Abstract
Repeated nicotine administration induces tyrosine hydroxylase (TH) mRNA in rat midbrain. In this study we investigate the mechanisms responsible for this response using two models of midbrain dopamine neurons, rat ventral midbrain slice explant cultures and mouse MN9D cells. In both models nicotine stimulates TH gene transcription rate in a dose-dependent manner. However, this stimulation is short-lived, lasting for 1 hr, but less than 3 hr, and is not sufficient to induce TH mRNA or TH protein. Nicotine elevates circulating glucocorticoids, which induce TH expression in some model systems. We tested the hypothesis that the effect of nicotine on midbrain TH mRNA is mediated by the glucocorticoid receptor. When rats are administered the glucocorticoid receptor antagonist mifepristone, the induction of TH mRNA by nicotine in both substantia nigra and ventral tegmentum is inhibited. Furthermore, the glucocorticoid receptor agonist dexamethasone stimulates TH gene transcription for sustained periods of time in both midbrain slices and MN9D cells, leading to induction of TH mRNA and TH protein. Our results are consistent with the hypothesis that nicotine induces TH mRNA in midbrain by elevating glucocorticoids, which then act on glucocorticoid receptors in dopamine neurons leading to transcriptional activation of the TH gene.
Keywords: tyrosine hydroxylase, midbrain dopamine neurons, glucocorticoid, nicotine, MN9D cells, ventral midbrain explant cultures
Tyrosine hydroxylase (TH) is a highly regulated enzyme, which catalyzes the first and rate-limiting step in catecholamine biosynthesis. It plays a key role in maintaining appropriate concentrations of dopamine, norepinephrine and epinephrine in neurons and adrenal medulla (AM). TH gene regulation has been most highly studied under in vivo conditions in AM and locus coeruleus (LC), using stress, reserpine and nicotine as stimuli (Kumer and Vrana 1996; Sabban and Kvetnansky 2001; Wong and Tank 2007). In AM, TH is induced primarily by transcriptional mechanisms. In contrast, both transcriptional and post-transcriptional mechanisms participate in regulating TH expression in LC neurons.
Fewer studies have investigated the regulation of TH expression in midbrain dopamine neurons. These neurons participate in motor control, reinforcement, cognition, mood development and drug addiction. Loss or dysregulation of these neurons is associated with Parkinson’s disease and schizophrenia. Hence, an understanding of the mechanisms that control TH expression in dopamine neurons is highly significant to these physiological and pathological states. Early studies suggested that stress and reserpine do not induce TH in midbrain; however, subsequent studies have shown that these stimuli selectively induce TH in ventral tegmentum (VTA), and less so in substantia nigra (SN) (for reviews see (Sabban and Kvetnansky 2001; Wong and Tank 2007)). More recently, Serova and Sabban (2002) have reported that chronic nicotine administration for 3 days is associated with induction of TH mRNA in both the SN and VTA and that these responses are inhibited by the alpha-7 nicotinic acetylcholine receptor (nAChR) antagonist, methyllycaconitine (MLA). This study clearly implicates nAChRs in the induction of midbrain TH expression; however, it remains unclear whether this induction of TH mRNA is mediated by transcriptional mechanisms, whether it leads to induction of TH protein, and whether it is due to a direct effect of nicotine on nAChRs on midbrain dopamine neurons.
In the present report we address aspects of these issues. We test whether nicotine stimulates TH gene transcription and induces TH mRNA and TH protein in two models of cultured midbrain dopamine neurons; ventral midbrain slice explant cultures and MN9D cells. Our results demonstrate that nicotine activates TH gene transcription in both models and that this activation is blocked by MLA. However, surprisingly, TH gene transcriptional activation is not sufficient to significantly induce TH mRNA or TH protein. Hence, we have tested the hypothesis that glucocorticoids, which are elevated by nicotine and induce TH in some model systems, participate in this nicotine-mediated response. Our results demonstrate that the glucocorticoid receptor (GR) antagonist mifepristone (RU38486) inhibits the induction of midbrain TH mRNA elicited by nicotine administration to rats. Furthermore, we show that the GR agonist dexamethasone induces TH gene expression in both midbrain slice cultures and MN9D cells. These results argue that the induction of midbrain TH mRNA by nicotine is not due solely to the direct interaction of the drug with midbrain nAChRs, but is at least partially mediated by a nicotine-induced rise of glucocorticoids.
EXPERIMENTAL PROCEDURES
Midbrain slice explant cultures and MN9D cell cultures
Ventral midbrain slices were cultured as described by Chen et al (2008). Briefly, brains were removed from Sprague-Dawley rat pups on postnatal day 7–10 and immediately placed in oxygenated (95% O2 and 5% CO2), ice-cold artificial cerebrospinal fluid (ACSF; 125 mM NaCl, 26 mM NaHCO3, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2, 1 mM MgSO4 (anhydrous) and 25 mM glucose, pH 7.4). Coronal midbrain slices (350–400 um) were obtained using an Integraslice 7550 PSDS Vibraslicer (Campden Instruments) and perfused in ice-cold oxygenated ACSF for 1–2 hr. The slices from each rat pup were placed on millicell inserts (Millipore) in a single well of a 6-well plate and cultured at 35°C in an atmosphere of 95% air and 5% CO2 in medium containing 50% Earles minimal essential medium, 25 mM Hepes, 25% Hanks basal salt solution (with phenol red), 2 mM L-glutamine, 0.01 ug/ml penicillin/streptomycin, 25% heat-inactivated horse serum (GIBCO) and 6.4 g/l glucose. The slices were cultured for 1 day in vitro prior to experimentation.
MN9D cells are a hybrid cell line derived from the fusion of mouse neuroblastoma N18TG2 cells with embryonic mouse mesencephalic neurons (Choi et al. 1992). The cells were cultured in DMEM supplemented with 10% fetal bovine serum (Invitrogen) in an atmosphere of 95% air and 5% CO2.
Administration of nicotine to rats
Male Sprague-Dawley rats (175–250 gm) purchased from Charles-River were used in this study. Rats were injected subcutaneously with 0.8 mg/ml nicotine (expressed in terms of nicotine free base, even though the bitartrate salt was used for injections). These injections were made twice daily with injections spaced ~12 hr apart. Nicotine bitartrate was dissolved in phosphate-buffered saline (150 mM NaCl and 10 mM potassium phosphate) and the solutions were buffered to pH 7.5. The injections were made using a volume of 1 ml/kg. Control rats were injected with the same volume of phosphate-buffered saline. Rats were also injected subcutaneously with 10 mg/kg mifepristone or control vehicle (sesame oil). These injections (volume of 1 ml/kg) were made 15 min prior to each injection of either saline or nicotine. Rats were injected for three days and one final time on the morning of the fourth day (7 injection periods). Rats were euthanized using an overdose of sodium pentobarbital (150 mg/kg, injected intraperitoneally) 3 hr after the final injection of nicotine or saline. Adrenal glands were removed while the animals were anesthetized prior to death. Brains were removed following anesthetization and decapitation, and SN and VTA were dissected or punched out. All tissues were immediatedly frozen on dry ice and stored at −80°C. All procedures and drug administrations with rats were performed in accordance with the guidelines and approval of the University of Rochester Committee on Animal Resources.
TH mRNA and TH RNA primary transcipt measurements
The experiments that were performed first in this study utilized a semiquantitative RT-PCR assay to measure the relative levels of TH mRNA and TH RNA primary transcripts in midbrain slices and MN9D cells (data in Figure 1, Figure 2 and Figure 4). This assay was used in previous papers from our laboratory to successfully measure changes in TH mRNA and TH RNA primary transcripts (Sun et al. 2004; Chen et al. 2008). More recently, we developed a quantitative RT-PCR assay and this assay was used in subsequent experiments (Figure 3 and Figure 5). In test samples that were assayed using both procedures, almost identical results were obtained.
Figure 1. Nicotine stimulated TH gene transcription in midbrain slice explant cultures and MN9D cells.
(A) Ventral midbrain slices from 7–10 day old mouse pups were cultured for 1 day in vitro and then treated with different concentrations of nicotine for 1 hr. MN9D cells were treated with different concentrations of nicotine for 15 min. Total cellular RNA was extracted and semiquantitative RT-PCR was used to measure changes in TH RNA primary transcripts that expressed intron 2 sequences. The levels of 28S rRNA were also measured using this assay and these values were used for normalization purposes. The autoradiogram depicts representative assays of RNA isolated from either midbrain explant cultures or MN9D cells. RNA from each sample was assayed in the presence (+RT) or absence (−RT) of RT, to verify that the amplified cDNA products were derived from RNA, not genomic DNA. (B) The bar graph represents the means ± SE from 4 slice cultures or 6 dishes of MN9D cells. (C) Ventral midbrain slice cultures or MN9D cells were treated with 100 uM nicotine for different periods of time and TH RNA primary transcripts were measured as described above. The bar graph represents the means ± SE from 3–4 slice cultures and 3–6 dishes of MN9D cells. (D) Ventral midbrain slice cultures or MN9D cells were treated for 1 hr with 100 uM nicotine ± 1 uM methyllycaconitine (MLA). The bar graph represents means ± SE from 3–5 slice cultures or dishes of MN9D cells.
a: p < .05 compared to controls.
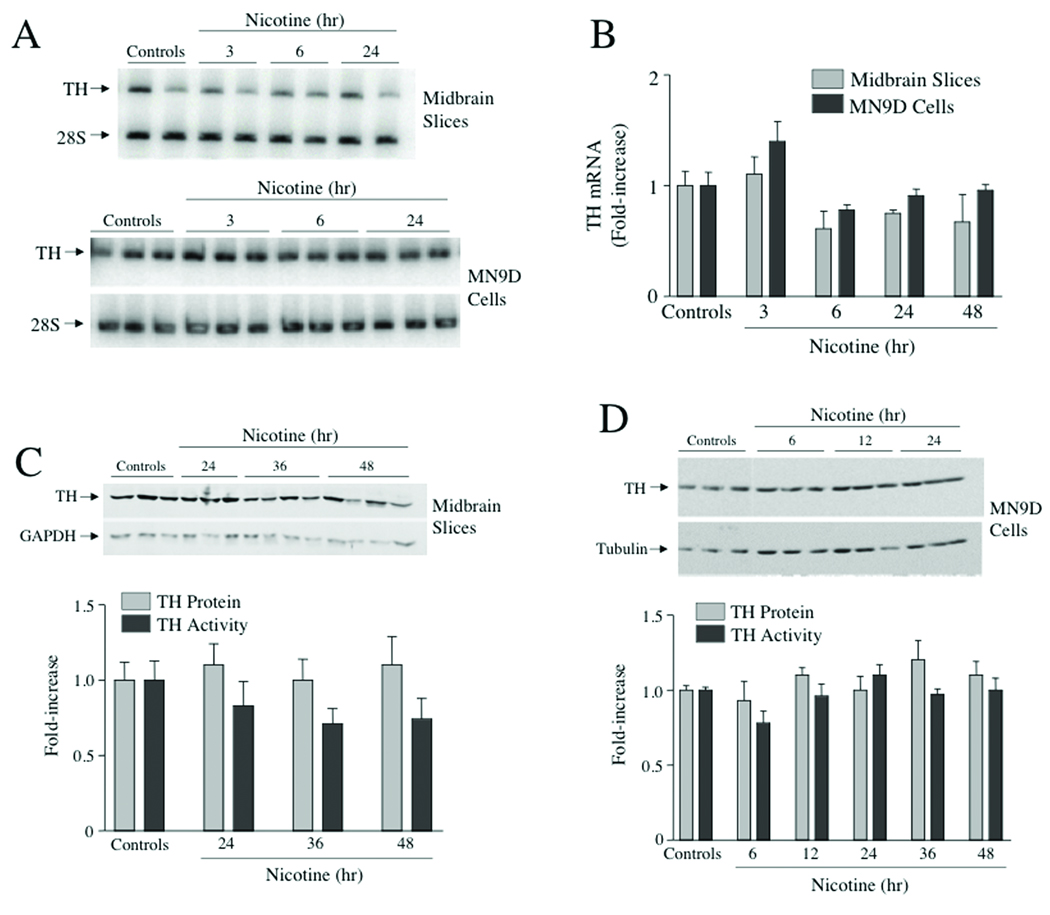
Figure 2. Nicotine did not induce TH mRNA, TH protein or TH activity in midbrain slice explant cultures or MN9D cells.
(A) Ventral midbrain slices from 7–10 day old mouse pups were cultured for 1 day in vitro and then treated with 100 uM nicotine for different periods of time up to 48 hr. MN9D cells were treated with 100 uM nicotine for similar durations of time. Total cellular RNA was extracted and semiquantitative RT-PCR was used to measure changes in TH RNA levels; 28S rRNA levels were also measured using this assay and these values were used for normalization purposes. The autoradiogram depicts representative assays of RNA isolated from either midbrain slice cultures or MN9D cells. (B) The bar graph depicts the means ± SE from 3 explant cultures or 3 dishes of MN9D cells. These results are from a single experiment, and similar results were obtained from at least two other experiments. (C) Midbrain slice explant cultures were treated with 100 uM nicotine for 24, 36 or 48 hr and then assayed for TH protein using western analysis and TH activity using saturating cofactor concentration (4 mM 6MPH4). The data represent means ± SE from 9–12 cultures. (D) MN9D cells were treated with 100 uM nicotine for the designated periods of time and TH protein and TH activity were assayed as in panel C. The data represent the means ± SE from 6–12 dishes.
Figure 4. Dexamethasone stimulated TH gene transcription and induced TH mRNA and TH protein in midbrain slice explant cultures.
Ventral midbrain slices from 7–10 day old rat pups were cultured for 1 day in vitro and then treated with 0.1 uM dexamethasone for 24 hr. The relative levels of TH mRNA, TH RNA primary transcripts and 28S rRNA were measured using semiquantitative RT-PCR. TH protein was measured using western analysis. Autoradiograms depicting representative assays are presented. TH activity was assayed using 4 mM 6MPH4. The bar graph represents the means ± SE from 3–5 slice cultures.
a: p < .05 compared to control values
Figure 3. Mifepristone inhibited the induction of TH mRNA elicited by nicotine administration to rats in adrenal medulla and midbrain.
Rats were injected subcutaneously with 0.8 mg/kg nicotine twice per day for 3 days with injections spaced ~12 hr apart. On the morning of the fourth day, the rats were injected one more time with 0.8 mg/kg nicotine and euthanized using an overdose of sodium pentobarbital (150 mg/kg) 3 hr after this final injection. Control animals were injected with the same volume of saline (1 ml/kg) at the same time points. When appropriate, 10 mg/kg mifepristone (MIF) (suspended in sesame oil) was injected subcutaneously 15 min prior to each injection of either saline or nicotine. The same volume of sesame oil (1 ml/kg) was used to inject animals that were not administered the GR antagonist. Adrenal glands were removed while the animals were anesthetized and then the animals were decapitated and SN and VTA were dissected. TH mRNA, DAT mRNA and GAPDH mRNA were measured using quantitative RT-PCR. GAPDH mRNA values were used to normalize TH mRNA values in adrenal samples, whereas DAT mRNA values were used for normalization in midbrain samples. The data represent the means ± SE from 10 adrenal samples, 7–10 SN samples and 4–5 VTA samples. The experiments were performed at least two times with similar results.
a: p < .05 compared to controls
Figure 5. Dexamethasone stimulated TH gene transcription and induced TH mRNA and TH protein in MN9D cells.
(A) MN9D cells were treated with different concentrations of dexamethasone for 24 hr and TH mRNA and TH primary transcripts were measured using semiquantitative RT-PCR (for the autoradiogram) and quantitative RT-PCR (for the complete concentration-response curve). The data represent the means ± SE from 3 dishes. Dexamethasone concentrations of 30 uM or greater yielded significant increases in both TH mRNA and TH RNA primary transcripts (p < .05). (B) MN9D cells were treated with 0.1 uM dexamethasone for different periods of time and TH mRNA and TH RNA primary transcripts were measured using quantitative RT-PCR. The data represent the means ± SE from 6 dishes. (D) MN9D cells were treated with 0.1 uM dexamethasone for different periods of time and TH protein was measured using western analysis and TH activity was assayed using 4 mM 6MPH4. The data represents the means ± SE from 3 dishes. (D) MN9D cells were treated with 0.1 uM dexamethasone in the presence or absence of different concentrations of mifepristone for 24 hr. TH RNA primary transcripts expressing genomic intron-2 sequences (filled squares) and mature TH mRNA transcripts (filled circles) were measured using quantitative RT-PCR. GAPDH mRNA transcripts were also measured using this assay and these values were used for normalization purposes. Values for TH RNA primary gene transcripts in control and dexamethasone-treated cells (without mifepristone) and expressed as fold-increases) were 1.0 ± 0.1 and 1.9 ± 0.2, respectively (p < .05). Values for TH mRNA transcripts in control and dexamethasone-treated cells were 1.0 ± 0.1 and 2.5 ± 0.2, respectively (p < .01). The data represent the means ± SE from 5–6 dishes.
a: p < .05 compared to control values.
The semiquantitative assay was performed as described in Chen et al. (2008). Briefly, 0.2–0.8 µg of total cellular RNA isolated from midbrain slices or MN9D cells were subjected to RT using random hexamer primers. Aliquots of the resulting single-stranded cDNA products were used along with the appropriate primers (see Table 1) in the PCR to incorporate 32P-dATP (0.5 µCi per reaction) into double-stranded products encoding 518 bp TH cDNA, 162 bp TH intron-2 genomic sequence or 295 bp 28S cDNA. For each experiment, the linearity of the RT-PCR was assessed with respect to both µg RNA added to the RT reaction and the number of PCR cycles. Densitometric values of bands corresponding to TH mRNA, TH intron-2 primary transcript and 28S rRNA PCR products observed on the dried-down electrophoretic gels were quantified using Phosphorimager analysis. TH mRNA and TH intron-2 primary transcript values were normalized to 28S rRNA values for each sample. These normalized values were then expressed as fold-increases over control values for each experiment and presented as such in Figure 1, Figure 2 and Figure 4.
Table 1.
PCR primers for semiquantitative and quantitative RT-PCR assays.
| Semiquantitative RT-PCR Assay | Quantitative RT-PCR Assay | |||
|---|---|---|---|---|
| TH mRNA (Rat) | Forward – 390 Reverse - 908 |
5’-ccccacctggagtactttgtg-3’ 5’-ccagatgacaggcgggcacta-3’ |
Forward – 620 Reverse - 695 |
5’-tcggaagctgattgcagaga-3’ 5’-ttccgctgtgtattccacatg-3’ |
| TH mRNA (Mouse) | Forward – 390 Reverse - 908 |
5’-ccccacctggagtactttgtg-3’ 5’-ccagatgacaggcgggcacta-3’ |
Forward – 1016 Reverese - 1076 |
5’-tgttggctgaccgcacat-3’ 5’-gcccccagagatgcaagtc-3’ |
| TH Intron-2 | Forward – 72 Reverse - 234 |
5’-ccagggctgaaatatggctg-3’ 5’-caggtcactagccagagcaac-3’ |
Forward – 215 Reverse - 284 |
5’-gttgctctggctagtgacctg-3’ 5’-gggtcttagctgtcggaccgg-3’ |
| 28S rRNA | Forward – 1 Reverse - 295 |
5’-gtgaacagcagttgaacatggg-3’ 5’-aactgcgacgctttccaaggc-3’ |
||
| GAPDH mRNA | Forward – 507 Reverse - 591 |
5’-ggaagggctcatgaccacagt-3’ 5’-acctttcgacaccgcactacc-3’ |
||
| DAT mRNA | Forward – 1588 Reverse - 1656 |
5’-ccagcaattcagtgatgacatca-3’ 5’-cagcatagccgccagtacag-3’ |
||
The quantitative RT-PCR assay employed an Applied Biosystems Prism 7000 real-time PCR cycler. Initial experiments were performed with RNA extracts from MN9D cells or midbrain regions to determine the linearity of TH mRNA, TH intron-2 primary transcript and GAPDH mRNA signals with respect to total cellular RNA concentrations added to the RT reactions; linearity was achieved using total cellular RNA concentrations between 0.05 to 0.2 µg. We routinely used 0.1 µg total cellular RNA in each assay. The RT reaction was performed as described by Chen et al. (2008). Real-time PCR was performed as recommended by Applied Biosystems using a 2 µl aliquot of the cDNA produced in the 20 µl RT reaction and SYBR green indicator to measure the amount of cDNA product formed in each PCR cycle. Forward and reverse primers were present at 1 µM in the PCR reactions. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was measured in each adrenal sample for normalization purposes, using sample cDNA transcribed from the same RT reaction as that used for measuring TH mRNA or TH intron-2 primary transcripts. Similarly, dopamine transporter (DAT) mRNA was measured in each SN and VTA sample for normalization purposes. The primers were as shown in Table 1. Different forward and reverse primers were used to measure TH mRNA in rat midbrain samples and mouse MN9D cells, as noted in the table. TH intron-2 primary transcript and GAPDH mRNA primers were the same for both rat and mouse samples. Cloned TH cDNA, TH genomic, GAPDH and DAT cDNA sequences encompassing the PCR-amplified regions were transcribed in vitro using an Ambion MAXIScript Transcription kit. Known amounts (pmols) of the in vitro transcribed mRNAs were used to construct standard curves using the same RT-PCR conditions employed for the sample mRNAs. The CT values derived from the real-time PCRs for different concentrations of standard TH mRNA, TH genomic, GAPDH or DAT mRNA were plotted against the known concentrations of the standard mRNAs used in the RT reaction to construct these curves. The CT values for the samples were then compared to the CT values on the standard curves to obtain pmol of TH mRNA, TH intron-2 primary transcripts, GAPDH or DAT mRNA. The TH mRNA and TH primary transcript values were then normalized to the GAPDH or DAT mRNA values for each sample and presented as fold-increases over controls.
Enzyme assays and TH protein measurement
TH activity was assayed as described by Chen et al. (2008), using 4 mM 6-methyl-5,6,7,8-tetrahydropterin (6MPH4) as cofactor. TH activity was expressed as pmols of 14CO2 formed per minute divided by mg of protein. TH protein was measured using western analysis as described previously (Chen et al. 2008). Protein determinations were made by the method of Bradford (1976), using bovine serum albumin as standard.
Statistical analyses
The results were analyzed by one-way analysis of variance, using the computer program INSTAT. Comparisons between groups were made using the Dunnett multiple comparisons test or the Student’s t-test, if only two groups were analyzed. A level of p <.05 was considered statistically significant.
RESULTS
Nicotine stimulated TH gene transcription in ventral midbrain slice explant cultures and MN9D cells
In our first set of studies we tested whether nicotine stimulated TH gene transcription in two different models of midbrain dopamine neurons, ventral midbrain slice explant cultures and cultured MN9D cells (Figure 1). In these experiments we utilized a semiquantitative RT-PCR assay to measure changes in the relative levels of TH RNA primary transcripts that express TH genomic intron-2 sequences. This assay is an indirect measure of TH gene transcription rate and has been used successfully to measure changes in TH transcription in both cell culture and in vivo systems (Sun et al. 2004; Chen et al. 2008). An autoradiogram depicting representative assays for TH intron-2 primary transcripts in RNA samples from both ventral midbrain slice cultures and MN9D cells is presented in Figure 1A. In these assays the RT step for each RNA sample was always run in the presence (+RT) or absence (−RT) of RT, to control for any signal that was due to amplification of genomic DNA. The −RT signals were not observed in samples isolated from MN9D cells, whereas faint −RT signals were sometimes observed in the midbrain slice samples. TH primary transcript values were only used for further analysis, if these −RT signals were either nonexistent or minor relative to the +RT signals. 28S rRNA signals were also measured and were used for normalization purposes.
Concentration-response curves were constructed for both model systems. As can be observed visually from the autoradiograms in Figure 1A and from the complete data set presented in Figure 1B, treatment of either midbrain slice cultures or MN9D cells with 100 µM or 300 µM nicotine elicited significant increases in TH RNA primary transcripts. These increases were 3–4 fold in midbrain slice cultures, but were only slightly greater than 2-fold in MN9D cells. In some experiments, small increases in TH RNA primary transcript levels were also observed at lower concentrations of nicotine; however, these increases were small, variable and did not reach statistical significance. This concentration-response curve was very similar to that observed for the effect of nicotine on TH mRNA levels in PC12 cells (Hiremagalur et al. 1993).
Time course studies showed that these responses to nicotine were relatively short-lived in both midbrain slice cultures and MN9D cells (Figure 1C). In both models TH gene transcription was stimulated at the 15 min and 1 hr time points, but no responses were observed after 3, 6 or 24 hr of treatment with 100 µM nicotine. The alpha-7 nAChR-selective antagonist MLA (1 µM) was effective in blocking the transcriptional response in both model systems (Figure 1D).
Nicotine did not induce TH mRNA or TH protein in ventral midbrain slice cultures or MN9D cells
We next tested whether nicotine induced TH mRNA and/or TH protein in ventral midbrain slice cultures or MN9D cells (Figure 2). In the first set of studies, slice cultures or cells were treated for different periods of time up to 48 hr with 100 µM nicotine and TH mRNA levels were measured using semiquantitative RT-PCR. Representative autoradiograms depicting these time courses are shown in Figure 2A and bar graphs depicting the quantitative data are shown in Figure 2B. TH mRNA levels were not induced by nicotine at any time point tested in either model system. In a second set of studies, TH protein and enzymatic activity were measured after different durations of treatment with 100 µM nicotine. Similarly, nicotine did not induce TH protein or activity at any time point tested in either midbrain slices or MN9D cells (Figures 2C and 2D).
Mifepristone inhibited the induction of TH mRNA in AM and midbrain elicited by nicotine administration to rats
The results of the previous experiments suggest that even though nicotine transiently stimulates TH gene transcription in both midbrain slices and MN9D cells via nAChRs, the stimulation is not sufficient to induce either TH mRNA or TH protein. However, Serova and Sabban (2002) have shown that treatment of rats with nicotine induces midbrain TH mRNA. One possible explanation for these discrepant results is that nicotine acts indirectly under in vivo conditions, by elevating humoral factors that induce midbrain TH mRNA via mechanisms that do not involve nAChRs present on midbrain dopamine neurons. One possible humoral factor is glucocorticoid.
To test the hypothesis that the induction of midbrain TH mRNA by nicotine under in vivo conditions is mediated by elevated glucocorticoids, we used mifepristone to block GRs in rats. Nicotine was administered according to the same protocol employed by Serova and Sabban (2002) and tissues were removed and dissected 3 hr after the final injection. We used quantitative RT-PCR to assay TH mRNA levels in adrenals, SN and VTA. In initial experiments we performed dose-response curves for nicotine to confirm the results of Serova and Sabban (2002). Our results basically agreed with theirs, in that we found 2–3 fold increases in VTA TH mRNA levels and ~1.5-fold increases in SN TH mRNA levels, using doses between 0.18 and 0.8 mg/kg nicotine (data not shown). However, we observed substantial inter-animal variability in these responses in both midbrain regions. In these initial experiments, we measured GAPDH mRNA for each sample to which TH mRNA values were normalized. Since inconsistent dissections might lead to variable amounts of dopaminergic and non-dopaminergic tissue included in each sample, values for GAPDH mRNA might not be representative of RNA isolated from dopamine neurons present in each tissue sample. Hence, we subsequently measured DAT mRNA in each midbrain sample for normalization purposes, since this value would more accurately assess the RNA derived from dopamine neurons. Ferrari et al (2002) reported no change in DAT mRNA in ventral midbrain after a single nicotine (0.4 mg/kg) injection, whereas Li et al (2004) reported a small increase in DAT mRNA in SN, but not in VTA after very high doses (~12 mg/kg/day) of nicotine administered orally for 4 weeks. It should be noted that this increase in SN DAT mRNA was only observed using in situ hybridization; no nicotine-induced changes were observed when DAT mRNA was measured using RNase protection assays. In our studies we did not detect significant changes in DAT mRNA levels in either SN or VTA after 3 days of treatment with nicotine as described above. Hence, the use of DAT mRNA for normalization purposes appears to be appropriate under our assay conditions and in our hands, greatly decreased the variability between midbrain samples. DAT mRNA was used in all the experiments reported in this paper for normalizing SN and VTA TH mRNA. GAPDH mRNA values were used to normalize TH mRNA values in adrenal samples.
In previous studies (Sun et al. 2004) we showed that nicotine induced TH mRNA in adrenal gland, when administered according to the paradigm used in these experiments. Hence, we first measured the response in the adrenal as a control, to assess the effectiveness of nicotine in these groups of rats. As expected, seven injections of nicotine (0.8 mg/kg) spaced ~12 hr apart induced TH mRNA by ~2-fold in adrenal medulla (Figure 3). However, surprisingly, this induction was almost completely blocked in animals pretreated with mifepristone. By itself, mifepristone had no effect on adrenal TH mRNA. The reason for the unexpected blockade of the nicotine-mediated induction of adrenal TH mRNA by mifepristone requires further investigation; however, these results confirm that both the nicotine injections and mifepristone treatments were effective in these animals.
As observed in the previous report (Serova and Sabban 2002), seven injections of nicotine (0.8 mg/kg) administered over 3–4 days elicited induction of TH mRNA in both SN and VTA (Figure 3). Treatment with mifepristone by itself did not produce significant changes in midbrain TH mRNA levels. However, mifepristone inhibited the nicotine-mediated induction of TH mRNA observed in both midbrain regions. The response to nicotine was almost totally blocked in the SN and was inhibited by 50–60% in VTA.
Dexamethasone stimulated TH gene transcription and induced TH mRNA and TH protein in midbrain slice cultures and MN9D cells
In the next set of studies we tested whether the GR agonist dexamethasone induced TH gene expression in midbrain slice cultures (Figure 4). When midbrain slice explant cultures were treated for 24 hr with 0.1 µM dexamethasone, TH mRNA levels increased 2–3 fold. Midbrain slice TH protein levels increased ~1.5-fold and TH activity increased ~2.3-fold after 24 hr of dexamethasone treatment. The apparent larger increase in TH activity relative to TH protein may be due to enzyme activation; however, this hypothesis was not further investigated.
A more extensive analysis of this dexamethasone response was performed using MN9D cells. In the first experiment we tested whether a 24 hr treatment with dexamethasone induced TH mRNA and TH RNA primary transcripts in MN9D cells, using semiquantitative RT-PCR (autoradiogram in Figure 5A). It is obvious from the autoradiogram that both these parameters increased after dexamethasone treatment. Subsequent studies utilized quantitative RT-PCR assays to measure changes in TH mRNA and TH primary transcripts in MN9D cells. The concentration-response curves in Figure 5A showed that maximal or near-maximal increases in both parameters were achieved using 30 µM dexamethasone. Time course studies are depicted in Figure 5B. Neither TH mRNA, nor TH RNA primary transcripts increased after 15 min of dexamethasone treatment. TH RNA primary transcripts were induced significantly after 1 hr of treatment and were near-maximally induced 1.8-2 fold after 3 hr of treatment. These increases in TH RNA primary transcripts were sustained for at least 48 hr of dexamethasone treatment. TH mRNA induction occurred at a much slower rate, with ~2-fold increases observed at the 24 and 48 hr time points. Next, we tested whether dexamethasone induced TH protein and TH activity in MN9D cells (Figure 5C). TH activity increased by ~1.5-fold after 24 hr of dexamethasone treatment and remained elevated for up to 72 hr. In contrast, TH protein increased 1.5-2 fold after 48 and 72 hr of treatment, but was not elevated at the 24 hr time point. As in the midbrain slice experiments, this result suggested that dexamethasone treatment might be associated with TH enzyme activation after 24 hr in dopamine neurons, but more studies are needed to confirm this conclusion.
In order to test whether the effect of dexamethasone was mediated by GRs, we performed a concentration-response curve for mifepristone in MN9D cells (Figure 5D). Mifepristone inhibited the dexamethasone-mediated effects on both TH gene transcription and TH mRNA induction in a dose-dependent manner. When using 0.1 µM dexamethasone as an inducing agent, almost total blockade was achieved using concentrations of mifepristone of 100 nM or greater and half-maximal inhibition was observed at ~30 nM mifepristone.
DISCUSSION
Information on TH gene regulation stems mainly from studies performed in models derived from epinephrine- or norepinephrine-secreting cells, like AM, sympathetic ganglia and LC. These studies have shown that TH is induced after prolonged stress, treatment with catecholamine-depleting drugs, like reserpine, or chronic treatment with cholinergic agonists (for reviews see (Kumer and Vrana 1996; Sabban and Kvetnansky 2001; Wong and Tank 2007)). In AM and LC, these stimuli activate TH gene transcription rate and at least in the adrenal the response is dependent on presynaptic input. These studies have led to the hypothesis that induction of TH protein occurs after transsynaptic depolarization of catecholaminergic neurons and is due to an initial activation of the TH gene, followed by an increase in TH mRNA and subsequently an increase in TH protein. Other studies have provided evidence that post-transcriptional mechanisms and humoral factors may also participate in these responses.
There is much less information on TH gene regulation in midbrain dopamine neurons. Early reports suggested that many stressors that induce TH in AM, sympathetic ganglia and LC do not increase TH expression in midbrain. However, more recent reports have shown that some stressors elicit small increases in midbrain TH mRNA, but this response is highly dependent on the strain of the animal and it is not clear whether these increases lead to induction of TH protein (Sabban and Kvetnansky 2001; Wong and Tank 2007). Treatment of rats with the catecholamine-depleting drug, reserpine, leads to induction of TH mRNA in VTA neurons, but not SN neurons, suggesting that TH regulation differs in these two midbrain regions (Pasinetti et al. 1990; Ortiz et al. 1996). Even more puzzling is the finding that midbrain TH mRNA and TH gene transcription are not robustly induced by neurotoxins that deplete striatal dopamine, such as MPTP, 6-hydroxydopamine and ampthetamines (Pasinetti et al. 1992; Sherman and Moody 1995; Bowyer et al. 1998; Rothblat et al. 2001). In the periphery, depletion of norepinephrine from sympathetic nerve terminals leads to relatively large compensatory increases in TH mRNA and TH protein in sympathetic ganglia and AM (Kumer and Vrana 1996). Why this type of compensatory response does not occur in nigrostriatal neurons is not clear. It is possible that appropriate signals are not transmitted to midbrain dopamine neurons to elicit a compensatory induction of TH mRNA when striatal nerve terminals are destroyed. However, it is difficult to test this hypothesis, because so little is known about the signaling mechanisms that control midbrain TH expression in response to extracellular stimuli. This lack of knowledge is unfortunate, because similar findings (small or insignificant changes in TH mRNA in surviving midbrain dopamine neurons) are observed in autopsy samples from Parkinsonian patients (Javoy-Agid et al. 1990; Kastner et al. 1993). Furthermore, it is possible that inappropriate regulation of TH gene expression may play a role in drug addiction or schizophrenia, diseases that are mediated by VTA neurons.
Interestingly, Serova and Sabban (2002) have presented evidence that nicotine induces TH gene expression in rat midbrain. They have shown that repeated injections of low doses of nicotine for three days leads to induction of TH mRNA in both SN and VTA, with a greater response observed in VTA. Previous to this report, Smith et al (1991) had shown that nicotine injected according to a similar paradigm induces TH activity in midbrain, but not in striatum. In the present study we have used cell culture models to further delineate the mechanisms responsible for this nicotine response.
Nicotine stimulates TH gene transcription, but does not induce TH mRNA or TH protein in cultured midbrain model systems
Our results show that nicotine stimulates TH gene transcription in a dose-dependent manner in both midbrain explant slice cultures and MN9D cells. Nicotine elicits a 2–4 fold stimulation of the TH gene and this response is totally blocked by the nAChR antagonist MLA. This latter finding is in agreement with the results of Serova and Sabban (2002), who demonstrate that MLA blocks the induction of midbrain TH mRNA by nicotine under in vivo conditions. At the concentration used in our studies (1 µ M), MLA effectively blocks nAChRs, but not muscarinic AChRs or other types of receptors; however, it is not selective for alpha-7 nAChRs at this concentration (Davies et al. 1999). We did not perform a detailed pharmacological analysis of the nAChRs responsible for this transcriptional effect, because it does not lead to enhanced TH expression. Nevertheless, the results are consistent with a model in which nicotine acts directly on nAChRs in midbrain to stimulate TH gene transcription. Furthermore, the results using the MN9D cells argue that at least part of this response may be mediated by nAChRs present on dopamine neurons.
A second important finding is that in both culture model systems transcriptional activation by nicotine is relatively transient, lasting for at least 1 hr, but less than 3 hr. No increases in transcription are observed at later time points. The kinetics of this transcriptional activation has important consequences. Nicotine activates TH gene transcription for a relatively brief period of time, and this transient activation does not lead to significant increases in TH mRNA or protein. This latter finding is consistent with results from previous studies investigating the effect of nicotine on TH gene expression in rat AM (Fossom et al. 1991). When nicotine is administered once to rats, adrenal TH gene transcription is activated 2–3 fold for up to 1 hr, but less than 3 hr. This activation is not associated with TH mRNA induction. In contrast, when nicotine is administered repeatedly to rats over a three hr period, TH gene transcription is activated for 3–6 hr and this leads to induction of TH mRNA and TH protein. These results suggest that TH gene transcription must be increased for a period of time greater than 1–3 hr in order to produce enough TH mRNA to measure its accumulation. The results in the midbrain models are in agreement with this hypothesis. In contrast, these midbrain results differ from those observed in PC12 cells treated with nicotine (Gueorguiev et al. 2000). In PC12 cells nicotine elicits a sustained increase in intracellular calcium, which leads to a sustained induction of TH mRNA. The reason for the disparate results between PC12 cells and midbrain models remains unclear. It is possible that midbrain nAChRs are desensitized after sustained exposure to nicotine. Alternatively, nicotine may activate different downstream signaling pathways and/or transcription factors. More work is needed to clarify this issue.
Nicotine-mediated induction of TH mRNA in midbrain in rats is dependent on the GR
Under in vivo conditions, nicotine may be acting by multiple mechanisms to stimulate TH gene transcription in midbrain neurons. It may be interacting directly with nAChRs present on dopamine neurons or on presynaptic nerve terminals of afferent neurons that innervate dopamine cell bodies or dendrites. Nicotine may also be acting at other sites in the brain, activating stimulatory input or disinhibiting inhibitory input into the midbrain. All of these possibilities may activate signaling pathways in dopamine neurons, leading to stimulation of the TH gene. Another possible mechanism by which nicotine may act in vivo is via elevation of humoral factors that act on dopamine neurons to induce TH. We have tested the hypothesis that glucocorticoids may participate as a humoral factor in this response.
It is well-established that nicotine increases circulating glucocorticoids in both rodents and humans (Fuxe et al. 1990; Caggiula et al. 1998; Reuter and Hennig 2003). In rats, circulating corticosterone increases 2–4 fold after administration of nicotine using the concentrations (0.087 to 0.35 mg/kg) employed by Serova and Sabban (2002) in their study on TH induction in midbrain. Similarly, in mice plasma corticosterone levels rise from ~100 ng/ml (or less) in controls to 400–500 ng/ml in animals treated with 1–2 mg/kg nicotine (Freund et al. 1988; Martin and Wehner 1988). It should be noted that the extent of these increases in circulating corticosterone is highly dependent on age, sex, rat or mouse strain and environmental context (Freund et al. 1988; Martin and Wehner 1988; Rhodes et al. 2001; Cruz et al. 2005; Faraday et al. 2005; Lutfy et al. 2006). These increases are almost identical to or greater than those observed after some types of stress (Freund et al. 1988; Hsu et al. 2007).
It is also well-established that glucocorticoids induce TH gene expression in cultured neuroblastoma and pheochromocytoma cells (Tank and Weiner 1982; Fossom et al. 1992). However, it is not clear whether glucocorticoids induce TH in other model systems and the in vivo response to glucocorticoids remains ambiguous. Early evidence showed that glucocorticoids induce TH in sympathetic ganglia (Otten and Thoenen 1975, 1976). However, subsequent studies have contradicted these findings. Sze and Hendrick (1983) have shown that glucocorticoids other than dexamethasone do not induce TH in sympathetic ganglia and that dexamethasone may be working by stimulating release of acetylcholine from preganglionic neurons. Sabban and coworkers (Nankova et al. 1996; Serova et al. 2008) have demonstrated that administration of cortisol to adrenalectomized rats does not induce TH in sympathetic ganglia and have presented evidence that the ganglionic response to stress is due to the direct action of ACTH, not glucocorticoids.
The role of glucocorticoids in regulating TH gene expression in brain is also unclear. An early report suggested that glucocorticoids induce TH in LC (Markey et al. 1982). However, subsequent studies have concluded that glucocorticoids do not induce TH mRNA in LC, nor do they play an important role in the response of LC to stress (Markey and Sze 1984; Makino et al. 2002). In regard to midbrain, McArthur et al (2007) have shown that perinatal exposure to dexamethasone in the drinking water alters the size and morphology of midbrain dopamine neurons into adulthood. Furthermore, it has been shown that glucocorticoids increase stimulated-dopamine release, alter dopamine turnover in midbrain neurons and modify dopamine-induced behaviors (Slotkin et al. 1992; Piazza et al. 1996; Rouge-Pont et al. 1999). However, to our knowledge, the present study is the first to directly demonstrate that glucocorticoids induce TH gene expression in cell model systems derived at least partially from midbrain origins. Our results clearly establish that the synthetic glucocorticoid dexamethasone stimulates TH gene transcription rate and induces TH mRNA and TH protein in both midbrain slice cultures and MN9D cells. The transcriptional response is sustained for at least 24 hr. The fact that mifepristone blocks these responses are consistent with it being mediated by GRs present in the MN9D cells. These results support the hypothesis that glucocorticoids are capable of inducing TH gene expression in midbrain and consequently may participate in the nicotine-mediated response.
A major finding in the present study is that the induction of midbrain TH mRNA by nicotine under in vivo conditions is inhibited by mifepristone. The nicotine response in the SN is totally blocked, whereas that in the VTA is inhibited by 50–60% by the GR antagonist. These results support the idea that under in vivo conditions the nicotine-mediated induction of midbrain TH mRNA is dependent at least partially on GRs. A number of methodological and conceptual considerations need to be discussed. First, the dose of mifepristone (10–20 mg/kg per day) used in these experiments has been shown to inhibit GR-mediated central nervous system effects in numerous reports (Joels et al. 2003; Yang et al. 2004; Wong and Herbert 2005; Avital et al. 2006; Dong et al. 2006). Furthermore, doses as high as 25 mg/kg have been shown to inhibit GR in brain without inhibiting the mineralocorticoid receptor (MR) (Wong and Herbert 2005; Avital et al. 2006). Secondly, we chose to administer the drug 15 min prior to each nicotine injection, because it has a relatively short half-life (~6–8 hr) in rats (He et al. 2007). Thirdly, GRs have been colocalized with TH in midbrain neurons (Harfstrand et al. 1986); consequently, it is feasible that glucocorticoids may act directly on these GRs in dopamine neurons to produce their effects on TH gene transcription. Finally, mifepristone is a highly specific antagonist of GRs, progesterone receptors and androgen receptors (Lu et al. 2006); consequently, it may be blocking all three of these receptors in our in vivo studies. However, to our knowledge, there is no evidence that nicotine elevates circulating levels of either progesterone or testosterone in male rats. Hence, even though it is possible that progesterone and/or androgen receptors may play a role in regulating TH gene expression under basal conditions or during development, it seems unlikely that these receptors mediate the observed response to nicotine in midbrain. It is also possible that MRs may participate in the nicotine response, since these receptors have high affinity for corticosterone (de Kloet et al. 2008). Mifepristone does not bind to MRs with high affinity (Lu et al. 2006); however, the drug is a substrate for the P-glycoprotein extrusion pump in the blood-brain barrier (de Kloet et al. 2008). Hence, it is difficult to predict what the concentration of mifepristone is at its site of action in brain and at high concentrations it is possible that MRs are blocked (Tanaka et al. 1997; de Kloet et al. 2008). MRs are primarily localized in limbic structures and prefrontal cortex (de Kloet et al. 2008) and to our knowledge, have not been definitively localized in midbrain dopamine neurons. Nevertheless, the involvement of mineralocorticoid receptors in TH gene regulation has not been investigated and our results do not rule out their potential participation in this response.
Taken together, one explanation for our results is that nicotine elevates circulating glucocorticoids, which pass into the brain and directly induce TH gene expression in midbrain dopamine neurons by interaction with GRs in these neurons and the consequent stimulation of TH gene transcription rate. This hypothesis is consistent with the results obtained using the cultured slice and MN9D cell model systems and the fact that midbrain dopamine neurons are known to express GR receptors (Harfstrand et al. 1986; Ronken et al. 1994). This hypothesis would also explain the discrepancy between the in vivo results, in which nicotine induces midbrain TH mRNA, and the cell culture results, in which nicotine does not induce TH mRNA in midbrain slices or MN9D cells. However, there are other models that also explain the available data. Glucocorticoids may be acting on afferent neurons or nearby glial cells to stimulate the synthesis and release of other factors that induce TH in midbrain dopamine neurons. Alternatively, glucocorticoids may be having a permissive effect, inducing factors in dopamine neurons, which are necessary for TH induction by other unknown extracellular stimuli. For instance, glucocorticoids may induce intracellular factors that respond to the activation of nAChRs by nicotine. On the other hand, glucocorticoids may be acting peripherally to elevate an unknown humoral factor, which then passes into brain and produces the response in midbrain neurons. These different possibilities need to be investigated. However, our findings suggest that nicotine’s effects on midbrain TH gene expression are not solely due to the actions of nicotine on midbrain nAChRs, but require the elevation of glucocorticoids and subsequent activation of GRs.
Understanding nicotine’s effects on TH gene expression in midbrain dopamine neurons is important in a number of contexts. Nicotine’s addictive properties are at least partially mediated by its ability to stimulate dopamine release from mesocorticolimbic neurons. Furthermore, it is well-established that smokers have a lower incidence of Parkinson’s disease, possibly because the nicotine in cigarettes promotes dopamine release from nigrostriatal neurons. In both these contexts, it would be expected that chronic exposure to nicotine would induce TH gene expression as an adaptive mechanism, to maintain dopamine levels in these neurons. Hence, an understanding of the mechanisms that regulate TH expression in these midbrain neurons in response to nicotine may be useful for treating these disorders. Furthermore, schizophrenia is associated with excessive release of dopamine from mesocorticolimbic neurons and Parkinson’s disease is due to the loss of nigrostriatal neurons. Since very little is known about the mechanisms that control TH gene expression in midbrain dopamine neurons, the results of this study may help to provide new therapeutic avenues to either down-regulate or up-regulate TH expression and hence dopamine biosynthesis during these diseases.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants DA05014 and NS39415 (to A.W.T.). P.R. was supported by training grants ES07026 and DA07232.
Abbreviations are as follows:
- TH
tyrosine hydroxylase
- SN
substantia nigra
- VTA
ventral tegmental area
- LC
locus coeruleus
- AM
adrenal medulla
- 6MPH4
6-methyl-5,6,7,8-tetrahydropterin
- nAChR
nicotinic acetylcholine receptor
- MLA
methyllycaconitine
- DAT
dopamine transporter
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GR
glucocorticoid receptor
- MR
mineralocorticoid receptor
REFERENCES
- Avital A, Segal M, Richter-Levin G. Contrasting roles of corticosteroid receptors in hippocampal plasticity. J Neurosci. 2006;26:9130–9134. doi: 10.1523/JNEUROSCI.1628-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Frame LT, Clausing P, Nagamoto-Combs K, Osterhout CA, Sterling CR, Tank AW. The long-term effects of amphetamine neurotoxicity on tyrosine hydroxylase mRNA and protein in aged rats. J Pharmacol Exp Thera. 1998;286:1074–1085. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Epstein LH, Sved AF, Knopf S, Rose C, McAllister CG, Antelman SM, Perkins KA. The role of corticosteroids in nicotine's physiological and behavioral effects. Psychoneuroendocrinology. 1998;23:143–159. doi: 10.1016/s0306-4530(97)00078-4. [DOI] [PubMed] [Google Scholar]
- Chen X, Xu L, Radcliffe P, Sun B, Tank AW. Activation of tyrosine hydroxylase mRNA translation by cAMP in midbrain dopaminergic neurons. Mol Pharmacol. 2008;73:1816–1828. doi: 10.1124/mol.107.043968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HK, Won L, Roback JD, Wainer BH, Heller A. Specific modulation of dopamine expression in neuronal hybrid cells by primary cells from different brain regions. Proc Natl Acad Sci U S A. 1992;89:8943–8947. doi: 10.1073/pnas.89.19.8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Delucia R, Planeta CS. Differential behavioral and neuroendocrine effects of repeated nicotine in adolescent and adult rats. Pharmacol Biochem Behav. 2005;80:411–417. doi: 10.1016/j.pbb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Davies AR, Hardick DJ, Blagbrough IS, Potter BV, Wolstenholme AJ, Wonnacott S. Characterisation of the binding of [3H]methyllycaconitine: a new radioligand for labelling alpha 7-type neuronal nicotinic acetylcholine receptors. Neuropharmacology. 1999;38:679–690. doi: 10.1016/s0028-3908(98)00221-4. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, de Jong IEM, Oitzl MS. Neuropharmacology of glucocorticoids: Focus on emotion, cognition and cocaine. Eur J Pharmacol. 2008;585:472–482. doi: 10.1016/j.ejphar.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Dong Z, Zhong W, Tian M, Han H, Cao J, Xu T, Luo J, Xu L. Stress evoked by opiate withdrawal facilitates hippocampal LTP in vivo. Hippocampus. 2006;16:1017–1025. doi: 10.1002/hipo.20234. [DOI] [PubMed] [Google Scholar]
- Faraday MM, Blakeman KH, Grunberg NE. Strain and sex alter effects of stress and nicotine on feeding, body weight, and HPA axis hormones. Pharmacol Biochem Behav. 2005;80:577–589. doi: 10.1016/j.pbb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Le Novere N, Picciotto MR, Changeux JP, Zoli M. Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur J Neurosci. 2002;15:1810–1818. doi: 10.1046/j.1460-9568.2001.02009.x. [DOI] [PubMed] [Google Scholar]
- Fossom LH, Carlson CD, Tank AW. Stimulation of tyrosine hydroxylase gene transcription rate by nicotine in rat adrenal medulla. Mol Pharmacol. 1991;40:193–202. [PubMed] [Google Scholar]
- Fossom LH, Sterling CR, Tank AW. Regulation of tyrosine hydroxylase gene transcription rate and tyrosine hydroxylase mRNA stability by cyclic AMP and glucocorticoid. Mol Pharmacol. 1992;42:898–908. [PubMed] [Google Scholar]
- Freund RK, Martin BJ, Jungschaffer DA, Ullman EA, Collins AC. Genetic differences in plasma corticosterone levels in response to nicotine injection. Pharmacol Biochem Behav. 1988;30:1059–1064. doi: 10.1016/0091-3057(88)90139-6. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Jansson A, von Euler G, Tanganelli S, Andersson K, Eneroth P. Regulation of endocrine function by the nicotinic cholinergic receptor. Ciba Found Symp. 1990;152:113–127. doi: 10.1002/9780470513965.ch7. discussion 127–130. [DOI] [PubMed] [Google Scholar]
- Gueorguiev VD, Zeman RJ, Meyer EM, Sabban EL. Involvement of alpha7 nicotinic acetylcholine receptors in activation of tyrosine hydroxylase and dopamine beta-hydroxylase gene expression in PC12 cells. J Neurochem. 2000;75:1997–2005. doi: 10.1046/j.1471-4159.2000.0751997.x. [DOI] [PubMed] [Google Scholar]
- Harfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikstrom AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, et al. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci U S A. 1986;83:9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Horn SW, Hussain MD. Improved bioavailability of orally administered mifepristone from PLGA nanoparticles. Int J Pharm. 2007;334:173–178. doi: 10.1016/j.ijpharm.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Hiremagalur B, Nankova B, Nitahara J, Zeman R, Sabban EL. Nicotine increases expression of tyrosine hydroxylase gene. Involvement of protein kinase A-mediated pathway. J Biol Chem. 1993;268:23704–23711. [PubMed] [Google Scholar]
- Hsu HR, Chen TY, Chan MH, Chen HH. Acute effects of nicotine on restraint stress-induced anxiety-like behavior, c-Fos expression, and corticosterone release in mice. Eur J Pharmacol. 2007;566:124–131. doi: 10.1016/j.ejphar.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Javoy-Agid F, Hirsch EC, Dumas S, Duyckaerts C, Mallet J, Agid Y. Decreased tyrosine hydroxylase messenger RNA in the surviving dopamine neurons of the substantia nigra in Parkinson's disease: an in situ hybridization study. Neuroscience. 1990;38:245–253. doi: 10.1016/0306-4522(90)90389-l. [DOI] [PubMed] [Google Scholar]
- Joels M, Velzing E, Nair S, Verkuyl JM, Karst H. Acute stress increases calcium current amplitude in rat hippocampus: temporal changes in physiology and gene expression. Eur J Neurosci. 2003;18:1315–1324. doi: 10.1046/j.1460-9568.2003.02845.x. [DOI] [PubMed] [Google Scholar]
- Kastner A, Hirsch EC, Herrero MT, Javoy-Agid F, Agid Y. Immunocytochemical quantification of tyrosine hydroxylase at a cellular level in the mesencephalon of control subjects and patients with Parkinson's and Alzheimer's disease. J Neurochem. 1993;61:1024–1034. doi: 10.1111/j.1471-4159.1993.tb03616.x. [DOI] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Li S, Kim KY, Kim JH, Park MS, Bahk JY, Kim MO. Chronic nicotine and smoking treatment increases dopamine transporter mRNA expression in the rat midbrain. Neurosci Lett. 2004;363:29–32. doi: 10.1016/j.neulet.2004.03.053. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, Giguere V, Hochberg RB, McKay L, Renoir JM, Weigel NL, Wilson EM, McDonnell DP, Cidlowski JA. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev. 2006;58:782–797. doi: 10.1124/pr.58.4.9. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Brown MC, Nerio N, Aimiuwu O, Tran B, Anghel A, Friedman TC. Repeated stress alters the ability of nicotine to activate the hypothalamic-pituitary-adrenal axis. J Neurochem. 2006;99:1321–1327. doi: 10.1111/j.1471-4159.2006.04217.x. [DOI] [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW. Regulatory role of glucocorticoids and glucocorticoid receptor mRNA levels on tyrosine hydroxylase gene expression in the locus coeruleus during repeated immobilization stress. Brain Res. 2002;943:216–223. doi: 10.1016/s0006-8993(02)02647-1. [DOI] [PubMed] [Google Scholar]
- Markey KA, Sze PY. Influence of ACTH on tyrosine hydroxylase activity in the locus coeruleus of mouse brain. Neuroendocrinology. 1984;38:269–275. doi: 10.1159/000123902. [DOI] [PubMed] [Google Scholar]
- Markey KA, Towle AC, Sze PY. Glucocorticoid influence on tyrosine hydroxylase activity in mouse locus coeruleus during postnatal development. Endocrinology. 1982;111:1519–1523. doi: 10.1210/endo-111-5-1519. [DOI] [PubMed] [Google Scholar]
- Martin BJ, Wehner JM. Influence of genotype on nicotine-induced increases of plasma corticosterone in mice as a result of acute nicotine pretreatment. Pharmacol Biochem Behav. 1988;30:1065–1070. doi: 10.1016/0091-3057(88)90140-2. [DOI] [PubMed] [Google Scholar]
- McArthur S, McHale E, Gillies GE. The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex-region- and time-specific manner. Neuropsychopharmacology. 2007;32:1462–1476. doi: 10.1038/sj.npp.1301277. [DOI] [PubMed] [Google Scholar]
- Nankova B, Kvetnansky R, Hiremagalur B, Sabban B, Rusnak M, Sabban EL. Immobilization stress elevates gene expression for catecholamine biosynthetic enzymes and some neuropeptides in rat sympathetic ganglia: effects of adrenocorticotropin and glucocorticoids. Endocrinology. 1996;137:5597–5604. doi: 10.1210/endo.137.12.8940389. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology. 1996;14:443–452. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- Otten U, Thoenen H. Circadian rhythm of tyrosine hydroxylase induction by short-term cold stress: modulatory action of glucocorticoids in newborn and adult rats. Proc Natl Acad Sci U S A. 1975;72:1415–1419. doi: 10.1073/pnas.72.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten U, Thoenen H. Selective induction of tyrosine hydroxylase and dopamine beta-hydroxylase in sympathetic ganglia in organ culture: role of glucocorticoids as modulators. Mol Pharmacol. 1976;12:353–361. [PubMed] [Google Scholar]
- Pasinetti GM, Morgan DG, Johnson SA, Millar SL, Finch CE. Tyrosine hydroxylase mRNA concentration in midbrain dopaminergic neurons is differentially regulated by reserpine. J Neurochem. 1990;55:1793–1799. doi: 10.1111/j.1471-4159.1990.tb04970.x. [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, Osterburg HH, Kelly AB, Kohama S, Morgan DG, Reinhard JF, Stellwagon RH, Finch CE. Slow changes of tyrosine hydroxylase gene expression in dopaminergic brain neurons after neurotoxin lesioning: a model for neuron aging. Mol Brain Res. 1992;13:63–73. doi: 10.1016/0169-328x(92)90045-d. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Barrot M, Rouge-Pont F, Marinelli M, Maccari S, Abrous DN, Simon H, Le Moal M. Suppression of glucocorticoid secretion and antipsychotic drugs have similar effects on the mesolimbic dopaminergic transmission. Proc Natl Acad Sci U S A. 1996;93:15445–15450. doi: 10.1073/pnas.93.26.15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Hennig J. Cortisol as an indicator of dopaminergic effects on nicotine craving. Hum Psychopharmacol. 2003;18:437–446. doi: 10.1002/hup.503. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, O'Toole SM, Czambel RK, Rubin RT. Male-female differences in rat hypothalamic-pituitary-adrenal axis responses to nicotine stimulation. Brain Res Bull. 2001;54:681–688. doi: 10.1016/s0361-9230(01)00488-9. [DOI] [PubMed] [Google Scholar]
- Ronken E, Mulder AH, Schoffelmeer AN. Glucocorticoid and mineralocorticoid receptors differentially modulate cultured dopaminergic neurons of rat ventral mesencephalon. Eur J Pharmacol. 1994;263:149–156. doi: 10.1016/0014-2999(94)90535-5. [DOI] [PubMed] [Google Scholar]
- Rothblat DS, Schroeder JA, Schneider JS. Tyrosine hydroxylase and dopamine transporter expression in residual dopaminergic neurons: Potential contributors to spontaneous recovery from experimental Parkinsonism. J Neurosci Res. 2001;65:254–266. doi: 10.1002/jnr.1149. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Abrous DN, Le Moal M, Piazza PV. Release of endogenous dopamine in cultured mesencephalic neurons: influence of dopaminergic agonists and glucocorticoid antagonists. Eur J Neurosci. 1999;11:2343–2350. doi: 10.1046/j.1460-9568.1999.00650.x. [DOI] [PubMed] [Google Scholar]
- Sabban EL, Kvetnansky R. Stress-triggered activation of gene expression in catecholaminergic systems: dynamics of transcriptional events. Tr Neurosci. 2001;24:91–98. doi: 10.1016/s0166-2236(00)01687-8. [DOI] [PubMed] [Google Scholar]
- Serova L, Sabban EL. Involvement of alpha 7 nicotinic acetylcholine receptors in gene expression of dopamine biosynthetic enzymes in rat brain. J Pharmacol Exp Ther. 2002;303:896–903. doi: 10.1124/jpet.102.039198. [DOI] [PubMed] [Google Scholar]
- Serova LI, Gueorguiev V, Cheng SY, Sabban EL. Adrenocorticotropic hormone elevates gene expression for catecholamine biosynthesis in rat superior cervical ganglia and locus coeruleus by an adrenal independent mechanism. Neuroscience. 2008;153:1380–1389. doi: 10.1016/j.neuroscience.2008.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman TG, Moody CA. Alterations in tyrosine hydroxylase expression following partial lesions of the nigrostriatal bundle. Mol Brain Res. 1995;29:285–296. doi: 10.1016/0169-328x(94)00259-h. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Lappi SE, McCook EC, Tayyeb MI, Eylers JP, Seidler FJ. Glucocorticoids and the development of neuronal function: effects of prenatal dexamethasone exposure on central noradrenergic activity. Biol Neonate. 1992;61:326–336. doi: 10.1159/000243761. [DOI] [PubMed] [Google Scholar]
- Smith KM, Mitchell SN, Joseph MH. Effects of chronic and subchronic nicotine on tyrosine hydroxylase activity in noradrenergic and dopaminergic neurones in the rat brain. J Neurochem. 1991;57:1750–1756. doi: 10.1111/j.1471-4159.1991.tb06377.x. [DOI] [PubMed] [Google Scholar]
- Sun B, Chen X, Xu L, Sterling C, Tank AW. Chronic nicotine treatment leads to induction of tyrosine hydroxylase in locus ceruleus neurons: the role of transcriptional activation. Mol Pharmacol. 2004;66:1011–1021. doi: 10.1124/mol.104.001974. [DOI] [PubMed] [Google Scholar]
- Sze PY, Hedrick BJ. Effects of dexamethasone and other glucocorticoid steroids on tyrosine hydroxylase activity in the superior cervical ganglion. Brain Res. 1983;265:81–86. doi: 10.1016/0006-8993(83)91336-7. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Fujita H, Matsuda S, Toku K, Sakanaka M, Maeda N. Glucocorticoid- and mineralocorticoid receptors in microglial cells: the two receptors mediate differential effects of corticosteroids. Glia. 1997;20:23–37. [PubMed] [Google Scholar]
- Tank AW, Weiner N. Induction of tyrosine hydroxylase by glucocorticoids in mouse neuroblastoma cells. Enhancement of the induction by cyclic AMP. Mol Pharmacol. 1982;22:421–430. [PubMed] [Google Scholar]
- Wong DL, Tank AW. Stress-induced catecholaminergic function: transcriptional and post-transcriptional control. Stress. 2007;10:121–130. doi: 10.1080/10253890701393529. [DOI] [PubMed] [Google Scholar]
- Wong EY, Herbert J. Roles of mineralocorticoid and glucocorticoid receptors in the regulation of progenitor proliferation in the adult hippocampus. Eur J Neurosci. 2005;22:785–792. doi: 10.1111/j.1460-9568.2005.04277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zheng X, Wang Y, Cao J, Dong Z, Cai J, Sui N, Xu L. Stress enables synaptic depression in CA1 synapses by acute and chronic morphine: possible mechanisms for corticosterone on opiate addiction. J Neurosci. 2004;24:2412–2420. doi: 10.1523/JNEUROSCI.5544-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]