Abstract
Follicular helper T (TFH) cells are the class of effector TH cells that regulates the stepwise development of antigen-specific B cell immunity in vivo. Deployment of CXCR5+ TFH cells to B cell zones of lymphoid tissues and stable cognate interactions with B cells are central to the delivery of antigen-specific TFH function. Recent advances help to unravel distinctive elements of developmental programming for TFH cells and unique effector TFH functions focused on antigen-primed B cells. Understanding the regulatory functions of TFH cells in the germinal center and the subsequent regulation of memory B cell responses to antigen recall represent the frontiers of this research area with the potential to alter fundamentally the design of future vaccines.
INTRODUCTION
Clonal selection and the programmed development of antigen-specific immune function are the central defining characteristics of adaptive immunity. Classical studies identified the cells of the immune system as the fundamental unit of clonal selection (Burnet, 1957; Talmage, 1957) and evidence for the production of antibodies (Fagraeus, 1948) with single specificities by individual lymphocytes (Nossal, 1959; Raff et al., 1973) as the operative effector mechanism. At least two types of lymphocytes (Miller, 1961) comprised the responding cellular compartment with T cells enhancing the production of antibodies by B cells (Claman et al., 1966; Miller and Mitchell, 1967). Early studies using hapten-carrier conjugates (Katz et al., 1970; Mitchison, 1971; Paul et al., 1966) postulated the existence of an antigen-bridge for T–B co-operation (Rajewsky et al., 1969) and helped to establish the basic tenets of ‘cognate’ help for antigen-specific B cell immunity.
It is now clear that antigen-specific TH cell development can proceed in multiple directions depending on the nature of the antigenic assault. The original TH1/TH2 paradigm (Mosmann and Coffman, 1989) identified distinguishable TH cell functional programs based on differential cytokine production with distinct cellular targets of action in vivo (Zhu and Paul, 2008). More recently, multiple subsets of regulatory TH (Treg) cells have been described as negative regulators of immune responsiveness to inhibit self-reactivity or guard against over-reactivity to pathogens (Sakaguchi, 2004). The TH17 cell subset adds a new layer to this complex system of immune regulation identifying separable developmental programs and cytokine profiles associated with chronic inflammatory disease and autoimmunity (Korn et al., 2009). There also exist less well-defined TH cell subsets capable of modifying DC maturation in ways that impact the development of effective CD8+ T cell memory (Janssen et al., 2003). In this context, follicular helper T (TFH) cells can be considered a separable TH cell subset specialized to regulate the evolution of effector and memory B cell responses (Fazilleau et al., 2007c; King et al., 2008; Vinuesa et al., 2005b). How the TFH cell compartment develops in vivo and differs from other subsets of effector TH cells is the subject of the current review.
Recent evidence suggests that TFH cells constitute a separate lineage of effector TH cells with distinct developmental programming and distinguishable effector function. There is also evidence to suggest that deployment of all effector TH cell subsets to appropriate follicular locations defines a unique set of effector TFH cell functions. We will present both positions and suggest that TH lineage differentiation and the programming of follicular location define multiple subsets of effector TFH cells needed to regulate antigen-specific B cell immunity. The regulation of antibody isotype across both effector and memory B cell development is one heterogeneous facet of TFH function that will be discussed in more detail. Furthermore, the distinction between pre-germinal center (GC) effector TFH and GC TFH cell function needs more clarity and is an important area of current research that will be discussed below. Finally, the maintenance and function of antigen-specific memory TFH cells that regulate memory B cell responses is a new emerging area of research that will be discussed in the last section of the review.
TH CELL REGULATED B CELL IMMUNITY
It is important to consider the temporal and spatial cellular dynamics that accompanies TH cell regulated B cell immunity (MacLennan, 1994; McHeyzer-Williams and McHeyzer-Williams, 2005). Specific recognition of peptide MHCII (pMHCII) complexes with threshold TCR affinity and adequate co-stimulation (Checkpoint IA) controls antigen-specific TH clonal selection, responder TH cell expansion and effector TH cell differentiation. Naive B cells will also encounter antigen in draining LNs very early after initial antigen priming (Checkpoint IB) (Batista and Harwood, 2009). Antigen-specific B cells will internalize antigen, process and present pMHCII complexes and move to the T–B borders of LNs to receive help from pMHCII-specific TH cells (Checkpoint II) (Allen et al., 2007a). Under the cognate control of effector TH cells, antigen-specific B cell development then divides into two major pathways, plasma cell (PC) versus germinal center (GC) development. The spectrum of effector TH cell activities delivered at this major developmental juncture belongs to the pMHCII-specific effector TFH cell compartment. The GC supports somatic diversification of BCR and selection of high affinity variants into the memory B cell compartment (MacLennan, 1994; McHeyzer-Williams and McHeyzer-Williams, 2005). Follicular dendritic cells (FDC) present antigen to survey variant BCR for effective antigen binding affinity (Checkpoint IIIA) while pMHCII-specific GC TFH cells form stable cognate conjugates with antigen-specific GC B cells (Checkpoint IIIB) (Allen and Cyster, 2008; Hauser et al., 2007b). The functions of pMHCII-specific GC TFH cells are fundamentally distinct from pre-GC effector TFH cells and thought to regulate clonal selection GC B cells and entry into the memory B cell compartment.
CXCR5+ FOLLICULAR HELPER T CELLS
As the name implies, the cardinal characteristic of all TFH cells is their re-positioning into the follicular regions of secondary lymphoid tissues. The chemokine receptor CXCR5 is highly expressed on B cells and is largely responsible for B cell partitioning into CXCL13 rich follicular areas in LNs (Ansel et al., 2000; Forster et al., 1996; Gunn et al., 1998a). In contrast, naive TH cells express high levels of CCR7, promoting extra-follicular placement into CCL19 and CCL21 rich areas in lymphoid tissue (Forster et al., 1999; Gunn et al., 1998b). CXCR5 was first reported on TH cells as the expression of an orphan receptor BLR1 on a small sub-population of CD4+CD45RO+ resting memory phenotype TH cells (CD44hiCD62LloIL2Rlo) in the peripheral blood of humans (Forster et al., 1994). Cells with this phenotype are also greatly enriched in human tonsils and distributed in the follicular areas and germinal centers (GC) of these inflamed tissues (Forster et al., 1994). In vitro, co-stimulation of CD4+ T cells through OX-40 and CD28 also induced CXCR5 expression and IL-4 secretion (Flynn et al., 1998). Importantly, blocking TH cell activation in vivo with CTLA4-Ig or in CD28 deficient animals also blocked OX-40, CXCR5-expressing TH cells and the development of germinal centers (Walker et al., 1999). At the same time, rapid CXCR5-expression was demonstrated directly on antigen-specific TH cells following priming in vivo (Ansel et al., 1999). These antigen-specific CXCR5+ TH cells also lost CCL19/21 responsiveness and migrated to follicular areas and GCs after priming. Hence, up-regulation of CXCR5 and down-regulation of CCR7 help to increase the probability of antigen-specific contact between pMHCII-specific TH cells and antigen-primed pMHCII+ B cells in draining lymphoid tissue.
TFH function
In these earliest reports of TFH cells, B helper cell function was assessed using CXCR5+ TH cells from human tonsils (Breitfeld et al., 2000; Schaerli et al., 2000). The CXCR5+ TH cell compartment contained all the capacity to support IgG and IgA production when co-cultured with B cells from the same tonsils. Functional assessment of cytokine production by in vitro re-stimulated CXCR5+ TH cells from human peripheral blood indicated some IL-2, IFN-γ and IL-10 production with negligible TH2 cytokines IL4, IL-5 or IL-13 (Schaerli et al., 2000). Antigen-specific TCR transgenic transfer experiments first demonstrated the concurrent and divergent development of tissue homing versus antibody promoting effector TH cells (Campbell et al., 2001). In contrast, to the tissue homing TH cells, the B cell helper subset was responsive to CXCL13 and not CCL21 with higher levels of IFN-γ and IL-4 upon re-stimulation in vitro. Both effector TH cell subsets expressed similar levels of ICOS and CD40L indicating the presence of shared effector potential (Campbell et al., 2001). Hence, CXCR5hiCCR7lo antigen-primed TH cells define a distinguishable subset of TH cells now called follicular B helper T cells or more commonly, follicular helper T cells (TFH).
TFH CELLS AS A SEPARATE TH LINEAGE
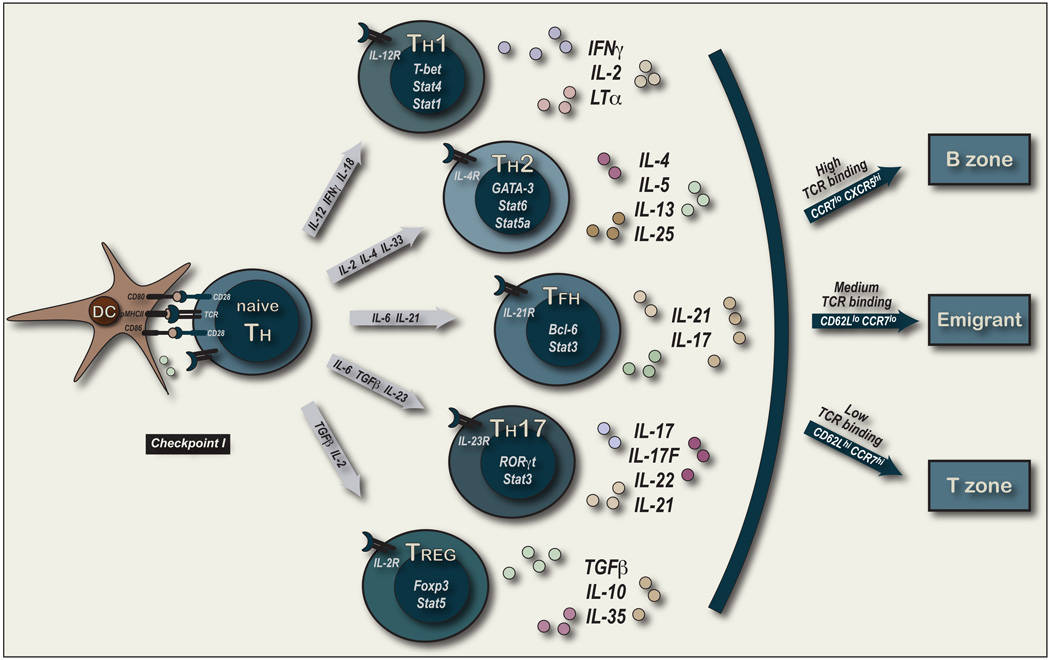
The range of effector functions associated with TH cells is extensive and the organization and developmental programming of these functions remains a focus of intense research. Changes in co-stimulation and cytokine delivery upon first contact with pMHCII-expressing DC have a major impact on TH cell fate and function (depicted in Figure 1). Manipulating these signals in vitro and in vivo have provided important insights into the derivation of different effector TH lineages and is the basis for most studies on TH cell lineage development (Zhu and Paul, 2008). Antigen-specific naive TH cells use initial instructive signals to drive cell-intrinsic developmental programs in their clonal progeny to define separable effector TH cell lineages.
Figure 1. TH Lineage Commitment and Micro-environmental Location.
This figure depicted the first contact between pMHCII+ DC and naive pMHCII-specific TH cells (referred to as Checkpoint 1A). Intercellular cognate contact involves TCR-pMHCII interactions in the context of a range of co-stimulatory that include CD80/86 and CD28 interactions. The extent and quality of co-stimulation will be controlled by the inflammatory context of antigen exposure for the DC at immunization. The cytokines produced by the activated DC as well as the initial cytokines produced by the activated TH cells will regulate the TH cell lineage that develops after clonal selection, expansion and differentiation in effector function. Some of the major known influences to the developmental of separate TH cell lineages are depicted to contrast to what is currently understood about effector TFH development. The arc on the right depicts a time-point after clonal expansion and effector TH cell differentiation when TH cells from all lineages either 1) remain in the T cell areas of LNs or 2) migrate to the B cell areas or 3) leave the LNs to distal tissues and not return during the first week after priming. The migratory division and cell surface phenotype are predictions based on our recent study using protein vaccination in an adjuvant containing a TLR-4 agonist and the use of an FTY720 analog to block LN emigration after priming (Fazilleau et al., 2009).
TH1/TH2 cells
The original TH1/TH2 paradigm identified differential programs of cytokine production thought to regulate cell-mediated immunity versus the production of antibody by B cells (Mosmann et al., 1986; Mosmann and Coffman, 1989). TH1 cells mediate immune responses to intracellular pathogens (Paul and Seder, 1994). IL-12 and IFN-γ are the principle inducers of the TH1 program with a synergistic role for IL-18. Induction of IL-12Rβ2 upon TCR activation (Szabo et al., 1997) increases Stat4-dependent IL-12 responsiveness in this pathway (Kaplan et al., 1996c). Stat1-dependent IFN-γ signals induce the TH1 master regulator T-bet that not only induces further IFN-γ production, but also inhibits IL-4 expression (Szabo et al., 2003). In contrast, TH2 cells mediate immune responses to extracellular parasites through the production of IL-4, IL-5, IL-13 and IL-25. Amplification of the TH2 program occurs through expression of IL-4R and Stat6 transduction of IL-4 signals that lead to the expression of the TH2 master regulator, GATA-3 (Kaplan et al., 1996b; Zheng and Flavell, 1997). GATA-3 is required for TH2 differentiation but also blocks the TH1 program. IL-2 can also potentiate TH2 cytokine production in a Stat5a–dependent mechanism (Zhu et al., 2003). In this manner, TH1 and TH2 can be considered alternate and non-overlapping cell fates that define separate TH lineages with each program also extinguishing the alternate cell fate.
TH17/Treg cells
More recently, a separable TH17 cell lineage has been described (Bettelli et al., 2006; Harrington et al., 2005; Mangan et al., 2006; Park et al., 2005; Veldhoen et al., 2006). TH17 cells were initially associated with autoimmune disease and chronic inflammation but have also been shown to mediate responses to extracellular bacteria and fungi through the production of IL17, IL-17F and IL22 (Korn et al., 2009). TGF-β and IL-6 were shown to induce IL-17 production from naive TH cells (Bettelli et al., 2006; Veldhoen et al., 2006). Importantly, TGF-β alone promoted Treg cell differentiation while addition of IL-6 not only induced high levels of IL-17 but also blocked the FoxP3 dependent development of Treg cells. Addition of IL-6 promoted expression of the orphan nuclear receptor RORγt in a Stat3-dependent manner. RORγt was necessary for TH17 development (Ivanov et al., 2006) with a role for IRF-4 (Brustle et al., 2007) and IRF4-binding protein (Chen et al., 2008) in the control of its expression. RORγt and the related RORα can bind FoxP3 and antagonize its function as one means for the reciprocal regulation of these two cell fates (Yang et al., 2008; Zhou et al., 2008). IL-6 also induces IL23R to promote survival and maintenance of TH17 cell function in the presence of the innate cytokine IL-23 (Yang et al., 2007; Zhou et al., 2007). Hence, TH17 and Treg cells constitute separate lineages of TH cells with unique transcriptional programs of development and distinct sets of effector cytokines from each other and the original TH1/TH2 options.
TFH cells
IL-21 is an interesting new member of the IL-2 family of cytokines that plays an important role in TH lineage development (Ozaki et al., 2000; Parrish-Novak et al., 2000; Spolski and Leonard, 2008). TH17 cells, TFH cells and TH2 cells are all known to produce IL-21. In TH17 development, IL-6 is a strong inducer of IL-21 in a Stat3 dependent mechanism (Nurieva et al., 2007; Zhou et al., 2007). Both IL-21 and IL21R deficient animals are defective in TH17 development suggesting an autocrine loop for TH17 cells with potential to amplify this particular lineage decision (Korn et al., 2007; Nurieva et al., 2007). IL21 and IL21R deficiency also impacts the development of B cell immunity with impaired isotype switch and deficient GC development (Ozaki et al., 2002; Spolski and Leonard, 2008). In this context, TFH cells are known to produce abundant levels of IL-21 (Nurieva et al., 2008; Vogelzang et al., 2008) creating potential autocrine action with concomitant expression of IL21R (Chtanova et al., 2004; Rasheed et al., 2006; Vinuesa et al., 2005a). Importantly, IL-21R expression only on TH cells partially rescued the B cell defect in IL21R deficient animals (Vogelzang et al., 2008). These studies also demonstrate a role for IL21 in the development of CXCR5+ICOShi TFH cells that was further enhanced by contact with ICOSL on B cells in vitro (Vogelzang et al., 2008) and in vivo (Nurieva et al., 2008). At the same time, IL-6 was also shown to induce IL-21 in TFH cells in a Stat3-dependent manner (Nurieva et al., 2008). TGF-β was not required for effector TFH induction and RORγt was not expressed by TFH cells or needed for TFH development in vivo (Nurieva et al., 2008). Together, these studies provide strong evidence for the differential programming events that control TH17 and TFH development into separable TH cell lineages.
Earlier microarray analyses of TFH cells also indicated separable gene expression programs for the TFH compartment. Using CD57 and CXCR5 expression to distinguish human tonsillar GC TFH cells (Kim et al., 2001) emphasized high levels of the chemokine CXCL13 as a major attribute of TFH cells (Kim et al., 2004). A separate study compared CD57+ and CD57− CXCR5+ tonsillar T cells to TH1 and TH2 cells polarized from cord blood in vitro, with central and effector memory TH cells from human peripheral blood (Chtanova et al., 2004). This comprehensive analysis highlighted ICOS and IL-21 as an abundant product of CD57+ TFH cells. The SLAM family member CD84 (Schwartzberg et al., 2009) and CD200 were highlighted as preferentially expressed by the TFH compartment with evidence for the differential expression of the transcriptional repressor, Bcl-6 (King et al., 2008; Vinuesa et al., 2005b). The use of CD57 was later called into question by a separate study that indicated CXCR5hiICOShi cells had the greatest B cell helper activity in the human tonsil (Rasheed et al., 2006). Nevertheless, this latter study also highlighted CXCL13 expression, IL21 and IL21R expression as well as low levels of GATA3 as distinctive in TFH cells (Rasheed et al., 2006). This study implicated TH2-associated transcription factors such as c-maf as being involved in TFH programming. In mouse, gene expression studies of TFH cells associated with over-expression of ICOS also identified IL-21 and CD200 as preferentially abundant in the dysregulated TFH cells (Vinuesa et al., 2005a) with TFH gene expression clustering distinctly when compared to TH1, TH2 and TH17 cells (Nurieva et al., 2008). These expression studies strongly supports the existence of a unique developmental program that leads to effector TFH cell functions separable from the other known TH cell subsets.
TFH LOCATION AS A DEVELOPMENTAL PROGRAM
While the recent data on separable lineage development are compelling, there are exceptions to the notion of a separate TH cell lineage for the TFH compartment. Both TH1 and TH2 cytokines are known to regulate antibody class switch (McHeyzer-Williams and McHeyzer-Williams, 2005; Vinuesa et al., 2005b) with IL-4 and IFN-γ being the earliest examples inducing IgG1/E and IgG2a respectively (Kuhn et al., 1991; Snapper and Paul, 1987; Takeda et al., 1996). TGF-β and IL-10 are implicated in the switch to IgA (Cazac and Roes, 2000; Defrance et al., 1992; Dullaers et al., 2009) although the cellular source of these factors during an immune response has not been well described. In vitro polarized TH1 and TH2 cells can both support B cell responses upon adoptive transfer (Secord et al., 1996; Smith et al., 2000). Furthermore, both TH1 and TH2 cells primed in vivo can enter follicular areas and interact with antigen-specific B cells (Pape et al., 2003; Smith et al., 2004; Toellner et al., 1998). IL-17 has also been implicated in the induction of autoreactive antibody responses (Nakae et al., 2003). A recent study localized IL-17 producing TH cells to the spontaneous and exaggerated splenic GC responses of BXD2 autoimmune prone mice, implicating the TH17 cells in the regulation of autoantibody (Hsu et al., 2008). Further to this notion, IL-17 production was induced preferentially from CXCR5+ICOShi TFH cells following MOG peptide in CFA immunization (Bauquet et al., 2008). ICOS regulated IL-21 production through c-Maf was implicated in this pathway to IL-17 production. These data contrast the lack of IL-17 production from TCR transgenic TH cells exposed to protein antigen in CFA (Nurieva et al., 2008) and may reflect the inherent plasticity in TFH programming or the impact of antigen specificity and TCR signal strength in this process. Finally, CD25+ Tregs have been reported within GC of human tonsils that interfere with T cell help and the induction of antibody (Lim et al., 2004). There are also reports of direct suppression of antibody production by FoxP3+ GC Tregs (Lim et al., 2005). Thus, all known TH lineages can migrate to follicular areas and into the GC to participate in the regulation of B cell immunity.
Polyclonal antigen-specific TFH cells
Directly monitoring antigen-specific TH cells in polyclonal immune systems (McHeyzer-Williams and Davis, 1995) and the advent of pMHCII tetramer technology (Altman et al., 1996; McHeyzer-Williams et al., 1996) provides experimental access to the polyclonal immune system. These new technologies allow direct assessment of antigen-specific TH cell responses to model antigens (Fazilleau et al., 2005; Rees et al., 1999; Savage et al., 1999), infectious disease (Malherbe et al., 2000; Stetson et al., 2002) and local antigen persistence (McLachlan et al., 2009). Our early studies using these strategies allowed access to antigen-specific TH cell repertoire (Fazilleau et al., 2007a; Malherbe et al., 2000; Malherbe et al., 2004; Malherbe et al., 2008; McHeyzer-Williams et al., 1999), TCR responsiveness (Bikah et al., 2000), effector TH cell function in vivo (Panus et al., 2000).
Our recent studies focused on the local development of antigen-specific CXCR5+ CD44hiCD62Llo effector TFH in the draining LNs (Fazilleau et al., 2007b). Examining the mechanism underlying this assortment of effector TH cell function, we demonstrated that the strength of TCR-pMHCII binding influenced the migratory behavior of antigen-specific effector TH cells (Fazilleau et al., 2009). The highest pMHCII-binding TCR assorted with the differentiation of effector TFH cells and localization to the B cell areas (CXCR5+CCR7lo). Under the same priming conditions, intermediate pMHCII binding promoted emigrant TH cells (CCR7loCD62Llo) that left the LN and low pMHCII binding promoted T zone localized TH cells (CCR7hiCD62Lhi)(depicted in Figure 1). In this polyclonal model, IL-2, IFN-γ, IL-10 and T-bet were expressed at equivalent levels by all subsets of pMHCII-specific effector TH cells at day 7 after priming. Cytokines more typically associated with TFH cells, IL-4 and IL-21 were present in all TH subsets but at higher levels in the TFH compartment. OX-40, CD69 and Bcl-6 were uniquely present in the effector TFH compartment. Hence, there was evidence for multiple sub-types of TH cells with separable developmental programs associated with the pMHCII-specific effector TFH cell compartment in this model system.
Therefore, deployment of separable TH cell lineages to follicular regions may represent an over-riding secondary developmental program for the antigen-specific regulation of distinct B cell fates. In this context, the nature of the innate stimulus would dictate the balance of effector TH cell subsets and a subset of these responders can relocate to the follicular regions to regulate effector and memory B cell functions. For example, CXCR5+CCR7− IFN-γ producing, T-bet expressing pMHCII-specific TH1 cells could be considered to be a TFH1 subtype of effector TFH cell that regulate B cell fate at Checkpoint II in vivo.
PRE-GERMINAL CENTER EFFECTOR TFH CELLS
Pre-GC effector TFH function is delivered in an antigen-specific manner to pMHCII-expressing B cells but how these differential functions develop in vivo remains poorly resolved. Under static conditions in vitro, successful pMHCII-TCR interactions involve the ordered formation of a stable immunological synapse (Bromley et al., 2001; Grakoui et al., 1999; Monks et al., 1998). Inter-cellular adhesion facilitates the scanning for adequate TCR-pMHCII interactions, central clustering and rearrangement of the cellular interface into a stable synapse (Bromley et al., 2001; Grakoui et al., 1999; Monks et al., 1998). Real-time imaging using two-photon laser scanning technology has provided outstanding clarity to these early cellular interactions in vivo (Miller et al., 2002). Studies labeling antigen-primed DC and pMHCII-specific TH cells indicate an early phase of transient 5–10 min contacts followed by longer duration interactions lasting many hours. Altered cell motility promotes serial DC engagement that leads to cell clustering and swarming behavior (Miller et al., 2004). The duration of DC-T cell contact during this later phase impacts cell fate (Celli et al., 2005; Celli et al., 2007; Shakhar et al., 2005) with at least 6hr of contact required to induce clonal expansion in CD4+ TH cells. Nevertheless, the impact of these early events on the development of effector and memory TH functions is less clear and remains an important focus of current research.
Effector TFH contact duration
First contact between antigen-specific TH and antigen-primed B cells has also been captured through dynamic imaging (Okada et al., 2005). Stable ‘monogamous’ interactions between one antigen-specific TH cell and one B cell can last for 10–60 min duration in the follicular regions of the LNs. These interactions were accompanied by highly dynamic movements with the B cells migrating extensively leading the TH cells (Okada et al., 2005). A more recent study demonstrated that expression of the adaptor molecule SAP was needed to form long duration contacts with antigen-primed B cells (Qi et al., 2008). There was no role for SAP in early TH-DC contacts and the SAP deficient TH cells reached the follicular regions and expressed all the hallmarks of effector TFH cells (CXCR5hi, CD40L+, ICOShi and OX40hi) (Qi et al., 2008). However, in the absence of SAP, TFH cells were still capable of cytokine production (Cannons et al., 2006), but unable to promote GC formation (Crotty et al., 2003; Schwartzberg et al., 2009). Importantly, effective contact duration between pre-GC effector TFH cells and pre-GC B cells promoted the entry of TFH cells into the GC reaction (Qi et al., 2008). Cognate interactions were also required for effective contact duration and would not occur if the TH cell antigen and the B cell antigen were unlinked (Qi et al., 2008). These studies clearly establish that duration of pre-GC pMHCII-specific effector TFH contact with pre-GC B cells is needed to promote normal GC formation. Furthermore, pre-GC effector TFH cell contact with pMHCII-expressing antigen-primed B cells may also be required for aspects of GC TFH functional developmental.
EFFECTOR TFH CELL FUNCTION
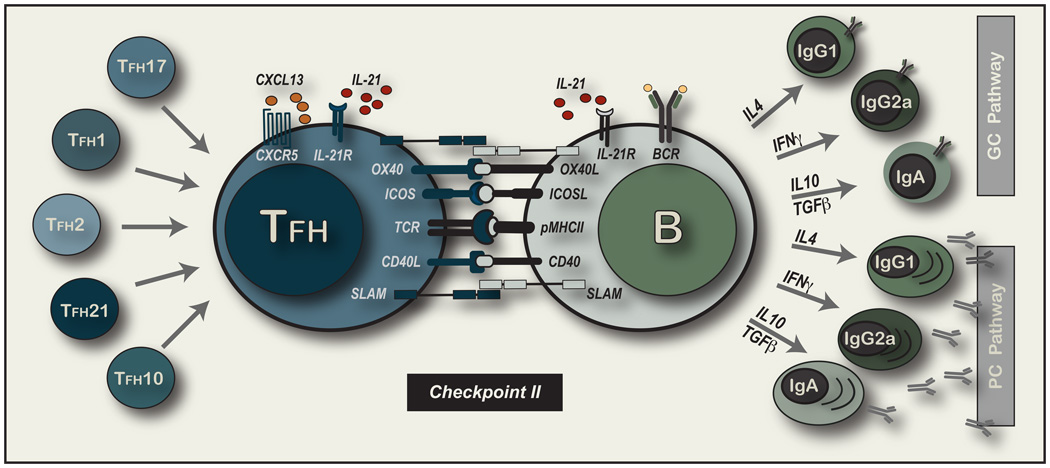
While location and cognate contact are important, it is important to consider in more detail the driving forces and consequences of Checkpoint II interactions (depicted in Figure 2). Antigen-activated B cells up-regulate CCR7 while retaining CXCR5 levels to counterbalance migration to the T–B borders (Ebert et al., 2004; Ohl et al., 2003; Reif et al., 2002). CXCR5+ effector TFH cells must lose CCR7 expression to achieve follicular placement. Further, CXCR5 expression on TH cells is not required for follicular placement but aspects of TFH activity such as isotype switch and GC formation are decreased in its absence (Arnold et al., 2007; Haynes et al., 2007). Increased CXCL13 expression by effector TFH cells must serve to attract CXCR5+ B cells (Kim et al., 2004). There is also evidence for IL-21 producing extra-follicular TH activity that controls self-reactive IgG production and is associated with CXCR4 expression and the loss of P-selectin ligand 1 binding (PSGL-1)(Odegard et al., 2008). Hence, B cell helper function may not only reside in the follicular areas but with pMHCII-specific TH cells that balance chemokine receptor expression for appropriate placement in vivo.
Figure 2. Delivery of Cognate Effector TFH function to pMHCII-expressing B cells.
The main focus of this display are the molecular interactions known to be involved at the cellular interface upon first contact of an effector TFH cell and an antigen-primed B cells. Cell surface molecules are emphasized with the presence of IL-21 depicted as one of many cytokine exchanges that may ensue. On the left hand side of the figure, we depict multiple TFH cell subsets that would arise as TH cells of known lineages that adopt the developmental program of follicular placement. This program is largely defined as the loss of CCR7 and expression of CXCR5 but relies on appropriate positioning in the follicular regions and subsequent contact with an antigen-primed B cell. ON the riight hand side of the figure, we depict the two major outcomes for B cells after receipt of antigen-specific T cell help: 1) Entry into the extra-follicular short-lived PC pathway to terminal differentiation or 2) movement into the follicular areas to rapidly expand and form the GC in the pathway to memory B cell development. Isotype switch proceeds in both pathways controlled by the cytokine mixture and cell contact received at this critical second developmental checkpoint.
Modifying cognate interactions
Cognate contact between effector TFH cells and pMHCII-expressing B cells defines a critical initiating event in the development of effective B cell immunity that may be modified by a multitude of cell surface expressed molecules (depicted in Figure 2). CD28 deficiency or treatment with blocking anti-CD28 antibodies leads to profound defects in B cell immunity (Shahinian et al., 1993). CD28 is required early to initiate naive TH cell responsiveness to pMHCII+ CD80 and CD86 expressing DC (Janeway and Bottomly, 1994; Matzinger, 1994). In contrast, CD40-CD40L interactions are central to the delivery of T cell help to B cells (Armitage et al., 1992; Lederman et al., 1992; Noelle et al., 1992). Mice and humans deficient in CD40 or CD40L are unable to efficiently class switch, form GCs or promote memory B cell responses (Kawabe et al., 1994; Xu et al., 1994). Interfering with OX40-OX40L interactions prevents TFH accumulation in follicular regions of LNs suggesting that early engagement with OX40L on activated DC (Brocker et al., 1999) or on a CD3−CD4+ accessory cell population (Kim et al., 2003) was needed to facilitate appropriate TFH migration. The continued expression of OX40 on effector TFH cells (Fazilleau et al., 2009; Qi et al., 2008) also suggests a role in the modulation of cell fate at effector TFH-B cell interactions that may extend to within the GC reaction. There are reports of other TNFR/L expression such as CD30 (Kim et al., 2003) and BTLA (Nurieva et al., 2007) that can play a role in the development of TFH cells and the delivery of TFH functions in vivo.
The CD28 family member inducible co-stimulator molecule ICOS (Hutloff et al., 1999) has been implicated in TFH cell function in pre-GC and the GC interactions with pMHCII-expressing B cells (Fazilleau et al., 2007c; King et al., 2008; Vinuesa et al., 2005b). ICOS deficient humans (Grimbacher et al., 2003) and mice (Dong et al., 2001; McAdam et al., 2001; Tafuri et al., 2001) and ICOS-L deficient mice (Mak et al., 2003) have marked deficits in antibody production, isotype switch and GC formation. ICOS deficiency is associated with decreased TFH cell development (Bossaller et al., 2006) and ICOS is considered an important molecule in the delivery of effector TFH function (Nurieva et al., 2008; Vogelzang et al., 2008). The highest levels of ICOS are expressed on GC TFH cells with the greatest capacity to support antibody production in vitro (Rasheed et al., 2006). Conversely, over-expression of ICOS in mice with a regulatory defect in ICOS expression (Yu et al., 2007) over-produced CXCR5+ TFH cells, displayed exuberant GC reactions with breakthrough autoimmune disease (Vinuesa et al., 2005a). Recent studies indicate that ICOS can substitute for CD28 and rescue the TFH defects and B cell defects in CD28 deficient mice (Linterman et al., 2009a). Furthermore, the abundant CXCR5+ TFH cells in this model act in a T cell autonomous manner to promote autoantibody production (Linterman et al., 2009b). Another CD28 family member PD-1 that has been implicated in the negative regulation of chronically activated T cells has also been demonstrated on GC TFH cells in human tonsil (Iwai et al., 2002) and mouse (Haynes et al., 2007). Positive and negative influences of co-stimulation are balanced in ways that remain poorly understood in vivo.
Overall, the precise function of different co-stimulatory molecules and their combinatorial impact on antigen-specific B cell fate remains an exciting area of current interest. Quantitative differences in cell surface molecules in combination with mixtures of cytokines will likely synergize in pre-determined ways to skew pMHCII-expressing B cells into separate pathways of B cell immunity. Unraveling these molecular combinations will help to define the rules of molecular control for antigen-specific B cell immunity.
PC versus GC development
Effectively regulated pMHCII-expressing B cells will either enter the PC or GC pathway at this juncture in development. The signaling lymphocyte activation molecule (SLAM) family of molecules plays an important role at the effector TFH-B interface revealed through the action of the signal transducer SAP (Schwartzberg et al., 2009). Adoptive transfer studies indicate that the SAP defect in B cell immune responsiveness is primarily a T cell defect (Veillette et al., 2008). When effective duration TFH-B contact cannot be sustained (Qi et al., 2008), the GC reaction and memory B cell development is compromised. In contrast, the extra-follicular pathway to plasma cell development remains intact in the SAP deficient animals with evidence for acute antibody responses and isotype switch (Crotty et al., 2003). SAP deficient TH cells reconstituted with a Fyn recruiting mutant of SAP can promote cytokine production but still do not support GC formation (Cannons et al., 2006). Taken together, these data suggest that different subsets of effector TFH cells may regulate entry into the extra-follicular pathway to short-lived PCs versus the GC pathway to memory B cell development. Alternatively, these separate developmental ‘decisions’ may require separable signals from the same cohort of effector TFH cells (such as distinct affinity TCR-pMHCII interactions). Understanding and controlling the balance of early antibody responses versus affinity-matured memory B cell responses may provide useful and separate means for modifying the adaptive immune response in future immunotherapeutic strategies.
Antibody Class Switch
Developmental progression in antigen-specific PCs and GC B cells both involve the switch to downstream antibody isotypes (McHeyzer-Williams and McHeyzer-Williams, 2005)(depicted in Figure 2). Early studies indicated that switch occurred in extra-follicular sites and within the GC at the same time (Jacob et al., 1991). Thus, it is possible that some systemic factor controlled these events in a co-ordinate way. Alternatively, pMHCII-specific TFH cells within both environments may actively and simultaneously instruct these developmental outcomes. It is also plausible, that first contact with effector TFH cells induced the commitment to switch to a particular antibody isotype in antigen-primed B cells, a process that requires multiple cell divisions to complete (Tangye et al., 2003). In the latter model, commitment to isotype switch would be imprinted by the cytokine signals delivered in a cognate manner at first contact with effector TFH cells. As a corollary of this model, commitment to a particular antibody isotype may also define a separable lineage of antigen-experienced B cells with distinct origins and requirements for survival, function and long-term propagation in vivo.
Based on what is understood about the cytokine control of isotype switch there is evidence for the existence of multiple effector TFH subsets (depicted in Figure 2). CXCR5+ TH1 cells that produce primarily IFN-γ would promote IgG2a production in the progeny of contacted antigen-specific B cells. We could consider this follicular homing subset, TFH1 cells. In the same manner, TFH2 would produce primarily IL-4 and promote switch to IgG1 and IgE in the clonal progeny of the primed B cells (Snapper and Paul, 1987). Antigen-specific inducible Treg producing TGF-β and IL-10 could direct IgA switch as TFH10 cells (Cazac and Roes, 2000; Defrance et al., 1992; Dullaers et al., 2009). There has also been a recent report of a TGF-β producing TFH cell compartment that promotes not only IgA switch but also plasma cells migration to mucosal tissue by up-regulating CCR10 expression (Dullaers et al., 2009). IL-21 is another important secreted factor in the regulation and/or enhancement of antibody isotype switch (Bryant et al., 2007; Spolski and Leonard, 2008). The absence of IL-21 alone reduced IgG1 production with elevated IgE, while IL-4 and IL-21 DKO mice display decreases in all antibodies in the serum (Ozaki et al., 2002). Hence, pMHCII-specific effector TFH cells may direct antibody isotype commitment through cognate and non-cognate contact modified by the cytokine combinations they deliver.
Bcl-6 and Blimp-1
Identifying antagonizing transcription factors allowed the more detailed molecular analysis of TH lineage commitment. In the TFH arena, the transcriptional repressor Bcl-6 has been used to distinguish the TFH compartment from other TH cell subsets as discussed above (Chtanova et al., 2004; Fazilleau et al., 2009; Nurieva et al., 2008; Vinuesa et al., 2005b; Vogelzang et al., 2008). This transcriptional repressor is highly expressed in GC B cells (Cattoretti et al., 1995; Onizuka et al., 1995) and has been demonstrated in a fraction of GC TFH cells (Ree et al., 1999). Bcl-6 deficient mice have defective B cell responses with an absence of GC and memory development (Dent et al., 1997; Shaffer et al., 2000; Ye et al., 1997). In our recent analysis of TFH cell development to protein vaccination, we reported the expression of Blimp-1 in the CCR7hiCXCR5lo T zone resident effector TH cell compartment (Fazilleau et al., 2009). Blimp-1 was first described as a master regulator for plasma cells (Turner et al., 1994) but has now found roles in natural Treg development (Kallies et al., 2006; Kaplan et al., 1996a; Martins et al., 2006), the differentiation of TH1 cells (Cimmino et al., 2008). Interestingly, these Bcl-6 and Blimp-1 are known to cross block (Martins and Calame, 2008) providing a means to extinguish the alternate cell fate in a feedback regulatory manner. This pair of transcription factors may represent antagonizing control of LN effector functions and used to balance the T zone versus B zone effector TH cells that emerge in response to antigenic assault.
GERMINAL CENTER TFH CELLS
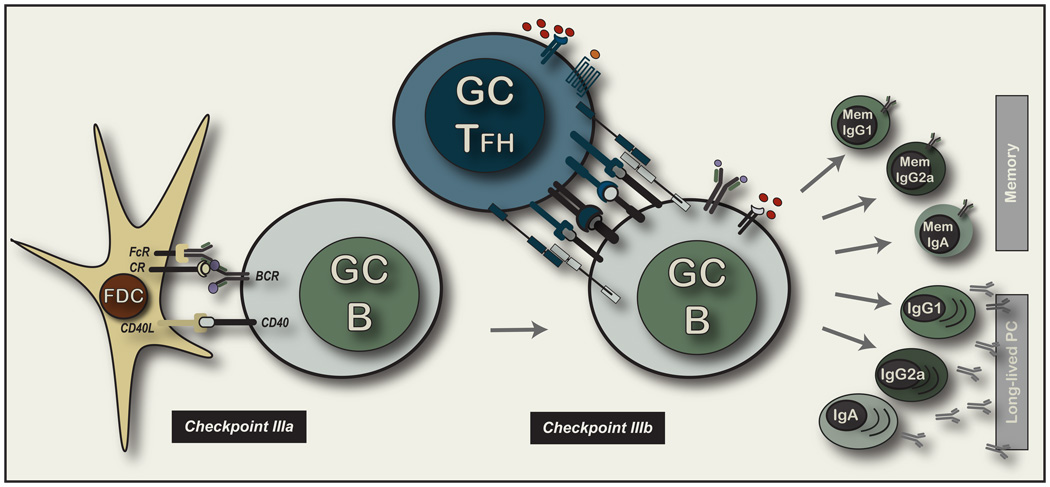
It must be emphasized that defects in either the priming of naive TH cells and B cells or the ineffective cognate interactions at the first contact between these two facets of the adaptive immune system will result in GC defects. Hence, the lack of GC formation is an unreliable phenotype for unraveling the impact of GC interactions themselves. Nevertheless, the GC functions of TFH cells remain of intense interest and central importance to the understanding of high-affinity antigen-specific memory B cell generation (depicted in Figure 3).
Figure 3. Selection of GC B cells and the cognate control of GC B cell.
The left side of this figure depicts the molecular interactions associated with continuous movement of variant GC B cells along the stromal network of mature FDC that have coated immune complexes on their dense light zone processes through FcR and complement receptors. The right side of the figure depicts the intercellular engagement of a GC TFH cell and a GC B cell. We have not labeled the molecules as there is still very little information on the molecular nature of this interaction regarding cell surface molecules or secreted cytokines. The phenotype that has been reported is largely similar between effector TFH cells and their GC TFH counterparts. The far right of figure depicts the predicted cellular outcomes of Checkpoint IIIA and IIIB interactions based on the known output of the GC reaction. There are broadly two classes of memory B cell that exits the GC 1) high affinity precursors for the memory response to antigen re-challenge that exist in a variety of physiological states and 2) long-lived PCs that contribute high affinity antibodies to the circulation of the animal largely for life. The latter compratment is not considered able to react to antigen re-challenge but is nevertheless considered a component of long-term B immunity. Finally, each post-GC pathway potentially comprises of all antibody isotypes that we propose may be considered to be separable lineages of memory B cells. For example, it is likely that IgG1 memory B cells have diverged in development from IgA memory B cells and not only received distinct inducing signals to develop in the first place (at Checkpoint II), but reside in separable microenvironmental niches around the body with distinguishable migratory phenotype, but also require unique activation requirements upon antigen re-challenge.
The T cells within GC are predominantly CD4+ and reside mainly in the light zones (Bowen et al., 1991; Fuller et al., 1993; MacLennan, 1994). Antigen-specific TH cells have been shown to enter the GC in adoptive transfer models (Garside et al., 1998) and during the course of an immune response in polyclonal systems (Gulbranson-Judge and MacLennan, 1996; Zheng et al., 1996). Our early studies in protein vaccination noted the dynamics of movement between pre-GC follicular regions at day 7 to predominantly GC by day 9 after priming (McHeyzer-Williams et al., 1999). This sequential positioning of antigen-specific TH cells was also found within the spleen of the polyclonal responders (Gulbranson-Judge and MacLennan, 1996). However, not all antigen-specific TH cells entered the GC with evidence for particular sets of the responsive TCR repertoire not assorting into the GC (Mikszta et al., 1999). Importantly, these studies also monitored memory development for additional non-GC clonotypes to demonstrate that GC entry was not required for memory TH cell development. Static analysis of GC TH cell in situ suggested that GCs were open environments for TH cells with evidence from micro-dissection studies for movement between different GC (Zheng et al., 1996).
The function of GC TFH cells has been more difficult to ascertain. Interfering with CD28-CD86 or CD40-CD40L interactions leads to dissolution of established GC (Han et al., 1995). Using high frequencies of high affinity BCR transgenic B cells and multivalent forms of antigen can promote GC formation without TH cells and in the absence of CD40 or CD28 signals (de Vinuesa et al., 2000). TH cell independent GCs are histologically similar to typical TH cell regulated GCs, containing immune complexes, FDC and B cells with a GC phenotype (Gaspal et al., 2006) but dissimilar global gene expression patterns (Yu et al., 2008). Importantly, TI GCs collapse early after priming with no evidence for somatic diversification of the BCR (de Vinuesa et al., 2000; Gaspal et al., 2006; Toellner et al., 2002). Thus, the presence of GC TFH cells does not appear to be necessary for the generation of GC microenvironments but they appear important for the later events of GC B cell selection and the production of high-affinity B cell memory.
GC cellular dynamics
Recent dynamic imaging of the GC reaction in situ has provided outstanding clarity to the kinetics of GC B cell (Hauser et al., 2007a; Schwickert et al., 2007) and GC TFH cell movements and interactions (Allen et al., 2007b). There was evidence for inter-zonal movement of GC B cells indicating bi-directional migration between light and dark zones (Allen et al., 2007b; Hauser et al., 2007a; Hauser et al., 2007b; Schwickert et al., 2007) with one study emphasizing the majority of movement to be intra-zonal (Hauser et al., 2007a; Hauser et al., 2007b). Surprisingly, cell division appeared in both zones of the GC in contrast to classical models (Allen et al., 2007b; Hauser et al., 2007a; Schwickert et al., 2007). There was also evidence for the ability of naive B cells to traverse the GC environment with evidence that high-affinity B cells could also enter and dominate existing GCs (Schwickert et al., 2007). All studies identified highly motile GC B cell movements with evidence for continuous uninterrupted movement over FDC processes. This movement contrasted with the capacity of GC B cells to form frequent short-term contacts but infrequent stable contacts with GC TFH cells (Allen et al., 2007b). The latter group suggested that these infrequent cognate contacts play a dominant role in the selection of high-affinity BCR expressing GC B cells (Allen et al., 2007a). Hence, a new model for affinity-based selection emerges with uninterrupted access of GC B cells to antigen-coated FDC followed by the ability of “re-primed” B cells to express pMHCII complexes to levels that gain competitive access to pMHCII-specific GC TFH cells.
It is important to emphasize that the functions of the pMHCII-specific GC TFH cells appear broadly distinct from the effector TFH counterparts. First, entry of TFH into the GC environment appears dependent on prior interactions with pre-GC B cells (Qi et al., 2008). Entry of effector TFH into the GC may not be the only pathway of GC entry, but appears to be one method of gaining access into this microenvironment. Extensive clonal expansion ensues for the B cells within the GC environment (MacLennan, 1994) and therefore precludes continued contact with the effector TFH cells at the time-point of entry. Furthermore, it is unclear whether there are developmental changes that occur within the effector TFH cells between the point of entry and the capacity to form stable contacts with GC B cells after BCR diversification. Controlling GC B cell exit and the development of high affinity B cell memory is also correlated with the presence of GC TFH cells (Toellner et al., 2002). It is possible that the GC TFH cells promote and support antibody isotype switch within the GC. However, it is also likely that commitment to isotype switch has already occurred and does not require these secondary contact events as discussed above. Alternatively, there may exist subsets of GC TFH cells specialized to select and support GC B cells of particular isotypes. Within this context, the major differential outcome of the GC interaction would be a major division represented by entry into the “reactive” memory B cell compartment or entry into long-lived plasma cell pathway (depicted in Figure 3). Both pathways contribute to long-term immune protection and represent two broad layers of antigen-specific B cell memory.
MEMORY TFH CELLS
The original CXCR5+ TFH cells were identified in human peripheral blood as circulating sub-populations of resting TH cells with a memory phenotype (Forster et al., 1994). Functional analysis of these circulating TFH cells distinguishes their activity from tonsillar TFH cells with reduced capacity for promoting antibody production by B cells (Kim et al., 2001). Nevertheless, the circulating TFH compartment may play a differential role in the regulation of memory B cells compared with other the TH cell memory compartment (Sallusto et al., 2004). The organization of antigen-specific memory TFH cells and the regulation of memory B cell responses is an important but poorly studied facet of this research area.
In the polyclonal model, we recently characterized the presence of a CXCR5+ TFH compartment that remained resident within the LNs, had decreased levels of ICOS and cytokine mRNA in vivo, but rapid and differential induction of IL-4, IL-10 and IL-21 upon re-stimulation with antigen in vitro (Fazilleau et al., 2007b). We proposed that these cells were the memory counterpart of the effector TFH population that emerged after initial priming. We provided evidence that local memory B cell responses arose more rapidly in their presence than elsewhere after antigen re-challenge. The continued expression of CD69 on the memory TFH compartment suggested recent TCR triggering with pMHCII complexes. Adoptive transfer of naive TCR transgenic TH cells located pMHCII depots in the draining LNs and not in the non-draining LNs or the spleen of previously immunized mice (Fazilleau et al., 2007b). These data suggested the presence of a resident B zone localized memory TFH compartment able to rapidly regulate pMHCII-expressing memory B cells upon re-challenge with antigen. We predict that cognate interactions that define the regulation of memory B cells (Checkpoint IV) would be qualitatively and quantitatively distinct from the interactions that define cell fate after initial priming. Understanding and then controlling these molecular interactions will provide new impetus and novel targets for the basic strategies used in typical prime-boost vaccines regimes.
CONCLUSIONS
TFH cells are a class of regulatory TH cells that specialize in the cognate control of antigen-specific B cell immunity. Upon antigen-specific activation, the emergence and movement of CXCR5+CCR7− pMHCII-specific TFH cells into the follicular regions is the major attribute of TFH cells. These TFH cells express CD40L, ICOS and SAP to form stable contacts with antigen-primed B cells with evidence for IL-4, IFN-γ, IL-10 and IL-21 as soluble factors to modify B cell fate. Distinct control of IL-21 production and the expression of Bcl-6 help to distinguish TFH cells from other polarized TH cell subsets and may define a separable TH cell lineage. However, we favor a model in which all known TH lineages can be deployed to the follicular regions to regulate a spectrum of outcomes in B cell immunity. In this model, both lineage and location would define the regulatory function of pMHCII-specific TFH cells.
There are three major junctures in the delivery of cognate TFH function in vivo. After TH clonal selection and differentiation of effector function, first contact with antigen-primed B cells occurs pre-GC in the follicular regions. We propose that these effector TFH cells control 1) commitment to antibody isotype and 2) entry into short-lived PC versus GC pathway to memory. Entry into the GC defines a distinct compartment of GC TFH cells that control 1) GC B cell selection and survival and 2) entry into the long-lived memory B cell and PC compartments. Long-term persistence in LNs defines a separate resting memory TFH compartment that control 1) memory B cell expansion and 2) rapid PC differentiation upon antigen re-challenge in vivo. Understanding the cellular development and molecular control of antigen-specific B cell immunity will provide new targets for the immunotherapeutic management of many clinical diseases with the potential to change the basic design of future vaccine strategies.
ACKNOWLEDGEMENTS
In honor of Joanne Fanelli-Panus (1967–2009) and her contributions to our understanding of antigen-specific T helper cell function in vivo. This review is supported by the National Institutes of Health (AI047231, AI040215 and AI059475 to MMW) and LM is a recipient of a Wenner-Grenn Fellowship.
REFERENCES
- Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20:14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007a;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007b;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, Anderson DM, Gimpel SD, Davis-Smith T, Maliszewski CR, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Arnold CN, Campbell DJ, Lipp M, Butcher EC. The germinal center response is impaired in the absence of T cell-expressed CXCR5. Eur J Immunol. 2007;37:100–109. doi: 10.1002/eji.200636486. [DOI] [PubMed] [Google Scholar]
- Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho C-I, Sharpe AH, Vuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular helper T cells anf TH-17 cells. Nature Immunology advance online publication. 2008 doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bikah G, Pogue-Caley RR, McHeyzer-Williams LJ, McHeyzer-Williams MG. Regulating T helper cell immunity through antigen responsiveness and calcium entry. Nat Immunol. 2000;1:402–412. doi: 10.1038/80841. [DOI] [PubMed] [Google Scholar]
- Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, Durandy A, Baumann U, Schlesier M, Welcher AA, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006;177:4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- Bowen MB, Butch AW, Parvin CA, Levine A, Nahm MH. Germinal center T cells are distinct helper-inducer T cells. Hum Immunol. 1991;31:67–75. doi: 10.1016/0198-8859(91)90050-j. [DOI] [PubMed] [Google Scholar]
- Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker T, Gulbranson-Judge A, Flynn S, Riedinger M, Raykundalia C, Lane P. CD4 T cell traffic control: in vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur J Immunol. 1999;29:1610–1616. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- Burnet FM. A modification of Jerne's theory of antibody production using the concept of clonal selection. Aust J Sci. 1957;20:67–69. doi: 10.3322/canjclin.26.2.119. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Kim CH, Butcher EC. Separable effector T cell populations specialized for B cell help or tissue inflammation. Nat Immunol. 2001;2:876–881. doi: 10.1038/ni0901-876. [DOI] [PubMed] [Google Scholar]
- Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, Anderson S, Cheever AW, Sher A, Schwartzberg PL. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoretti G, Chang CC, Cechova K, Zhang J, Ye BH, Falini B, Louie DC, Offit K, Chaganti RS, Dalla-Favera R. BCL-6 protein is expressed in germinal-center B cells. Blood. 1995;86:45–53. [PubMed] [Google Scholar]
- Cazac BB, Roes J. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity. 2000;13:443–451. doi: 10.1016/s1074-7613(00)00044-3. [DOI] [PubMed] [Google Scholar]
- Celli S, Garcia Z, Bousso P. CD4 T cells integrate signals delivered during successive DC encounters in vivo. J Exp Med. 2005;202:1271–1278. doi: 10.1084/jem.20051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli S, Lemaitre F, Bousso P. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 2007;27:625–634. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Chen Q, Yang W, Gupta S, Biswas P, Smith P, Bhagat G, Pernis AB. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29:899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- Cimmino L, Martins GA, Liao J, Magnusdottir E, Grunig G, Perez RK, Calame KL. Blimp-1 attenuates Th1 differentiation by repression of ifng, tbx21, and bcl6 gene expression. J Immunol. 2008;181:2338–2347. doi: 10.4049/jimmunol.181.4.2338. [DOI] [PubMed] [Google Scholar]
- Claman HN, Chaperon EA, Triplett RF. Thymus-marrow cell combinations. Synergism in antibody production. Proc Soc Exp Biol Med. 1966;122:1167–1171. doi: 10.3181/00379727-122-31353. [DOI] [PubMed] [Google Scholar]
- Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, Wabl M, Klaus GG, MacLennan IC. Germinal centers without T cells. J Exp Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrance T, Vanbervliet B, Briere F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992;175:671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- Dullaers M, Li D, Xue Y, Ni L, Gayet I, Morita R, Ueno H, Palucka KA, Banchereau J, Oh S. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity. 2009;30:120–129. doi: 10.1016/j.immuni.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert LM, Horn MP, Lang AB, Moser B. B cells alter the phenotype and function of follicular-homing CXCR5+ T cells. Eur J Immunol. 2004;34:3562–3571. doi: 10.1002/eji.200425478. [DOI] [PubMed] [Google Scholar]
- Fagraeus A. The plasma cell reaction and its relation to the formation of antibodies in vitro. J Immunol. 1948;58:1–3. [PubMed] [Google Scholar]
- Fazilleau N, Cabaniols JP, Lemaitre F, Motta I, Kourilsky P, Kanellopoulos JM. Valpha and Vbeta public repertoires are highly conserved in terminal deoxynucleotidyl transferase-deficient mice. J Immunol. 2005;174:345–355. doi: 10.4049/jimmunol.174.1.345. [DOI] [PubMed] [Google Scholar]
- Fazilleau N, Delarasse C, Motta I, Fillatreau S, Gougeon ML, Kourilsky P, Pham-Dinh D, Kanellopoulos JM. T cell repertoire diversity is required for relapses in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. J Immunol. 2007a;178:4865–4875. doi: 10.4049/jimmunol.178.8.4865. [DOI] [PubMed] [Google Scholar]
- Fazilleau N, Eisenbraun MD, Malherbe L, Ebright JN, Pogue-Caley RR, McHeyzer-Williams LJ, McHeyzer-Williams MG. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat Immunol. 2007b;8:753–761. doi: 10.1038/ni1472. [DOI] [PubMed] [Google Scholar]
- Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Local development of effector and memory T helper cells. Curr Opin Immunol. 2007c;19:259–267. doi: 10.1016/j.coi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell receptor binding. Nature Immunology. 2009 doi: 10.1038/ni.1704. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J Exp Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein--coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Fuller KA, Kanagawa O, Nahm MH. T cells within germinal centers are specific for the immunizing antigen. J Immunol. 1993;151:4505–4512. [PubMed] [Google Scholar]
- Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- Gaspal FM, McConnell FM, Kim MY, Gray D, Kosco-Vilbois MH, Raykundalia CR, Botto M, Lane PJ. The generation of thymus-independent germinal centers depends on CD40 but not on CD154, the T cell-derived CD40-ligand. Eur J Immunol. 2006;36:1665–1673. doi: 10.1002/eji.200535339. [DOI] [PubMed] [Google Scholar]
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R, Eibel H, Fischer B, Schaffer AA, Mages HW, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- Gulbranson-Judge A, MacLennan I. Sequential antigen-specific growth of T cells in the T zones and follicles in response to pigeon cytochrome c. Eur J Immunol. 1996;26:1830–1837. doi: 10.1002/eji.1830260825. [DOI] [PubMed] [Google Scholar]
- Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998a;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A. 1998b;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Hathcock K, Zheng B, Kepler TB, Hodes R, Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hauser AE, Junt T, Mempel TR, Sneddon MW, Kleinstein SH, Henrickson SE, von Andrian UH, Shlomchik MJ, Haberman AM. Definition of germinal-center B cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity. 2007a;26:655–667. doi: 10.1016/j.immuni.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Hauser AE, Shlomchik MJ, Haberman AM. In vivo imaging studies shed light on germinal-centre development. Nat Rev Immunol. 2007b;7:499–504. doi: 10.1038/nri2120. [DOI] [PubMed] [Google Scholar]
- Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Okazaki T, Nishimura H, Kawasaki A, Yagita H, Honjo T. Microanatomical localization of PD-1 in human tonsils. Immunol Lett. 2002;83:215–220. doi: 10.1016/s0165-2478(02)00088-3. [DOI] [PubMed] [Google Scholar]
- Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- Kallies A, Hawkins ED, Belz GT, Metcalf D, Hommel M, Corcoran LM, Hodgkin PD, Nutt SL. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat Immunol. 2006;7:466–474. doi: 10.1038/ni1321. [DOI] [PubMed] [Google Scholar]
- Kaplan MH, Boyer SN, Grusby MJ. Genomic organization of the murine CTL lipase gene. Genomics. 1996a;35:606–609. doi: 10.1006/geno.1996.0407. [DOI] [PubMed] [Google Scholar]
- Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996b;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996c;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- Katz DH, Paul WE, Goidl EA, Benacerraf B. Carrier function in anti-hapten immune responses. I. Enhancement of primary and secondary anti-hapten antibody responses by carrier preimmunization. J Exp Med. 1970;132:261–282. doi: 10.1084/jem.132.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104:1952–1960. doi: 10.1182/blood-2004-03-1206. [DOI] [PubMed] [Google Scholar]
- Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Gaspal FM, Wiggett HE, McConnell FM, Gulbranson-Judge A, Raykundalia C, Walker LS, Goodall MD, Lane PJ. CD4(+)CD3(−) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–654. doi: 10.1016/s1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009 doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Lederman S, Yellin MJ, Inghirami G, Lee JJ, Knowles DM, Chess L. Molecular interactions mediating T–B lymphocyte collaboration in human lymphoid follicles. Roles of T cell-B-cell-activating molecule (5c8 antigen) and CD40 in contact-dependent help. J Immunol. 1992;149:3817–3826. [PubMed] [Google Scholar]
- Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- Lim HW, Hillsamer P, Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Invest. 2004;114:1640–1649. doi: 10.1172/JCI22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong R, Silva D, Withers D, Anderson G, Verma NK, Brink R, Hutloff A, Goodnow CC, Vineusa CG. Roquin differentiates the specialized functions of duplicated T cell costimulatory receptor genes cd28 and Icos. Immunity Advanced On Line. 2009a doi: 10.1016/j.immuni.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong R, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Waters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J Exp Med Advanced online. 2009b doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Mak TW, Shahinian A, Yoshinaga SK, Wakeham A, Boucher LM, Pintilie M, Duncan G, Gajewska BU, Gronski M, Eriksson U, et al. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell-dependent B cell responses. Nat Immunol. 2003;4:765–772. doi: 10.1038/ni947. [DOI] [PubMed] [Google Scholar]
- Malherbe L, Filippi C, Julia V, Foucras G, Moro M, Appel H, Wucherpfennig K, Guery JC, Glaichenhaus N. Selective activation and expansion of high-affinity CD4+ T cells in resistant mice upon infection with Leishmania major. Immunity. 2000;13:771–782. doi: 10.1016/s1074-7613(00)00075-3. [DOI] [PubMed] [Google Scholar]
- Malherbe L, Hausl C, Teyton L, McHeyzer-Williams MG. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity. 2004;21:669–679. doi: 10.1016/j.immuni.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Malherbe L, Mark L, Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Vaccine adjuvants alter TCR-based selection thresholds. Immunity. 2008;28:698–709. doi: 10.1016/j.immuni.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Martins G, Calame K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol. 2008;26:133–169. doi: 10.1146/annurev.immunol.26.021607.090241. [DOI] [PubMed] [Google Scholar]
- Martins GA, Cimmino L, Shapiro-Shelef M, Szabolcs M, Herron A, Magnusdottir E, Calame K. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol. 2006;7:457–465. doi: 10.1038/ni1320. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams LJ, Panus JF, Mikszta JA, McHeyzer-Williams MG. Evolution of antigen-specific T cell receptors in vivo: preimmune and antigen-driven selection of preferred complementarity-determining region 3 (CDR3) motifs. J Exp Med. 1999;189:1823–1838. doi: 10.1084/jem.189.11.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHeyzer-Williams MG, Altman JD, Davis MM. Enumeration and characterization of memory cells in the TH compartment. Immunol Rev. 1996;150:5–21. doi: 10.1111/j.1600-065x.1996.tb00693.x. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams MG, Davis MM. Antigen-specific development of primary and memory T cells in vivo. Science. 1995;268:106–111. doi: 10.1126/science.7535476. [DOI] [PubMed] [Google Scholar]
- McLachlan JB, Catron DM, Moon JJ, Jenkins MK. Dendritic Cell Antigen Presentation Drives Simultaneous Cytokine Production by Effector and Regulatory T Cells in Inflamed Skin. Immunity. 2009 doi: 10.1016/j.immuni.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikszta JA, McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-driven selection of TCR In vivo: related TCR alpha-chains pair with diverse TCR beta-chains. J Immunol. 1999;163:5978–5988. [PubMed] [Google Scholar]
- Miller JF. Immunological function of the thymus. Lancet. 1961;2:748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- Miller JF, Mitchell GF. The thymus and the precursors of antigen reactive cells. Nature. 1967;216:659–663. doi: 10.1038/216659a0. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- Mitchison NA. The carrier effect in the secondary response to hapten-protein conjugates. I. Measurement of the effect with transferred cells and objections to the local environment hypothesis. Eur J Immunol. 1971;1:10–17. doi: 10.1002/eji.1830010103. [DOI] [PubMed] [Google Scholar]
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal GJV. Antibody production by single cells. III. The histology of antibody production. Br J Exp Pathol. 1959;40:301–311. [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard JM, Marks BR, DiPlacido LD, Pohelek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. The Journal of Experimental Medicine. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl L, Henning G, Krautwald S, Lipp M, Hardtke S, Bernhardt G, Pabst O, Forster R. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J Exp Med. 2003;197:1199–1204. doi: 10.1084/jem.20030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O’Garra A, Cahalan MD, Cyster JG. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol 3, e150. 2005 doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onizuka T, Moriyama M, Yamochi T, Kuroda T, Kazama A, Kanazawa N, Sato K, Kato T, Ota H, Mori S. BCL-6 gene product, a 92- to 98-kD nuclear phosphoprotein, is highly expressed in germinal center B cells and their neoplastic counterparts. Blood. 1995;86:28–37. [PubMed] [Google Scholar]
- Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci U S A. 2000;97:11439–11444. doi: 10.1073/pnas.200360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- Panus JF, McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific T helper cell function: differential cytokine expression in primary and memory responses. J Exp Med. 2000;192:1301–1316. doi: 10.1084/jem.192.9.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape KA, Kouskoff V, Nemazee D, Tang HL, Cyster JG, Tze LE, Hippen KL, Behrens TW, Jenkins MK. Visualization of the genesis and fate of isotype-switched B cells during a primary immune response. J Exp Med. 2003;197:1677–1687. doi: 10.1084/jem.20012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- Paul WE, Siskind GW, Benacerraf B. Studies on the effect of the carrier molecule on antihapten antibody synthesis. II. Carrier specificity of anti-2,4-dinitrophenyl-poly-l-lysine antibodies. J Exp Med. 1966;123:689–705. doi: 10.1084/jem.123.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T–B cell interactions underlie germinal center formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Feldmann M, De Petris S. Monospecificity of bone marrow-derived lymphocytes. J Exp Med. 1973;137:1024–1030. doi: 10.1084/jem.137.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K, Schirrmacher V, Nase S, Jerne NK. The requirement of more than one antigenic determinant for immunogenicity. J Exp Med. 1969;129:1131–1143. doi: 10.1084/jem.129.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- Ree HJ, Kadin ME, Kikuchi M, Ko YH, Suzumiya J, Go JH. Bcl-6 expression in reactive follicular hyperplasia, follicular lymphoma, and angioimmunoblastic T-cell lymphoma with hyperplastic germinal centers: heterogeneity of intrafollicular T-cells and their altered distribution in the pathogenesis of angioimmunoblastic T-cell lymphoma. Hum Pathol. 1999;30:403–411. doi: 10.1016/s0046-8177(99)90115-6. [DOI] [PubMed] [Google Scholar]
- Rees W, Bender J, Teague TK, Kedl RM, Crawford F, Marrack P, Kappler J. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc Natl Acad Sci U S A. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, Cyster JG. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–99. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg PL, Mueller KL, Qi H, Cannons JL. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat Rev Immunol. 2009;9:39–46. doi: 10.1038/nri2456. [DOI] [PubMed] [Google Scholar]
- Schwickert TA, Lindquist RL, Shakar G, Livshits G, Skokos D, Kosco-Vilbois MH, Dustin ML, Nussenzweig MC. In vivo imaging of germinal center reveals a dynamic open structure. Nature. 2007;XX:XX. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- Secord EA, Rizzo LV, Barroso EW, Umetsu DT, Thorbecke GJ, DeKruyff RH. Reconstitution of germinal center formation in nude mice with Th1 and Th2 clones. Cell Immunol. 1996;174:173–179. doi: 10.1006/cimm.1996.0307. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, Dustin ML. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–714. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Brewer JM, Rush CM, Riley J, Garside P. In vivo generated Th1 cells can migrate to B cell follicles to support B cell responses. J Immunol. 2004;173:1640–1646. doi: 10.4049/jimmunol.173.3.1640. [DOI] [PubMed] [Google Scholar]
- Smith KM, Pottage L, Thomas ER, Leishman AJ, Doig TN, Xu D, Liew FY, Garside P. Th1 and Th2 CD4+ T cells provide help for B cell clonal expansion and antibody synthesis in a similar manner in vivo. J Immunol. 2000;165:3136–3144. doi: 10.4049/jimmunol.165.6.3136. [DOI] [PubMed] [Google Scholar]
- Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- Stetson DB, Mohrs M, Mallet-Designe V, Teyton L, Locksley RM. Rapid expansion and IL-4 expression by Leishmania-specific naive helper T cells in vivo. Immunity. 2002;17:191–200. doi: 10.1016/s1074-7613(02)00363-1. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- Talmage DW. Allergy and immunology. Annu Rev Med. 1957;8:239–256. doi: 10.1146/annurev.me.08.020157.001323. [DOI] [PubMed] [Google Scholar]
- Tangye SG, Avery DT, Hodgkin PD. A division-linked mechanism for the rapid generation of Ig-secreting cells from human memory B cells. J Immunol. 2003;170:261–269. doi: 10.4049/jimmunol.170.1.261. [DOI] [PubMed] [Google Scholar]
- Toellner KM, Jenkinson WE, Taylor DR, Khan M, Sze DM, Sansom DM, Vinuesa CG, MacLennan IC. Low-level hypermutation in T cell-independent germinal centers compared with high mutation rates associated with T cell-dependent germinal centers. J Exp Med. 2002;195:383–389. doi: 10.1084/jem.20011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toellner KM, Luther SA, Sze DM, Choy RK, Taylor DR, MacLennan IC, Acha-Orbea H. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med. 1998;187:1193–1204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Jr, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Veillette A, Zhang S, Shi X, Dong Z, Davidson D, Zhong MC. SAP expression in T cells, not in B cells, is required for humoral immunity. Proc Natl Acad Sci U S A. 2008;105:1273–1278. doi: 10.1073/pnas.0710698105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005a;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005b;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Walker LS, Gulbranson-Judge A, Flynn S, Brocker T, Raykundalia C, Goodall M, Forster R, Lipp M, Lane P. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, de Waard R, Leung C, Nouri-Shirazi M, Orazi A, Chaganti RS, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet. 1997;16:161–170. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
- Yu D, Cook MC, Shin DM, Silva DG, Marshall J, Toellner KM, Havran WL, Caroni P, Cooke MP, Morse HC, et al. Axon growth and guidance genes identify T-dependent germinal centre B cells. Immunol Cell Biol. 2008;86:3–14. doi: 10.1038/sj.icb.7100123. [DOI] [PubMed] [Google Scholar]
- Yu D, Tan AH, Hu X, Athanasopoulos V, Simpson N, Silva DG, Hutloff A, Giles KM, Leedman PJ, Lam KP, et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature. 2007;450:299–303. doi: 10.1038/nature06253. [DOI] [PubMed] [Google Scholar]
- Zheng B, Han S, Kelsoe G. T helper cells in murine germinal centers are antigen-specific emigrants that downregulate Thy-1. J Exp Med. 1996;184:1083–1091. doi: 10.1084/jem.184.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]