Abstract
Context: The human glucocorticoid receptor α (GRα) is a nuclear hormone receptor that regulates multiple physiological and pathophysiological processes. There are large variations in both physiological and therapeutic response to glucocorticoids. Multiple previous studies suggested that genetic polymorphisms in GRα (NR3C1) might play an important role.
Objective: The aim of the study was to identify and determine the functional implications of common genetic variation in NR3C1.
Design: We resequenced the NR3C1 gene using 240 DNA samples from four ethnic groups, followed by functional characterization of the effects of selected polymorphisms.
Results: A total of 108 polymorphisms were identified in GRα, including nine nonsynonymous coding single nucleotide polymorphisms (cSNPs) and four synonymous cSNPs with a minor allele frequency greater than 5%. Functional studies showed that SNPs encoding Phe(65)Val and Asp(687)Glu displayed slightly increased levels of protein compared with WT, and Asp(687)Glu also caused increased GRα receptor number. In addition, Ala(229)Thr and Ile(292)Val showed slightly decreased ligand binding affinity in COS-1 cells. A genotype-phenotype association study of NR3C1 gene expression in 240 lymphoblastoid cell lines identified one SNP, Cm746T>C, located 5′-upstream of noncoding exon 1C, and one haplotype, Cm237delC/Cm238C>T/Cm240G>C in exon 1C of the gene that were associated with GRα mRNA expression and a trend with GRα number.
Conclusions: These results represent a step toward understanding the functional role of common sequence variation in the GRα gene (NR3C1) and the potential application of those SNPs in translational studies.
We identified 108 polymorphisms in the GRα gene by resequencing 240 DNA samples from 4 ethnic groups, and these efforts followed by functional characterization and genotype-phenotype association studies represent a step toward understanding the potential impact of GRα polymorphisms on GRα function.
The glucocorticoid receptor (GR), encoded by NR3C1, belongs to the nuclear hormone receptor superfamily. GRα is the predominant alternatively spliced product of the NR3C1 gene and is expressed in the cytoplasm of most cells (1). GRβ is another alternatively spliced product of NR3C1 differing only in the final exon, exon 9. In this paper, we focused mainly on the GRα, realizing that the same single nucleotide polymorphisms (SNPs) could also play a role in GRβ function. GRα can influence gene transcription through glucocorticoid response elements (GRE) upon binding glucocorticoid (2). Synthetic glucocorticoids such as dexamethasone and prednisone have a variety of actions (3). However, serious side effects can occur after long-term treatment with these agents (4). In addition, GRα might influence drug-drug interactions by altering drug metabolism through the regulation of cytochrome P450 gene expression (5). Therefore, GRα pharmacogenomics might be medically important.
NR3C1 encodes a 94-kDa protein that includes three classical domains: the N-terminal domain, the central DNA binding domain, and the C-terminal ligand binding domain (6). It contains eight coding exons (exon 2–9) and at least nine tissue-specific noncoding exon 1s with alternative splice sites (7,8). Previous studies have shown that polymorphisms in the coding and regulatory regions are associated with disease risk and response to glucocorticoids (9,10,11,12,13,14,15,16,17,18). To characterize further the genetic variation within this important gene, we resequenced NR3C1 using 240 DNA samples from four ethnic groups, followed by functional genomics. We also performed genotype-phenotype association studies with GRα expression levels in the same lymphoblastoid cell lines from which DNA used to resequence NR3C1 was obtained. These studies represent a step toward determining the functional implications of common genetic variation in GRα and their possible role in disease pathophysiology and drug treatment response.
Materials and Methods
DNA samples and lymphoblastoid cell lines
DNA samples and lymphoblastoid cell lines from 60 Caucasian-American (CA), 60 African-American (AA), 60 Han Chinese-American (HCA), and 60 Mexican-American (MA) subjects (sample sets HD100CAU, HD100AA, HD100CHI, and HD100MEX) were purchased from the Coriell Cell Repository (Camden, NJ). These samples had been anonymized by the National Institute of General Medical Sciences, and all subjects had provided written consent for their experimental use. This study was reviewed and approved by the Mayo Clinic Institutional Review Board.
GRα gene (NR3C1) resequencing
NR3C1 (NT_029289.10) was resequenced for coding exons 2–9 (GRα), nine upstream noncoding exons, approximately 1000 bp upstream of exons 1A and 1D and intron sequence between the remainder of the noncoding exon 1s. We also resequenced exon-intron splice junctions, a portion of the 3′-untranslated region (UTR), and two regions in introns 1A3 and 7 with high sequence homology among primates. Primers used for gene resequencing are listed in Supplemental Table 1 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Amplicons were sequenced on both strands with an ABI 3730 DNA sequencer (Applied Biosystems, Foster City, CA). Independent amplifications were performed for samples in which a SNP was observed only once or any sample with an ambiguous chromatogram. The chromatograms were analyzed with Mutation Surveyor (SoftGenetics, State College, PA).
NR3C1 expression and exon array analysis
Expression array analyses were performed using Affymetrix U133 Plus 2.0 GeneChips as described previously (19). Exon array analysis was performed with Affymetrix Human Exon 1.0 ST Array chips using total RNA extracted from eight lymphoblastoid cell lines, two from each ethnic group. Expression array data were normalized by GC Robust Multi-array Average background adjustment (20).
Expression constructs
The wild-type (WT) plasmids, pRShGRα expressing human GR (hGR) α (NM_000176) and pRShMR expressing the mineralcorticoid receptor (NM_000901), were provided by Dr. Ronald Evans (Salk Institute, La Jolla, CA). Variant nucleotides were introduced by site-directed mutagenesis with pRShGRα as template. WT and variant open reading frames (ORFs) were cloned into pcDNA3.1/V5-His-TOPO® (Invitrogen, San Diego, CA) and were used in the following transfections. The primers used to perform site-directed mutagenesis are listed in Supplemental Table 1. We also obtained a pMMTV-luc plasmid (American Type Culture Collection, Manassas, VA) that contains a GRE upstream of a firefly luciferase ORF. The phRL-CMV vector encoding Renilla luciferase and the pSV40-β-galactosidase encoding β-galactosidase were purchased from Promega (Madison, WI).
Cell culture
COS-1 cells and lymphoblastoid cells were cultured as described previously (21). Human Raji and Jurkat cells were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum.
Western blot analyses
COS-1 cells in 12-well plates were transfected with 5 μg expression constructs together with 1 μg pSV40-β-galactosidase as a control for transfection efficiency using 7 μl Lipofectamine 2000 (Invitrogen). Cell supernatants, after correction for β-galactosidase activity, were loaded on 12% sodium dodecyl sulfate gels. Proteins were detected with anti-GR P20 (1:200) polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and secondary antibody. Results were quantified with the AMBIS Radioanalytic Imaging System (Ambis, Inc., San Diego, CA). Data were expressed as percentages of the intensity of the WT GRα protein band.
Intact cell dexamethasone binding assays
COS-1 cells transfected with 1 μg GRα expression construct were incubated for 1 h at 37 C with seven different concentrations (0.8 to 50 nm) of [1,2,4,6,7-H3]-dexamethasone (Amersham Pharmacia Biotech, Little Chalfont, UK) with or without a 500-fold excess of nonradioactive dexamethasone. Binding assays were also performed with lymphoblastoid cells in 96-well plates with six different dexamethasone concentrations (0.8 to 25 nm). After washing with PBS, the cells were harvested, and radioactivity was measured. Specific binding was calculated by subtracting nonspecific binding after correction for the empty vector background signal. Scatchard analysis was performed, and apparent dissociation constants (Kd) and receptor number were calculated.
Transactivation assays
COS-1 cells in 24-well plates were transfected with 0.2 μg GRα WT, variants, or empty vector, together with 5 μg pMMTV-luc and 0.6 pg phRL-CMV. Twenty-four hours later, cells were treated for an additional 24 h with 0, 0.1, 0.3, 1, and 500 nm dexamethasone (Steraloids, Newport, RI). Cells were lysed, and luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega).
EMSA
Nuclear extracts from Raji and Jurkat cells were isolated (21) and quantified by the method of Bradford. Biotin-labeled oligonucleotides were used to perform EMSA. Probe sequences are listed in Supplemental Table 2. EMSAs were performed with the LightShift Chemiluminescent EMSA Kit (Pierce, Rockford, IL). A 400-fold excess of unlabeled probe was added for the competition assays.
Data analysis
Linkage disequilibrium (LD) was determined by calculating D′ and r2 values for all possible pairwise combinations of polymorphisms (22). LD plots for all SNPs with minor allele frequencies (MAFs) higher than 0.001 were generated using Haploview version 4.1 (23). Haplotypes were “inferred” computationally (24). The association between SNPs and GRα mRNA expression array data was determined with PLINK (http://pngu.mgh.harvard.edu/purcell/plink/) (25). Least square regression was used to perform the association analysis. Kd values were calculated by nonlinear regression analysis using a one-site binding model, and differences between groups were determined by Student’s t test with unequal variance using the Prism program (GraphPad Software Inc., San Diego, CA).
Results
NR3C1 gene resequencing
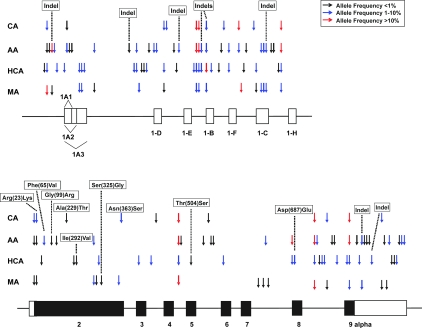
NR3C1 was resequenced as described in Materials and Methods using 240 DNA samples obtained from four ethnic groups. These resequencing data have been deposited in PharmGKB (www.pharmgkb.org) with submission no. PS207914. Figure 1 shows locations of the polymorphisms observed, and individual polymorphisms are listed in Table 1. A total of 108 polymorphisms, 57 not found in public databases (www.ncbi.nlm.nih.gov), were identified, including 21 coding SNPs (cSNPs), nine of which were nonsynonymous (Table 1). Five of the nonsynonymous cSNPs were reported previously, with Arg(23)Lys and Asn(363)Ser studied widely (9,10,11,12,13,14,15,16,26,27). MAFs for all of the nonsynonymous cSNPs were less than 5% in any of the four ethnic groups, except for Asn(363)Ser, which had a MAF of 5.8% in CAs (Table 1). Among the 12 synonymous cSNPs, four had MAFs greater than 5% in at least one ethnic group: 879G>A, 1764C>T, 2034C>T, and 2298T>C. All polymorphisms were in Hardy-Weinberg equilibrium except for A2744delT in AAs (P = 0.02521) and Cm240G>C, Cm238C>T, Cm237delC in MAs (P = 0.0084). Large differences were observed among ethnic groups in polymorphisms and allele frequencies (Table 1).
Figure 1.
hGRα gene (NR3C1) structure and polymorphisms. Schematic representation of hGRα gene (NR3C1). Exons are represented as rectangles, with black rectangles indicating the ORF and white rectangles indicating UTRs. Arrows indicate the locations of polymorphisms, with colors representing minor allele frequencies. Red arrows indicate polymorphisms with frequencies of more than 10%, whereas blue arrows and black arrows indicate polymorphisms with frequencies from 1 to 10% and of less than 1%, respectively.
Table 1.
Human GRα gene (NR3C1) resequencing
| No. | Location | Nucleotide | SNPs | Ancestra→variant sequence change | Amino acid change | Minor allele frequencies
|
rs (dbSNP) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| AA | CA | HCA | MA | |||||||
| 1 | 5′FR of exon 1A1 | −1141 | Am1141 | G→C | 0.000 | 0.000 | 0.008 | 0.000 | ||
| 2 | 5′FR of exon 1A1 | −1133 | Am1133 | G→A | 0.008 | 0.075 | 0.092 | 0.225 | rs41400245 | |
| 3 | 5′FR of exon 1A1 | −968 | Am968 | A→T | 0.008 | 0.000 | 0.000 | 0.000 | ||
| 4 | 5′FR of exon 1A1 | −857 | Am857 | Deletion of A | 0.108 | 0.000 | 0.000 | 0.008 | rs61757424 | |
| 5 | 5′FR of exon 1A1 | −438 | Am438 | C→T | 0.075 | 0.000 | 0.000 | 0.000 | rs6868190 | |
| 6 | 5′FR of exon 1A1 | −226 | Am226 | C→T | 0.000 | 0.000 | 0.017 | 0.000 | ||
| 7 | Exon 1A1 | −176 | Am176 | G→T | 0.000 | 0.000 | 0.017 | 0.000 | ||
| 8 | Exon 1A1 | −175 | Am175 | Deletion of G or SNP of T | 0.017 | 0.008 | 0.000 | 0.000 | rs60488854 | |
| 9 | Exon 1A1 | −112 | Am112 | T→C | 0.008 | 0.000 | 0.000 | 0.000 | ||
| 10 | Exon 1A2 | −16 | Am16 | T→G | 0.008 | 0.000 | 0.000 | 0.000 | ||
| 11 | Exon 1A3 | −607 | Am607 | G→A | 0.000 | 0.000 | 0.025 | 0.017 | rs61757433 | |
| 12 | Exon 1A3 | −603 | Am603 | T→G | 0.000 | 0.000 | 0.017 | 0.017 | rs61757434 | |
| 13 | Exon 1A3 | −101 | Am101 | G→A | 0.000 | 0.000 | 0.017 | 0.000 | rs61757436 | |
| 14 | Intron 1A3 | 35 | p35 | G→A | 0.017 | 0.000 | 0.000 | 0.008 | ||
| 15 | 5′FR of exon 1-D | −988 to −987 | Dm988 | Deletion of AT | 0.008 | 0.000 | 0.000 | 0.000 | ||
| 16 | 5′FR of exon 1-D | −987 | Dm987 | T→C | 0.000 | 0.000 | 0.025 | 0.000 | ||
| 17 | 5′FR of exon 1-D | −925 | Dm925 | A→G | 0.042 | 0.000 | 0.025 | 0.000 | ||
| 18 | 5′FR of exon 1-D | −742 | Dm742 | C→T | 0.000 | 0.000 | 0.000 | 0.017 | ||
| 19 | 5′FR of exon 1-D | −693 | Dm693 | A→G | 0.000 | 0.000 | 0.008 | 0.000 | ||
| 20 | 5′FR of exon 1-D | −531 | Dm531 | T→C | 0.000 | 0.000 | 0.008 | 0.000 | ||
| 21 | 5′FR of exon 1-D | −459 | Dm459 | C→G | 0.000 | 0.000 | 0.017 | 0.000 | ||
| 22 | 5′FR of exon 1-D | −317 | Dm317 | G→C | 0.008 | 0.000 | 0.000 | 0.000 | ||
| 23 | Exon 1-D | −140 | Dm140 | A→T | 0.025 | 0.000 | 0.000 | 0.000 | ||
| 24 | Exon 1-D | −33 | Dm33 | G→C | 0.025 | 0.000 | 0.000 | 0.000 | rs35722527 | |
| 25 | Intron 1-D | 102 | p102 | G→A | 0.000 | 0.017 | 0.017 | 0.008 | rs10482603 | |
| 26 | Intron 1-D | 157 | p157 | G→A | 0.000 | 0.017 | 0.000 | 0.000 | rs61759003 | |
| 27 | 5′FR of exon 1-E | −139 | Em139 | G→A | 0.008 | 0.000 | 0.000 | 0.000 | rs61759005 | |
| 28 | 5′FR of exon 1-E | −133 | Em133 | A→G | 0.000 | 0.000 | 0.008 | 0.000 | ||
| 29 | 5′FR of exon 1-E | −58 | Em58 | Insertion of A | 0.042 | 0.000 | 0.000 | 0.000 | ||
| 30 | 5′FR of exon 1-B | −237 | Bm237 | A→G | 0.000 | 0.000 | 0.025 | 0.000 | ||
| 31 | 5′FR of exon 1-B | −189 | Bm189 | T→G | 0.164 | 0.175 | 0.058 | 0.050 | rs3806855 | |
| 32 | 5′FR of exon 1-B | −187 | Bm187 | T→C | 0.164 | 0.175 | 0.058 | 0.050 | rs3806854 | |
| 33 | 5′FR of exon 1-B | −174 to −172 | Bm174 | Deletion of GCC | 0.034 | 0.000 | 0.017 | 0.000 | ||
| 34 | Exon 1-B | −10 | Bm10 | Insertion of C | 0.083 | 0.075 | 0.175 | 0.017 | rs5871845 | |
| 35 | Exon 1-B | −9 | Bm9 | G→A | 0.000 | 0.000 | 0.017 | 0.000 | rs36208514 | |
| 36 | 5′FR of exon 1-F | −208 | Fm208 | C→G | 0.000 | 0.000 | 0.008 | 0.000 | ||
| 37 | 5′FR of exon 1-F | −175 | Fm175 | T→C | 0.008 | 0.000 | 0.000 | 0.000 | ||
| 38 | 5′FR of exon 1-F | −137 | Fm137 | G→A | 0.000 | 0.017 | 0.000 | 0.000 | rs36208517 | |
| 39 | Exon 1-F | −52 | Fm52 | A→G | 0.042 | 0.000 | 0.025 | 0.000 | rs10482604 | |
| 40 | 5′FR of exon 1-C | −746 | Cm746 | T→C | 0.075 | 0.183 | 0.000 | 0.133 | rs10482605 | |
| 41 | 5′FR of exon 1-C | −710 | Cm710 | G→A | 0.000 | 0.000 | 0.008 | 0.000 | ||
| 42 | 5′FR of exon 1-C | −475 | Cm475 | C→T | 0.008 | 0.000 | 0.000 | 0.000 | rs61759010 | |
| 43 | Exon 1-C | −312 | Cm312 | T→G | 0.000 | 0.017 | 0.017 | 0.008 | rs10482609 | |
| 44 | Exon 1-C | −240 | Cm240 | G→C | 0.058 | 0.000 | 0.000 | 0.017 | ||
| 45 | Exon 1-C | −238 | Cm238 | C→T | 0.058 | 0.000 | 0.000 | 0.017 | rs10482611 | |
| 46 | Exon 1-C | −237 | Cm237 | Deletion of C | 0.058 | 0.000 | 0.000 | 0.017 | ||
| 47 | 5′FR of exon 1-H | −455 | Hm455 | C→T | 0.008 | 0.000 | 0.000 | 0.000 | rs61759017 | |
| 48 | 5′FR of exon 1-H | −371 | Hm371 | T→A | 0.008 | 0.000 | 0.000 | 0.000 | rs61759019 | |
| 49 | 5′FR of exon 1-H | −338 | Hm338 | T→C | 0.000 | 0.000 | 0.008 | 0.000 | ||
| 50 | 5′FR of exon 1-H | −236 | Hm236 | G→A | 0.167 | 0.167 | 0.058 | 0.050 | rs10482614 | |
| 51 | Exon 2 | 66 | E2p66 | G→A | 0.008 | 0.017 | 0.000 | 0.008 | rs6189 | |
| 52 | Exon 2 | 68 | E2p68 | G→A | Arg (23) Lys | 0.008 | 0.017 | 0.000 | 0.008 | rs6190 |
| 53 | Exon 2 | 72 | E2p72 | A→G | 0.000 | 0.000 | 0.008 | 0.000 | ||
| 54 | Exon 2 | 193 | E2p193 | T→G | Phe (65) Val | 0.042 | 0.000 | 0.000 | 0.000 | rs6192 |
| 55 | Exon 2 | 295 | E2p295 | G→A | Gly (99) Arg | 0.008 | 0.000 | 0.000 | 0.000 | |
| 56 | Exon 2 | 624 | E2p624 | G→A | 0.008 | 0.000 | 0.000 | 0.000 | ||
| 57 | Exon 2 | 685 | E2p685 | G→A | Ala (229) Thr | 0.000 | 0.008 | 0.000 | 0.000 | |
| 58 | Exon 2 | 804 | E2p804 | C→T | 0.000 | 0.000 | 0.008 | 0.000 | rs6199 | |
| 59 | Exon 2 | 874 | E2p874 | A→G | Ile (292) Val | 0.000 | 0.000 | 0.008 | 0.000 | |
| 60 | Exon 2 | 879 | E2p879 | G→A | 0.092 | 0.000 | 0.000 | 0.017 | rs10482622 | |
| 61 | Exon 2 | 885 | E2p885 | G→A | 0.000 | 0.000 | 0.000 | 0.008 | ||
| 62 | Exon 2 | 897 | E2p897 | A→G | 0.000 | 0.000 | 0.025 | 0.000 | rs13306588 | |
| 63 | Exon 2 | 973 | E2p973 | A→G | Ser (325) Gly | 0.000 | 0.000 | 0.000 | 0.008 | |
| 64 | Exon 2 | 1088 | E2p1088 | A→G | Asn (363) Ser | 0.000 | 0.058 | 0.000 | 0.017 | rs6195 |
| 65 | Exon 3 | 1242 | E3p1242 | A→G | 0.000 | 0.000 | 0.025 | 0.000 | ||
| 66 | Intron 3 | −57 | I3m57 | T→G | 0.000 | 0.000 | 0.025 | 0.000 | rs3822376 | |
| 67 | Intron 3 | −46 | I3m46 | G→C | 0.000 | 0.008 | 0.000 | 0.000 | rs61753484 | |
| 68 | Intron 4 | −16 | I4m16 | G→T | 0.300 | 0.350 | 0.083 | 0.175 | rs6188 | |
| 69 | Intron 4 | −9 | I4m9 | T→A | 0.008 | 0.000 | 0.000 | 0.000 | ||
| 70 | Exon 5 | 1510 | E5p1510 | A→T | Thr (504) Ser | 0.000 | 0.000 | 0.008 | 0.000 | |
| (Continued) | ||||||||||
Table 1A.
Continued
| No. | Location | Nucleotide | SNPs | Ancestra→variant sequence change | Amino acid change | Minor allele frequencies
|
rs (dbSNP) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| AA | CA | HCA | MA | |||||||
| 71 | Intron 5 | 89 | I5p89 | A→T | 0.008 | 0.000 | 0.000 | 0.000 | ||
| 72 | Intron 5 | 123 | I5p123 | A→G | 0.000 | 0.008 | 0.000 | 0.000 | ||
| 73 | Intron 5 | −124 | I5m124 | T→C | 0.008 | 0.000 | 0.000 | 0.000 | rs10482684 | |
| 74 | Intron 5 | −71 | I5m71 | C→A | 0.008 | 0.000 | 0.000 | 0.000 | ||
| 75 | Exon 6 | 1764 | E6p1764 | C→T | 0.000 | 0.000 | 0.058 | 0.000 | rs6194 | |
| 76 | Intron 7 | 7384 | I7p7384 | G→A | 0.000 | 0.000 | 0.000 | 0.008 | ||
| 77 | Intron 7 | 7446 | I7p7446 | G→A | 0.000 | 0.000 | 0.000 | 0.008 | ||
| 78 | Intron 7 | 7470 | I7p7470 | G→C | 0.025 | 0.000 | 0.000 | 0.008 | ||
| 79 | Exon 8 | 2034 | E8p2034 | C→T | 0.167 | 0.000 | 0.058 | 0.000 | rs258751 | |
| 80 | Exon 8 | 2061 | E8p2061 | T→G | Asp (687) Glu | 0.000 | 0.000 | 0.017 | 0.000 | |
| 81 | Intron 8 | 244 | I8p244 | T→C | 0.292 | 0.367 | 0.083 | 0.175 | rs258750 | |
| 82 | Intron 8 | −236 | I8m236 | C→G | 0.042 | 0.000 | 0.000 | 0.000 | rs9324911 | |
| 83 | Intron 8 | −184 | I8m184 | C→T | 0.000 | 0.000 | 0.008 | 0.000 | ||
| 85 | Intron 8 | −158 | I8m158 | T→C | 0.000 | 0.000 | 0.025 | 0.000 | ||
| 86 | Intron 8 | −157 | I8m157 | G→T | 0.000 | 0.017 | 0.000 | 0.008 | rs10482704 | |
| 87 | Intron 8 | −146 | I8m146 | G→A | 0.008 | 0.000 | 0.000 | 0.000 | rs10482705 | |
| 88 | Intron 8 | −43 | I8m43 | G→C | 0.008 | 0.000 | 0.000 | 0.000 | rs61628130 | |
| 89 | Intron 8 | −9 | I8m9 | C→G | 0.000 | 0.000 | 0.025 | 0.000 | ||
| 90 | Exon 9 α | 2250 | E9p2250 | C→T | 0.000 | 0.000 | 0.025 | 0.000 | ||
| 91 | Exon 9 α | 2298 | E9p2298 | T→C | 0.167 | 0.183 | 0.058 | 0.050 | rs6196 | |
| 92 | 3′UTR α | 2352 | pA2352 | G→A | 0.008 | 0.000 | 0.000 | 0.000 | ||
| 93 | 3′UTR α | 2624 | pA2624 | A→C | 0.000 | 0.000 | 0.025 | 0.000 | ||
| 94 | 3′UTR α | 2744 | pA2744 | Deletion of T | 0.025 | 0.000 | 0.000 | 0.025 | rs10482707 | |
| 95 | 3′UTR α | 3170 | pA3170 | C→A | 0.008 | 0.000 | 0.000 | 0.000 | rs61753502 | |
| 96 | 3′UTR α | 3271 | pA3271 | C→A | 0.008 | 0.000 | 0.000 | 0.000 | ||
| 97 | 3′UTR α | 3559 | pA3559 | T→C | 0.008 | 0.000 | 0.000 | 0.000 | rs6197 | |
| 98 | 3′UTR α | 3560 | pA3560 | Insertion of ACTGAT | 0.000 | 0.000 | 0.025 | 0.000 | ||
| 99 | 3′UTR α | 3583 | pA3583 | Insertion of ATGT | 0.000 | 0.017 | 0.000 | 0.008 | rs10482710 | |
| 100 | 3′UTR α | 3808 | pA3808 | T→C | 0.008 | 0.000 | 0.000 | 0.000 | ||
| 101 | 3′UTR α | 3940 | pA3940 | A→G | 0.000 | 0.000 | 0.000 | 0.008 | ||
| 102 | 3′UTR α | 4214 | pA4214 | A→G | 0.042 | 0.000 | 0.000 | 0.000 | rs10043662 | |
| 103 | 3′UTR α | 4265 | pA4265 | C→T | 0.000 | 0.000 | 0.008 | 0.000 | ||
| 104 | 3′UTR α | 4332 | pA4332 | G→T | 0.000 | 0.000 | 0.008 | 0.000 | ||
| 105 | 3′UTR α | 4376 | pA4376 | C→T | 0.000 | 0.000 | 0.025 | 0.000 | rs13306586 | |
| 106 | 3′UTR α | 4378 | pA4378 | G→C | 0.008 | 0.000 | 0.000 | 0.000 | rs61753505 | |
| 107 | 3′UTR α | 4522 | pA4522 | A→G | 0.083 | 0.000 | 0.000 | 0.000 | rs6193 | |
| 108 | 3′UTR α | 4633 | pA4633 | A→T | 0.025 | 0.000 | 0.000 | 0.000 | ||
Locations of polymorphisms, alterations in nucleotide and amino acid sequences, as well as observed minor allele frequencies of each SNP in four different ethnic groups are listed. The numbering for polymorphisms in individual noncoding exon 1s and upstream 5′-FRs is based on their distance from the 3′-splice junction for that exon. Negative or positive numbers are located 5′ or 3′ from this position, respectively. Letters represents individual noncoding upstream exon. Numbering for polymorphisms in coding exons 2–9α and downstream of the 3′-UTR begins from the A of the translation initiation codon in exon 2. Variants in introns are numbered based on their distance to the nearest splice site, with positive numbers for 3′ and negative numbers for 5′ splice site. rs numbers are listed when they have been assigned. SNPs used to perform functional studies are highlighted by shading. For SNP designation, m or p indicates minus or plus. The least common allele in AA subjects was defined as the ″minor allele.″
We also calculated “nucleotide diversity,” a quantitative measure of genetic variation, adjusted for the number of alleles studied (28). As shown in Supplemental Table 3, DNA from AA subjects showed greater apparent “diversity” in sequence than did that from other ethnic groups, probably reflecting the greater antiquity of these sequences. In addition, Tajima’s D, a test of the “neutral” mutation hypothesis (29), was also estimated for each population. Under conditions of neutrality, Tajima’s D should equal zero. Only the HCA samples had a value that differed significantly from zero (P = 0.014) (Supplemental Table 3).
NR3C1 haplotype and LD analysis
Haplotype analysis was performed, and it identified 59 haplotypes, 11 observed and 48 inferred, with frequencies greater than 1% (Table 2). Haplotype frequencies also showed significant ethnic differences. A total of 31, 8, 5, and 3 haplotypes were specific for AA, CA, HCA, and MA subjects, respectively. However, we did not observe clearly defined haplotype blocks in any of the ethnic groups except the HCAs, in which a small block was detected (Supplemental Fig. 1).
Table 2.
hGRα gene (NR3C1) haplotypes with frequencies ≥1%
| AA | CA | HCA | MA | Am1141 | Am1133 | Am857 | Am175 | Am607 | Am603 | Am101 | Dm925 | Dm742 | Dm531 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *1A | 0.44577 | 0.52277 | 0.60201 | 0.47831 | G | G | I | G | G | T | G | A | C | T |
| *1B | 0.04353 | — | — | — | G | G | I | G | G | T | G | A | C | T |
| *1C | 0.02613 | 0.01511 | 0.08956 | 0.01667 | G | G | I | G | G | T | G | A | C | T |
| *1D | 0.02554 | — | — | — | G | G | I | G | G | T | G | A | C | T |
| *1E | 0.025 | — | — | — | G | G | I | G | G | T | G | A | C | T |
| *1F | 0.01701 | 0.12015 | — | 0.075 | G | G | I | G | G | T | G | A | C | T |
| *1G | 0.01667 | — | — | 0.00833 | G | G | I | G | G | T | G | A | C | T |
| *1H | 0.01667 | — | — | — | G | G | I | G | G | T | G | A | C | T |
| *1I | 0.01649 | — | — | — | G | G | D | G | G | T | G | A | C | T |
| *1J | 0.00833 | — | — | 0.025 | G | G | I | G | G | T | G | A | C | T |
| *1K | — | 0.11395 | — | 0.04167 | G | G | I | G | G | T | G | A | C | T |
| *1L | — | 0.01755 | — | — | G | A | I | G | G | T | G | A | C | T |
| *1M | — | 0.01667 | 0.01667 | 0.00833 | G | G | I | G | G | T | G | A | C | T |
| *1N | — | 0.01337 | — | — | G | G | I | G | G | T | G | A | C | T |
| *1O | — | 0.01066 | — | — | G | G | I | G | G | T | G | A | C | T |
| *1P | — | 0.00833 | 0.02458 | — | G | A | I | G | G | T | G | A | C | T |
| *1Q | — | — | 0.05875 | 0.18002 | G | A | I | G | G | T | G | A | C | T |
| *1R | — | — | 0.02925 | — | G | G | I | G | G | T | G | A | C | T |
| *1S | — | — | 0.01667 | — | G | G | I | G | G | T | A | A | C | T |
| *1T | — | — | 0.00979 | 0.01667 | G | G | I | G | A | G | G | A | C | T |
| *1U | — | — | — | 0.01667 | G | A | I | G | G | T | G | A | C | T |
| *1V | — | — | — | 0.01336 | G | G | I | G | G | T | G | A | T | T |
| *2A | 0.00833 | — | — | — | G | G | D | G | G | T | G | A | C | T |
| *2B | — | 0.00878 | — | 0.00833 | G | G | I | G | G | T | G | A | C | T |
| *3 | — | 0.00789 | — | — | G | G | I | G | G | T | G | A | C | T |
| *4A | 0.00407 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4B | 0.00393 | — | — | — | G | G | D | G | G | T | G | G | C | T |
| *4C | 0.00361 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4D | 0.00319 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4E | 0.00303 | — | — | — | G | G | D | G | G | T | G | G | C | T |
| *4F | 0.00292 | — | — | — | G | G | I | T | G | T | G | G | C | T |
| *4G | 0.00284 | — | — | — | G | G | I | T | G | T | G | G | C | T |
| *4H | 0.00239 | — | — | — | G | G | I | T | G | T | G | G | C | T |
| *4I | 0.00226 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4J | 0.00182 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4K | 0.00163 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4L | 0.00122 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4M | 0.00112 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4N | 0.00111 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4O | 0.00103 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4P | 0.001 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4Q | 0.00093 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4R | 0.00086 | — | — | — | G | G | D | G | G | T | G | G | C | T |
| *4S | 0.00074 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4T | 0.00061 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4U | 0.00046 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4V | 0.00035 | — | — | — | G | G | I | G | G | T | G | G | C | T |
| *4W | 0.00034 | — | — | — | G | G | D | G | G | T | G | G | C | T |
| *4X | 0.00018 | — | — | — | G | G | I | T | G | T | G | G | C | T |
| *5 | 0.00833 | — | — | — | G | G | I | G | G | T | G | A | C | T |
| *6 | — | 0.00833 | — | — | G | G | I | G | G | T | G | A | C | T |
| *7 | — | — | 0.00833 | — | G | G | I | G | G | T | G | A | C | C |
| *8 | — | — | — | 0.00833 | G | A | I | G | G | T | G | A | C | T |
| *9A | — | 0.02633 | — | 0.01667 | G | G | I | G | G | T | G | A | C | T |
| *9B | — | 0.00833 | — | — | G | A | I | G | G | T | G | A | C | T |
| *9C | — | 0.00833 | — | — | G | G | I | D | G | T | G | A | C | T |
| *9D | — | 0.00745 | — | — | G | A | I | G | G | T | G | A | C | T |
| *10 | — | — | 0.00833 | — | C | G | I | G | G | T | G | A | C | T |
| *11 | — | — | 0.01667 | — | G | G | I | G | G | T | G | A | C | T |
| (Continued) | ||||||||||||||
Table 2A.
Continued
| p102 | Em58 | Bm189 | Bm187 | Bm174 | Bm10 | Fm208 | Fm137 | Fm52 | Cm746 | Cm312 | Cm240 | Cm238 | Cm237 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | D | T | T | I | D | C | G | A | T | T | G | C | I |
| G | D | G | C | I | D | C | G | A | T | T | G | C | I |
| G | D | T | T | I | I | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | T | T | C | T | D |
| G | D | T | T | I | D | C | G | A | C | T | G | C | I |
| G | D | G | C | I | D | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | T | T | G | C | I |
| G | D | G | C | I | D | C | G | A | T | T | G | C | I |
| G | D | G | C | I | D | C | G | A | T | T | G | C | I |
| A | D | T | T | I | D | C | G | A | T | G | G | C | I |
| G | D | G | C | I | I | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | A | A | T | T | G | C | I |
| G | D | T | T | I | I | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | T | T | G | C | I |
| G | D | G | C | I | D | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | C | T | G | C | I |
| G | D | T | T | I | D | C | G | A | C | T | G | C | I |
| G | D | T | T | I | D | C | G | A | C | T | G | C | I |
| G | I | G | T | D | D | C | G | G | T | T | G | C | I |
| G | I | T | T | D | D | C | G | G | T | T | G | C | I |
| G | I | T | T | D | D | C | G | G | T | T | G | C | I |
| G | I | T | T | D | D | C | G | G | T | T | G | C | I |
| G | I | G | T | D | D | C | G | G | T | T | G | C | I |
| G | I | T | C | D | D | C | G | G | T | T | G | C | I |
| G | I | T | T | D | D | C | G | G | T | T | G | C | I |
| G | I | G | C | D | D | C | G | G | T | T | G | C | I |
| G | I | T | C | D | D | C | G | G | T | T | G | C | I |
| G | I | T | C | D | I | C | G | G | T | T | G | C | I |
| G | I | G | T | D | D | C | G | G | T | T | G | C | I |
| G | I | T | C | I | I | C | G | G | T | T | G | C | I |
| G | I | G | T | I | I | C | G | G | T | T | G | C | I |
| G | I | G | C | I | I | C | G | G | T | T | G | C | I |
| G | I | G | C | D | I | C | G | G | T | T | G | C | I |
| G | I | G | C | D | D | C | G | G | T | T | G | C | I |
| G | I | T | T | I | I | C | G | G | T | T | G | C | I |
| G | I | T | C | D | D | C | G | G | T | T | G | C | I |
| G | I | G | T | D | I | C | G | G | T | T | G | C | I |
| G | I | T | C | D | D | C | G | G | T | T | G | C | I |
| G | I | G | C | D | D | C | G | G | T | T | G | C | I |
| G | I | T | T | D | I | C | G | G | T | T | G | C | I |
| G | I | G | C | D | D | C | G | G | T | T | G | C | I |
| G | I | G | T | D | D | C | G | G | T | T | G | C | I |
| G | D | G | C | I | D | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | C | T | G | C | I |
| G | D | T | T | I | D | G | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | C | T | G | C | I |
| G | D | G | C | I | D | C | G | A | T | T | G | C | I |
| G | D | G | C | I | D | C | G | A | T | T | G | C | I |
| G | D | G | C | I | D | C | G | A | T | T | G | C | I |
| G | D | T | T | I | D | C | G | A | T | T | G | C | I |
| (Continued) | |||||||||||||
Table 2B.
Continued
| AA | CA | HCA | MA | Hm236 | E2p66 | E2p68 | E2p193 | E2p295 | E2p624 | E2p685 | E2p874 | E2p879 | E2p973 | E2p1088 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *1A | 0.44577 | 0.52277 | 0.60201 | 0.47831 | G | G | G | T | G | G | G | A | G | A | A |
| *1B | 0.04353 | — | — | — | A | G | G | T | G | G | G | A | G | A | A |
| *1C | 0.02613 | 0.01511 | 0.08956 | 0.01667 | G | G | G | T | G | G | G | A | G | A | A |
| *1D | 0.02554 | — | — | — | G | G | G | T | G | G | G | A | G | A | A |
| *1E | 0.025 | — | — | — | G | G | G | T | G | G | G | A | G | A | A |
| *1F | 0.01701 | 0.12015 | — | 0.075 | G | G | G | T | G | G | G | A | G | A | A |
| *1G | 0.01667 | — | — | 0.00833 | A | G | G | T | G | G | G | A | G | A | A |
| *1H | 0.01667 | — | — | — | G | G | G | T | G | G | G | A | G | A | A |
| *1I | 0.01649 | — | — | — | G | G | G | T | G | G | G | A | A | A | A |
| *1J | 0.00833 | — | — | 0.025 | G | G | G | T | G | G | G | A | G | A | A |
| *1K | — | 0.11395 | — | 0.04167 | A | G | G | T | G | G | G | A | G | A | A |
| *1L | — | 0.01755 | — | — | A | G | G | T | G | G | G | A | G | A | A |
| *1M | — | 0.01667 | 0.01667 | 0.00833 | G | G | G | T | G | G | G | A | G | A | A |
| *1N | — | 0.01337 | — | — | A | G | G | T | G | G | G | A | G | A | A |
| *1O | — | 0.01066 | — | — | G | G | G | T | G | G | G | A | G | A | A |
| *1P | — | 0.00833 | 0.02458 | — | G | G | G | T | G | G | G | A | G | A | A |
| *1Q | — | — | 0.05875 | 0.18002 | G | G | G | T | G | G | G | A | G | A | A |
| *1R | — | — | 0.02925 | — | A | G | G | T | G | G | G | A | G | A | A |
| *1S | — | — | 0.01667 | — | G | G | G | T | G | G | G | A | G | A | A |
| *1T | — | — | 0.00979 | 0.01667 | G | G | G | T | G | G | G | A | G | A | A |
| *1U | — | — | — | 0.01667 | G | G | G | T | G | G | G | A | A | A | A |
| *1V | — | — | — | 0.01336 | G | G | G | T | G | G | G | A | G | A | A |
| *2A | 0.00833 | — | — | — | G | A | A | T | G | G | G | A | A | A | A |
| *2B | — | 0.00878 | — | 0.00833 | G | A | A | T | G | G | G | A | G | A | A |
| *3 | — | 0.00789 | — | — | G | A | A | T | G | G | G | A | G | A | G |
| *4A | 0.00407 | — | — | — | A | G | G | G | G | G | G | A | G | A | A |
| *4B | 0.00393 | — | — | — | G | G | G | G | G | G | G | A | A | A | A |
| *4C | 0.00361 | — | — | — | G | G | G | G | G | G | G | A | A | A | A |
| *4D | 0.00319 | — | — | — | A | G | G | G | G | G | G | A | G | A | A |
| *4E | 0.00303 | — | — | — | G | G | G | G | G | G | G | A | A | A | A |
| *4F | 0.00292 | — | — | — | G | G | G | G | G | G | G | A | G | A | A |
| *4G | 0.00284 | — | — | — | G | G | G | G | G | G | G | A | G | A | A |
| *4H | 0.00239 | — | — | — | G | G | G | G | G | G | G | A | G | A | A |
| *4I | 0.00226 | — | — | — | G | G | G | G | G | G | G | A | A | A | A |
| *4J | 0.00182 | — | — | — | G | G | G | G | G | G | G | A | G | A | A |
| *4K | 0.00163 | — | — | — | G | G | G | G | G | G | G | A | A | A | A |
| *4L | 0.00122 | — | — | — | G | G | G | G | G | G | G | A | G | A | A |
| *4M | 0.00112 | — | — | — | G | G | G | G | G | G | G | A | G | A | A |
| *4N | 0.00111 | — | — | — | G | G | G | G | G | G | G | A | G | A | A |
| *4O | 0.00103 | — | — | — | G | G | G | G | G | G | G | A | G | A | A |
| *4P | 0.001 | — | — | — | G | G | G | G | G | G | G | A | A | A | A |
| *4Q | 0.00093 | — | — | — | G | G | G | G | G | G | G | A | G | A | A |
| *4R | 0.00086 | — | — | — | G | G | G | G | G | G | G | A | A | A | A |
| *4S | 0.00074 | — | — | — | G | G | G | G | G | G | G | A | G | A | A |
| *4T | 0.00061 | — | — | — | A | G | G | G | G | G | G | A | G | A | A |
| *4U | 0.00046 | — | — | — | A | G | G | G | G | G | G | A | G | A | A |
| *4V | 0.00035 | — | — | — | G | G | G | G | G | G | G | A | G | A | A |
| *4W | 0.00034 | — | — | — | G | G | G | G | G | G | G | A | A | A | A |
| *4X | 0.00018 | — | — | — | G | G | G | G | G | G | G | A | G | A | A |
| *5 | 0.00833 | — | — | — | A | G | G | T | A | A | G | A | G | A | A |
| *6 | — | 0.00833 | — | — | G | G | G | T | G | G | A | A | G | A | A |
| *7 | — | — | 0.00833 | — | G | G | G | T | G | G | G | G | G | A | A |
| *8 | — | — | — | 0.00833 | G | G | G | T | G | G | G | A | G | G | A |
| *9A | — | 0.02633 | — | 0.01667 | G | G | G | T | G | G | G | A | G | A | G |
| *9B | — | 0.00833 | — | — | G | G | G | T | G | G | G | A | G | A | G |
| *9C | — | 0.00833 | — | — | A | G | G | T | G | G | G | A | G | A | G |
| *9D | — | 0.00745 | — | — | A | G | G | T | G | G | G | A | G | A | G |
| *10 | — | — | 0.00833 | — | G | G | G | T | G | G | G | A | G | A | A |
| *11 | — | — | 0.01667 | — | G | G | G | T | G | G | G | A | G | A | A |
| (Continued) | |||||||||||||||
All haplotypes with frequencies of 1% or greater in at least one group are listed. Haplotypes containing a variant amino acid sequence were also included in the table even if their frequencies were less than 1%. Nucleotide locations are numbered as described in the legend for Table 1. Haplotypes (*1, *2... *11) were designated on the basis of variant amino acids, beginning at the N terminus and proceeding to the C terminus of the encoded protein, with the WT as *1. Subsequent letter designations were based on haplotype frequencies. Variant nucleotides compared with ″reference sequence″ (i.e. the most common sequence in AA subjects) are highlighted by shading. Dashes represent lack of that haplotype in the population indicated.
Table 2C.
Continued
| I4m16 | E5p1510 | I5m71 | E6p1764 | I7p7470 | E8p2034 | E8p2061 | I8p244 | I8m236 | I8m157 | I8m43 | E9p2298 | pA2744 | pA3271 | pA3583 | pA4214 | pA4522 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| T | A | C | C | G | T | T | C | C | G | G | C | I | C | D | A | G |
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| G | A | C | C | G | T | T | T | C | G | G | T | I | C | D | A | A |
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| T | A | C | C | G | C | T | C | C | G | G | T | I | C | D | A | A |
| T | A | C | C | C | C | T | C | C | G | G | C | I | C | D | A | A |
| T | A | C | C | G | T | T | C | C | G | G | C | I | C | D | A | A |
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| G | A | C | C | G | C | T | T | C | G | G | T | D | C | D | A | A |
| T | A | C | C | G | C | T | C | C | G | G | C | I | C | D | A | A |
| T | A | C | C | G | C | T | C | C | G | G | C | I | C | D | A | A |
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| T | A | C | C | G | C | T | C | C | G | G | C | I | C | D | A | A |
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| T | A | C | T | G | T | T | C | C | G | G | C | I | C | D | A | A |
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| T | A | C | C | G | C | T | C | C | G | G | T | I | C | D | A | A |
| T | A | C | C | G | C | T | C | C | T | G | T | I | C | D | A | A |
| T | A | C | C | G | C | T | C | C | T | G | T | I | C | D | A | A |
| T | A | A | C | G | C | T | C | G | G | G | T | I | A | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | A | C | G | C | T | C | G | G | G | T | I | A | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | A | C | G | C | T | C | G | G | G | T | I | A | D | G | A |
| T | A | A | C | G | C | T | C | G | G | G | T | I | A | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | G | G | G | T | I | C | D | G | A |
| T | A | C | C | G | C | T | C | C | G | C | C | I | C | D | A | A |
| T | A | C | C | G | C | T | C | C | G | G | T | I | C | D | A | A |
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| G | A | C | C | G | C | T | T | C | G | G | T | I | C | D | A | A |
| T | A | C | C | G | C | T | C | C | G | G | T | I | C | I | A | A |
| T | A | C | C | G | C | T | C | C | G | G | C | I | C | D | A | A |
| T | A | C | C | G | C | T | C | C | G | G | C | I | C | D | A | A |
| T | T | C | T | G | T | T | C | C | G | G | C | I | C | D | A | A |
| G | A | C | C | G | C | G | T | C | G | G | T | I | C | D | A | A |
Pairwise LD analysis for all the SNPs was performed by calculating D′ and r2 values (22). Among the cSNPs, pairwise analysis indicated strong LD between 66G>A and Arg23Lys in the AA, CA, and MA groups (D′ = 1; r2 = 1; P < 0.001). HCA samples did not contain these two SNPs. We also observed strong LD between two synonymous cSNPs, 2034C>T and 2298T>C in HCA (D′ = 1; r2 = 1; P < 0.001) and AA subjects (D′ = 0.685; r2 = 0.469; P < 0.001).
Functional studies of cSNPs
Nine nonsynonymous cSNPs and four common synonymous cSNPs with MAFs greater than 5% were identified. Gly(99)Arg and Ala(229)Thr were located in the transactivation domain of the NR3C1 gene (30). Thr(504)Ser was in the region that interacts with activator protein-1/nuclear factor-κB, whereas Asp(687)Glu and three synonymous cSNPs, 1764C>T, 2034C>T, and 2298T>C, were in the ligand binding domain, a region that is also important for protein-protein interaction (6). To determine the potential functional impact of common cSNPs in NR3C1, we performed functional assays using COS-1 cells. Sixteen mammalian expression constructs were created, including WT, nine nonsynonymous, and four common synonymous cSNPs. We also created constructs for SNPs that were tightly linked with each other, 66G>A/Arg(23)Lys, and 2034C>T/2298T>C. After transient expression in COS-1 cells, protein expression level, whole cell receptor binding affinity (Kd), and the transactivation activity of GRα were evaluated.
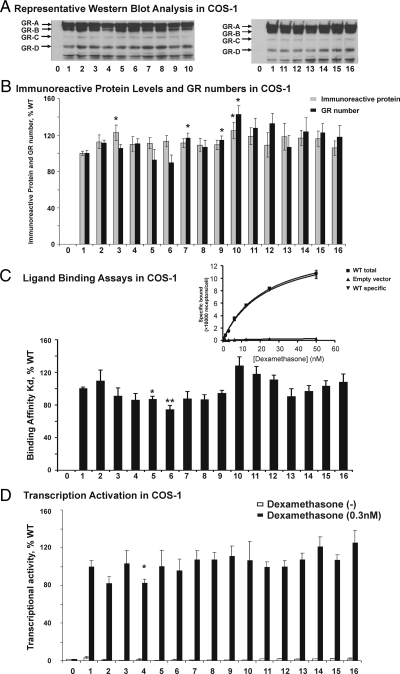
Representative quantitative Western blots for GRα expression constructs are shown in Fig. 2A. Previous studies identified eight GRα isoforms that result from alternative translation initiation in COS-1 cells overexpressing hGRα using antibodies directed against the N (amino acids 346-367) and C (amino acids 755-771) terminals (31). In the present study, we used a polyclonal antibody targeting amino acids 720 to 770 using lysates from COS-1 cells transfected with different constructs, after correction for transfection efficiency. The same GRα isoforms were observed with all of the constructs (Fig. 2A). Because of low intensity, we only quantified the bands for the GRα-A and -B isoforms (Fig. 2B). Levels of protein for GRα variants, expressed as a percentage of WT, ranged from 106 to 125%. Levels for the Phe(65)Val and Asp(687)Glu variants were slightly higher than WT (P = 0.022 and 0.020). Because previous results showed that 66G>A/Arg(23)Lys can alter GRα-A/-B (12), we also quantified the ratio between the two isoforms. No significant difference was found, perhaps due to high expression levels and the close position of the two bands on the gel. To determine the effects of these SNPs on Kd and GRα number, we also performed whole-cell dexamethasone binding studies. Although the assay has been used in previous studies (32), we used mineralcorticoid receptor as a positive control to make sure that the assay was adequately sensitive (Supplemental Fig. 2, A and B). A representative whole-cell dexamethasone binding assay is shown in Fig. 2C. The Kd for GRα WT was 19.9 ± 5.5 nm (mean ± sd), and Ala(229)Thr and Ile(292)Val showed significantly lower Kd values (P = 0.019 and 0.006) (Fig. 2C). Ser(325)Gly, Thr(504)Ser, and Asp(687)Glu displayed elevated GRα number (Bmax) (P = 0.039, 0.037, 0.011) (Fig. 2B). To validate these results from COS-1 overexpression studies, we selected several cell lines to perform whole-cell dexamethasone binding assays, based on NR3C1 genotype for significant SNPs from the 240 lymphoblastoid cell lines from which the DNA used for resequencing was extracted, including Phe(65)Val, Ser(325)Gly, Ala(229)Thr, Ile(292)Val, Thr(504)Ser, and Asp(687)Glu as well as a widely studied SNP and haplotype, 66G>A/Arg(23)Lys and Asn(363)Ser. Only Ala(229)Thr showed a lower Kd than WT (P = 0.03), although with only one heterozygous cell line. However, no significant difference was detected for the GRα number.
Figure 2.
Functional studies of cSNPs in hGRα gene (NR3C1). Level of transcriptional activity, binding affinity, and immunoreactive protein of the recombinant human NR3C1 variants are shown in the bar graphs. The numbers on the x-axis indicate recombinant WT or coding variant constructs for GRα gene (NR3C1): zero for empty vector as a negative control, 1 for WT, 2 for Arg(23)Lys, 3 for Phe(65)Val, 4 for Gly(99)Arg, 5 for Ala(229)Thr, 6 for Ile(292)Val, 7 for Ser(325)Gly, 8 for Asn(363)Ser, 9 for Thr(504)Ser, 10 for Asp(687)Glu, 11 for E2p879G>A, 12 for E6p1764C>T, 13 for E8p2034C>T, 14 for E9p2298T>C, 15 for E2p66G>A/Arg(23)Lys, and 16 for E8p2034C>T/E9p2298T>C. The value for WT was set to 100%, and other constructs are expressed relative to the WT value. Each bar represents the average of at least nine independent transfections for luciferase assay and Western blots or three binding assays (mean ± sem). *, P < 0.05; **, P < 0.01 compared with WT of GRα. A, A representative Western blot for NR3C1 variants in COS-1 cell. B, Immunoreactive protein levels and GRα numbers of NR3C1 variants in COS-1 cell. C, Representative binding assay for WT GRα and Kd (binding affinity) values for NR3C1 variants in COS-1 cell. WT-specific binding was calculated by subtracting nonspecific binding of empty vector from WT total binding. D, Transcriptional activities of NR3C1 variants at dexamethasone, 0.3 nm in COS-1 cell.
Finally, we performed luciferase assays with COS-1 cells overexpressing different constructs in the presence of increasing concentrations of dexamethasone to determine the effect of the SNPs on GRα transactivation activity. Firefly luciferase activity in response to various dexamethasone concentrations was measured to determine the activity of the glucocorticoid-responsive mouse mammary tumor virus promoter. Renilla luciferase was used to correct transfection efficiency. With increasing concentration of dexamethasone (0–500 nm), the transactivation of GRα showed a dose-dependent change (Supplemental Fig. 3). Renilla luciferase activities were significantly repressed with higher concentrations of dexamethasone (>10 nm), consistent with previous reports (33). However, repression levels showed no differences between WT and variants except at 500 nm (data not shown). Fig. 2D shows transcriptional activity at 0.3 nm for WT and variants. No significant differences at 0.3 nm were observed between WT and variants. However, SNP Gly(99)Arg showed slightly lower transcriptional activities in the presence of 0.1, 0.3, and 1 nm dexamethasone (P = 0.028, 0.038, 0.012). 66G>A/Arg(23)Lys and Asn(363)Ser also resulted in slightly lower activity in the presence of 0.1 nm and 1 nm dexamethasone, respectively (63.4 and 82.3% of WT; P = 0.0007 and 0.0497), consistent with previous reports (11,12,13,14,15). In addition to nonsynonymous cSNPs, SNPs in regulatory regions can also have functional consequences. Therefore, we performed an association study to identify SNPs that might have a significant impact on NR3C1 expression.
NR3C1 genotype-phenotype association analysis for mRNA expression
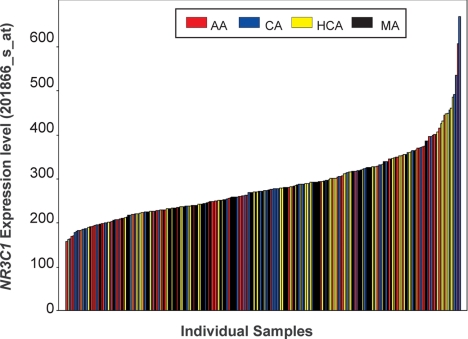
RNA isolated from the 240 lymphoblastoid cells from which DNA was extracted for resequencing was used to perform expression array analysis (19). GRα expression levels for three Affymetrix probe sets (201865_x_at, 201866_s_at, and 211671_s_at) correlated well (r ≥ 0.75; P < 0.0001). NR3C1 expression varied approximately 6-fold in these cells as determined by probe, 201866_s_at (Fig. 3). Because NR3C1 contains multiple exon 1s, we also performed Affymetrix Human Exon 1.0 ST Array assay using eight randomly selected lymphoblastoid cells. The probe sets for NR3C1 on these exon arrays hybridize with all of the noncoding exon 1s except for exons 1D and 1H. The exon array data indicated that exon 1C was most highly expressed in these lymphoblastoid cells compared with other exon 1s (data not shown).
Figure 3.
mRNA expression levels of NR3C1 in human lymphoblastoid cell lines. Each bar represents the mRNA expression level of individual sample using Affymetrix U133 plus 2.0 expression microarray analysis (Probe sets 201866_s_at). Data are colored by ethnic group.
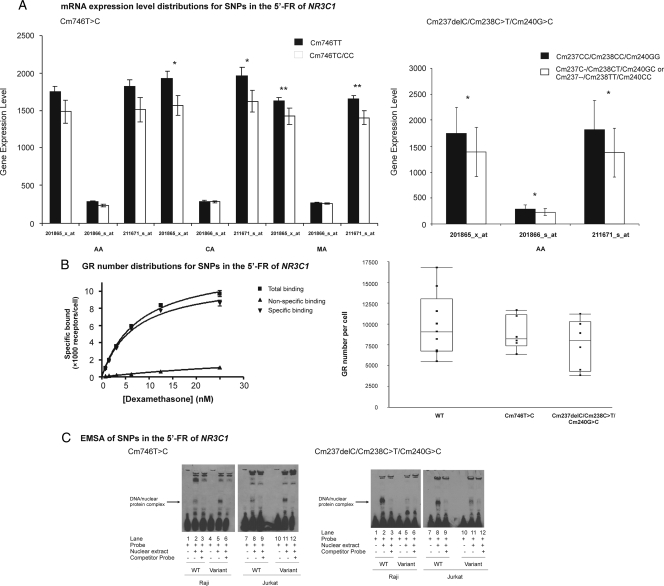
To determine whether SNPs in NR3C1 might be associated with NR3C1 expression, we performed a genotype-phenotype correlation study using PLINK analysis (25). The analysis was performed for each ethnic group using all three GRα expression probe sets. One SNP, Cm746T>C, located in the 5′-flanking region upstream of exon 1C, with a MAF of 13.3% in MAs, showed a significant association with NR3C1 gene expression in that population and remained significant after Bonferroni correction. Expression of the TT genotype was significantly higher than CT or CC, as shown in Fig. 4A with probe sets 201865_x_at and 211671_s_at (P < 0.001). The frequency of this SNP was 18.3% in CAs and 7.5% in AAs, and significant differences were also observed in CA subjects (P < 0.05). In addition, three polymorphisms, deletion of C at nucleotide 237 in exon 1, Cm238C>T and Cm240G>C in exon 1C showed tight LD (D′ = 1; r2 = 1; P < 0.001) in AA subjects. The frequency of this haplotype was 5.8% in AAs. The expression level for CC/CC/GG was significantly higher than those for the C-/CT/GC or –/TT/CC haplotypes for all three probe sets (P < 0.05) in AAs (Fig. 4A). Although P values for this haplotype were not significant after Bonferroni correction because it was associated with all three NR3C1 probe sets, we performed EMSA together with Cm746T>C. We also performed whole-cell dexamethasone binding studies using lymphoblastoid cell lines to determine whether these four SNPs that resulted in alterations in mRNA expression level might also change receptor number. We selected six cell lines of Cm746T>C carriers, six cell lines for Cm237delC/Cm238C>T /Cm240G>C carriers, and nine WT (without any observed SNPs). Representative binding assay and genotype-phenotype associations for these SNPs are shown in Fig. 4B. Cm237delC/Cm238C>T /Cm240G>C and Cm746T>C showed a trend toward lower receptor number compared with WT.
Figure 4.
Functional studies of SNPs in the exon 1C and 5′-FR of exon 1C. A, SNP-NR3C1 expression association. **, P < 0.001; *, P < 0.05. SNP Cm746T>C showed in three ethnic groups, and haplotype Cm237insC/Cm238C>T/Cm240G>C showed in AA subjects with all three probe sets. Forty-eight, 39, and 45 Cm746TT carriers and 8, 21, and 15 Cm746TC/CC carriers were observed in AA, CA, and MA subjects, respectively. Fifty homozygous WT for Cm237insC/Cm238C>T/Cm240G>C as well as six heterozygous or homozygous variants for this haplotype were detected in AAs. B, Representative whole-cell dexamethasone binding assay for lymphoblastoid cell line and SNP-GR number association study performed with selected lymphoblastoid cell lines. Each dot represented GR number for each sample. The box plot indicates the quartiles of GR number. C, EMSA of SNPs in the exon 1C and 5′-FR of exon 1C. EMSA were performed using biotin-labeled probes containing WT or variant sequences for the common Cm746T>C SNP and for the haplotype Cm237insC/Cm238C>T/Cm240G>C using nuclear extract from Raji and Jurkat cells. Competition reactions were performed with 400-fold excess of unlabeled probes.
EMS assays
To determine the effect of the Cm746T>C SNP and the Cm237delC/Cm238C>T /Cm240G>C haplotype on protein binding patterns, we performed EMSA using nuclear extracts from Raji and Jurkat cells. EMSA indicated that the WT for Cm746T had lower nuclear protein binding than did the variant Cm746C, whereas WT for Cm237C/Cm238C /Cm240G had higher nuclear protein binding than did the variant haplotype Cm237delC/Cm238T/Cm240C (Fig. 4C). These results supported possible differential effects of these polymorphisms on transcription.
Discussion
The GR plays an important role in multiple physiological and pathophysiological processes (2). Alternative splicing of transcripts for the human GR gene in exon 9 generates two isoforms, α and β (34). GRα is expressed in most human tissues and represents the “classical” hGR that functions as a ligand-dependent transcription factor (1,2). Glucocorticoids are also widely used as therapeutic agents to treat a variety of diseases (3). Genetic variation in NR3C1 has been shown to affect both disease pathophysiology and response to glucocorticoid therapy, which supported the hypothesis that SNPs might play a role in receptor function (6). Therefore, in this study, we resequenced NR3C1 using 240 DNA samples from four ethnic groups to identify common genetic polymorphisms. We identified 108 polymorphisms, 51 of which were publicly available (Table 1 and Fig. 1). Some SNPs identified during previous resequencing efforts with different ethnic groups were also observed during our studies. For example, 21, 23, and 32 SNPs identified in studies performed by Hawkins et al. (27), Koyano et al. (35), and Chung et al. (26) were also found in our study. However, none of the previously identified rare functional mutations in the GRα gene were observed during our resequencing studies (32,36).
We then performed functional studies with all nine nonsynonymous SNPs, four common synonymous SNPs, and two common coding region haplotypes. Our Western blot analysis showed that multiple isoforms were present in COS-1 cells overexpressing GRα, and protein levels for the Phe(65)Val and Asp(687)Glu variants were slightly higher than WT (Fig. 2B). Asp(687)Glu also showed higher receptor number in COS-1 cells (Fig. 2B). Apparent Kd values for Ala(229)Thr and Ile(292)Val were 13 and 26% lower than WT, respectively (Fig. 2C). Whole-cell dexamethasone binding assays in lymphoblastoid cell lines also indicated that Kd of Ala(229)Thr was significantly lower than WT. Transactivation capacity with a GRE-LUC reporter did not show significant differences at 0.3 nm among the variants, although several SNPs showed significant effects at individual dexamethasone concentration (Fig. 2D). Obviously, these results do not exclude the possibility that other GRE-containing promoters might show different effects. Several SNPs have been studied intensively, including 66G>A and Arg23Lys, Asn(363)Ser, and Thr(504)Ser (11,12,13,14,15,16). 66G>A and Arg(23)Lys have been reported to be involved in glucocorticoid resistance (9), and Asn(363)Ser has been associated with increased glucocorticoid sensitivity and coronary artery disease (37). Previous studies with 66G>A and Arg(23)Lys showed no effect on dexamethasone binding capacity, mRNA, or protein expression levels (12,13) but did report effect on ratio of isoforms A and B (12). We did not observe a significant difference in the A/B ratio between WT and this variant. This might be due to high expression levels and close positions of the two isoforms on the gel. The effect of these SNPs on transactivation remains controversial (11,12,13). We observed a significant effect of 66G>A and Arg(23)Lys on dexamethasone-dependent transactivation activity in the presence of 0.1 nm dexamethasone. Finally, we failed to observe significant effects of Asn(363)Ser or Thr(504)Ser on GRα expression level, ligand binding, or transactivation capacity (Fig. 2, B–D), consistent with previous findings (13,14,15,16).
To identify SNPs in regulatory regions that might affect GRα expression, we performed genotype-phenotype association studies using GRα expression array data obtained with the 240 cell lines from which DNA for resequencing had been extracted. Obviously, expression patterns are tissue-specific. However, because one of the major functions of glucocorticoids when used as immunosuppressants involves effects on lymphocytes (38), lymphoblastoid cell lines represent a reasonable system for hypothesis testing. Using exon arrays, we found that exon 1C is highly expressed in these cell lines, consistent with previous results (8,39). However, exon 1C is expressed in both GRα and GRβ, so its levels are not specific for GRα. Therefore, we used probe sets specific to the α isoform to perform the association study. Our association study identified one SNP, Cm746T>C in the 5′-FR of exon 1C, that was significantly associated with NR3C1 expression level in MA subjects (Fig. 4A). We also observed a significant association with the Cm237delC/Cm238C>T/Cm240G>C haplotype in exon 1C (Fig. 4A). These SNPs also showed a trend toward lower GRα number than WT determined by the whole cell binding assays using lymphoblastoid cell lines (Fig. 4B). Furthermore, EMSAs for both Cm746T>C and Cm237delC/Cm238C>T/Cm240G>C showed differential protein binding patterns (Fig. 4C). A search for possible transcription factors binding at these two sites using AliBaba 2.1 (http://darwin.nmsu.edu/∼molb470/fall2003/Projects/solorz/aliBaba_2_1.htm) identified potential SP1 binding sites for Cm746T>C and Cm237delC/Cm238T/Cm240C. Recently, Kumsta et al. (40) reported that the variant C allele for Cm746T>C showed reduced transcriptional activity in reporter gene assay, consistent with our expression array data, and the C allele was also associated with recurrent major depression in a Belgian study (18), also suggesting possible functional effects of this SNP. Obviously, these results obtained from the microarray data, realizing the limitation of microarray data, could be further confirmed by quantitative RT-PCR analysis. Overall, these observations suggest that future studies might focus on these SNPs and their possible influence on clinical phenotypes related to GRα function.
In conclusion, this comprehensive series of studies provides new insight into the GRα pharmacogenetics and might help us to understand better the role of genetic variation in NR3C1 on response to glucocorticoids.
Supplementary Material
Acknowledgments
We thank Luanne Wussow for her assistance with the preparation of this manuscript.
Footnotes
This work was supported by National Institutes of Health Grants U01 GM61388 (the Pharmacogenetics Research Network), K22 CA130828, and R01 CA138461; an American Society for Pharmacology and Experimental Therapeutics-Astellas Award; and a PhRMA Foundation “Center of Excellence in Clinical Pharmacology” Award.
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 12, 2009
Abbreviations: AA, African-American; CA, Caucasian-American; cSNP, coding SNP; GR, glucocorticoid receptor; GRE, glucocorticoid response element; HCA, Han Chinese-American; hGR, human GR; LD, linkage disequilibrium; MA, Mexican-American; MAF, minor allele frequency; ORF, open reading frame; SNP, single nucleotide polymorphism; UTR, untranslated region; WT, wild-type.
References
- Duma D, Jewell CM, Cidlowski JA 2006 Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J Steroid Biochem Mol Biol 102:11–21 [DOI] [PubMed] [Google Scholar]
- Kumar R, Thompson EB 2005 Gene regulation by the glucocorticoid receptor: structure:function relationship. J Steroid Biochem Mol Biol 94:383–394 [DOI] [PubMed] [Google Scholar]
- Löwenberg M, Stahn C, Hommes DW, Buttgereit F 2008 Novel insights into mechanisms of glucocorticoid action and the development of new glucocorticoid receptor ligands. Steroids 73:1025–1029 [DOI] [PubMed] [Google Scholar]
- Rosen J, Miner JN 2005 The search for safer glucocorticoid receptor ligands. Endocr Rev 26:452–464 [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Gerbal-Chaloin S, Drocourt L, Maurel P, Vilarem MJ 2003 The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim Biophys Acta 1619:243–253 [DOI] [PubMed] [Google Scholar]
- DeRijk RH, Schaaf M, de Kloet ER 2002 Glucocorticoid receptor variants: clinical implications. J Steroid Biochem Mol Biol 81:103–122 [DOI] [PubMed] [Google Scholar]
- Encío IJ, Detera-Wadleigh SD 1991 The genomic structure of the human glucocorticoid receptor. J Biol Chem 266:7182–7188 [PubMed] [Google Scholar]
- Turner JD, Muller CP 2005 Structure of the glucocorticoid receptor (NR3C1) gene 5′ untranslated region: identification, and tissue distribution of multiple new human exon 1. J Mol Endocrinol 35:283–292 [DOI] [PubMed] [Google Scholar]
- Bray PJ, Cotton RG 2003 Variations of the human glucocorticoid receptor gene (NR3C1): pathological and in vitro mutations and polymorphisms. Hum Mutat 21:557–568 [DOI] [PubMed] [Google Scholar]
- Rosmond R, Radulovic V, Holm G 2006 A brief update of glucocorticoid receptor variants and obesity risk. Ann NY Acad Sci 1083:153–164 [DOI] [PubMed] [Google Scholar]
- Russcher H, Smit P, van den Akker EL, van Rossum EF, Brinkmann AO, de Jong FH, Lamberts SW, Koper JW 2005 Two polymorphisms in the glucocorticoid receptor gene directly affect glucocorticoid-regulated gene expression. J Clin Endocrinol Metab 90:5804–5810 [DOI] [PubMed] [Google Scholar]
- Russcher H, van Rossum EF, de Jong FH, Brinkmann AO, Lamberts SW, Koper JW 2005 Increased expression of the glucocorticoid receptor-A translational isoform as a result of the ER22/23EK polymorphism. Mol Endocrinol 19:1687–1696 [DOI] [PubMed] [Google Scholar]
- de Lange P, Koper JW, Huizenga NA, Brinkmann AO, de Jong FH, Karl M, Chrousos GP, Lamberts SW 1997 Differential hormone-dependent transcriptional activation and repression by naturally occurring human glucocorticoid receptor variants. Mol Endocrinol 11:1156–1164 [DOI] [PubMed] [Google Scholar]
- Huizenga NA, Koper JW, De Lange P, Pols HA, Stolk RP, Burger H, Grobbee DE, Brinkmann AO, De Jong FH, Lamberts SW 1998 A polymorphism in the glucocorticoid receptor gene may be associated with an increased sensitivity to glucocorticoids in vivo. J Clin Endocrinol Metab 83:144–151 [DOI] [PubMed] [Google Scholar]
- Jewell CM, Cidlowski JA 2007 Molecular evidence for a link between the N363S glucocorticoid receptor polymorphism and altered gene expression. J Clin Endocrinol Metab 92:3268–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano S, Saito Y, Nagano M, Maekawa K, Kikuchi Y, Murayama N, Fujino T, Ozawa S, Nakajima T, Matsumoto K, Saito H, Sawada J 2003 Functional analysis of three genetic polymorphisms in the glucocorticoid receptor gene. J Pharmacol Exp Ther 307:110–116 [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, Cidlowski JA 2002 AUUUA motifs in the 3′UTR of human glucocorticoid receptor α and β mRNA destabilize mRNA and decrease receptor protein expression. Steroids 67:627–636 [DOI] [PubMed] [Google Scholar]
- van West D, Van Den Eede F, Del-Favero J, Souery D, Norrback KF, Van Duijn C, Sluijs S, Adolfsson R, Mendlewicz J, Deboutte D, Van Broeckhoven C, Claes S 2006 Glucocorticoid receptor gene-based SNP analysis in patients with recurrent major depression. Neuropsychopharmacology 31:620–627 [DOI] [PubMed] [Google Scholar]
- Li L, Fridley B, Kalari K, Jenkins G, Batzler A, Safgren S, Hildebrandt M, Ames M, Schaid D, Wang L 2008 Gemcitabine and cytosine arabinoside cytotoxicity: association with lymphoblastoid cell expression. Cancer Res 68:7050–7058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Irizarry RA 2004 Preprocessing of oligonucleotide array data. Nat Biotechnol 22:656–658; author reply, 658 [DOI] [PubMed] [Google Scholar]
- Moyer AM, Salavaggione OE, Wu TY, Moon I, Eckloff BW, Hildebrandt MA, Schaid DJ, Wieben ED, Weinshilboum RM 2008 Glutathione s-transferase p1: gene sequence variation and functional genomic studies. Cancer Res 68:4791–4801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick PW 2000 Genetics of populations. 2nd ed. Sudbury, MA: Jones and Bartlett Publishers [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ 2005 Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265 [DOI] [PubMed] [Google Scholar]
- Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA 2002 Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet 70:425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC 2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CC, Shimmin L, Natarajan S, Hanis CL, Boerwinkle E, Hixson JE 2009 Glucocorticoid receptor gene variant in the 3′ untranslated region is associated with multiple measures of blood pressure. J Clin Endocrinol Metab 94:268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins GA, Amelung PJ, Smith RS, Jongepier H, Howard TD, Koppelman GH, Meyers DA, Bleecker ER, Postma DS 2004 Identification of polymorphisms in the human glucocorticoid receptor gene (NR3C1) in a multi-racial asthma case and control screening panel. DNA Seq 15:167–173 [DOI] [PubMed] [Google Scholar]
- Fullerton SM, Clark AG, Weiss KM, Nickerson DA, Taylor SL, Stengârd JH, Salomaa V, Vartiainen E, Perola M, Boerwinkle E, Sing CF 2000 Apolipoprotein E variation at the sequence haplotype level: implications for the origin and maintenance of a major human polymorphism. Am J Hum Genet 67:881–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima F 1989 Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Cidlowski JA 2005 The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids 70:407–417 [DOI] [PubMed] [Google Scholar]
- Lu NZ, Cidlowski JA 2005 Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell 18:331–342 [DOI] [PubMed] [Google Scholar]
- Charmandari E, Kino T, Ichijo T, Zachman K, Alatsatianos A, Chrousos GP 2006 Functional characterization of the natural human glucocorticoid receptor (hGR) mutants hGRαR477H and hGRαG679S associated with generalized glucocorticoid resistance. J Clin Endocrinol Metab 91:1535–1543 [DOI] [PubMed] [Google Scholar]
- Ibrahim NM, Marinovic AC, Price SR, Young LG, Fröhlich O 2000 Pitfall of an internal control plasmid: response of Renilla luciferase (pRL-TK) plasmid to dihydrotestosterone and dexamethasone. BioTechniques 29:782–784 [DOI] [PubMed] [Google Scholar]
- Oakley RH, Sar M, Cidlowski JA 1996 The human glucocorticoid receptor β isoform. Expression, biochemical properties, and putative function. J Biol Chem 271:9550–9559 [DOI] [PubMed] [Google Scholar]
- Koyano S, Saito Y, Sai K, Kurose K, Ozawa S, Nakajima T, Matsumoto K, Saito H, Shirao K, Yoshida T, Minami H, Ohtsu A, Saijo N, Sawada J 2005 Novel genetic polymorphisms in the NR3C1 (glucocorticoid receptor) gene in a Japanese population. Drug Metab Pharmacokinet 20:79–84 [DOI] [PubMed] [Google Scholar]
- (Likó I, Igaz P, Patócs A, Tóth S, Pázmány T, Tóth M, Rácz K 2004 Sequence variants of the ligand-binding domain of the glucocorticoid receptor gene and their functional consequences on the three-dimensional protein structure. Curr Med Chem 11:3229–3237 [DOI] [PubMed] [Google Scholar]
- Lin RC, Wang XL, Morris BJ 2003 Association of coronary artery disease with glucocorticoid receptor N363S variant. Hypertension 41:404–407 [DOI] [PubMed] [Google Scholar]
- Tissing WJ, Meijerink JP, den Boer ML, Pieters R 2003 Molecular determinants of glucocorticoid sensitivity and resistance in acute lymphoblastic leukemia. Leukemia 17:17–25 [DOI] [PubMed] [Google Scholar]
- Pedersen KB, Vedeckis WV 2003 Quantification and glucocorticoid regulation of glucocorticoid receptor transcripts in two human leukemic cell lines. Biochemistry 42:10978–10990 [DOI] [PubMed] [Google Scholar]
- Kumsta R, Moser D, Streit F, Koper JW, Meyer J, Wüst S 2009 Characterization of a glucocorticoid receptor gene (GR, NR3C1) promoter polymorphism reveals functionality and extends a haplotype with putative clinical relevance. Am J Med Genet B Neuropsychiatr Genet 150B:476–482 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.