Abstract
A quantitative enzyme-linked immunosorbent assay was used for identification of selected enteroviruses: poliovirus type 1, echovirus type 6, coxsackievirus A type 9, and coxsackievirus B types 1 through 6. Partially purified viral antigens or virus-specific antibodies were adsorbed to polystyrene spectrophotometer cuvettes, which permitted the assays to be reported and compared in terms of enzyme units specifically reacting. Both the adsorbed antigen and the adsorbed antibody methods were approximately equal in terms of sensitivity and specificity of reaction. By use [14C]leucine-labeled enteroviruses, the amount of virus that bound to the plastics used was shown to be dependent on the purity of the virus preparation used, but it was higher than the amount that was bound by plastics coated with viral antibody. Diluents which contained 0.15% (vol/vol) Tween 20 and 2.0% (wt/vol) bovine serum albumin in phosphate-buffered saline, pH 7.2, were found to be the most effective in inhibiting nonspecific adsorption of immunoreagents. However, the presence of these inhibitors in phosphate-buffered saline solutions also caused desorption of virus or viral antibody during immunoassays; the amount of virus desorption varied with the type of preparation used, and antibody desorption was dependent on the concentration of antibody initially used for adsorption. For specific identification of a given enterovirus type by the enzyme-linked immunosorbent assay method used, approximately 10(5) plaque-forming units of virus per assay tube were required.
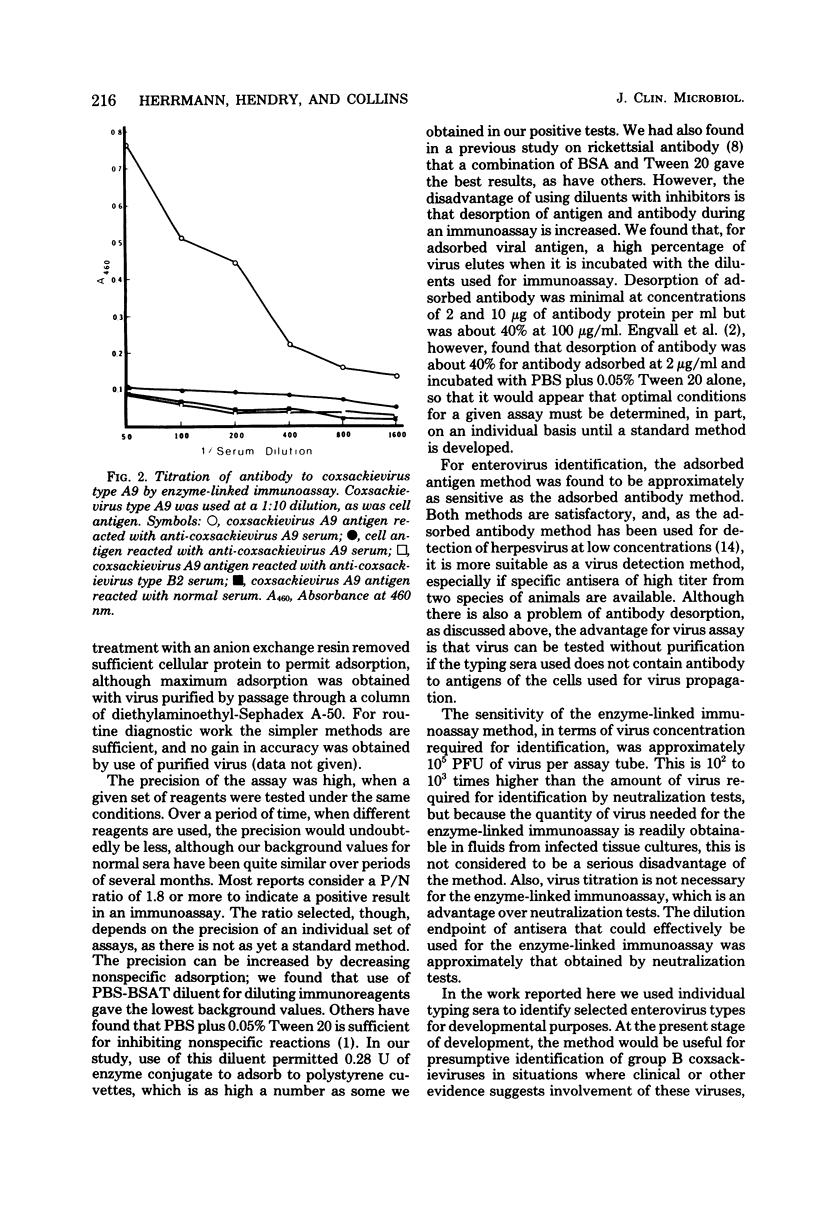
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bullock S. L., Walls K. W. Evaluation of some of the parameters of the enzyme-linked immunospecific assay. J Infect Dis. 1977 Oct;136 (Suppl):S279–S285. doi: 10.1093/infdis/136.supplement_2.s279. [DOI] [PubMed] [Google Scholar]
- Engvall E., Jonsson K., Perlmann P. Enzyme-linked immunosorbent assay. II. Quantitative assay of protein antigen, immunoglobulin G, by means of enzyme-labelled antigen and antibody-coated tubes. Biochim Biophys Acta. 1971 Dec 28;251(3):427–434. doi: 10.1016/0005-2795(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- French M. L., Schmidt N. J., Emmons R. W., Lennette E. H. Immunofluorescence staining of group B coxsackieviruses. Appl Microbiol. 1972 Jan;23(1):54–61. doi: 10.1128/am.23.1.54-61.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRON D. J., HELIMAN A. PURIFICATION OF POLIOVIRUS BY DEAE 'SEPHADEX A-25'. Nature. 1964 Oct 17;204:263–264. doi: 10.1038/204263a0. [DOI] [PubMed] [Google Scholar]
- Herrmann J. E., Cliver D. O. Rapid method to determine labeling specificity of radioactive enteroviruses. Appl Microbiol. 1973 Feb;25(2):313–314. doi: 10.1128/am.25.2.313-314.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. E., Collins M. F. Quantitation of immunoglobulin adsorption to plastics. J Immunol Methods. 1976;10(4):363–366. doi: 10.1016/0022-1759(76)90030-2. [DOI] [PubMed] [Google Scholar]
- Herrmann J. E., Hollingdale M. R., Collins M. F., Vinson J. W. Enzyme immunoassay and radioimmunoprecipitation tests for the detection of antibodies to Rochalimaea (Rickettsia) quintana. Proc Soc Exp Biol Med. 1977 Feb;154(2):285–288. doi: 10.3181/00379727-154-39655. [DOI] [PubMed] [Google Scholar]
- Herrmann J. E., Morse S. A., Collins M. F. Comparison of techniques and immunoreagents used for indirect immunofluorescence and immunoperoxidase identification of enteroviruses. Infect Immun. 1974 Jul;10(1):220–226. doi: 10.1128/iai.10.1.220-226.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. C., Axelrad M. A. An improved assay for haemolytic plaque-forming cells. Immunology. 1971 Feb;20(2):253–257. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mathiesen L. R., Feinstone S. M., Wong D. C., Skinhoej P., Purcell R. H. Enzyme-linked immunosorbent assay for detection of hepatitis A antigen in stool and antibody to hepatitis A antigen in sera: comparison with solid-phase radioimmunoassay, immune electron microscopy, and immune adherence hemagglutination assay. J Clin Microbiol. 1978 Feb;7(2):184–193. doi: 10.1128/jcm.7.2.184-193.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda Q. R., Bailey G. D., Fraser A. S., Tenoso H. J. Solid-phase enzyme immunoassay for herpes simplex virus. J Infect Dis. 1977 Oct;136 (Suppl):S304–S310. doi: 10.1093/infdis/136.supplement_2.s304. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- RIGGS J. L., BROWN G. C. Application of direct and indirect immunofluorescence for identification of enteroviruses and titrating their antibodies. Proc Soc Exp Biol Med. 1962 Aug-Sep;110:833–837. doi: 10.3181/00379727-110-27664. [DOI] [PubMed] [Google Scholar]
- Ruitenberg E. J., Steerenberg P. A., Brosi B. J., Buys J. Reliability of the enzyme-linked immunosorbent assay (ELISA) for the serodiagnosis of Trichinella spiralis infections in conventionally raised pigs. J Immunol Methods. 1976;10(1):67–83. doi: 10.1016/0022-1759(76)90008-9. [DOI] [PubMed] [Google Scholar]
- Saunders G. C., Clinard E. H. Rapid micromethod of screening for antibodies to disease agents using the indirect enzyme-labeled antibody test. J Clin Microbiol. 1976 Jun;3(6):604–608. doi: 10.1128/jcm.3.6.604-608.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Dennis J., Lennette E. H. Antibody responses of rhesus (Macaca mulatta) monkeys experimentally infected with coxsackieviruses of group B and group A, type 9. II. Heterotypic antibody responses to echoviruses, polioviruses and reovirus type 1. J Immunol. 1967 May;98(5):1060–1066. [PubMed] [Google Scholar]
- Taber L. H., Mirkovic R. R., Adam V., Ellis S. S., Yow M. D., Melnick J. L. Rapid diagnosis of enterovirus meningitis by immunofluorescent staining of CSF leukocytes. Intervirology. 1973;1(2):127–134. doi: 10.1159/000148839. [DOI] [PubMed] [Google Scholar]
- Voller A., Bartlett A., Bidwell D. E., Clark M. F., Adams A. N. The detection of viruses by enzyme-linked immunosorbent assay (ELISA). J Gen Virol. 1976 Oct;33(1):165–167. doi: 10.1099/0022-1317-33-1-165. [DOI] [PubMed] [Google Scholar]
- Wolters G., Kuijpers L., Kacaki J., Schuurs A. Solid-phase enzyme-immunoassay for detection of hepatitis B surface antigen. J Clin Pathol. 1976 Oct;29(10):873–879. doi: 10.1136/jcp.29.10.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Kim H. W., Clem T., Wyatt R. G., Kalica A. R., Chanock R. M., Kapikian A. Z. Enzyme-linked immunosorbent assay (ELISA) for detection of human reovirus-like agent of infantile gastroenteritis. Lancet. 1977 Aug 6;2(8032):263–267. doi: 10.1016/s0140-6736(77)90951-5. [DOI] [PubMed] [Google Scholar]


