast cells from the rat lung and intestine were first shown to release VIP when stimulated with calcium ionophores [35], and in another early study VIP was found to induce erythema and pruritis when injected into human skin and to stimulate release of histamine by rat peritoneal mast cells [36], which suggests that VIP may have an inflammatory effect. However, a more recent study has shown that VIP protects rat myenteric neurones from the lethal effect of peritoneal mast cell degranulation [37], and taken together these studies suggest that VIP may have inhibitory or excitatory effects on mast cells, although in both cases the action of VIP occurred via VPAC2 only. The amino acid structure of VIP released by mast cells also differs from the common mammalian VIP structure, with a reported variation of the classical VIP(1–28) amino acid structure to a truncated VIP(10–28) produced by murine peritoneal mast cells, bone marrow derived mast cells and in rat basophilic leukaemia cells [38]. It has been shown that when murine mast cells are cultured with immunoglobulin (Ig)E, this stimulates the release of truncated VIP(10–28) together with histamine [38]. Moreover, studies using human cord blood-derived mast cells have also reported that interaction of IgE with mast cell high-affinity IgE receptor (FCεRI) stimulates up-regulation of VPAC2 expression on the mast cell surface and that culture of human mast cells with VIP stimulates production of a wide range of inflammatory cytokines, including monocyte chemoattractant protein-1, inducible protein-10, interleukin (IL)-8, tumour necrosis factor (TNF) and IL-3 [27]. These studies indicate that mast cells not only produce VIP in conjunction with histamine via a classical IgE-mediated pathway, but they also respond to VIP by degranulating in a non-classical fashion (via VIP receptors) and that the release of VIP may have a detrimental effect by inducing atopy. Further evidence to support this hypothesis has been reported in murine studies which show that hyperexpression of VPAC2 leads to increased type I hypersensitivity reactions, while VPAC2 null mice exhibit decreased type I hypersensitivity reactions [39].
Neutrophils and eosinophils
A study by O'Dorisio et al.[40] was the first to show that VIP was detected in neutrophils which had been isolated from leukaemic patients. However, relatively little is known about the expression of VIP by neutrophils or the effect of VIP on neutrophil function. In some studies VIP reportedly primes the oxidative response of neutrophils to formyl-methionyl-leucyl-phenylalanine (FMLP) [41] and phorbol myristate acetate (PMA) [42]. Neither of these studies detected a VIP receptor in the neutrophil plasma membrane, which suggested a receptor-independent mechanism, although expression of VPAC1 has now been detected on the plasma membrane of neutrophils [28]. However, an inhibitory effect of VIP on the biology of human neutrophils has also been reported by Palermo et al.[43]. In that study, VIP blocked the interferon (IFN)-γ-stimulated up-regulation of neutrophil FC gamma receptor and inhibited antibody-dependent cellular cytotoxicity. It is therefore possible that VIP may have either inhibitory or excitatory effects upon neutrophils, as it does on mast cells, and it is possible that different VIP thresholds may induce different effects on neutrophils acting through VPAC1 (rather than VPAC2 as with mast cells). However, to date, relatively few in vivo studies have been published regarding the effect of VIP on neutrophils.
Published data are also rather scant regarding the effect of VIP on eosinophils. VIP is increased in eosinophils isolated from patients with colitis compared to healthy human volunteers [44], but it is not known whether this has any relationship to pathology, or whether up-regulation of VIP in these cells is an attempt to control inflammation homeostatically within the intestinal microenvironment. Dunzendorfer et al.[45] have indicated that VIP has an anti-inflammatory effect on eosinophils, reporting that VIP inhibited eosinophil migration and production of IL-16 in vitro[46], which subsequently inhibited chemotaxis of lymphocytes. VIP may therefore inhibit chemotaxis of lymphocytes and monocytes into immune compartments in which eosinophils have migrated in response to parasites or allergens. However, most studies investigating diseases in which eosinophils are active (e.g. allergic or parasitic disease) have generally only made an association between eosinophil accumulation and increased innervation of affected tissues by VIP-ergic neurones. The interaction of VIP with eosinophils is therefore another area of VIP biology which is vastly under-studied.
Monocytes and macrophages
Substantial evidence now exists to indicate that VIP has an excitatory effect on monocytes and macrophages but inhibits LPS-induced or IFN-γ-induced inflammatory pathways in these cells. In the late 1990s studies began to emerge which reported that an analogue of VIP (RO25-1553) inhibited production of TNF-α, IL-6 and IL-12 by LPS-stimulated human monocytes [47], and in vitro studies also reported that VIP inhibited LPS-induced IL-12 and nitric oxide (NO) production by murine macrophages via a cAMP-dependent mechanism [48]. Although this latter study reported no effect on TNF-α, the inhibitory effect of VIP on LPS-induced TNF-α production has now been established firmly in murine microglial cells [49], murine macrophages [50,51] and human monocytes [52]. Following the study by Xin and Sriram [48], Delgado et al.[32] also reported that VIP inhibited LPS-induced inflammatory pathways in monocytes and macrophages via cAMP-dependent or independent mechanisms (Fig. 1).
Fig. 1.
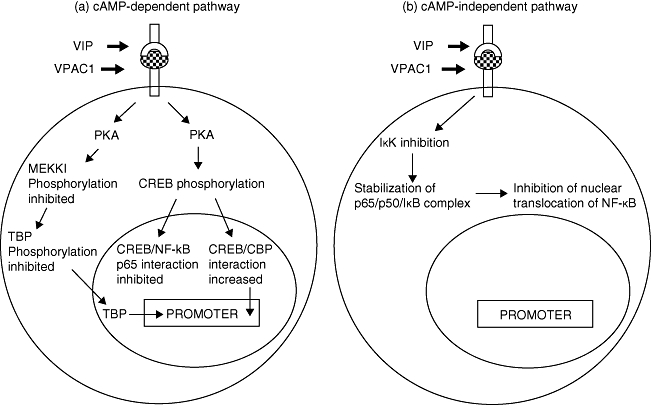
Vasoactive intestinal peptide (VIP) ligation of VPAC1 induces both (a) cyclic adenosine 5′-phosphate (cAMP)-dependent and (b) cAMP-independent signalling cascades.
The cAMP-dependent pathway and the subsequent activity of phosphokinase A (PKA) has two different downstream effects. The first effect is to phosphorylate the cAMP response element binding protein (CREB) which then binds to the co-factor, CREB binding protein (CBP), and prevents its interaction with nuclear factor κB (NFκB) [53], thus reducing the activity of NFκB [54]. Secondly, the cAMP-dependent pathway inhibits phosphorylation of MAP/ERK kinase (MEK) kinase 1 (MEKK1) which in turn inhibits the MEKK3/6/p38 pathway and ultimately the phosphorylation of another NFκB co-factor, the TATA-box binding protein (TBP) [53], which then has reduced affinity for both NFκB and DNA (Fig. 1a). The cAMP-dependent pathway also inhibits the production of IFN-γ-induced inflammatory cytokines and nitric oxide by murine macrophages due to inhibited phosphorylation of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway. Thus, VIP acting via VPAC1 may inhibit both the inflammatory response to LPS and activation by IFN-γ. The cAMP-independent pathway inhibits the activity of inhibitory κB kinase (IκK) which prevents phosphorylation of the IκB, and increases the stabilization of IκB/NFκB complexes which prevents nuclear translocation of NFκB subunits [53] (Fig. 1b).
Similarly, modulation of murine IL-12 by VIP can also be cAMP-dependent or -independent depending on the transcriptional regulators involved [55,56]. These studies were the first to show that VIP inhibited transcriptional regulation of cytokine and inducible nitric oxide synthase (iNOS) genes, but that VIP also affected other transcription factors such as activator protein 1 (AP-1). In studies in which VIP inhibited the inflammatory response of LPS-stimulated murine microglial cells, not only was AP-1 binding to DNA inhibited but also the heterodimeric composition of AP-1 was altered, changing from a c-JUN/c-FOS to a JUN-B/c-FOS [57]. Our studies have also shown that VIP inhibits LPS-induced nuclear translocation of both NFκB and c-JUN in human monocytic THP1 cells [52]. Studies which have shown the dual inhibitory effect of VIP on both inflammatory cytokine production and IFN-γ responsiveness are of great significance, as IFN-γ stimulates a rapid killing of bacterially infected murine macrophages by enhancing LPS-activated reactive oxygen species (ROS) pathways [58]. Our studies show that VIP inhibits ROS production by Salmonella enterica serovar typhimurium-infected macrophages and that the effect is maintained when cells are cultured with IFN-γ. However, the anti-inflammatory effect of VIP leads to increased intracellular multiplication of wild-type S. typhimurium and increased survival of S. typhimurium PhoP mutants (Fig. 2) [59], which are normally highly attenuated, and are unable to survive in murine macrophages [60] and are avirulent in mice [61]. Our studies, therefore, indicate that although VIP has significant anti-inflammatory properties (due probably to the inhibition of transcription factors which up-regulate a large number of proinflammatory genes) the therapeutic use of VIP may encourage opportunistic bacterial infection or may possibly reactivate latent infections. However, this potentially very significant disadvantage also needs to be considered when using currently available immunomodulatory drugs.
Fig. 2.

Vasoactive intestinal peptide (VIP) inhibits production of reactive oxygen species (ROS) and promotes survival of wild-type Salmonella and avirulent Salmonella PhoP mutants in macrophages co-cultured with interferon (IFN)-γ. (a) Optical density measured following nitroblue tetrazolium (NBT) reduction assay in murine macrophages infected with S. typhimurium 14 028; S. typhimurium 4/74 or S. typhimurium 14028 PhoP mutants over 2–24 h post-infection (multiplicity of infection = 10) and co-cultured with IFN-γ (100 U/ml) with or without VIP (10−10 M);  = 2 h;
= 2 h;  = 7 h; □ = 24 h post-infection. Each bar represents the mean of five replicate experiments performed on three separate occasions. *Significant decrease (P < 0·05) in ROS production by VIP cultured cells compared to relevant corresponding treatment without VIP. (b) The number of salmonella colony-forming units recovered from murine macrophages co-cultured with IFN-γ (100 U/ml) with or without VIP (10−10 M) 2–24 h post-infection;
= 7 h; □ = 24 h post-infection. Each bar represents the mean of five replicate experiments performed on three separate occasions. *Significant decrease (P < 0·05) in ROS production by VIP cultured cells compared to relevant corresponding treatment without VIP. (b) The number of salmonella colony-forming units recovered from murine macrophages co-cultured with IFN-γ (100 U/ml) with or without VIP (10−10 M) 2–24 h post-infection;  = 2 h;
= 2 h;  = 7 h; □ = 24 h post-infection. Each bar represents the mean of five replicate experiments performed on three separate occasions.
= 7 h; □ = 24 h post-infection. Each bar represents the mean of five replicate experiments performed on three separate occasions.
It is possible that the difference between the overall inhibitory action of VIP on monocytes and macrophages compared to the excitatory effect of VIP on mast cells may be largely the result of expression of VPAC1 on monocytes and macrophages, but not mast cells (which express only VPAC2). Evidence to support this has been reported in studies which show that the inhibitory effect of VIP on LPS-induced inflammatory responses by murine monocytes and macrophages occurs via VPAC1 ligation, even though both VPAC1 and VPAC2 are expressed under such conditions [62].
Some interesting studies have shown that the action of VIP may be due not only to the inhibition of inflammatory factors but also, at least in part, by the stimulation of anti-inflammatory cytokines such as IL-10 [63], thus indicating that VIP is truly an immune modulator rather than an immune inhibitor. We therefore began to study the relationship between IL-18 and its inhibitory peptide IL-18 binding protein (BP) in human monocytic THP1 cells. Four isoforms of IL-18BP are known (BPa-BPd) [64] and of these, IL-18BPa is produced in the highest quantity by monocytes and is elevated significantly in the sera of septic patients [65].
We hypothesized that VIP would inhibit IL-18 production by human monocytic THP1 cells, but that this may be due to increased stimulation of IL-18Bpa. Our results show that during a constant 48-h period of exposure to LPS from the oral pathogen Porphyromonas gingivalis or the enteric pathogen Escherichia coli the amount of IL-18BPa produced by the monocytes increased, but did not increase enough to prevent a concurrent elevation of IL-18 [66]. However, the addition of VIP to the culture media not only inhibited production of IL-18 but also IL-18BPa over the 48-h period (Fig. 3). In light of these results, we may hypothesize that the release of potent broad-ranging anti-inflammatory peptides, such as VIP, in immune compartments may occur at times when specific inhibitors, such as IL-18BPa, are not produced in high enough concentrations to be effective, although much greater study will be needed to confirm this hypothesis.
Fig. 3.

Enzyme-linked immunosorbent assay (ELISA) analysis showing that vasoactive intestinal peptide (VIP) inhibits production of both interleukin (IL)-18 and IL-18Bpa in human THP1 monocytes stimulated with Escherichia coli lipopolysaccharide (LPS). Data shows the effect of VIP (10−8 M) on IL-18 and IL-18BPa production by human monocytic THP1 cells stimulated with E. coli LPS (100 ng/ml). Each bar represents the mean of five replicate experiments performed on three separate occasions. *Significant decrease (P < 0·05) in cytokine production when VIP is added to culture compared to corresponding response without VIP.
Dendritic cells
Dendritic cells (DCs) are paramount in the initiation of immunity and tolerance. DCs are able to present antigen in orders of magnitude greater than macrophages [67], but also have the ability to induce immune tolerance [68]. Studies which investigate the effect of VIP on DC biology may therefore have significant implications for the therapeutic use of VIP and, in this regard, some recent studies have reported exciting data. Studies by Delneste et al.[33] were the first to show that human monocyte-derived dendritic cells (MDDC) express VPAC1 constitutively and, after 6 days in culture, VPAC2. This study also showed that VIP itself (10−6 M) had little effect on maturation of immature MDDC but synergized with suboptimal levels of TNF-α to induce mature MDDCs, as determined by increased expression of CD40, CD54, CD80, human leucocyte antigen D-related (HLA-DR) and production of IL-12. This would suggest that VIP induces an immune competent DC phenotype which (via the action of IL-12) should drive naive T lymphocytes towards T helper type 1 (Th1) differentiation. However, other studies have indicated that VIP has a differential effect on murine bone marrow-derived DCs (BMDCs). In studies by Delgado et al.[34], 6-day-old BMDCs which were stimulated with VIP (10−8 M) prior to culturing with naive T lymphocytes induced the development of Th2 cells. Furthermore, this study also showed that VIP down-regulated the expression of co-stimulatory molecules induced by E. coli LPS and subsequently inhibited proliferation of CD4+ T lymphocytes, and that although murine BMDCs expressed both VPAC1 and VPAC2, the inhibitory effect of VIP occurred via VPAC1.
This study was then followed by others which showed that when BMDCs were differentiated in the presence of VIP, they induced IL-10/transforming growth factor (TGF)-β-producing regulatory T cells (Treg) in vivo[69]. Gonzalez-Rey et al. showed that the addition of VIP to culture media early in the differentiation of human monocytes to MDDC stimulated differentiation of DCs which polarized naive CD4+ T cells to IL-10/TGF-β-producing Tregand also tolerogenic CD8+ T cells [70]. The CD8+ T cells produced IL-10 and expressed a CD28−/cytotoxic T lymphocyte antigen (CTLA)4+ phenotype, and both the CD4+ and CD8+ Tregs generated by VIP inhibited allogeneic immune responses [70]. Therefore, VIP may have different effects upon DCs depending at which point along the differentiation pathway it interacts with the developing DCs. It is also possible that the temporal expression of VPAC receptors augments this response, so that early ligation of VIP with VPAC1 produces an inhibitory DC phenotype and that later ligation of VIP with VPAC2 may act synergistically with cytokines, such as TNF-α, to induce a mature and immunogenic DC phenotype.
Specific effect of VIP on Toll-like receptors (TLRs)
Recent work has indicated that VIP has an inhibitory effect on myeloid immune responses at the initial point of pathogen recognition. Gomariz et al.[71] reported that daily intraperitoneal administration of VIP (1 nM) down-regulated TLR-2 and TLR-4 expression in colonic extracts obtained from a murine trinitrobenzene sulphonic acid (TNBS) model of human Crohn's disease and that TLR-4 expression was decreased on the surface of macrophages, DCs and lymphocytes within the mesenteric lymph nodes of these mice. This group then went on to show that VIP administration inhibited expression of Th1 cytokines in the colon and restored Treg populations to control levels [72]. Up-regulation of TLR-4 by LPS in human rheumatoid synovial fibroblast has also been shown to be inhibited by VIP, although the high constitutive expression of TLR-2 and TLR-4 by these cells was unaffected by VIP [73]. Initially it was speculated that the mechanism behind TLR modulation may be via inhibition of NFκB [71]. However, although murine TLR-2 gene expression is modulated via NFκB [74], and VIP inhibits LPS-induced DNA binding of NFκB in murine RAW 264·7 macrophages [75] and human monocytic THP1 cells [52,76], an NFκB promoter sequence has not been detected in either murine TLR-4 gene or human TLR-2 or TLR-4 genes [77]. Therefore, VIP-induced inhibition of NFκB could not explain the inhibitory effect of VIP on up-regulation of expression of human TLR-2 and TLR-4, or expression of murine TLR-4. We investigated the effect of VIP on the ets family transcription factor PU.1, which is required for expression of human TLR-2 [78] and TLR-4 [79] and the differentiation of macrophages from monocytes [80]. Our data showed that VIP inhibited nuclear translocation of PU.1 and subsequent expression of one of its downstream gene target proteins (monocyte colony stimulating factor receptor) [81], and that this effect occurred in conjunction with a significant decrease in P. gingivalis LPS-induced up-regulation of TLR-2 and also up-regulation of TLR-4 following stimulation of cells by E. coli LPS (Fig. 4) [82]. Results which also indicated the inhibitory effect of PU.1 by VIP were observed by decreased, LPS-induced differentiation of monocytes to macrophages [82]. This discovery was repeated by studies using a TNBS-induced mouse model of human colitis, which showed that VIP decreased PU.1 binding to DNA and that mutation of PU.1 prevented the inhibitory effect of VIP on TLR-4 up-regulation [83].
Fig. 4.

Fluorescence activated cell sorter (FACS) dot plots showing that vasoactive intestinal peptide (VIP) inhibits expression of Toll-like receptor (TLR)-2 and TLR-4 on the cell membrane of human monocytic (vit D3 differentiated) THP1 cells stimulated with bacterial lipopolysaccharide (LPS). (a) TLR-4 expression by unstimulated THP1 cells cultured for 48 h represents only 2·65% of the THP1 population. (b) Increased expression of TLR-4 in 38% of cell population (arrow) following culture with Escherichia coli LPS (100 ng/ml) for 48 h. (c) TLR-4 expressing THP1 population is reduced to 10·9% (arrow) when cultured with both E. coli LPS and VIP (10−8 M). (d) TLR-2 expression by unstimulated THP1 cells cultured for 48 h represents only 2·5% of the THP1 population. (e) Increased expression of TLR-2 in 27·83% of cell population (arrow) following culture with Porphyromonas gingivalis LPS (100 ng/ml) for 48 h. (f) TLR-2 expressing THP1 population is reduced to 13·24% (arrow) when cultured with both P. gingivalis LPS and VIP (10−8 M). (g) Isotype control, immunoglobulin (Ig)G2a, phycoerythrin (PE) (CD14) and IgG2a, fluorescein isothiocyanate (FITC) (TLR-2/TLR-4). Dot plots are representative of data obtained on at least 10 separate occasions.
Thus, VIP not only inhibits the production of inflammatory mediators by monocytes and macrophages via inhibition of NFκB and AP-1, it also subsequently inhibits the ability of these cells to enhance their detection of environmental LPS by preventing up-regulation of TLR-2 and TLR-4 via inhibition of PU.1. The inhibitory effect of VIP on TLR-2 and TLR-4 may have significant therapeutic value in sepsis, but also highlights the intriguing possibility that VIP may inhibit other TLRs or other pathogen-associated molecular patterns expressed by different cells and may, therefore, have even broader potential than is currently envisaged.
VIP as a novel therapeutic
Given the many inhibitory effects of VIP on the function of innate immune cells, the probable potential of VIP as an anti-inflammatory is obviously significant. However, this potential is enhanced by studies which have investigated the effect of VIP administration during animal models of human disease.
Although there is evidence to suggest that the release of VIP may have a pathological effect on tissues via mast cell degranulation, other studies have reported a significant therapeutic effect of VIP. Using a murine model of pancreatitis, Kojima et al.[31] have shown that administration of VPAC1 agonist reduced production of TNF-α, IL-6 and serum amylase with a subsequent reduction in histopathological damage associated with disease, but that TNF-α, IL-6 and serum amylase levels were increased by VPAC2 agonists. Therefore, this study indicates once again that VIP may have either an inhibitory or excitatory effect (acting via VPAC1 and VPAC2) on cells and tissues which express both VPAC1 and VPAC2 receptors and that the use of selective VPAC1 agonists (rather than VIP itself) may be of greater therapeutic benefit and may be administered more safely, as VPAC1 selectivity will prevent pathology via VPAC2 ligation. However, some excellent studies using animal models of disease have indicated that VIP (rather than VPAC1 agonists) also has significant therapeutic potential. In studies which investigated the murine model of human Crohn's disease (TNBS-induced colitis), VIP ameliorated completely the clinical signs of disease and the histopathological lesions associated with it [84]. The therapeutic potential of VIP was shown further by this model when clinical signs of Crohn's disease were also ameliorated following reconstitution of experimental animals with BMDCs which were stimulated with VIP prior to administration [85]. In this latter study the amelioration of pathology due to VIP-cultured BMDDCs coincided with a significant rise in Treg populations in vivo. VIP also has potential in the rapid treatment of bacterial sepsis. Studies by Delgado et al.[86] have shown that VIP injection increases murine survival rate by 15–20% in animals in which sepsis has been induced by injected with lethal doses (400–600 µg) of LPS. This study also showed that the effect of VIP is most likely to be due to suppression of elevated levels of inflammatory cytokines associated with sepsis, in particular TNF-α, by VIP. The use of VIP in cases of septic shock may, therefore, be beneficial when administered as an adjunctive therapy together with antibiotics, because VIP may decrease quickly the level of systemic inflammatory cytokines, while the use of antibiotic will not only kill the bacterial infection but will decrease the likelihood that bacterial will overgrow, which could occur if VIP was administered alone. VIP also has therapeutic value in murine models of human disease with no known infectious aetiology, such as rheumatoid arthritis (RA). In murine (collagen-induced) models of human arthritis, VIP (5 × 10−8 M) administered every other day prevented chronic cartilage damage and joint remodelling associated with disease [87]. Disease remission in this study was still evident 2 weeks after cessation of VIP treatment. This study has even greater significance in light of new evidence which indicates that RA patients have VPAC1 polymorphisms, suggesting a role for VIP in homeostatic control of inflammation and prevention of RA [88].
Many studies have reported the neuroprotective effect of VIP which may have applications in a number of different neurological diseases and spinal trauma. VIP inhibits microglia-induced neurodegeneration [89] and the production of inflammatory cytokines associated with spinal cord injury in rats [90]. Although some mechanisms occurring during neurological protection by VIP are due most probably to pathways already stated (e.g. inhibition of transcription factor pathways and nuclear translocation), one study has also shown that VIP increased expression of activity-dependent neuroprotective protein in RAW 264·7 cells (murine macrophages) [91] and, as such, indicates further a dual mechanism by which VIP may have therapeutic effect.
The key to the therapeutic use of VIP in human disease appears to be in its delivery. VIP is degraded quickly by enzymes, and delivery systems which protect its integrity have been investigated with great success. VIP incorporated into phospholipids has been used successfully in animal models of pulmonary hypertension [92]. The effect of phospholipid on VIP is actually twofold, as it not only protects the peptide from degradation but changes its biochemical conformation from a random coil to an α-helix [93], and this conformational change increases the affinity of VIP with VPAC receptors, as shown by studies in which VIP was incorporated into liposomes [94,95]. VIP-protective delivery systems such as this are the most likely delivery systems to be utilized in future, and it is possible to envisage that the extended release of VIP by formulation of slow-release biodegradeable microspheres may have very significant therapeutic (and economic) benefits in the treatment of chronic inflammatory diseases.
In conclusion, many more studies need to be performed to investigate the effect of VIP on the biology of important myeloid immune cells such as neutrophils and eosinophils. More studies also need to be performed to dissect the cross-talk between VPAC1 and VPAC2 under different conditions and the effect of VIP in animals which have currently active or dormant microbial infections. However, in vivo studies indicate that VIP has enormous therapeutic potential in the treatment of inflammatory disease and in the treatment of pathology induced by physical trauma and there are many possible diseases, not discussed in this review, in which VIP will almost certainly have potential as an anti-inflammatory drug.
Disclosure
None.
References
- 1.Said SI, Mutt V. A peptide fraction from lung tissue with prolonged vasodilatory activity. Scand J Clin Lab Invest. 107(Suppl 1969):51–6. [PubMed] [Google Scholar]
- 2.Said SI, Mutt V. Isolation from porcine intestinal wall of a vasoactive octacosapeptide related to secretin and to glucagon. Eur J Biochem. 1972;28:199–204. doi: 10.1111/j.1432-1033.1972.tb01903.x. [DOI] [PubMed] [Google Scholar]
- 3.Said SI, Rosenberg RN. Vasoactive intestinal polypeptide: abundant immunoreactivity in neuronal cell lines and normal nervous tissues. Science. 1976;192:907–8. doi: 10.1126/science.1273576. [DOI] [PubMed] [Google Scholar]
- 4.Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res. 2001;49:27–37. doi: 10.1016/s0008-6363(00)00229-7. [DOI] [PubMed] [Google Scholar]
- 5.D'Amato M, De Beurme FA, Lefebvre RA. Comparison of the effect of vasoactive intestinal polypeptide and non-adrenergic non-cholinergic neurone stimulation in the cat gastric fundus. Eur J Pharmacol. 1988;152:71–82. doi: 10.1016/0014-2999(88)90837-0. [DOI] [PubMed] [Google Scholar]
- 6.Grider JR, Rivier JR. Vasoactive intestinal peptide (VIP) as transmitter of inhibitory motor neurones of the gut: evidence from the use of selective VIP antagonists and VIP antiserum. J Pharmacol Exp Ther. 1990;253:738–42. [PubMed] [Google Scholar]
- 7.Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- 8.Nishizawa M, Hayakawa Y, Yanaihara N, Okamoto H. Nucleotide sequence divergence and functional constraint in VIP mRNA evolution between human and rat. FEBS Lett. 1985;183:55–9. doi: 10.1016/0014-5793(85)80953-4. [DOI] [PubMed] [Google Scholar]
- 9.Itoh N, Obata K, Yanaihara N, Okamoto H. Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM-27. Nature. 1983;304:547–9. doi: 10.1038/304547a0. [DOI] [PubMed] [Google Scholar]
- 10.Du B-H, Eng J, Hulmes JD, Chang M, Pan EY-C, Yalow RS. Guinea pig has a unique mammalian VIP. Biochem Biophys Res Commun. 1985;128:1093–8. doi: 10.1016/0006-291x(85)91052-6. [DOI] [PubMed] [Google Scholar]
- 11.Chartrel N, Wang Y, Fournier A, Vaudry H, Conlon MJ. Frog vasoactive intestinal peptide and galanin: primary structures and effects on pituitary adenylate cyclase. Endocrinology. 1995;136:3079–86. doi: 10.1210/endo.136.7.7540547. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Conlon JM. Neuroendocrine peptides (NPY, GRP, VIP, somatostatin) from the brain and stomach of the alligator. Peptides. 1993;14:573–9. doi: 10.1016/0196-9781(93)90147-9. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson A. Structure of the vasoactive intestinal octacosapeptide from chicken intestine. The amino acid sequence. FEBS Lett. 1975;60:322–6. doi: 10.1016/0014-5793(75)80740-x. [DOI] [PubMed] [Google Scholar]
- 14.Thwaites DT, Young J, Thorndyke MC, Dimaline R. The isolation and chemical characterization of a novel vasoactive intestinal peptide-related peptide from a teleost fish, the cod, Gadus morhua. Biochim Biophys Acta. 1989;999:217–20. doi: 10.1016/0167-4838(89)90221-5. [DOI] [PubMed] [Google Scholar]
- 15.Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–19. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- 16.Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- 17.Couvineau A, Rouyer-Fessard C, Darmoul D, et al. Cloning and functional expression of two cDNA encoding proteins with different N-terminal domains. Biochem Biophys Res Commun. 1994;200:769–76. doi: 10.1006/bbrc.1994.1517. [DOI] [PubMed] [Google Scholar]
- 18.Harmar AJ, Arimura A, Gozes I, et al. International union of pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–70. [PMC free article] [PubMed] [Google Scholar]
- 19.Laburthe M, Couvineau A. Molecular pharmacology and structure of VPAC receptors for VIP and PACAP. Regul Pept. 2002;108:165–73. doi: 10.1016/s0167-0115(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 20.Campbell RM, Scanes CG. Evolution of the growth hormone-releasing factor (GRF) family of peptides. Growth Regul. 1992;2:175–91. [PubMed] [Google Scholar]
- 21.Segre GV, Goldring SR. Receptors for secretin, calcitonin, parathyroid hormone (PTH)/PTH-related peptide, glucagon-like peptide 1, growth hormone-releasing hormone, and glucagon belong to a newly discovered G-protein-linked receptor family. Trends Endocrinol Metab. 1993;4:309–14. doi: 10.1016/1043-2760(93)90071-l. [DOI] [PubMed] [Google Scholar]
- 22.Rawlings SR, Hezareh M. Pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP/vasoactive intestinal polypeptide receptors: actions on the anterior pituitary gland. Endocr Rev. 1996;17:4–29. doi: 10.1210/edrv-17-1-4. [DOI] [PubMed] [Google Scholar]
- 23.Laburthe M, Rousset M, Boissard C, Chevalier G, Zweibaum A, Rosselin G. Vasoactive intestinal peptide: a potent stimulator of adenosine 3′:5′-cyclic monophosphate accumulation in gut carcinoma cell lines in culture. Proc Natl Acad Sci USA. 1978;75:2772–5. doi: 10.1073/pnas.75.6.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salomon R, Couvineau A, Rouyer-Fessard C, et al. Characterization of a common VIP-PACAP receptor in human small intestinal epithelium. Am J Physiol. 1993;264:294–300. doi: 10.1152/ajpendo.1993.264.2.E294. [DOI] [PubMed] [Google Scholar]
- 25.MacKenzie CJ, Lutz EM, McCulloch DA, Mitchell R, Harmar AJ. Phospholipase C activation by VIP1 and VIP2 receptors expressed in COS 7 cells involves a pertussis toxin-sensitive mechanism. Ann NY Acad Sci. 1996;26:579–84. doi: 10.1111/j.1749-6632.1996.tb17523.x. [DOI] [PubMed] [Google Scholar]
- 26.Boissard C, Marie JC, Hejblum G, Gespach C, Rosselin G. Vasoactive intestinal peptide receptor regulation and reversible desensitization in human colonic carcinoma cells in culture. Cancer Res. 1986;46:4406–13. [PubMed] [Google Scholar]
- 27.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harfi I, D'Hondt S, Corazza F, Sariban E. Regulation of human polymorphonuclear leukocytes functions by the neuropeptide pituitary adenylate cyclase-activating polypeptide after activation of MAPKs. J Immunol. 2004;173:4154–63. doi: 10.4049/jimmunol.173.6.4154. [DOI] [PubMed] [Google Scholar]
- 29.Lara-Marquez ML, O'Dorisio MS, Karacay B. Vasoactive intestinal peptide (VIP) receptor type 2 (VPAC2) is the predominant receptor expressed in human thymocytes. Ann NY Acad Sci. 2000;921:45–54. doi: 10.1111/j.1749-6632.2000.tb06950.x. [DOI] [PubMed] [Google Scholar]
- 30.El Zein N, Badran B, Sariban E. VIP differentially activates beta2 integrins, CR1, and matrix metalloproteinase-9 in human monocytes through cAMP/PKA, EPAC, and PI-3K signaling pathways via VIP receptor type 1 and FPRL1. J Leukoc Biol. 2008;83:972–81. doi: 10.1189/jlb.0507327. [DOI] [PubMed] [Google Scholar]
- 31.Kojima M, Ito T, Oono T, et al. VIP attenuation of the severity of experimental pancreatitis is due to VPAC1 receptor-mediated inhibition of cytokine production. Pancreas. 2005;30:62–70. [PubMed] [Google Scholar]
- 32.Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide prevent inducible nitric oxide synthase transcription in macrophages by inhibiting NF-kappa B and IFN regulatory factor 1 activation. J Immunol. 1999;162:4685–96. [PubMed] [Google Scholar]
- 33.Delneste Y, Herbault N, Galea B, et al. Vasoactive intestinal polypeptide synergizes with TNF-α in inducing human dendritic cell maturation. J Immunol. 1999;163:3071–5. [PubMed] [Google Scholar]
- 34.Delgado M, Reduta A, Sharma V, Ganea D. VIP/PACAP oppositely affects immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity for CD4(+) T cells. J Leukoc Biol. 2004;75:1122–30. doi: 10.1189/jlb.1203626. [DOI] [PubMed] [Google Scholar]
- 35.Cutz E, Chan E, Track NS, Goth A, Said SI. Release of vasoactive intestinal polypeptide in mast cells by histamine liberators. Nature. 1978;275:661–2. doi: 10.1038/275661a0. [DOI] [PubMed] [Google Scholar]
- 36.Fjellner B, Hägermark O. Studies on pruritogenic and histamine-releasing effects of some putative peptide neurotransmitters. Acta Derm Venereol. 1981;61:245–50. [PubMed] [Google Scholar]
- 37.Rijnierse A, Nijkamp FP, Kraneveld A. Mast cells and nerve tickle in the tummy. Implications for inflammatory bowel disease and irritable bowel syndrome. Pharmacol Ther. 2007;116:207–35. doi: 10.1016/j.pharmthera.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Wershil BK, Turck CW, Sreedharan SP, et al. Variants of vasoactive intestinal peptide in mouse mast cells and rat basophilic leukaemia cells. Cell Immunol. 1993;151:369–78. doi: 10.1006/cimm.1993.1246. [DOI] [PubMed] [Google Scholar]
- 39.Voice JK, Grinninger C, Kong Y, Bangale Y, Paul S, Goetzl EJ. Roles of vasoactive intestinal peptide (VIP) in the expression of different immune phenotypes by wild-type mice and T cell-targeted type II VIP receptor transgenic mice. J Immunol. 2003;170:308–14. doi: 10.4049/jimmunol.170.1.308. [DOI] [PubMed] [Google Scholar]
- 40.O'Dorisio MS, O'Dorisio TM, Cataland S, Balcerzak SP. Vasoactive intestinal polypeptide as a biochemical marker for polymorphonuclear leukocytes. J Lab Clin Med. 1980;96:666–72. [PubMed] [Google Scholar]
- 41.Pedrera C, Lucas M, Bellido L, López-González MA. Receptor-independent mechanisms are involved in the priming of neutrophil's oxidase by vasoactive intestinal peptide. Regul Pept. 1994;54:505–11. doi: 10.1016/0167-0115(94)90548-7. [DOI] [PubMed] [Google Scholar]
- 42.López-González MA, Lucas M. Priming effect of vasoactive intestinal peptide on the respiratory burst of neutrophils non-mediated by plasma membrane receptors. Experientia. 1994;50:486–8. doi: 10.1007/BF01920753. [DOI] [PubMed] [Google Scholar]
- 43.Palermo MS, Vermeulen ME, Giordano MN. Human antibody-dependent cellular cytotoxicity mediated by interferon gamma-activated neutrophils is impaired by vasoactive intestinal peptide. J Neuroimmunol. 1996;69:123–8. doi: 10.1016/0165-5728(96)00078-1. [DOI] [PubMed] [Google Scholar]
- 44.Metwali A, Blum A, Ferraris L, Klein J, Fiocchi C, Weinstock J. Eosinophils within the healthy or inflamed human intestine produce substance P and vasoactive intestinal peptide. J Neuroimmunol. 1994;52:69–78. doi: 10.1016/0165-5728(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 45.Dunzendorfer S, Meierhofer C, Wiedermann CJ. Signalling in neuropeptide-induced migration of human eosinophils. J Leukoc Biol. 1998;64:828–34. doi: 10.1002/jlb.64.6.828. [DOI] [PubMed] [Google Scholar]
- 46.Dunzendorfer S, Feistritzer C, Enrich B, Wiedermann CJ. Neuropeptide-induced inhibition of IL-16 release from eosinophils. Neuroimmunomodulation. 2002;10:217–23. doi: 10.1159/000068324. –2003. [DOI] [PubMed] [Google Scholar]
- 47.Dewit D, Gourlet P, Amraoui Z, et al. The vasoactive intestinal peptide analogue RO25-1553 inhibits the production of TNF and IL-12 by LPS-activated monocytes. Immunol Lett. 1998;60:57–60. doi: 10.1016/s0165-2478(97)00129-6. [DOI] [PubMed] [Google Scholar]
- 48.Xin Z, Sriram S. Vasoactive intestinal peptide inhibits IL-12 and nitric oxide production in murine macrophages. J Neuroimmunol. 1998;89:206–12. doi: 10.1016/s0165-5728(98)00140-4. [DOI] [PubMed] [Google Scholar]
- 49.Delgado M. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit the MEKK1/MEK4/JNK signaling pathway in endotoxin-activated microglia. Biochem Biophys Res Commun. 2002;293:771–6. doi: 10.1016/S0006-291X(02)00283-8. [DOI] [PubMed] [Google Scholar]
- 50.Delgado M, Pozo D, Martinez C, et al. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit endotoxin-induced TNF-alpha production by macrophages: in vitro and in vivo studies. J Immunol. 1999;162:2358–67. [PubMed] [Google Scholar]
- 51.Foster N, Hulme SD, Barrow PA. Inhibition of IFN-gamma-stimulated proinflammatory cytokines by vasoactive intestinal peptide (VIP) correlates with increased survival of Salmonella enterica serovar typhimurium phoP in murine macrophages. J Interferon Cytokine Res. 2005;25:31–42. doi: 10.1089/jir.2005.25.31. [DOI] [PubMed] [Google Scholar]
- 52.Foster N, Cheetham J, Taylor JJ, Preshaw PM. VIP inhibits Porphyromonas gingivalis LPS-induced immune responses in human monocytes. J Dent Res. 2005;84:999–1004. doi: 10.1177/154405910508401106. [DOI] [PubMed] [Google Scholar]
- 53.Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase activating polypeptide inhibit expression of Fas ligand in activated T lymphocytes by regulating c-Myc, NFκB, NF-AT, and early growth factors 2/3. J Immunol. 2001;166:1028–40. doi: 10.4049/jimmunol.166.2.1028. [DOI] [PubMed] [Google Scholar]
- 54.Yang X-J, Ogyzko VV, Nishikawa J, Howard BH, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–24. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 55.Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. VIP and PACAP inhibit IL-12 production in LPS-stimulated macrophages. Subsequent effect on IFN gamma synthesis by T cells. J Neuroimmunol. 1999;96:167–81. doi: 10.1016/s0165-5728(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 56.Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit interleukin-12 transcription by regulating nuclear factor kappaB and Ets activation. J Biol Chem. 1999;274:31930–40. doi: 10.1074/jbc.274.45.31930. [DOI] [PubMed] [Google Scholar]
- 57.Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase activating polypeptide inhibit the MEKK1/MEK4/JNK signaling pathway in LPS-stimulated macrophages. J Neuroimmunol. 2000;110:97–105. doi: 10.1016/s0165-5728(00)00359-3. [DOI] [PubMed] [Google Scholar]
- 58.Foster N, Hulme SD, Barrow PA. Induction of antimicrobial pathways during early-phase immune response to Salmonella spp. in murine macrophages: gamma interferon (IFN-gamma) and upregulation of IFN-gamma receptor alpha expression are required for NADPH phagocytic oxidase gp91-stimulated oxidative burst and control of virulent Salmonella spp. Infect Immun. 2003;71:4733–41. doi: 10.1128/IAI.71.8.4733-4741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foster N, Hulme SD, Barrow PA. Vasoactive intestinal peptide (VIP) prevents killing of virulent and phoP mutant Salmonella typhimurium by inhibiting IFN-gamma stimulated NADPH oxidative pathways in murine macrophages. Cytokine. 2006;36:134–40. doi: 10.1016/j.cyto.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Fields PI, Groisman EA, Heffron F. A Salmonella locus that controls resistance to microbicida proteins from phagocytic cells. Science. 1989;243:1059–62. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- 61.Miller SI, Kukral AM, Mekalanos JJ. A two componenet regulatory system (phoP-phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–8. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ganea D, Delgado M. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) as modulators of both innate and adaptive immunity. Crit Rev Oral Biol Med. 2002;13:229–37. doi: 10.1177/154411130201300303. [DOI] [PubMed] [Google Scholar]
- 63.Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide enhance IL-10 production by murine macrophages: in vitro and in vivo studies. J Immunol. 1999;162:1707–16. [PubMed] [Google Scholar]
- 64.Kim SH, Eisenstein M, Reznikov L, et al. Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc Natl Acad Sci USA. 2000;97:1190–5. doi: 10.1073/pnas.97.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Novick D, Schwartsburd B, Pinkus R, et al. A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine. 2001;14:334–42. doi: 10.1006/cyto.2001.0914. [DOI] [PubMed] [Google Scholar]
- 66.Foster N, Andreadou K, Jamieson L, Preshaw PM, Taylor JJ. VIP inhibits P. gingivalis LPS-induced IL-18 and IL-18BPa in monocytes. J Dent Res. 2007;86:883–7. doi: 10.1177/154405910708600915. [DOI] [PubMed] [Google Scholar]
- 67.Inaba K, Steinman RM. Protein-specific helper T-lymphocyte formation initiated by dendritic cells. Science. 1985;229:475–9. doi: 10.1126/science.3160115. [DOI] [PubMed] [Google Scholar]
- 68.Joffre O, Nolte MA, Spörri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–47. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 69.Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Pozo D, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc Natl Acad Sci USA. 2005;102:13562–7. doi: 10.1073/pnas.0504484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gonzalez-Rey E, Chorny A, Fernandez-Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood. 2006;107:3632–8. doi: 10.1182/blood-2005-11-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomariz RP, Arranz A, Abad C, et al. Time-course expression of Toll-like receptors 2 and 4 in inflammatory bowel disease and homeostatic effect of VIP. J Leukoc Biol. 2005;78:491–502. doi: 10.1189/jlb.1004564. [DOI] [PubMed] [Google Scholar]
- 72.Arranz A, Juarranz Y, Leceta J, Gomariz RP, Martínez C. VIP balances innate and adaptive immune responses induced by specific stimulation of TLR2 and TLR4. Peptides. 2008;29:948–56. doi: 10.1016/j.peptides.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 73.Gutiérrez-Cañas I, Juarranz Y, Santiago B, et al. VIP down-regulates TLR4 expression and TLR4-mediated chemokine production in human rheumatoid synovial fibroblasts. Rheumatology. 2006;45:527–32. doi: 10.1093/rheumatology/kei219. [DOI] [PubMed] [Google Scholar]
- 74.Musikacharoen T, Matsuguchi T, Kikuchi T, Yoshikai Y. NF-κ and STAT5 play important roles in the regulation of mouse Toll-like receptor 2 gene expression. J Immunol. 2001;166:4516–24. doi: 10.4049/jimmunol.166.7.4516. [DOI] [PubMed] [Google Scholar]
- 75.Delgado M, Munoz-Elias EJ, Kan Y, et al. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit tumor necrosis factor alpha transcriptional activation by regulating nuclear factor-kB and cAMP response element-binding protein/c-Jun. J Biol Chem. 1998;273:31427–36. doi: 10.1074/jbc.273.47.31427. [DOI] [PubMed] [Google Scholar]
- 76.Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit nuclear factor-κB-dependent gene activation at multiple levels in the human monocytic cell line THP-1. J Biol Chem. 2001;276:369–80. doi: 10.1074/jbc.M006923200. [DOI] [PubMed] [Google Scholar]
- 77.Rehli M. Of mice and men: species variation of Toll-like receptor variation. Trends Immunol. 2002;23:375–8. doi: 10.1016/s1471-4906(02)02259-7. [DOI] [PubMed] [Google Scholar]
- 78.Haehnel V, Schwarzfischer L, Fenton MJ, Rehli M. Transcriptional regulation of the human Toll-like receptor 2 gene in monocytes and macrophages. J Immunol. 2002;168:5629–37. doi: 10.4049/jimmunol.168.11.5629. [DOI] [PubMed] [Google Scholar]
- 79.Rehli M, Poltorak A, Schwarzfischer L, Krause SW, Andreesen R, Beutler B. PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J Biol Chem. 2000;275:9773–81. doi: 10.1074/jbc.275.13.9773. [DOI] [PubMed] [Google Scholar]
- 80.Shivdasani RA, Orkin SH. The transcriptional control of hematopoiesis. Blood. 1996;87:4025–39. [PubMed] [Google Scholar]
- 81.Zhang DE, Hetherington CJ, Chen HM, Tenen DG. The macrophage transcription factor PU.1 directs tissue-specific expression of the macrophage colony-stimulating factor receptor. Mol Cell Biol. 1994;14:373–81. doi: 10.1128/mcb.14.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Foster N, Lea SR, Preshaw PM, Taylor JJ. Pivotal advance: vasoactive intestinal peptide inhibits up-regulation of human monocyte TLR2 and TLR4 by LPS and differentiation of monocytes to macrophages. J Leukoc Biol. 2007;81:893–903. doi: 10.1189/jlb.0206086. [DOI] [PubMed] [Google Scholar]
- 83.Arranz A, Androulidaki A, Zacharioudaki V, et al. Vasoactive intestinal peptide suppresses toll-like receptor 4 expression in macrophages via Akt1 reducing their responsiveness to lipopolysaccharide. Mol Immunol. 2008;45:2970–80. doi: 10.1016/j.molimm.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 84.Arranz A, Abad C, Juarranz Y, Leceta J, Martinez C, Gomariz RP. Vasoactive intestinal peptide as a healing mediator in Crohn's disease. Neuroimmunomodulation. 2008;15:46–53. doi: 10.1159/000135623. [DOI] [PubMed] [Google Scholar]
- 85.Gonzalez-Rey E, Delgado M. Therapeutic treatment of experimental colitis with regulatory dendritic cells generated with vasoactive intestinal peptide. Gastroenterology. 2006;131:1799–811. doi: 10.1053/j.gastro.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 86.Delgado M, Martinez C, Pozo D, et al. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activation polypeptide (PACAP) protect mice from lethal endotoxemia through the inhibition of TNF-alpha and IL-6. J Immunol. 1999;162:1200–5. [PubMed] [Google Scholar]
- 87.Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–8. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- 88.Delgado M, Robledo G, Rueda B, et al. Genetic association of vasoactive intestinal peptide receptor with rheumatoid arthritis: altered expression and signal in immune cells. Arthritis Rheum. 2008;58:1010–19. doi: 10.1002/art.23482. [DOI] [PubMed] [Google Scholar]
- 89.Delgado MD, Ganea D. Vasoactive intestinal peptide prevents activated microglia-induced neurodegeneration under inflammatory conditions: potential therapeutic role in brain trauma. FASEB J. 2003;17:1922–4. doi: 10.1096/fj.02-1029fje. [DOI] [PubMed] [Google Scholar]
- 90.Kim WK, Kan Y, Ganea D, Hart RP, Gozes I, Jonakait GM. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit tumor necrosis factor-α production in injured spinal cord and in activated microglia via a cAMP-dependent pathway. J Neurosci. 2000;20:3622–30. doi: 10.1523/JNEUROSCI.20-10-03622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quintana FJ, Zaltzman R, Fernandez-Montesinos R, et al. NAP, a peptide derived from the activity-dependent neuroprotective protein, modulates macrophage function. Ann NY Acad Sci. 2006;1070:500–6. doi: 10.1196/annals.1317.069. [DOI] [PubMed] [Google Scholar]
- 92.Rubinstein I. Human VIP-alpha: an emerging biologic response modifier to treat primary pulmonary hypertension. Expert Rev Cardiovasc Ther. 2005;3:565–9. doi: 10.1586/14779072.3.4.565. [DOI] [PubMed] [Google Scholar]
- 93.Gololobov G, Noda Y, Sherman S, Rubinstein I, Baranowska-Kortylewicz J, Paul S. Stabilization of vasoactive intestinal peptide by lipids. J Pharmacol Exp Ther. 1998;285:753–8. [PubMed] [Google Scholar]
- 94.Sethi V, Onyüksel H, Rubinstein I. Liposomal vasoactive intestinal peptide. Methods Enzymol. 2005;391:377–95. doi: 10.1016/S0076-6879(05)91021-5. [DOI] [PubMed] [Google Scholar]
- 95.Hajos F, Stark B, Hensler S, Prassl R, Mosgoeller W. Inhalable liposomal formulation for vasoactive intestinal peptide. Int J Pharm. 2008;357:286–94. doi: 10.1016/j.ijpharm.2008.01.046. [DOI] [PubMed] [Google Scholar]


