SUMMARY
Follicular helper T (TFH) cells are a class of helper T cells specialized in the cognate control of antigen-specific B cell immunity. Upon first contact with antigen-primed B cells, pre-germinal center effector TFH cells promote B cell clonal expansion, antibody isotype switch, plasma cell differentiation and the induction of germinal centers. In contrast, within germinal centers, TFH cells regulate the fate of antigen-specific GC B cells expressing high-affinity variant B cell receptors to promote memory B cell and long-lived plasma cell development. Recent studies unravel multiple signals controlling TFH development and functional sub-types of antigen-specific TFH cells, including memory TFH cells that accelerate memory B cell responses to antigen re-challenge in vivo.
INTRODUCTION
Helper T cell regulated B cell immunity is considered the basis of long-term immune protection provided by most vaccines in use today. There are spatial and temporal constraints on cognate programming events following antigen-specific priming that serve as developmental checkpoints in clonal selection and the commitment to adaptive immune function [1,2]. The rules that govern information exchange at each developmental checkpoint define the molecular mechanisms of antigen-specific immune protection in this pathway and are the focus of the current review.
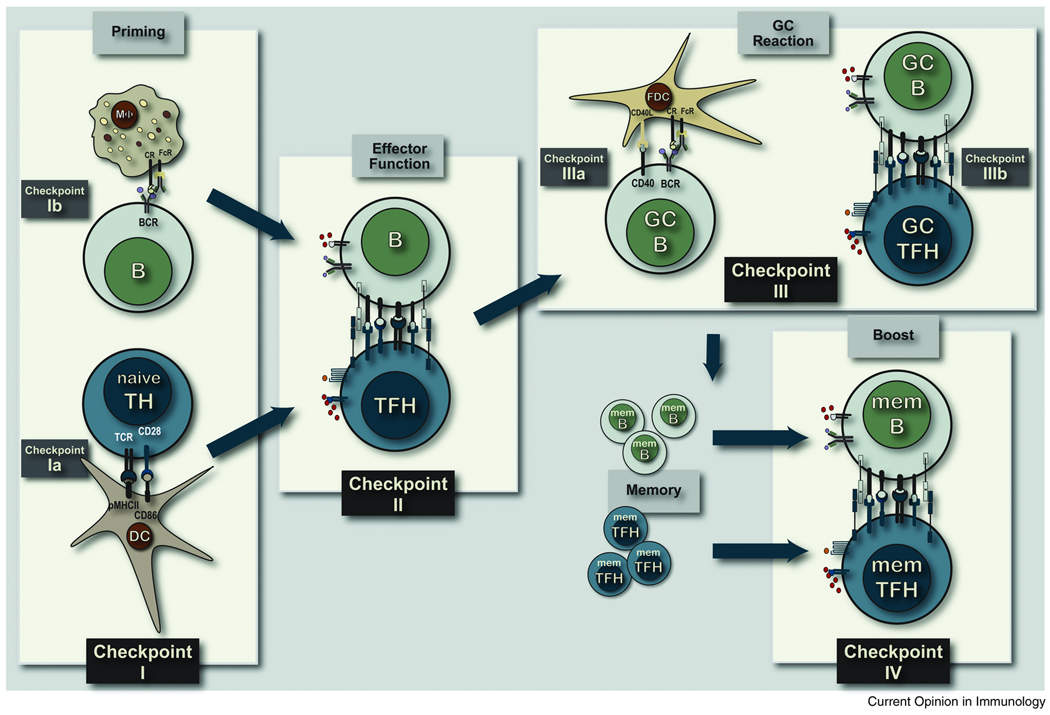
Follicular helper T cells are now recognized as the class of helper T cells that regulate the multiple stages of B cell immunity (Figure 1) [3–6]. After initial contact with antigen-experienced DC (Checkpoint I), antigen-specific effector TFH cells emerge as CXCR5+CCR7− TH cells that migrate to the follicular regions of lymphoid organs to form stable contacts with antigen-primed B cells (Checkpoint II). Subsequent to cognate B cell contact, a cohort of effector TFH cells migrate to germinal centers, form stable contacts with variant GC B cells (Checkpoint III) to regulate the development of antigen-specific memory B cell compartment in ways that remain poorly understood. Finally, memory TFH cells persist within the priming environment to regulate the antigen-specific memory B cell response to re-challenge (Checkpoint IV). We propose that the strength of antigen receptor binding, the duration of cellular contact and the molecular context of cognate interactions are the defining attributes of each developmental checkpoint in vivo.
FIGURE 1. COGNATE TH CELL REGULATION OF B CELL IMMUNITY.
Following local protein vaccination, mature antigen-experienced dendritic cells from the site of injection traffic to the draining lymphoid tissue to prime pMHCII-specific naive TH cells at Checkpoint Ia. Antigen can also be transported to the subcapsullar sinus by macrophage to present native cell associate antigen to B cells at Checkpoint Ib. Antigen-specific B cells will take up protein antigen, process and present pMHCII complexes and move towards the T-B borders to interact with pMHCII-specific effector TFH cells at Checkpoint II. Following stable cognate contact a cohort of antigen-specific B cells will move into the follicular regions, massively expand to form secondary follicles, somatically diversify their BCR, express the variant BCR and then traverse FDC networks in the light zone of germinal center at continuous Checkpoint IIIa interactions. GC B cells expressing high affinity variant BCR form stable contacts with GC TFH cells at Checkpoint IIIb prior to GC exit and entry into the memory B cell compartment as either memory response precursors or long-lived plasma cells. Antigen-specific memory TFH cells and memory B cells persist in the priming lymphoid tissue to interact upon secondary challenge with antigen at Checkpoint IV a requisite regulatory interaction for expansion of memory B cells and formation of memory response plasma cells.
INITIATING ADAPTIVE IMMUNITY: CHECKPOINT I
Vaccines provide foreign antigen within an inflammatory context to initiate dendritic cell (DC) maturation. Antigen-experienced DC will express peptide-MHC class II (pMHCII) complexes and a spectrum of secreted and surface-expressed molecules to recruit naive pMHCII-specific TH cells (Checkpoint Ia), promote TH clonal expansion and effector TH cell differentiation. The strength of TCR-pMHCII interactions and the extended molecular context of these cognate events impact antigen-specific TH cell fate and the acquisition of effector TH cell function. Our recent findings indicated the requirement of a threshold TCR affinity to reach maximal local clonal accumulation [7*]. Surprisingly, antigen dose did not alter the clonal selection threshold but changing the vaccine adjuvant altered clonal composition and pMHCII binding profiles of responder TH cells. More recently, we provided evidence for a casual link between TCR binding strength and the differentiation of effector TH cells [8**]. In this protein vaccination model, we identified three separable sub-types of antigen-specific effector TH cells expressing a hierarchy of TCR binding strength. T-zone localized effector TH cells expressed the lowest binding, emigrant effector TH cells an intermediate binding and the effector TFH cell compartment the highest binding to pMHCII complexes. Hence, adjuvant controls the threshold for clonal selection and strength of TCR-pMHCII binding regulates the deployment of effector TH cell function.
Naive B cells that can recognize soluble or cell-associated antigen with sufficient binding strength (Checkpoint 1b) will internalize antigen, process and present pMHCII complexes. Vaccine adjuvants can influence these early events in B cell priming through the engagement of innate receptors [9,10], however their mechanism of action and developmental consequence in vivo remains poorly resolved. Specific recognition by BCR will lead to increased co-stimulatory molecule expression and movement towards the T cell zones of secondary lymphoid tissue [11]. Here, the antigen-primed pMHCII-expressing B cells receive cognate help as a prerequisite for secondary developmental programming events. Without cognate help, protein antigen-primed B cells will largely die without expansion or plasma cell differentiation. Strength of germ-line encoded BCR binding, even at this earliest developmental checkpoint, may impact levels of pMHCII or co-stimulatory molecule expression by antigen-primed B cells, thereby, indirectly influencing subsequent B cell fate and function.
PRE-GC EFFECTOR TFH CELLS: CHECKPOINT II
The cardinal feature of antigen-specific CXCR5+ TFH cells is migration towards the B cell zones of secondary lymphoid tissue [12,13] and then placement within the GCs of an ongoing immune response [14,15]. Functional analysis in vitro [15] and then in vivo upon adoptive transfer [16] established the propensity of the CXCR5+ TH compartment to support antibody production by B cells. The presence of antigen-specific TH cells within the GC has been described in human tonsils [17,18] and across multiple murine models [19–22]. In this review, we will refer to the pre-GC compartment involved in checkpoint II interactions as “effector TFH cells” to distinguish them from the “GC TFH cells” associated with checkpoint III interactions (Figure 1). While it remains unclear whether functional and developmental differences exist between these two TFH cell types, it is important to approach current analysis with this possibility in mind.
First contact between pMHCII-specific effector TFH cells and antigen-primed pMHCII+ B cells occurs at the T-B borders [2,19,23]. Cyster and colleagues [24*] first captured dynamic ‘monogamous’ cognate interactions between individual TH cells and B cells demonstrating highly motile conjugates with the B cell leading movement. It was known that in the absence of SAP, an adaptor protein for signaling through the SLAM family of receptors [25], TFH cells were still able to secrete cytokines [26] but unable to promote a GC reaction [27]. Germain and colleagues [28**] recently connected these observations by interrogating the control, stability and consequence of effector TFH-B cell early events using dynamic imaging in vivo. This elegant study demonstrated the requirement for SAP in the formation of stable pre-GC TFH-B interactions. There was no role for SAP in the initial contact with pMHCII+ DC allowing all other known features of effector TFH cells to develop (expression of CXCR5, CD40L, ICOS and OX40). Importantly, the antigen-primed B cells had to express the pMHCII specificity of the TFH cells to form the stable contacts [28**]. Finally, the long duration interactions were required for subsequent entry of effector TFH cells into the GC reaction. Hence, it is likely that effector TFH cells produce the GC TFH cell compartment and may require signals from the antigen-primed B cells at this earlier developmental juncture.
EFFECTOR TFH FUNCTION: LINEAGE AND LOCATION
The spectrum of helper T cell functions with regulatory impact on different cellular targets is extensive and expanding [29*]. The programming and organization of these functions across different subtypes of effector TH cells is still an area of intense research. In this context, the assignment of cytokine production by effector TFH cell functions remains controversial. Early assessments of cytokine production by in vitro re-stimulated CXCR5+ TFH cells indicated IL-2, IFN-γ and IL-10 from human peripheral blood [15] with evidence for IL-4 and IFN-γ from TCR transgenic mouse TFH cells [16]. Molecules important in the development of normal B cell immunity were implicated in early studies. CD40L, ICOS and OX40 expression were candidates for the delivery of effector TFH cell function and shown to be expressed on antigen-specific TFH cells [30*]. Early microarray analyses suggested separable gene expression programs for TFH cells and other known TH cell subsets. CXCL13 was highlighted early [31] with evidence for ICOS, IL-21 [32,33], IL-21R [34] and the differential expression of Bcl-6 [32] being used as the most reliable attributes of TFH function in vivo. Thus, the acquisition of special effector TFH cell functions may be associated with the programming of a separate TH cell lineage.
IL-21 has been highlighted as an important cytokine in TFH development [5,35**,36**]. IL-21 and IL21R deficiency has a detrimental impact antibody isotype switch and GC formation [37]. Either deficiency also blocks the development of TH17 cells in studies that indicate IL-21 acts in an autocrine manner to amplify the TH17 cell subset [38,39]. Recent studies have highlighted the role of IL-21 in the generation of TFH cells as an autocrine factor [35**,36**] with further capacity to control B cell immunity as an effector molecule delivered to B cells. King and colleagues [36**] demonstrated that IL21R expression on TH cells partially rescued the B cell defect in IL-21R deficient animals. These studies indicated IL-21 enhanced co-stimulatory stimuli to developing TH cells that enhanced TFH induction. Dong and colleagues [35**] preferentially induced CXCR5+ Bcl-6+ TFH cells in vitro using IL-21 and antibodies to IL-4, IFN-γ and TGF-β blocking the development of TH1, TH2 and TH17 development. Importantly, both groups demonstrated the requirement of ICOS-L on B cells for induction of complete TFH cell development in vivo. These studies argue for a separate TFH lineage with IL-21 as the primary effector function and Bcl-6 as an important regulator of this program.
Antigen-specific B cell development proceeds in at least two separate pathways following checkpoint II interactions (Figure 2). A major developmental decision involves commitment to plasma cell differentiation without BCR diversification or entry into the germinal center pathway to memory B cell development. Both pathways support antibody class switch recombination that can vary depending on the cytokine milieu upon cognate TH cell contact. Our recent analysis of cytokine expression in vivo [8**], demonstrated the presence of IL-2, IFN-γ, IL-10 as well as IL-4 and IL-21 in the antigen-specific effector TFH compartment. Elegant studies by Locksley and colleagues using IL-4 and IFN-γ reporter mice [40**], demonstrated the production of IL-4 and IFN-γ by TFH cells at the T-B borders and within GCs after antigen-specific priming. Two further studies by Mohrs and colleagues [41*] and Pearce and colleagues [42*] using reporter mice provided further evidence for a connection between TH2 cells and TFH cell development. Together, these trends suggest that multiple TH cell functional subsets may express a secondary migration program to deploy them to the appropriate tissue location to deliver their local regulatory TFH functions.
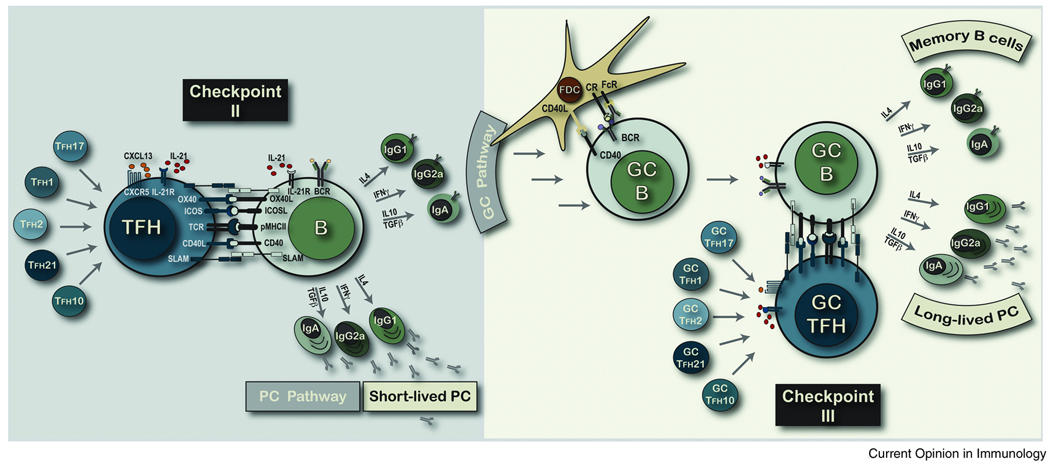
FIGURE 2. SEPARABLE ANTIGEN-SPECIFIC TFH AND B CELL LINEAGES.
We depict critical sets of molecular interactions at Checkpoint II that appear common to all antigen-specific effector TFH interactions with antigen-primed pMHCII-expressing B cells. We propose that all sub-types of functionally distinct TH cell lineages can also program the migration to the T-B borders and B cell zones to become TFH cells and regulate B cell fate and function. Further, these separate effector TFH lineages, depicted according to their original TH cell program, will promote distinct B cell lineage decisions at Checkpoint II. We propose that antibody isotype is a major ‘lineage’ decision for antigen-specific B cells and that within each isotype-specific lineage the secondary outcomes of plasma cell versus GC B cell fate may be controlled by the duration of contact at Checkpoint II. Entry into the GC pathway involves clonal expansion, somatic BCR diversification and affinity-based selection for all isotype-specific GC B cells at Checkpoint IIIa. GC B cells with a positive selection ‘imprint’ at Checkpoint IIIa will form stable cognate interactions with pMHCII-specific GC TFH cells at Checkpoint IIIb. We propose that there is some lineage maintenance between the effector TFH and the GC TFH creating distinct TFH lineages dedicated to the regulation of separate isotype-specific B cells. In this model, IL-4 expressing GC TFH will re-connect with IgG1+ GC B cells and IFN-γ+ TFH will re-connect with IgG2a+ GC B cells as demonstrated by Locksley and colleagues [40**]. It is plausible that the duration of intercellular contact regulates memory B cell versus long-lived plasma cell fate and function at this point of memory B lineage development.
GERMINAL CENTERS: CHECKPOINT III
The GC cycle of activity begins with recruitment and massive expansion of antigen-specific B cells in the B cell zones of secondary lymphoid tissue. There is evidence for BCR-affinity based selection for entry into the GC cycle [43]. It is curious to speculate that the strength of pMHCII-TCR contact at checkpoint II influences this developmental progression. In this model, the initial strength of BCR binding to antigen may be indirectly responsible for the duration of effector TFH cell pMHCII+ B cell contact at checkpoint II. Nevertheless, stable contact at checkpoint II impacts migration of effector TFH cells in the GC environment. Diversification of the BCR by somatic hypermutation accompanies expression of the variant BCR and then by some means that remains unclear, high affinity GC B cells are selected to enter the long-lived memory compartment. Selection coincides with developmental decisions to become memory response precursors or the long-lived plasma cells to provide long-term antigen-specific immune protection [44]. Antibody isotype also divides across these two major developmental pathways providing multiple layers of memory B cell function in vivo.
Three separate dynamic imaging studies provided breakthrough insights into our current appreciation of GC cellular dynamics in vivo [45–47**]. All three studies highlighted the zonal organization of the GC and documented movement of GC B cells between these areas in both directions. All groups reported the continuous movement of GC B cells along the antigen-laden processes of the follicular dendritic cells (FDC), the stromal element of the GC [48*]. Importantly, the GC B cells did not pause in a synapse-like manner on these FDC with constant velocities at checkpoint IIIa interaction. Nussenzweig and colleagues [47**] reported the sporadic appearance of naïve B cells traversing the GC structures and the capacity of high affinity antigen-specific B cells to join formed GC indicating a more open selection process than previously considered for B cells. Cyster and colleagues [45**,49], reported the transient and then stable contacts between GC B cells and GC TFH cells suggesting that the immune synapse at checkpoint IIIb might be the rate-limiting step in GC B cell selection. This fascinating proposal suggests that the TCR-pMHCII interactions or some other controller of checkpoint IIIb stability could indirectly discriminate the affinity of the GC B cell’s BCR for antigen.
GC TFH FUNCTION: SEPARATE B CELL LINEAGES
In their recent analysis of TFH-GC B cell doublets, Locksey and colleagues [40**] provided another surprising and potentially important insight into our understanding of GC cognate interactions. These studies isolated doublets from day 21 of an immune response and demonstrated the phenotype of TFH cells and phenotype/genotype of antigen-specific GC B cells. Most remarkably, IL-4 producing TFH cells paired with IgG1 expressing GC B cells and IFN-γ producing TFH cells paired with IgG2a expressing GC B cells. The authors suggested a continued requirement for these particular growth factors well beyond the initiation of antibody isotype switch. These data also provide evidence for separable cytokine-producing TFH cell lineages dedicated to the regulation of isotype-switched B cells (Figure 2). Thus, antibody isotype would also define separable functional lineages across the antigen-experienced B cell compartment. In this model, TFH cognate contact may control functional commitment in GC B cells and/or deployment of the memory compartment to the different locations in vivo.
Many different varieties of GC TFH have been reported. Kuchroo and colleagues [50*] recently demonstrate that TFH cells can produce IL-17, while Mountz and colleagues [51*] indicate IL-17 producing GC TFH cells within the GCs of animals that are prone to develop autoimmunity. Using FoxP3 reporter mice, Fagarasan and colleagues [52**] demonstrate that FoxP3 expressing TH cells were precursors of FoxP3neg TFH cells within the GCs of peyers patches (PP). Importantly, this TFH lineage was restricted to the PP with no capacity to form LN or splenic GC TFH cells. Hence, local environmental cues were highlighted as an important discriminating feature of the functional program in vivo. GC TFH populations have been demonstrated to produce TGF-β1 and IL-21 in ways that synergize to over-ride IgG switch and promote IgA and migration of plasma cells towards mucosal tissues [53]. There have also been recent reports of ICOS+ extra-follicular TH cells that do not express CXCR5 but may be required for the development/maintenance of the short-lived plasma cell pathway [54*]. Thus, heterogeneity of GC TFH function suggests that the priming context across different types of immune responses impacts the composition of TFH function.
Where there is somatic BCR diversification there must be mechanisms for selecting against self-reactivity. Recent clear evidence for this process came when Goodnow and colleagues [33] demonstrated the induction of autoimmunity in animals with dysregulated ICOS expression on TH cells. There were substantially higher numbers of TFH cells in these animals with exaggerated spontaneous GC formation that correlated with the induction of autoantibody and pathology. Recently, Vinuesa and colleagues [55*] demonstrated rescue of this model by altering TFH generation GC formation and abrogating disease. These data further support the role of GC TFH cells in the selection of the memory B cell compartment and highlight the possibility that too many GC TFH cells may cause problems in GC B cell selection and the maintenance of peripheral immune tolerance.
MEMORY B CELL RESPONSE: CHECKPOINT IV
CXCR5+ TH cells were first reported in the peripheral blood of humans [56] but were subsequently demonstrated to express reduced capacity to promote antibody production in vitro [31]. How these circulating CXCR5+ cells relate to the resident TFH compartment in peripheral LNs [4,30] has not been resolved. Our recent demonstration of a memory counterpart to the antigen-specific effector TFH population indicates a cohort of CXCR5+ TFH cells that persists locally to regulate accelerated memory B cell responses. These memory TFH cells re-capitulate the cytokine expression pattern of the primary response effector TFH compartment. Importantly, we provided clear evidence for persistent cell associated pMHCII complexes that may play a role in the local retention mechanism confirm earlier reports in the response to influenza virus infection [57]. Overall, the systemic organization of memory TFH cells suggests a substantial regional component for the cognate regulators of antigen-specific memory B cell responses. This critical arena of research has received very little attention in recent years but underlies the future rational design of vaccine boost strategies.
CONCLUSIONS
Follicular helper T cells define a class of regulatory TH cells specialized for the cognate control of all stages of antigen-specific B cell development. Recent advances in this field indicate that initial contact with pMHCII-expressing DC induces multiple subtypes of CXCR5+ effector TFH cells producing different cytokines involved in the programming of B cell immunity. Antibody isotype switch is one major facet of the B cell response regulated by cognate control that may define antigen-specific B lineage commitment throughout plasma cell and memory B cell differentiation. The mechanisms used by pMHCII-specific GC TFH cells to regulate GC B cells control the fate and function of the antigen-specific memory B cell compartment are now experimentally accessible but remain poorly resolved. Finally, pMHCII-specific memory TFH cells persist in the local priming environment for the cognate control of accelerated memory B cell responses to antigen re-challenge.
Understanding how to regulate these cognate regulators is the central design challenge for the next generation of protein vaccines. It is now feasible to select vaccine adjuvants and antigen delivery strategies to optimize different TFH functions and subsequent B cell fates. Valuable recent advances emphasize experimental models that are accessible to quantitative cellular and detailed molecular analysis in physiologically relevant settings. These studies allow us to devise complex but layered models for the regulation of antigen-specific B cell immunity that are experimentally tractable and promise mechanistic insight to the future vaccine effort.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES AND RECCOMMENDED READING
- 1.McHeyzer-Williams LJ, Malherbe LP, McHeyzer-Williams MG. Checkpoints in memory B-cell evolution. Immunol Rev. 2006;211:255–268. doi: 10.1111/j.0105-2896.2006.00397.x. [DOI] [PubMed] [Google Scholar]
- 2.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 3.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Local development of effector and memory T helper cells. Curr Opin Immunol. 2007;19:259–267. doi: 10.1016/j.coi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 5.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 6.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 7. Malherbe L, Mark L, Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Vaccine adjuvants alter TCR-based selection thresholds. Immunity. 2008;28:698–709. doi: 10.1016/j.immuni.2008.03.014. Our study that demonstrates the impact of adjuvant on TCR-affinity based clonal selection in the antigen-specific TH cell compartment. These studies laid the foundations for analysis of TFH function in vivo.
- 8. Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. TCR repertoire and pMHCII binding strength assorts with TH cell function indicating a central role for TCR signal in the development of regulatory funciton in vivo. Together with the study of Reinhardt et al (ref 40) provides evidence for multiple TH cell subtypes within the TFH compartment suggesting the lineage commitment and location together define antigen-specific TFH function.
- 9.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 11.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 12.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker LS, Gulbranson-Judge A, Flynn S, Brocker T, Raykundalia C, Goodall M, Forster R, Lipp M, Lane P. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell DJ, Kim CH, Butcher EC. Separable effector T cell populations specialized for B cell help or tissue inflammation. Nat Immunol. 2001;2:876–881. doi: 10.1038/ni0901-876. [DOI] [PubMed] [Google Scholar]
- 17.Fuller KA, Kanagawa O, Nahm MH. T cells within germinal centers are specific for the immunizing antigen. J Immunol. 1993;151:4505–4512. [PubMed] [Google Scholar]
- 18.Bowen MB, Butch AW, Parvin CA, Levine A, Nahm MH. Germinal center T cells are distinct helper-inducer T cells. Hum Immunol. 1991;31:67–75. doi: 10.1016/0198-8859(91)90050-j. [DOI] [PubMed] [Google Scholar]
- 19.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 20.Gulbranson-Judge A, MacLennan I. Sequential antigen-specific growth of T cells in the T zones and follicles in response to pigeon cytochrome c. Eur J Immunol. 1996;26:1830–1837. doi: 10.1002/eji.1830260825. [DOI] [PubMed] [Google Scholar]
- 21.McHeyzer-Williams LJ, Panus JF, Mikszta JA, McHeyzer-Williams MG. Evolution of antigen-specific T cell receptors invivo preimmune and antigen-driven selection of preferred complementarity-determining region 3 (CDR3) motifs. J Exp Med. 1999;189:1823–1838. doi: 10.1084/jem.189.11.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng B, Han S, Kelsoe G. T helper cells in murine germinal centers are antigen-specific emigrants that downregulate Thy-1. J Exp Med. 1996;184:1083–1091. doi: 10.1084/jem.184.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 24. Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O'Garra A, Cahalan MD, Cyster JG. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. The first dynamic imaging studies of pre-GC TH-B cell contacts indicating stable 'monogamous' interactions with the B cell leading the TH cell movement.
- 25.Schwartzberg PL, Mueller KL, Qi H, Cannons JL. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat Rev Immunol. 2009;9:39–46. doi: 10.1038/nri2456. [DOI] [PubMed] [Google Scholar]
- 26.Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, Anderson S, Cheever AW, Sher A, Schwartzberg PL. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 28. Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal center formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. Outstanding dynamic imaging analysis of the antigen-specific pre-GC TFH-B cell interactions demonstrating a requirement for SAP expression to form stable conjugates and that these cognate interactions are required for subsequent entry into the GC reaction. Initial DC-TH interactions were unaffected and many typical features of effector TFH cells were expressed in the absence of SAP, but these cells could not form stable contacts and subsequently enter the GC.
- 29. Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. A comprehensive review of the current thinking in the context of TH cell lineage committment and what is understood about progamming TH cell function.
- 30. Fazilleau N, Eisenbraun MD, Malherbe L, Ebright JN, Pogue-Caley RR, McHeyzer-Williams LJ, McHeyzer-Williams MG. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat Immunol. 2007;8:753–761. doi: 10.1038/ni1472. This is the first demonstration of an antigen-specific memory TFH compartment that persists within the initial priming environment to enhance local memory B cell responses to antigen rechallenge. Our studies in the latter half of this manuscript provide evidence for persistent pMHCII complexes and long-lived antigen presentation after standard protein vaccination.
- 31.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 33.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 34.Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 35. Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. A comprehensive study of TFh development following adoptive transfer in vivo and in vitro re-stimulation. These studies indicate independent development of TFH cells compared to TH1, TH2 and TH17 cells that is mediated by IL-21, IL-6 and STAT3 and not dependent on TGF-β and argue for a distinct lineage of TFH cells.
- 36. Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. Together with ref 35 demonstrates a central role for IL-21 in the development of TFH cells. IL-21 acts in an autocrine manner to amplify the TFH cell fate by increasing TCR co-stimulation upon initial antigen triggering as well as providing the effector function to IL21R-expressing B cells that supports isotype switch and GC formation.
- 37.Spolski R, Leonard WJ. Interleukin-21 basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 38.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 40. Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. An outstanding use of cytokine reporter mice demonstrating the capacity of TH1 and TH2 cells to become IFN-γ and IL-4 producing TFH cells respectively. These studies also focused on isolated TFH-GC B cell conjugates to demonstrate the pairing of IL-4 producing TFH cells with IgG1-expressing B cells indicated the existence of distinct TFH cell lineages that may be dedicated to the regulation of separate isotype-specific B cell lineages.
- 41.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. 2009 doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. 2009 doi: 10.1084/jem.20090303. These two studies also use the IL-4 reporter mice to connect the TH2 cell lineage to IL-4 producing TFH cells following helminth infection. The functions of TH2 cells and TFH cells were separately compartmentalized further emphasizing deployment of cells as an important facet of TH cell function.
- 43.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, Brink R. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med. 2006;203:2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 46.Hauser AE, Junt T, Mempel TR, Sneddon MW, Kleinstein SH, Henrickson SE, von Andrian UH, Shlomchik MJ, Haberman AM. Definition of germinal-center B cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity. 2007;26:655–667. doi: 10.1016/j.immuni.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 47. Schwickert TA, Lindquist RL, Shakar G, Livshits G, Skokos D, Kosco-Vilbois MH, Dustin ML, Nussenzweig MC. In vivo imaging of germinal center reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. These three exceptional papers provided the first dynamic imaging studies of the GC reaction. The studies highlighted the intrazonal movement of GC B cells and the continuous movement of GC B cells along FDC networks. Ref 45 emphasized the stable contact of GC B cells and GC TFH cells suggesting this may provide a rate limiting step in the selection of high affinity memory B cells in the GC.
- 48. Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers phenotype and function. Semin Immunol. 2008;20:14–25. doi: 10.1016/j.smim.2007.12.001. An up-to-date and timely review of our current understanding of FDC function in vivo.
- 49.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho C-I, Sharpe AH, Vuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular helper T cells anf TH-17 cells. Nature Immunology. 2008;10:167–175. doi: 10.1038/ni.1690. Evidence for IL-17 production by TFH cells and a connection between TH17 and TFH cell lineages through the expression of c-Maf.
- 51. Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. This study also provides evidence for IL-17 production by TFH cells in a model of spontaneous autoimmune disease and indicates a role for IL-17 in the GC environment.
- 52. Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. This analysis of FoxP3 reporter mice demonstrate the plasticity of FoxP3-expressing cells to lose FoxP3 in the peyers patches and become TFH cells that support local GC formation. Interestingly, this change only occurs within the PP and not within LNs and spleen. Local environments prime specialist functional programs from distinct TH cell precursors.
- 53.Dullaers M, Li D, Xue Y, Ni L, Gayet I, Morita R, Ueno H, Palucka KA, Banchereau J, Oh S. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity. 2009;30:120–129. doi: 10.1016/j.immuni.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Odegard JM, Marks BR, DiPlacido LD, Pohelek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. This study demonstrates the presence of B cell helper activity in ICOS expressing TH cells in extra-follicular regions of LNs in the context of autoimmune disease suggested that these B cell help functions may not be restricted to the B cell zones.
- 55. Linterman MA, Rigby RJ, Wong R, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Waters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 2009;206:561–576. doi: 10.1084/jem.20081886. These studies follow up on the original sanroque autoimmune mouse model to demonstrate that TFH dysfunction is a primary defect in the generation of spontaneous GC and induction of autoimmunity. These studies further highlight the possibility that TFH cells play a role in positive selection within the GC.
- 56.Forster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein-- coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- 57.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]