Abstract
Peptide neurotransmitters and peptide hormones, collectively known as neuropeptides, are required for cell-cell communication in neurotransmission and for regulation of endocrine functions. Neuropeptides are synthesized from protein precursors (termed proneuropeptides or prohormones) that require proteolytic processing primarily within secretory vesicles that store and secrete the mature neuropeptides to control target cellular and organ systems. This review describes interdisciplinary strategies that have elucidated two primary protease pathways for prohormone processing consisting of the cysteine protease pathway mediated by secretory vesicle cathepsin L and the well-known subtilisin-like proprotein convertase pathway that together support neuropeptide biosynthesis. Importantly, this review discusses important areas of current and future biomedical neuropeptide research with respect to biological regulation, inhibitors, structural features of proneuropeptide and protease interactions, and peptidomics combined with proteomics for systems biological approaches. Future studies that gain in-depth understanding of protease mechanisms for generating active neuropeptides will be instrumental for translational research to develop pharmacological strategies for regulation of neuropeptide functions. Pharmacological applications for neuropeptide research may provide valuable therapeutics in health and disease.
Keywords: proneuropeptides, prohormones, protease, cathepsin L, subtilisin-like proprotein convertases, peptide neurotransmitter, peptide hormones, secretory vesicles, peptide neurochemistry
INTRODUCTION
Neuropeptides for cell-cell communication in nervous and endocrine systems
Neuropeptides mediate neurotransmission as peptide neurotransmitters, and mediate cell-cell communication as peptide hormones for endocrine regulation of target cellular systems. The term ‘neuropeptides’ refers to this large, diverse class of peptide neurotransmitters and peptide hormones that typically consist of 3-40 residues. There are more than a hundred different neuropeptides, and new neuropeptides are yet to be discovered.
The unique primary sequence of each neuropeptide defines its selective and potent biological actions. The same neuropeptides often serve important functions in both the nervous system as neurotransmitters (figure 1) and as peptide hormones in peripheral endocrine systems (figure 2). For example, enkephalins function as neurotransmitters in brain and also are involved in peripheral actions including regulation of intestinal motility and immune cell functions (1-3). ACTH (adrenocorticotropin hormone) is present in brain where it functions as a neuromodulator; furthermore, ACTH is a prominent peptide hormone released from the pituitary gland for control of glucocorticoid production in the adrenal cortex (4, 5). Neuropeptides such as ß-endorphin, NPY (neuropeptide Y), galanin, CRF (corticotropin releasing factor), vasopressin, insulin, and numerous others (Table 1) mediate diverse physiological functions that include analgesia, feeding behavior and blood pressure regulation, cognition, stress, water balance, and glucose metabolism, respectively (6-14).
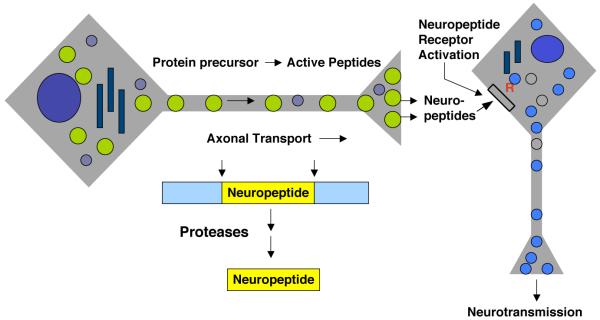
Figure 1. Peptide neurotransmitters in brain.
Neuropeptides in brain function as peptide neurotransmitters to mediate chemical communications among neurons. Neuropeptides are synthesized within secretory vesicles that are transported from the neuronal cell body via the axon to nerve terminals. The proneuropeptide (or prohormone) is packaged with the newly formed secretory vesicle in the cell body, and proteolytic processing of the precursor protein occurs during axonal transport and maturation of the secretory vesicle. Mature processed neuropeptides are contained within secretory vesicles at the synapse where activity-dependent, regulated secretion of neuropeptides occurs to mediate neurotransmission via neuropeptide activation of peptidergic receptors.
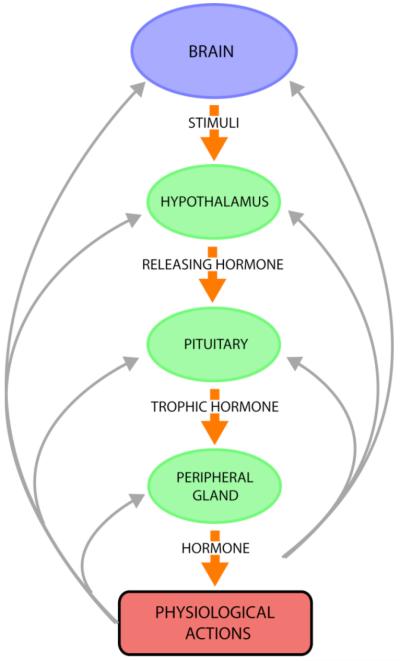
Figure 2. Peptide hormones in endocrine systems.
Neuropeptides function as peptide hormones to mediate cell-cell communication in peripheral endocrine systems. For example, the hypothalamo-neurohypophyseal system regulates the pituitary-adrenal axis by secretion CRF from the hypothalamus region of brain to induce secretion of the peptide hormone ACTH from the pituitary. Released ACTH targets the adrenal cortex for stimulation of glucocorticoid production; resultant increases in plasma glucocorticoid participates in feedback inhibition of CRF and ACTH to maintain constant levels of glucocorticoid. Numerous peptide hormones regulate physiological functions.
Table 1. Neuropeptides in the Nervous and Endocrine Systems.
| Neuropeptide |
Regulatory Function |
|---|---|
| enkephalin | analgesia, pain relief |
| beta-endorphin | analgesia, pain relief |
| ACTH | steroid production |
| α-MSH | skin pigmentation, appetite |
| CRF (corticotropin releasing factor) | ACTH secretion |
| insulin | glucose metabolism |
| glucagon | glucose metabolism |
| galanin | cognition |
| NPY | obesity, blood pressure |
| somatostatin | growth regulation |
| vasopressin | water balance |
| calcitonin | calcium regulation |
| cholecystokinin | learning, memory, appetite |
| PACAP | neuronal differentiation |
Peptide neurotransmitters and hormones are collectively termed neuropeptides. There are more than 100 neuropeptides, and many have yet to be identified. Neuropeptides typically consist of small peptides of approximately 3-40 residues. Examples of several neuropeptides and their regulatory functions are listed. Abbreviations are adrenocorticotropin hormone (ACTH), α-melanocyte stimulating hormone (α-MSH), and neuropeptide Y (NPY).
Elucidation of protease pathways in consideration of neuropeptide therapeutic strategies
Regulation of the actions of each neuropeptide will be desirable for modifying the specific biological functions of selected neuropeptides for potential therapeutic applications. Investigation of proteases that convert inactive protein precursors into active neuropeptides may lead to novel protease-targeted approaches for regulation of neuropeptide biosynthesis and function. This review will describe successful strategies utilized for identification of two primary protease pathways for neuropeptide production, and will address necessary areas of current and future research to explore properties of protease mechanisms that may provide specific drug targets for future therapeutic control of neuropeptide production and functions.
Proteolytic Processing is Required for the Biosynthesis of Neuropeptides
Proneuropeptide (prohormone) precursors of neuropeptides
Neuropeptides are derived from larger protein precursors known as proneuropeptides or prohormones. Proneuropeptides refers to protein precursors of peptide neurotransmitters as well as peptide hormones, while prohormones refer primarily to endocrine peptide hormone precursors. To encompass peptide functions in both the nervous and endocrine systems, the terminology of ‘neuropeptide’ and the respective ‘proneuropeptides’ will be utilized in this article to refer to ‘neuroendocrine’ functions of neuropeptides.
Proneuropeptide precursors share distinct and common features. Notably, the small active form of each neuropeptide is a segment present within its full-length precursor protein. A proneuropeptide may contain one copy of the active neuropeptide, as represented by proNPY, progalanin, and provasopressin (figure 3) (15-17). Alternatively, a precursor may contain multiple related copies of the active neuropeptide. For example, proenkephalin contains four copies of (Met)enkephalin, one copy of the related (Leu)enkephalin, and one copy each of the ME-Arg-Gly-Leu and ME-Arg-Phe (figure 3) (18, 19). Furthermore, one precursor may undergo tissue-specific processing to generate distinct neuropeptides in different tissue regions. For example, the POMC precursor (proopiomelanocortin) generates ACTH in anterior pituitary, but is converted to α-MSH (α-melanocyte-stimulating hormone) and ß-endorphin in the intermediate lobe of pituitary (20, 21). Proteolysis of these precursors, especially tissue-specific proteolytic mechanisms, is required for biologically active neuropeptides to be generated.
Figure 3. Proneuropeptides: structural features for proteolytic processing.
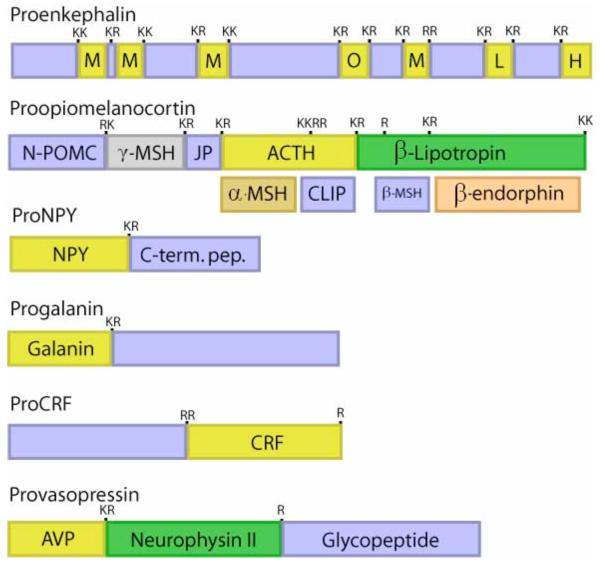
Neuropeptides are synthesized as proneuropeptide precursors, also known as prohormones, that require proteolytic processing to liberate the active neuropeptide. Proteolytic processing occurs at dibasic and monobasic sites, as well as at multibasic sites. The precursor proteins may contain one copy of the active neuropeptide, such as the proneuropeptides for NPY, galanin, CRF, and vasopressin. Some proneuropeptides such as proenkephalin contains multiple copies of the active neuropeptide; proenkephalin contains four copies of (Met)enkephalin (ME), one copy of (Leu)enkephalin (LE), and the related opioid peptides ME-Arg-Phe (H) and ME-Arg-Gly-Cleu (O). Certain precursors contain different peptide hormones within the same precursor, such as the POMC precursor which gives rise to the distinct peptide hormones ACTH, α-MSH, and ß-endorphin. The presence of ACTH in anterior pituitary, and the presence of α-MSH and ß-endorphin in intermediate pituitary illustrate that tissue-specific processing of the POMC prohormone occurs.
While each proneuropeptide precursor possesses a distinct primary sequence, proteolytic processing occurs at dibasic residue sites that commonly flank the NH2- and COOH-termini of neuropeptides within their precursors (figure 3). The dibasic residues Lys-Arg (KR) most often flank the neuropeptides; however, the dibasic sites Lys-Lys, Arg-Arg, and sometimes Arg-Lys also occur. Processing sometimes occurs at monobasic Arg sites, such as that in provasopressin, prosomatostatin, and other proneuropeptides. Or, processing at multi-basic residue sites may occur, such as at the tetrabasic residue processing site within the ACTH segment of POMC that leads to production of α-MSH. Processing at non-basic residues occurs occasionally, but this article will focus on proteolytic mechanisms for processing proneuropeptides and prohormones at basic residue sites. Overall, proteolytic processing is a key process required for the biosynthesis of numerous active neuropeptides from inactive precursors.
Proteolytic processing of proneuropeptides
Biosynthesis of neuropeptides begins with translation of the respective mRNAs to generate the preproneuropeptide or preprohormone precursors. Proteolytic processing begins cotranslationally at the rough endoplasmic reticulum (RER) where the NH2-terminal signal peptide of the preproneuropeptide is cleaved by signal peptidase. The resulting proneuropeptide or prohormone is routed through the Golgi apparatus and is packaged into newly formed secretory vesicles together with processing proteases. As the secretory vesicle matures, proteolytic processing occurs so that the mature secretory vesicle contains processed, biologically active neuropeptide that awaits cellular stimuli for regulated secretion.
Proteolytic processing at the dibasic or monobasic sites of proneuropeptides occurs primarily within regulated secretory vesicles (22-28). Cleavage at the COOH-terminal side of the paired basic residues results in peptide intermediates with basic residue extensions on their COOH-termini, which must then be removed by Lys/Arg carboxypeptidase to generate the mature neuropeptide. Alternatively, cleavage of the precursor at the NH2-terminal side of dibasic residue sites will generate peptide intermediates with basic residue extensions on their NH2-termini, which will then be removed by Arg/Lys aminopeptidase to generate the active neuropeptide. Processing may also occur between the dibasic residues, which will then require both carboxypeptidase and aminopeptidase exopeptidase activities to generate the final neuropeptides.
Neuropeptides may also undergo posttranslational modification that modifies the biological activities of peptides. Activities of the neuropeptides may be altered by disulfide bond formation, glycosylation, COOH-terminal α-amidation, phosphorylaton, sulfation, and acetylation (12, 29-34). This article, however, will focus on protease mechanisms for neuropeptide biosynthesis.
Criteria for identification of proteases for proneuropeptide processing
Elucidation of proteases in brain and neuroendocrine tissues is complicated due to the many different cell types and presence of proteases in many subcellular compartments of these cells. To insure that the main proteases are identified for producing an active peptide, the neuropeptide field has utilized key criteria for successful elucidation of proteases that generate peptide neurotransmitters and hormones. These criteria are (1) the processing protease must be present in the organelle site where production of the active peptide occurs, primarily in secretory vesicles, (2) the protease must possess the appropriate substrate cleavage specificity to generate the active peptide, and (3) protease inhibition or gene knockdown should reduce production of the active peptide. Application of these criteria has led to elucidation of the recently identified cysteine protease pathway and serine protease pathways, mediated by cathepsin L and proprotein convertases, respectively, for neuropeptide production.
Cysteine and Serine Protease Pathways for Processing Proneuropeptides (Prohormones)
Investigation for proteases that cleave at dibasic, as well as monobasic, processing sites within proneuropeptides have yielded identification of (1) the newly discovered cysteine protease pathway for processing the proenkephalin (as well as other proneuropeptides), consisting of cathepsin L followed by Arg/Lys aminopeptidase (aminopeptidase B), and (2) the well-established proprotein convertase (PC) family of subtilisin-like proteases that process proneuropeptides, prohormones, and other proproteins followed by the Arg/Lys carboxypeptidase step (carboxypeptidase ‘E’ or ‘H’) (figure 4). Elucidation of these two protease pathways resulted from application of the criteria required of processing proteases by ‘biochemical’ and ‘molecular’ approaches, respectively. With the new role for secretory vesicle cathepsin L in prohormone processing, discussion of these dual protease pathways for coordinate regulation of neuropeptide biosynthesis will be included.
Figure 4. Cysteine and subtilisin-like protease pathways for proneuropeptide processing.
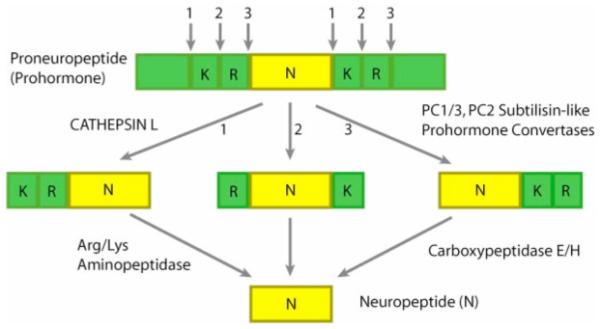
Distinct cysteine protease and subtilisin-like protease pathways have been demonstrated for proneuropeptide processing. Recent studies have identified secretory vesicle cathepsin L as an important processing enzyme for the production of the endogenous enkephalin opioid peptide. Preference of cathepsin L to cleave at the NH2-terminal side of dibasic residue processing sites yields peptide intermediates with NH2-terminal residues, which are removed by Arg/Lys aminopeptidase. The well-established subtilisin-like protease pathway involves several prohormone convertases (PC). PC1/3 and PC2 have been characterized as neuroendocrine processing proteases (processing in neuroendocrine tissues also involves PC5 (135)). The PC enzymes preferentially cleave at the COOH-terminal side of dibasic processing sites, which results in peptide intermediates with basic residue extensions at their COOH-termini that are removed by carboxypeptidase E/H.
Identification of neuropeptide protease pathway components in chromaffin granules, a model neuropeptide-containing secretory vesicle system
Chromaffin granules: model neurosecretory vesicles for proneuropeptide processing proteases
Elucidation of protease pathways for neuropeptide biosynthesis has been facilitated in the field with use of isolated chromaffin granules, a well-established model neurosecretory vesicle system utilized for investigation of enzymes that synthesize neuropeptides and small molecule neurotransmitters that function in brain and neuroendocrine tissues (35-37). These chromaffin granules were utilized to identify the primary proenkephalin-cleaving prohormone processing activity as cathepsin L using biochemistry and chemical biology approaches, as well as for studies of native in vivo prohormone convertases (38-41). Chromaffin granules contain proneuropeptide precursors that undergo proteolytic processing to generate several neuropeptides which include enkephalin, NPY, galanin, somatostatin, VIP, and others (38, 39, 42-44). An important advantage of using chromaffin granules is that they can be purified as a homogeneous preparation of secretory vesicles in high yield from adrenal medullary chromaffin cells (bovine), thus allowing purification of enzyme protein in adequate amounts for characterization and identification by mass spectrometry. Chromaffin granules represent a key model system for elucidating protease components of both the cathepsin L and proprotein convertase pathways for neuropeptide biosynthesis in neuronal and endocrine tissues.
Cathepsin L in secretory vesicles for proenkephalin and proneuropeptide processing identified by activity-based profiling
The major proenkephalin (PE) processing activity in chromaffin granules was found to consist of the ‘prohormone thiol protease’ complex (PTP) (38, 39). Using full-length recombinant enkephalin precursor as substrate, purification of PE-cleaving activity led to isolation of the high molecular weight PTP complex of approximately 180-200 kDa (39). The apparent molecular weight of PTP suggested the presence of several protein subunits, since proteases typically possess lower molecular masses than that of native PTP. PTP activity belonged to the cysteine protease family, based on its sensitivity to inhibition by cysteine protease inhibitors (39). Studies were then targeted to identify the catalytic subunit of PTP responsible for PE-cleaving activity.
Activity-based profiling of active cysteine proteases was instrumental for identification of the protease responsible for PE processing in chromaffin granules. The activity probe DCG-04, the biotinylated form of E64c that inhibits cysteine proteases, was utilized for specific affinity labeling of the 27 kDa protease enzyme of the PTP complex (28, 40). Two-dimensional gels resolved three primary DCG-04 labeled proteins of 27-29 kDa (figure 5), whose identification was indicated as cathepsin L by mass spectrometry of tryptic peptides. These findings suggested a new biological function for cathepsin L in secretory vesicles for producing the enkephalin neuropeptide. The secretory vesicle function of cathepsin L contrasts with the well known lysosomal function of cathepsin L for degradation of proteins.
Figure 5. Activity-based profiling for identification of proenkephalin cleaving activity as cathepsin L.

Activity-based profiling (APB) utilizes the strategy of labeling the active site of active proteases, often with an inhibitor-related probe, to identify proteolytic activity. Inhibition of proenkephalin cleaving activity by the cysteine protease inhibitor E64c in isolated chromaffin secretory vesicles (also known as chromaffin granules) allowed affinity labeling of the 27 kDa active protease enzyme proteins by a biotinylated form of E64 known as DCG-04 (panel a). The inhibitor-labeled proteins were separated by 2-D gels (panel b) and subjected to peptide sequencing by mass spectrometry, revealing the identity of the proneuropeptide processing activity as cathepsin L.
Expression of cathepsin L in secretory vesicles for enkephalin neuropeptide production
The criteria for colocalization with neuropeptides, appropriate cleavage specificity, and inhibition or gene knockdown were evaluated for cathepsin L as a proneuropeptide processing enzyme. Confirmation of the localization of cathepsin L within secretory vesicles (chromaffin granules) was achieved by immunoelectron microscopy (figure 6), and by colocalization with enkephalin and NPY neuropeptides in neuroendocrine chromaffin cells by fluorescence immunohistochemistry (figure 6). Cathepsin L was also found to undergo cosecretion with enkephalin whose secretion is stimulated by activation of the regulated secretory pathway in these cells (40).
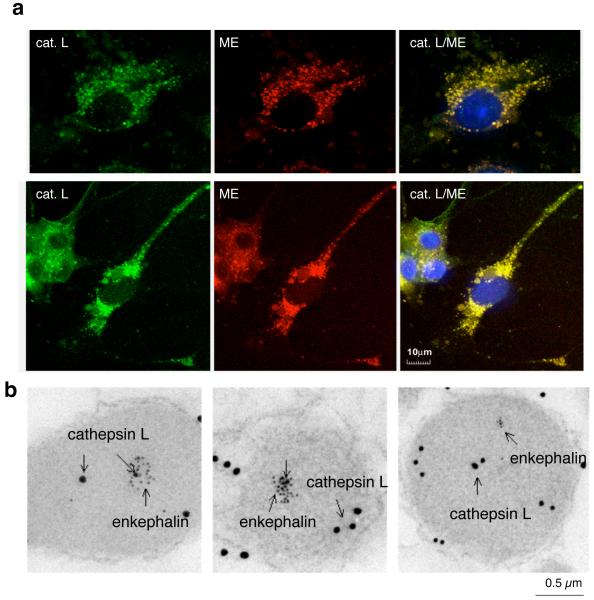
Figure 6. Localization of cathepsin L to neuropeptide-containing secretory vesicles.
(a) Colocalization of cathepsin L with enkephalin in chromaffin cells demonstrated by confocal immunofluorescence microscopy. Cathepsin L and (Met)enkephalin (green and red fluorescence, respectively) in chromaffin cells were visualized by immunofluorescence confocal microscopy. Excellent colocalization of cathepsin L and (Met)enkephalin was demonstrated by the merged images with colocalization indicated by yellow fluorescence. In chromaffin cells, the majority of cathepsin L is colocalized with (Met)enkephalin within secretory vesicles.
(b) Immunoelectron microscopy demonstrates colocalization of cathepsin L with the (Met)enkephalin neuropeptide in secretory vesicles. Cathepsin L localization was indicated by labeling with 15 nm colloidal gold-conjugated anti-rabbit, and (Met)enkephalin (ME) was detected as 6 nm gold particles conjugated to anti-mouse. The presence of both 15- and 6-nm cold particles within these vesicles demonstrated the colocalization of cathepsin L with the enkephalin neuropeptide in secretory vesicles.
Cellular routing and trafficking cathepsin L gene expression was demonstrated by coexpression of cathepsin L with proenkephalin in neuroendocrine PC12 cells (derived from rat adrenal medulla) (45). Expression of cathepsin L resulted in its trafficking to secretory vesicles that contain enkephalin and chromogranin A. Furthermore, cathepsin L expression resulted in cellular processing of proenkephalin into (Met)enkephalin that undergoes regulated secretion from PC12 cells. Cathepsin L generated high molecular weight PE-derived intermediates (of about 23, 18-19, 8-9, and 4.5 kDa) that were identical to those in vivo in chromaffin granules assessed by western blots (38). Such results demonstrated a cellular role for cathepsin L in the production of (Met)enkephalin in secretory vesicles for its regulated secretion.
Aminopeptidase B with cathepsin L for proneuropeptide processing
Studies of the cleavage specificity of cathepsin L demonstrated that this cysteine protease prefers to cleave on the NH2-terminal side of dibasic residue processing sites of enkephalin-containing peptide substrates BAM-22P and Peptide F (40), and as illustrated in figure 4. The cleavage specificity of cathepsin L results in enkephalin intermediate peptides with NH2-terminal basic residue extensions, which then require removal by Arg/Lys aminopeptidase activity. Secretory vesicles from adrenal medullary chromaffin cells (46) and from pituitary (47) contain Arg/Lys aminopeptidase activity for neuropeptide production.
Recent molecular cloning studies have identified aminopeptidase B in chromaffin secretory vesicles as an appropriate Arg/Lys aminopeptidase (48). Molecular cloning of the bovine aminopeptidase B (AP-B) cDNA defined its primary sequence that provided production of specific antisera to demonstrate localization of AP-B in secretory vesicles that contain cathepsin L with the neuropeptides enkephalin and NPY. The AP-B in neuropeptide-containin chromaffin secretory vesicles was demonstrated by immunoelectron microscopy. AP-B was also found in several neuroendocrine tissues by western blots. Recombinant bovine AP-B (48) and rat AP-B (49) were compared. Recombinant bovine AP-B showed preference for Arg-MCA substrate compared to Lys-MCA. AP-B was inhibited by arphamenine, an inhibitor of aminopeptidases. Bovine AP-B showed similar activities for Arg-(Met)enkephalin and Lys-(Met)enkephalin neuropeptide substrates to generate (Met)enkephalin, while rat AP-B preferred Arg-(Met)enkephalin. Furthermore, AP-B possesses an acidic pH optimum of 5.5-6.5 that is similar to the internal pH of secretory vesicles. The significant finding of the secretory vesicle localization of AP-B with neuropeptides and cathepsin L suggests a role for this exopeptidase in the biosynthesis of neuropeptides.
Molecular biological approaches for elucidation of the proprotein convertases
Proprotein convertase family of processing enzymes
The mammalian family of proprotein convertases that resemble yeast KEX2 gene and bacterial subtilisin was identified by molecular homology cloning based on predicted similarities of yeast kex2 with mammalian prohormone processing enzymes (22-27). The yeast KEX2 gene product is a Ca2+-dependent subtilisin-like serine protease that is required for processing the yeast pro-α-mating factor at paired basic residues (50). Homology cloning led to elucidation of the proprotein convertase family whose members consist of PC1/3, PC2, furin, PACE4, PC3, PC5/6, and PC7 for processing at basic residues (23-27). More recently, several related proteases that cleave at non-basic residues have been identified, consisting of the subtilisin/kexin-like isozyme-1 (SKI-1/SIP) and the neural apoptosis-regulated convertase-1 (PCSK9/NARC-1) (25-27). Several excellent reviews of the proprotein convertases have outlined research progress in this field (22-28, 50, 51). This limited review will more on the roles of neuroendocrine-specific proprotein convertases, consisting primarily of PC1/3 and PC2 for production of neuropeptides.
Among the PC members, PC1/3 and PC2 are most relevant to neuropeptide biosynthesis since PC1/3 and PC2 expression are primarily restricted to neuroendocrine tissues (23-27). Furthermore, the appropriate localization of PC1/3 and PC2 within secretory vesicles is consistent with their roles in proneuropeptide processing. Endogenous PC1/3 and PC2 activities have been characterized within secretory vesicles of pancreas (23), pituitary (52), and adrenal medulla (41) for processing proinsulin, POMC, and proenkephalin. PC1/3 and PC2 are known to be present in numerous neuroendocrine tissues that generate neuropeptides. Other members of the PC family (furin, PACE4, PC5/6, and others) show a more ubiquitous pattern of tissue distribution.
PC1/3 and PC2 endoproteases with the carboxypeptidase E exopeptidase for neuropeptide biosynthesis
Cleavage specificity studies indicate the distinct property for PC enzymes to cleave at the COOH-terminal side of paired basic residue processing sites within proneuropeptides and prohormones (figure 4). This cleavage specificity differs from cathepsin L which cleaves at the NH2-terminal side of such dibasic residue processing sites (40, 53, 54). PC processing yields neuropeptide intermediates with COOH-terminal basic residues (Arg or Lys) that are removed by the neuroendocrine-specific carboxypeptidase E (also known as carboxypeptidase H, and hence CPE/H) (23-28, 55). The PC subtilisin-like convertases combined with carboxypeptidase E represent an important protease pathway for the conversion of proneuropeptides in active peptide neurotransmitters and hormones.
Coordinate regulation of dual protease pathways in neuropeptide biosynthesis
The presence the distinct cathepsin L and proprotein convertase pathways for neuropeptide biosynthesis leads to the question of how may they be coordinately regulated? It will be of interest to investigate whether cells may switch from pathway to another under different conditions. Furthermore, what is the extent of participation of each pathway during increased or decreased production of a particular neuropeptide under different physiological or disease conditions? Future research that addresses these questions will be important to understand cellular mechanisms utilized in the control of these protease pathways.
Protease Knockout Mice for Evaluation of Proteases in Neuropeptide Production
Protease gene knockout studies in mice have indicated the biological roles of PC1/3, PC2, CPE/H, and cathepsin L proteases in neuropeptide production. Generally, selective effects on certain neuropeptides have been observed.
PC2 and PC1/3 deficient mice
Extensive comparisons of multiple neuropeptides in different tissues of PC2 null mice demonstrated the selective effects of PC2 (56-65). Among many neuropeptides examined (56-65), α-MSH (derived from POMC) was nearly absent in PC2 deficient mice (56). Results of neuropeptide studies in PC2 null mice revealed three characteristic types of findings. Firstly, while the tissue levels of several neuropeptides were altered in PC2 null mice, not all neuropeptides were modified, which indicated that some but not all neuropeptides were influenced by the absence of PC2 (57). Secondly, certain neuropeptides displayed tissue-specific differences in PC2 null mice (57). For example, NPY was decreased in ileum of PC2 null mice, but NPY in hypothalamus of brain was not altered. Thirdly, a particular tissue region often showed selective alterations among different neuropeptides. For example, the neuropeptide-rich hypothalamus region showed decreased (Met)enkephalin in PC2 deficient mice, but NPY, VIP, galanin, and CRF were not altered. These findings demonstrated the selective role of PC2 in the production of brain neuropeptides.
In peripheral endocrine organs, α-MSH in pituitary is nearly obliterated in the PC2 null mice (57); elevation of ACTH indicated its role as a substrate for PC2 in the production of α-MSH. Furthermore, pituitaries of PC2 null mice show increased levels of ß-endorphin1-31, which has been shown to be utilized as a PC2 substrate for the production of ß-endorphin1-27 (58).
Multiple peptide hormones involved in glucose metabolism utilize PC2 for their biosynthesis. For example, proinsulin processing is incomplete in PC2 null mice (59, 60). Furthermore, defective processing of proglucagon occurs in the PC2 deficient mice (64). Interestingly, impaired processing of proislet amyloid polypeptide by PC2 in pancreas leads to amyloid formation and cell death related to diabetes (66).
The PC1/3 enzyme is involved in neuropeptide production, often in conjunction with PC2. PC1/3 deficient mice show selective reduction of insulin (67, 68). PC1/3 null mice also show defects in processing proGHRH (pro-growth hormone releasing hormone) and POMC (67-69). The orexigenic hormone ghrelin is generated from its precursor by PC1/3, demonstrated from studies in the PC1/3 null mice (65). Interestingly, levels of peptides derived from POMC did not show substantial changes in the PC1/3 deficient mice (69).
These examples of neuropeptide studies in PC2 and PC1/3 deficient mice demonstrate the established roles of these neuroendocrine proprotein convertases in the production of peptide neurotransmitters and hormones. Further discussion of more recent peptidomic studies in these protease gene knockout mice are included in the section on ‘peptidomics’ of this article.
Cathepsin L deficient mice
Cathepsin L deficient mice show decreased levels of the enkephalin neuropeptide in brain that are reduced by approximately one-half (40). In addition, enkephalin brain levels in are also reduced by about one-half in PC2 deficient mice (57). These results support dual roles for both cathepsin L and PC2 in enkephalin production. Ongoing studies indicate multiple decreases in brain and endocrine neuropeptides in cathepsin L knockout mice (Hook et al., unpublished observations). With the alteration in brain neuropeptides, it will be of interest in future studies to assess the behavioral effects of the loss of neuropeptides in cathepsin L knockout mice. Cathepsin L knockout mice are viable and show phenotypes of hair loss and cardiac myopathy (70-72). The mechanism for these functional effects of cathepsin L deficiency could possibly involve neuropeptides. New and continued investigations of neuropeptides in cathepsin L knockout mice will provide knowledge of the relative roles of cathepsin L in the production of particular neuropeptides.
Carboxypeptidase E (CPE) deficient mice
The exopeptidase carboxypeptidase E has been examined in vivo as an inactive mutant CPE of the Cpefat/fat mice (73, 74) and in CPE gene knockout mice (75). Analyses of peptides representing CPE substrates demonstrated their accumulation in the Cpefat/fat mice. Notably, the fat/fat mice show the phenotype of obesity, indicating participation of neuropeptides in the fat/fat condition. Furthermore, knockout of the CPE gene results in endocrinological behavioral deficits, including more food intake, elevation of plasma glucose during fasting, and insulin resistance (75). Such findings implicate CPE in physiological roles of prohormone processing in physiological conditions.
Inhibitors and Modulators of Processing Enzymes
Endogenous and exogenous inhibitors for protease components involved in proneuropeptide and prohormone processing are highly desirable for investigating the regulation of the proteolytic steps in neuropeptide biosynthesis. Proteases in biological systems are typically regulated by endogenous protease inhibitors, as well as positive modulators of protease activities. Furthermore, exogenous inhibitors (often as small drug molecules) are important for investigating protease mechanisms and for therapeutic applications in health and disease. Future progress for development of specific inhibitors of processing proteases will be key for potential therapeutic applications.
The regulation of PC1/3 and PC2 is achieved by multiple in vivo factors including inhibitors and modulators, as well as by pH and calcium-dependence. Endogenous regulation of PC1/3 involves the propeptide of PC1/3 and inhibition by proSAAS-derived peptide. The propeptide segment inhibits PC1/3, as well as PC2 and furin (76, 77). Furthermore, PC1/3 is inhibited by proSAAS-related peptides (78). Notably, the 7B2-CT peptide inhibits the PC2 enzyme (79); furthermore, 7B2 itself is utilized for PC2 maturation and activation (80, 81).
Cathepsin L in neuropeptide-containing secretory vesicles of adrenal medullary chromaffin cells (bovine) has been found to be colocalized with an endogenous serpin protease inhibitor known as endopin 2C (82, 83). Endopin 2C displays excellent selectivity and potency for inhibition of cathepsin L compared to other cysteine cathepsins. Endopin 2C is also present in pituitary for regulation of cathepsin L. A related isoform endopin 1 is also present in chromaffin secretory vesicles (84, 85). Endopin 1 does not inhibit cathepsin L, but inhibits trypsin-like proteases cleaving at basic residues. Interestingly, endopin 1 does not inhibit PC1/3 or PC2. Effective inhibition of cathepsin L by endopin 2C will be useful for assessing neuropeptide biosynthesis achieved by cathepsin L.
Exogenous inhibitors as regulators of processing enzymes have been under much investigation (86-96), but few selective inhibitors exist. Specific active-site directed inhibitors of processing enzymes are desirable for mechanistic studies, but such inhibitors are unlikely to provide selective regulation for processing specific proneuropeptide or prohormones, since each protease appears to process multiple proneuropeptide substrates. Inhibition of a processing enzyme will may lead to changes in multiple neuropeptides. However, tissue-specific differences in the profile of processing proteases utilized for producing a particular neuropeptide may provide some selectivity for inhibitors of proneuropeptide processing proteases. Effective inhibitors that modulate proteolytic processing of proneuropeptide or prohormones will be valuable for understanding how proteases participate in the biosynthetic scheme for neuropeptide production.
Structural Biology of Proteases and Proneuropeptides (Prohormones)
The structural basis for protease and proneuropeptide interactions and processing is important for understanding mechanisms for processing diverse proneuropeptide structures by common processing enzymes of the cysteine and serine protease pathways (figure 4), as well as by related proteases. Structural studies have provided knowledge of processing protease active-site configurations and enzyme conformations (97-101). However, little is known about the structures of proneuropeptides. The primary structures of proneuropeptide are diverse and each proneuropeptide possesses a unique primary sequence. However, similarities of structural features of processing sites within proneuropeptides may be predicted based on their recognition and cleavage by several common protease processing pathways. Hence, the question of how proteases recognize different proprotein structures for specific cleavage of paired basic residues is important for understanding proneuropeptide processing.
Importantly, the structures of proneuropeptides and prohormones are largely unknown. Determination of their conformational organization will be key for defining binding site configuration(s) with processing enzymes at proprotein cleavage sites. Furthermore, structural features of proneuropeptide and prohormone interactions with processing proteases may reveal specific interactions. Investigation of the functional roles of specific proprotein-protease interactions may reveal strategies to disrupt such interactions, thereby resulting in selective inhibition of proneuropeptide or prohormone processing. Furthermore, the combination of protein structural studies by crystallography (102, 103), CD (104, 105), H-D exchange (hydrogen-deuterium exchange) (106, 107), and related approaches for defining protein structures will be fruitful for understanding structural features of how proneuropeptide interact with processing proteases for production of active neuropeptides.
Structural knowledge of the PC1/3 and PC2 have been provided by models based on the crystal structure of the related furin protease (97, 98). Furthermore, based on homology modeling, the catalytic domains of the PC members are quite homologous with similar structural features for the catalytic subsites. It is believed that further studies of the PC enzymes by crystallization with inhibitors will provide specific knowledge of the structural comparisons of active site and inhibitor configurations.
Structural studies of cathepsin L have provided knowledge of molecular mechanisms involved in cathepsin L function. The three-dimensional structure of human procathepsin L allowed determination of the binding characteristics to the prosegment that inhibits enzymatic activity of procathepsin L (99, 100), which utilizes a similar mode of inhibition of proteases of the papain superfamily by prosegments. Furthermore, determination of cathepsin L complexed with the inhibitor E-64 demonstrated that the active site of cathepsin L differs from that of cathepsin B (101); such information can facilitate design and development of specific inhibitors of cathepsin L compared to other cathepsin cysteine proteases.
Peptidomic Approaches for Identification of In Vivo Neuropeptides by Mass Spectrometry
Neuropeptidomics studied by mass spectrometry
Detection and identification of neuropeptides in the majority of neuropeptide research investigations have utilized specific radioimmunoassays. Such assays provide sensitive detection, but are limited since immunoassays do not define the primary neuropeptide sequences being detected. It is known that antisera may cross-react with related peptide structures; therefore, results from RIA data can indicate that the neuropeptide of interest and related peptides are detected.
Mass spectrometry is ideal for identification, quantitation, and simultaneous evaluation of multiple neuropeptides in biological samples; this approach has been termed ‘peptidomics’ for peptide research. Definitive identification of neuropeptides by mass spectrometry is required to define their active peptide sequences. Knowledge of the defined primary sequences of active neuropeptides is required for defining processing proteases that cleave at designated sites to generate the specified neuropeptides. Furthermore, quantitative mass spectrometry can be utilized to define relative levels of peptides in different cellular conditions so that regulatory mechanisms for neuropeptide production can be studied.
Importantly, there are many neuropeptides and intermediates derived from protein precursors that have not yet been identified. The diversity of neuropeptides has yet to be fully defined. Historically, active neuropeptides were purified from tissue extracts, tested in bioassays, and the final purified peptide was subjected to peptide sequencing for identification. This approach was used for identification of the first enkephalin opioid neuropeptide, achieved by HPLC purification, bioassay for opioid regulation of guinea pig ileum contraction, and peptide sequencing (108, 109). A second approach used to define neuropeptides has been to examine primary proneuropeptide sequences deduced from respective cDNAs (15-20) for prediction of active neuropeptides based on the positions of paired basic residues that typically flank active peptides within their precursors. Prediction of active peptides then often utilizes synthetic peptides for assessment of biological activities, and antisera generated against such synthetic peptides indicated the in vivo cellular localization of such predicted peptides. For example, the cDNA encoding procalcitonin predicted the presence of the related CGRP peptide (calcitonin gene related peptide) that was found in vivo (110, 111). While these purification and cDNA predictive approaches have identified many neuropeptides, the diversity generated through proteolytic processing of respective protein precursors should also be directly investigated from in vivo tissue sources. Direct identification of in vivo neuropeptides is best achieved with direct primary sequence determination by peptidomic technology using liquid chromatography (LC) coupled to on-line tandem mass spectrometry (LC-MS/MS).
Considerations for peptidomic studies of neuropeptides
Several issues should be addressed for effective application of peptidomic and proteomic approaches to study neuropeptide systems. First, appropriate sample preparation conditions must reflect the in vivo composition and levels of endogenous neuropeptides and hormones. Secondly, neuropeptides of wide ranges of abundances must be effectively identified and quantitated, including those of low abundance. Thirdly, peptidomic LC-MS/MS must be organized into relevant biological information.
Biological samples must be prepared to maintain the integrity of in vivo neuropeptides for neuropeptide analyses. In addition, consideration of multiple factors for experimental design must be optimized with respect to tissue regions, cell type(s), the secretory vesicle organelle where neuropeptide biosynthesis occurs, inactivation of endogenous proteases that modify neuropeptides, differential distribution of soluble and membrane components, enrichment, and other features to be addressed by the goal of the project.
Identification and quantitation of neuropeptides may utilize different mass spectrometry approaches including multiple-reaction-monitoring (MRM) for directed analysis of specific peptides, global analyses of peptide components by shotgun approaches, and analyses of large intact proteins by top-down analyses. MRM methods focus on analysis of a single peptide species, usually at a particular LC retention time, which provides exceptional sensitivity and quantitation of unlabeled peptides. MRM requires previous knowledge of the target peptide and requires rigorous analysis of standard peptides to optimize LC-MS conditions. Peptidomics of neuropeptide systems may or may not include proteolysis followed by LC-MS/MS. MS spectral data are compared to protein databases for scoring of the confidence levels of peptide identification. Tools for quantitative comparison of neuropeptides in different experimental conditions includes several types of isotopic labels (ICAT, iTRAQ, SILAC, TMAB) (112-115), absolute spiking (AQUA) (116), and spectral counting (NSAF) (117). In addition, analyses of protein precursors and high molecular weight intermediates derived from proneuropeptides may utilize top-down MS approaches with extremely high accuracy mass spectrometers such as Fourier-Transform Ion Cyclotron Resonance (FT-ICR) mass spectrometry (118). A variety of mass spectrometry disciplines can be utilized for structural and quantitative analyses of neuropeptides.
Neuropeptidomics of protease gene knockout mice for PC2 and PC1/3 reveals differential roles in neuropeptide biosynthesis
Quantitative LC-MS/MS approaches have provided fruitful knowledge of the roles of PC2 and PC1/3 proteases in neuropeptide production. For example, quantitative neuropeptidomic analyses with TMAB labeling of hypothalamic neuropeptides in PC2 deficient mice demonstrated that among identified neuropeptides (including those derived from proenkephalin, POMC, prodynorphin, proCCK, and many others), approximately one-third found in wild-type mice were not found in PC2 knockout mice (119). Another one-third of the neuropeptides were partially reduced by 25-75% of wild-type levels. Notably, comparison of cleavage sites implicated further knowledge of preferred amino acids at P1′ and P2′, as well as P3, positions.
Quantitative peptidomic evaluation of neuropeptides in brain and pituitary (69) demonstrated that certain peptides were influenced by the lack of PC1/3 (enkephalin, VGF, oxytocin, chromogranin A and B derived peptides), but some neuropeptides were not affected (neuropeptides derived from proSAAS, POMC, and provasopressin). Following the action of PC1/3 and PC2, removal of COOH-terminal basic residues is achieved by carboxypeptidase E (55) (also known as carboxypeptidase H, and hence CPE/H). Application of quantitative neuropeptidomics to the Cpefat/fat mice, which lack active carboxypeptidase E processing enzyme due to a mutation, led to identification of novel neuropeptides (120).
These examples of quantitative neuropeptidomics demonstrate the capabilities to analyze changes in neuropeptide systems in different physiological conditions.
Proteomics of Secretory Vesicles that Generate Neuropeptides
The secretory vesicle organelle is the primary site for neuropeptide biosynthesis. It is, therefore, important to understand the functional protein systems that allow neuropeptide to be produced. Knowledge of the secretory vesicle proteins and in vivo intravesicular protein conditions via proteomic approaches can advance our understanding of neuropeptide biosynthetic mechanisms. Recent examination of proteins in model chromaffin secretory vesicles revealed several functional protein categories that together support secretory vesicle production of neuropeptides and bioactive catecholamines for cell-cell communication (figure 7) (121).
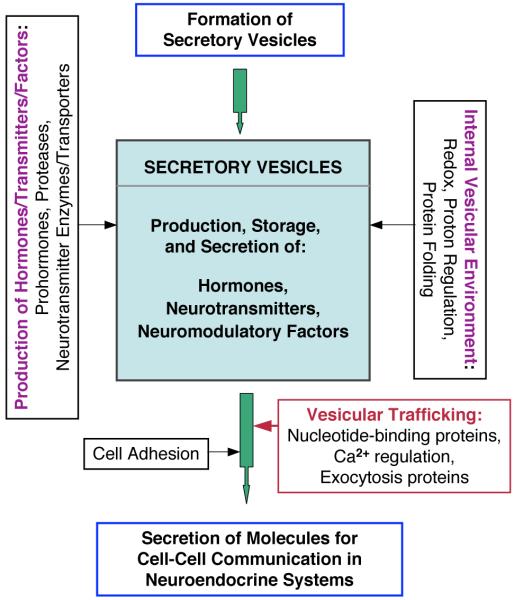
Figure 7. Proteomics reveals functional secretory vesicle protein systems for neuropeptide biosynthesis, storage, and secretion.
To explore the in vivo protein environment and composition of neuropeptide-synthesizing secretory vesicles, chromaffin secretory vesicles (also known as chromaffin granules) were isolated and subjected to proteomic analyses of proteins in the soluble and membrane components of the vesicles. Based on the knowledge that the primary function of the secretory vesicle organelle is to produce, store, and release active neuropeptides, proteins obtained from proteomic data were organized into functional categories to represent formation of secretory vesicles, neuropeptide biosynthesis, and exocytotic mechanisms for regulated secretion. Protein systems in secretory vesicle function consisted of those for (1) production of hormones, neurotransmitters, and neuromodulatory factors, (2) generating selected internal vesicular conditions for reducing condition, acidic pH conditions maintained by ATPases, and chaperones for protein folding, and (3) vesicular trafficking mechanisms to allow mobilization of secretory vesicles for exocytosis, which utilizes proteins for nucleotide-binding, calcium regulation, and vesicle exocytosis. These protein systems are coordinated to allow the secretory vesicle to synthesize and release of neuropeptides for cell-cell communication in the control of neuroendocrine functions.
Chromaffin granules represent model secretory vesicles for the produce, store, and secrete active enkephalin and related neuropeptides that function as peptide hormones and neurotransmitters for cell-cell communication. Protein systems involved in vesicular neuropeptide biosynthesis were examined in proteomic studies of soluble and membrane fractions of dense core secretory vesicles purified from neuroendocrine chromaffin cells. Proteins were separated by SDS-PAGE, and proteins from systematically sectioned gel lanes were identified by microcapillary LC-MS/MS (μLC-MS/MS) of tryptic peptides (121). Proteomic results revealed functional categories of prohormones, proteases, catecholamine neurotransmitter metabolism, protein folding, redox regulation, ATPases, calcium regulation, signaling components, exocytotic mechanisms, and related functions. Several novel secretory vesicle components involved in proteolysis were identified consisting of cathepsin B, cathepsin D, cystatin C, ubiquitin, and TIMP, as well carboxypeptidase E/H and proprotein convertases that are known to participate in prohormone processing. Significantly, the membrane fraction exclusively contained an extensive number of GTP nucleotide-binding proteins related to Rab, Rho, and Ras signaling molecules, together with SNARE-related proteins and annexins that are involved in trafficking and exocytosis of secretory vesicle components. Membranes also preferentially contained ATPases that regulate proton translocation. These results implicate membrane-specific functions for signaling and exocytosis that allow secretory vesicles to produce, store, and secrete active peptide hormones and neurotransmitters released from adrenal medulla for the control of physiological functions.
The protein systems utilized in these chromaffin vesicles, representing dense core secretory vesicles (121), resemble those of brain synaptic vesicles (122). Proteomic studies provides inference for secretory vesicle protein systems utilized for functions of these vesicles including their biogenesis (121, 123-125) that are required for production of enkephalin and related neuropeptides in brain and endocrine tissues.
Secretory vesicles at synaptic nerve terminals in brain are essential for chemical neurotransmission among neurons. Proteomic studies of synaptic proteins have revealed their regulation by brain injury (126), brain-derived neurotrophic factor (BDNF) (127), as well as drug regulation by morphine (128). The protein systems that support secretory vesicle exocytosis of peptide neurotransmitters and receptor activation at synaptic junctions of neurons function in concert to achieve neuropeptide-mediated communication in neural circuits.
Future Perspectives - Linking Neuropeptide Mechanisms for Translation into Therapeutic Applications
It is of high importance to apply knowledge of protease mechanisms for neuropeptide biosynthesis to small molecule strategies for development of therapeutic agents that can modulate the production of specific peptide neurotransmitters or hormones. Current and future research utilizing new approaches and tools, as discussed in this review, can provide insight into selective pharmacological approaches for exogenous therapeutic regulation of neuropeptide actions. Numerous health and disease conditions are regulated by neuropeptides.
Proteases are essential for the conversion of inactive proprotein precursors into the active neuropeptides. Two main protease pathways have been elucidated for processing proneuropeptides and hormones, consisting of the recently discovered cysteine protease cathepsin L with aminopeptidase B, and the well-established subtilisin-like serine proteases consisting of prohormone convertases 1 and 2 followed by carboxypeptidase E/H. Endogenous regulators modulate these two protease pathways as endogenous peptide inhibitors, activators, and in vivo secretory vesicle proteins. Neuropeptides in CSF (cerebrospinal fluid) in neurological diseases can monitor brain nervous activity since neuropeptides represent active neurotransmission (129, 130).
Knowledge of specific regulators for particular neuropeptides can lead to future translational research for small molecule regulation of prohormone convertases and cathepsin L pathways in the control of physiological functions. For example, regulation of opioid peptide production - enkephalin, ß-endorphin, and dynorphin - may lead to new drugs for analgesia and pain relief. Specific small molecule control of hypothalamic NPY in the control of feeding behavior may lead to improvement in obese conditions. Regulation of hypothalamic CRF and pituitary ACTH production is important for the control of steroid biosynthesis in adrenal cortex for metabolic regulation. PC related proteases have been implicated in sterol and lipid metabolism (131), tumor progression (132, 133), atherosclerosis (134), and other physiological and disease conditions.
Application of protease mechanisms to drug development for control of neuropeptides in health and disease is an exciting area and necessary area of research for neuropeptide regulation of neuroendocrine systems.
Acknowledgments
Support from the National Institutes of Health for this research is appreciated.
Terms/Definitions List
- Neuropeptides
Biologically active peptides that function as peptide neurotransmitters or peptide hormones. Neuropeptides typically consist of 3-40 amino acids in length.
- Proneuropeptide or Prohormone
The proprotein precursors of neuropeptides are referred to as proneuropeptides or prohormones.
- Processing Proteases
Protease enzymes cleave proneuropeptides or prohormones to generate the smaller biologically active neuropeptides.
- Neuropeptidomics
Study of the system of diverse peptides that function as neuropeptides, typically using integrated liquid chromatography, mass spectrometry, and bioinformatic approaches.
- Proteomics
Study of the system of proteins that function together in interacting networks that provide the basis for biological and physiological regulation.
- Regulated Secretory Vesicles
Neuropeptides are in large part synthesized in secretory vesicles of the regulated secretory pathway, also known as dense core secretory vesicles.
Footnotes
- Proteases are essential for proteolytic processing of proneuropeptide precursors into active peptide neurotransmitters and hormones.
- Secretory vesicles represent the primary subcellular site of neuropeptide biosynthesis, which are produced, stored, and secreted to mediate cell-cell communication.
- Protease pathways for proneuropeptide processing have been elucidated consisting of (a) the newly identified cysteine protease cathepsin L with aminopeptidase B in secretory vesicles, and (b) the well-established, proprotein convertase family that include the neuroendocrine-specific prohormone convertases 1 and 2 (PC1/3 and PC2) with carboxypeptidase E.
- Protease gene knockout experiments have validated the roles of PC1/3, PC2, as well as cathepsin L for the production of neuropeptides in nervous and endocrine tissues.
- Endogenous regulators consisting of inhibitors and activators participate in the in vivo control of processing enzyme functions.
- Structural biology of protease and proneuropeptides will be important to understand interacting mechanisms for proneuropeptide processing.
- Neuropeptidomics has recently been applied to investigations of neuropeptide systems for their primary sequence and structural identification, as well as quantitation by LC-MS/MS tandem mass spectrometry.
- Proteomic studies have revealed functional protein families that participate in secretory vesicle functions for the production, storage, and secretion of neuropeptides.
- Pharmacological evaluation of unique specificities among neuropeptide processing systems will be valuable for design of future strategies to develop selective small molecule modulators of processing enzymes for therapeutic applications in health and disease.
Future Issues: Areas of Neuropeptide Research for Exploration.
- How are cathepsin L and prohormone convertase protease pathways coordinately regulated?
- What is the proteolytic basis for tissue-specific processing of proneuropeptides, such as that for POMC?
- Selective and potent inhibitors of protease components for processing prohormones should be developed to facilitate basic and pharmacological research.
- What are the structural features of prohormone and protease interactions for functional processing?
- What are the full complement of in vivo neuropeptides that are biologically active at peptidergic receptors to regulate target cell functions?
- How do the different protein systems within secretory vesicles participate in the control of protease processing to generate active neuropeptides?
Literature Cited
- 1.Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. Ann. Re.v Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- 2.Law PY, Loh HH. Regulation of opioid receptor activities. J Pharmacol. Exp. Ther. 1999;289:607–624. [PubMed] [Google Scholar]
- 3.Roy S, Loh HH. Effects of opioids on the immune system. Neurochem. Res. 1996;21:1375–1386. doi: 10.1007/BF02532379. [DOI] [PubMed] [Google Scholar]
- 4.Felig Philip BJ, Frohman L. Endocrinology and Metabolism. McGraw-Hill Inc.; New York: 1981. pp. 293–297. [Google Scholar]
- 5.Norris D. Vertebrate Endocrinology. Academic Press; San Diego: 1997. pp. 137–141. [Google Scholar]
- 6.Brunton LL, Lazo JS, Parker KL. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. Mc-Graw-Hill; New York: 2006. pp. 547–590. [Google Scholar]
- 7.Eva C, Serra M, Mele P, Panzica G, Oberto A. Physiology and gene regulation of the brain NPY Y1 receptor. Frontiers in Neuroendocrinology. 2006;27:308–339. doi: 10.1016/j.yfrne.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Crawley JN. Biological actions of galanin. Regul. Peptides. 1995;59:1–16. doi: 10.1016/0167-0115(95)00083-n. [DOI] [PubMed] [Google Scholar]
- 9.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Ann. Rev. Pharmacol. Tox. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 10.Olson BR. Vasopressin-receptor antagonists: a new class of agents for the treatment of hyponatremia. Endocr. Metab. Immune Disord. Drug Targets. 2006;6:249–258. doi: 10.2174/187153006778249985. [DOI] [PubMed] [Google Scholar]
- 11.Steiner DF. New aspects of proinsulin physiology and pathophysiology. J. Pediatr. Endocrinol. Metab. 2000;13:229–239. doi: 10.1515/jpem.2000.13.3.229. [DOI] [PubMed] [Google Scholar]
- 12.Krieger D, Brownstein MJ, Martin JB. Brain Peptides. Wiley-Interscience; New York: 1983. [Google Scholar]
- 13.Siegel GJAB, Albers RW, Fisher SK, Uhler MD. Basic Neurochemistry. Lippincott Williams and Wilkins; Philadelphia: 1999. pp. 363–382. [Google Scholar]
- 14.Strand FL, Rose KJ, Zuccarelli LA, Kume J, Alves SE, Antonawich FJ, Garrett LY. Neuropeptide hormones as neurotrophic factors. Physiol. Rev. 1991;71:1017–1046. doi: 10.1152/physrev.1991.71.4.1017. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi H, Yang HY, Sabol SL. Rat neuropeptide Y precursor gene expression. mRNA structure, tissue distribution, and regulation by glucocorticoids, cyclic AMP, and phorbol ester. J. Biol. Chem. 1988;263:6288–6295. [PubMed] [Google Scholar]
- 16.Rokaeus A, Brownstein MJ. Construction of a porcine adrenal medullary cDNA library and nucleotide sequence analysis of two clones encoding a galanin precursor. Proc. Natl. Acad. Sci. USA. 1986;83:6287–6291. doi: 10.1073/pnas.83.17.6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson BG, D’Angio LA, Jr., Pasieka KB, Majzoub JA. Preprocorticotropin releasing hormone: cDNA sequence and in vitro processing. Molec. Cell. Endocrinol. 1989;61:175–180. doi: 10.1016/0303-7207(89)90128-7. [DOI] [PubMed] [Google Scholar]
- 18.Yoshikawa K, Williams C, Sabol S. Rat brain preproenkephalin mRNA, cDNA cloning, primary structure, and distribution in the central nervous system. J. Biol. Chem. 1984;259:14301–14308. [PubMed] [Google Scholar]
- 19.Comb M, Rosen H, Seeburg P, Adelman J, Herbert E. Primary structure of the human proenkephalin gene. DNA. 1983;2:213–229. doi: 10.1089/dna.1983.2.213. [DOI] [PubMed] [Google Scholar]
- 20.Roberts JL, Seeburg PH, Shine J, Herbert E, Baxter JD, Goodman HM. Corticotropin and beta-endorphin: construction and analysis of recombinant DNA complementary to mRNA for the common precursor. Proc. Natl. Acad. Sci. USA. 1979;76:2153–2157. doi: 10.1073/pnas.76.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi H, Teranishi Y, Nakanishi S, Numa S. Isolation and structural organization of the human corticotropoin-beta-lipotropin precursor gene. FEBS Lett . 1981;135:97–102. doi: 10.1016/0014-5793(81)80952-0. [DOI] [PubMed] [Google Scholar]
- 22.Docherty K, Steiner DF. Post-translational proteolysis in polypeptide hormone biosynthesis. Ann. Rev. Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- 23.Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway. J. Biol. Chem. 1999;274:20745–20748. doi: 10.1074/jbc.274.30.20745. [DOI] [PubMed] [Google Scholar]
- 24.Hook VY, Azaryan AV, Hwang SR, Tezapsidis N. Proteases and the emerging role of protease inhibitors in prohormone processing. FASEB J. 1994;8:1269–1278. doi: 10.1096/fasebj.8.15.8001739. [DOI] [PubMed] [Google Scholar]
- 25.Fugere M, Day R. Cutting back on pro-protein convertases: the latest approaches to pharmacological inhibition. Trends Pharmacol. Sci. 2005;26:294–301. doi: 10.1016/j.tips.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seidah NG, Prat A. Precursor convertases in the secretory pathway, cytosol and extracellular milieu. Essays Biochem. 2002;38:79–94. doi: 10.1042/bse0380079. [DOI] [PubMed] [Google Scholar]
- 27.Scamuffa N, Calvo F, Chretien M, Seidah NG, Khatib AM. Proprotein convertases: lessons from knockouts. FASEB J. 2006;20:1954–1963. doi: 10.1096/fj.05-5491rev. [DOI] [PubMed] [Google Scholar]
- 28.Hook V, Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Troutner K, Toneff T, Bundey R, Logvinova A, Reinheckel T, Peters C, Bogyo M. Cathepsin L and Arg/Lys aminopeptidase: a distinct prohormone processing pathway for the biosynthesis of peptide neurotransmitters and hormones. Biol. Chem. 2004;385:473–480. doi: 10.1515/BC.2004.055. [DOI] [PubMed] [Google Scholar]
- 29.Brange J, Langkjoer L. Insulin structure and stability. Pharm. Biotechnol. 1993;5:315–355. doi: 10.1007/978-1-4899-1236-7_11. [DOI] [PubMed] [Google Scholar]
- 30.Loh YP, Gainer H. The role of glycosylation on the biosynthesis, degradation, and secretion of the ACTH-beta-lipotropin common precursor and its ppetide products. FEBS Lett. 1979;96:269–272. doi: 10.1016/0014-5793(78)80415-3. [DOI] [PubMed] [Google Scholar]
- 31.Eipper BA, Stoffers DA, Mains RE. The biosynthesis of neuropeptides: peptide alpha-amidation. Annu. Rev. Neurosci. 1992;15:57–85. doi: 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- 32.Dass C, Mahalakshmi P. Amino acid sequence determination of phosphoenkephalins using liquid secondary ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1995;9:1148–1154. doi: 10.1002/rcm.1290091213. [DOI] [PubMed] [Google Scholar]
- 33.Vargas F, Frerot O, Brion F, Trung TMD, Lafitte A, Gulat-Marnay C. 3′-Phosphoadenosine 5′-phosphosulfate biosynthesis and the sulfation of cholecystokinin by the tyrosylprotein-sulfotransfferase in rat brain tissue. Chem. Biol. Interact. 1994;92:281–291. doi: 10.1016/0009-2797(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 34.Wilkinson CW. Roles of acetylation and other post-translational modification in melanocortin function and interactions with endorphins. Peptides. 2006;27:453–471. doi: 10.1016/j.peptides.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 35.Carmichael SW, Winkler H. The adrenal chromaffin cell. Sci. Am. 1985;253:40–49. doi: 10.1038/scientificamerican0885-40. [DOI] [PubMed] [Google Scholar]
- 36.Njus D, Kelley PM, Harnadek GJ. The chromaffin vesicle: a model secretory organelle. Physiologist. 1985;28:235–241. [PubMed] [Google Scholar]
- 37.Zinder O, Pollard HB. The chromaffin granule: recent studies leading to a functional model for exocytosis. Essays Neurochem. Neuropharmacol. 1980;4:125–162. [PubMed] [Google Scholar]
- 38.Schiller MR, Mende-Mueller L, Moran K, Meng M, Miller KW, Hook VY. “Prohormone thiol protease” (PTP) processing of recombinant proenkephalin. Biochemistry. 1995;34:7988–7995. doi: 10.1021/bi00025a004. [DOI] [PubMed] [Google Scholar]
- 39.Yasothornsrikul S, Aaron W, Toneff T, Hook VY. Evidence for the proenkephalin processing enzyme prohormone thiol protease (PTP) as a multicatalytic cysteine protease complex: activation by glutathione localized to secretory vesicles. Biochemistry. 1999;38:7421–7430. doi: 10.1021/bi990239w. [DOI] [PubMed] [Google Scholar]
- 40.Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Toneff T, Bundey R, et al. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc. Natl. Acad. Sci. USA. 2003;100:9590–9595. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azaryan AV, Krieger TJ, Hook VY. Purification and characteristics of the candidate prohormone processing proteases PC2 and PC1/3 from bovine adrenal medulla chromaffin granules. J. Biol. Chem. 1995;270:8201–8208. doi: 10.1074/jbc.270.14.8201. [DOI] [PubMed] [Google Scholar]
- 42.Spinazzi R, Andreis PG, Nussdorfer GG. Neuropeptide-Y and Y-receptors in the autocrine-paracrine regulation of adrenal gland under physiological and pathophysiological conditions. Intl. J. Molec. Med. 2005;15:3–13. [PubMed] [Google Scholar]
- 43.Rokaeus A, Pruss RM, Eiden LE. Galanin gene expression in chromaffin cells is controlled by calcium and protein kinase signaling pathways. Endocrinology. 1990;127:3096–3102. doi: 10.1210/endo-127-6-3096. [DOI] [PubMed] [Google Scholar]
- 44.Morel G, Leroux P, Garcia Caballero T, Beiras A, Gossard F. Ultrastructural distribution of somatostatin-14 and -28 in rat adrenal cells. Cell Tissue Res. 1990;261:517–524. doi: 10.1007/BF00313531. [DOI] [PubMed] [Google Scholar]
- 45.Hwang SR, Garza C, Mosier C, Toneff T, Wunderlich E, et al. Cathepsin L expression is directed to secretory vesicles for enkephalin neuropeptide biosynthesis and secretion. J. Biol. Chem. 2007;282:9556–9563. doi: 10.1074/jbc.M605510200. [DOI] [PubMed] [Google Scholar]
- 46.Yasothornsrikul S, Toneff T, Hwang SR, Hook VY. Arginine and lysine aminopeptidase activities in chromaffin granules of bovine adrenal medulla: relevance to prohormone processing. J. Neurochem. 1998;70:153–163. doi: 10.1046/j.1471-4159.1998.70010153.x. [DOI] [PubMed] [Google Scholar]
- 47.Gainer H, Russell JT, Loh YP. An aminopeptidase activity in bovine pituitary secretory vesicles that cleaves the N-terminal arginine from beta-lipotropin60-65. FEBS Lett. 1984;175:135–139. doi: 10.1016/0014-5793(84)80586-4. [DOI] [PubMed] [Google Scholar]
- 48.Hwang SR, O’Neill A, Bark S, Foulon T, Hook V. Secretory vesicle aminopeptidase B related to neuropeptide processing: molecular identification and subcellular localization to enkephalin- and NPY-containing chromaffin granules. J. Neurochem. 2007;100:1340–1350. doi: 10.1111/j.1471-4159.2006.04325.x. [DOI] [PubMed] [Google Scholar]
- 49.Cadel S, Foulon T, Viron A, Balogh A, Modol-Monnnet S, Noel N, Cohen P. Aminopeptidase B from the rat testis is a bifunctional enzyme structurall related to leukotriene-A4 hydrolase. Proc. Natl. Acad. Sci. USA. 1997;94:2963–2968. doi: 10.1073/pnas.94.7.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rockwell NC, Krysan DJ, Komiyama T, Fuller RS. Precursor processing by kex2/furin proteases. Chem. Rev. 2002;102:4525–4548. doi: 10.1021/cr010168i. [DOI] [PubMed] [Google Scholar]
- 51.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nature Rev. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Estivariz FE, Friedman TC, Chikuma T, Loh YP. Processing of adrenocorticotropin by two proteases in bovine intermediate lobe secretory vesicle membranes. A distinct acidic, tetrabasic residue-specific calcium-activated serine protease and a PC2-like enzyme. J. Biol. Chem. 1992;267:7456–7463. [PubMed] [Google Scholar]
- 53.Krieger TJ, Hook VY. Purification and characterization of a novel thiol protease involved in processing the enkephalin precursor. J. Biol. Chem. 1991;266:8376–8383. [PubMed] [Google Scholar]
- 54.Krieger TJ, Mende-Mueller L, Hook VY. Prohormone thiol protease and enkephalin precursor processing: cleavage at dibasic and monobasic sites. J. Neurochem. 1992;59:26–31. doi: 10.1111/j.1471-4159.1992.tb08871.x. [DOI] [PubMed] [Google Scholar]
- 55.Fricker LD. Carboxypeptidase E. Annu. Rev. Physiol. 1988;50:309–321. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- 56.Miller R, Aaron W, Toneff T, Vishnuvardhan D, Beinfeld MC, Hook VY. Obliteration of alpha-melanocyte-stimulating hormone derived from POMC in pituitary and brains of PC2-deficient mice. J. Neurochem. 2003;86:556–563. doi: 10.1046/j.1471-4159.2003.01856.x. [DOI] [PubMed] [Google Scholar]
- 57.Miller R, Toneff T, Vishnuvardhan D, Beinfeld M, Hook VY. Selective roles for the PC2 processing enzyme in the regulation of peptide neurotransmitter levels in brain and peripheral neuroendocrine tissues of PC2 deficient mice. Neuropeptides. 2003;37:140–148. doi: 10.1016/s0143-4179(03)00027-1. [DOI] [PubMed] [Google Scholar]
- 58.Allen RG, Peng B, Pellegrino MJ, Miller ED, Grandy DK, et al. Altered processing of pro-orphanin FQ/nociceptin and pro-opiomelanocortin-derived peptides in the brains of mice expressing defective prohormone convertase 2. J. Neurosci. 2001;21:5864–5870. doi: 10.1523/JNEUROSCI.21-16-05864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furuta M, Yano H, Zhou A, Rouille Y, Holst JJ, et al. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc. Natl. Acad. Sci. USA. 1997;94:6646–6651. doi: 10.1073/pnas.94.13.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Furuta M, Carroll R, Martin S, Swift HH, Ravazzola M, et al. Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2. J. Biol. Chem. 1998;273:3431–3437. doi: 10.1074/jbc.273.6.3431. [DOI] [PubMed] [Google Scholar]
- 61.Johanning K, Juliano MA, Juliano L, Lazure C, Lamango NS, et al. Specificity of prohormone convertase 2 on proenkephalin and proenkephalin-related substrates. J. Biol. Chem. 1998;273:22672–22680. doi: 10.1074/jbc.273.35.22672. [DOI] [PubMed] [Google Scholar]
- 62.Berman Y, Mzhavia N, Polonskaia A, Furuta M, Steiner DF, et al. Defective prodynorphin processing in mice lacking prohormone convertase PC2. J. Neurochem. 2000;75:1763–1770. doi: 10.1046/j.1471-4159.2000.0751763.x. [DOI] [PubMed] [Google Scholar]
- 63.Vishnuvardhan D, Connolly K, Cain B, Beinfeld MC. PC2 and 7B2 null mice demonstrate that PC2 is essential for normal pro-CCK processing. Biochem. Biophys. Res. Commun. 2000;273:188–191. doi: 10.1006/bbrc.2000.2915. [DOI] [PubMed] [Google Scholar]
- 64.Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, et al. Severe defect in proglucagon processing in islet A-cells of prohormone convertase 2 null mice. J. Biol. Chem. 2001;276:27197–202. doi: 10.1074/jbc.M103362200. [DOI] [PubMed] [Google Scholar]
- 65.Zhu X, Cao Y, Voogd K, Steiner DF. On the processing of proghrelin to ghrelin. J. Biol. Chem. 2006;281:38867–38870. doi: 10.1074/jbc.M607955200. [DOI] [PubMed] [Google Scholar]
- 66.Marzban L, Rhodes CJ, Steiner DF, Haataja L, Halban PA, Verchere CB. Impaired NH2-terminal processing of human proislet amyloid polypeptide by the prohormone convertase PC2 leads to amyloid formation and cell death. Diabetes. 2006;55:2192–2201. doi: 10.2337/db05-1566. [DOI] [PubMed] [Google Scholar]
- 67.Zhu X, Zhou A, Dey A, Norrbom C, Carroll R, et al. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc. Natl. Acad. Sci. USA. 2002;99:10293–10298. doi: 10.1073/pnas.162352599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu X, Orci L, Carroll R, Norrbom C, Ravazzola M, Steiner DF. Severe block in processing of proinsulin to insulin accompanied by elevation of des-64,65 proinsulin intermediates in islets of mice lacking prohormone convertase 1/3. Proc. Natl. Acad. Sci. USA. 2002;99:10299–10304. doi: 10.1073/pnas.162352799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pan H, Nanno D, Che FY, Zhu X, Salton SR, et al. Neuropeptide processing profile in mice lacking prohormone convertase-1. Biochemistry. 2005;44:4939–4948. doi: 10.1021/bi047852m. [DOI] [PubMed] [Google Scholar]
- 70.Tobin DJ, Foitzik K, Reinheckel T, Mecklenburg L, Botchkarev VA, et al. The lysosomal protease cathepsin L is an important regulator of keratinocyte and melanocyte differentiation during hair follicle morphogenesis and cycling. Am. J. Pathol. 2002;160:1807–1821. doi: 10.1016/S0002-9440(10)61127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reinheckel T, Deussing J, Roth W, Peters C. Towards specific functions of lysosomal cysteine peptidases: phenotypes of mice deficient for cathepsin B or cathepsin L. Biol. Chem. 2001;382:735–741. doi: 10.1515/BC.2001.089. [DOI] [PubMed] [Google Scholar]
- 72.Stypmann J, Glaser K, Roth W, Tobin DJ, Petermann I, et al. Dilated cardiomyopathy in mice deficient for the lysosomal cysteine peptidase cathepsin L. Proc. Natl. Acad. Sci. USA. 2002;99:6234–6239. doi: 10.1073/pnas.092637699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naggert KJ, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF, Carrol RJ, Paigen BJ, Leiter EH. Hyperproinsulinaema in obese fat/fat mice associated with a carboxypeptidse E mutation which reducecs enzyme activity. Nat. Genet. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- 74.Fricker LD, Berman YL, Leiter EH, Devi LA. Carboxypeptidase E activity is deficient in mice with the fat mutation. Effect on peptide processing. J. Biol. Chem. 1996;271:30619–30624. doi: 10.1074/jbc.271.48.30619. [DOI] [PubMed] [Google Scholar]
- 75.Cawley NX, Zhou J, Hill JM, Abebe D, Romboz S, Yanik T, Rodriguiz RM, Wetsel WC, Loh YP. The carboxypeptidase E knockout mouse exhibits endocrinological and behavioral deficits. Endocrinology. 2004;145:5807–5819. doi: 10.1210/en.2004-0847. [DOI] [PubMed] [Google Scholar]
- 76.Rabah N, Gauthier D, Wilkes BC, Gauthier DJ, Lazure C. Single amino acid substitution in the PC1/3 propeptide can induce significant modifications of its inhibitory profile toward its cognate enzyme. J. Biol. Chem. 2006;281:7556–7567. doi: 10.1074/jbc.M510607200. [DOI] [PubMed] [Google Scholar]
- 77.Boudreault A, Gauthier D, Lazure C. Proprotein convertase PC1/3-related peptides are potent slow tight-binding inhibitors of murine PC1/3 and Hfurin. J. Biol. Chem. 1998;273:31574–31580. doi: 10.1074/jbc.273.47.31574. [DOI] [PubMed] [Google Scholar]
- 78.Basak A, Koch P, Dupelle M, Fricker LD, Devi LA, et al. Inhibitory specificity and potency of proSAAS-derived peptides toward proprotein convertase 1. J. Biol. Chem. 2001;276:32720–32728. doi: 10.1074/jbc.M104064200. [DOI] [PubMed] [Google Scholar]
- 79.Fortenberry Y, Liu J, Lindberg I. The role of the 7B2 CT peptide in the inhibition of prohormone convertase 2 in endocrine cell lines. J. Neurochem. 1999;73:994–1003. doi: 10.1046/j.1471-4159.1999.0730994.x. [DOI] [PubMed] [Google Scholar]
- 80.Muller L, Zhu P, Juliano MA, Juliano L, Lindberg I. A 36-residue peptide contains all of the information required for 7B2-mediated activation of prohormone convertase 2. J. Biol. Chem. 1999;274:21471–21477. doi: 10.1074/jbc.274.30.21471. [DOI] [PubMed] [Google Scholar]
- 81.Westphal CH, Muller L, Zhou A, Zhu X, Bonner-Weir S, et al. The neuroendocrine protein 7B2 is required for peptide hormone processing in vivo and provides a novel mechanism for pituitary Cushing’s disease. Cell. 1999;96:689–700. doi: 10.1016/s0092-8674(00)80579-6. [DOI] [PubMed] [Google Scholar]
- 82.Hwang SR, Stoka V, Turk V, Hook VY. The novel bovine serpin endopin 2C demonstrates selective inhibition of the cysteine protease cathepsin L compared to the serine protease elastase, in cross-class inhibition. Biochemistry. 2005;44:7757–7767. doi: 10.1021/bi050053z. [DOI] [PubMed] [Google Scholar]
- 83.Hwang SR, Stoka V, Turk V, Hook V. Resistance of cathepsin L compared to elastase to proteolysis when complexed with the serpin endopin 2C, and recovery of cathepsin L activity. Biochem. Biophys. Res. Commun. 2006;340:1238–1243. doi: 10.1016/j.bbrc.2005.12.130. [DOI] [PubMed] [Google Scholar]
- 84.Hwang SR, Steineckert B, Yasothornsrikul S, Sei CA, Toneff T, et al. Molecular cloning of endopin 1, a novel serpin localized to neurosecretory vesicles of chromaffin cells. Inhibition of basic residue-cleaving proteases by endopin 1. J. Biol. Chem. 1999;274:34164–34173. doi: 10.1074/jbc.274.48.34164. [DOI] [PubMed] [Google Scholar]
- 85.Hook VY, Hwang SR. Novel secretory vesicle serpins, endopin 1 and endopin 2: endogenous protease inhibitors with distinct target protease specificities. Biol. Chem. 2002;383:1067–1074. doi: 10.1515/BC.2002.115. [DOI] [PubMed] [Google Scholar]
- 86.Cameron A, Appel J, Houghten RA, Lindberg I. Polyarginines are potent furin inhibitors. J. Biol. Chem. 2000;275:36741–36749. doi: 10.1074/jbc.M003848200. [DOI] [PubMed] [Google Scholar]
- 87.Kacprzak MM, Peinado JR, Than ME, Appel J, Henrich S, et al. Inhibition of furin by polyarginine-containing peptides: nanomolar inhibition by nona-D-arginine. J. Biol. Chem. 2004;279:36788–36794. doi: 10.1074/jbc.M400484200. [DOI] [PubMed] [Google Scholar]
- 88.Jean F, Stella K, Thomas L, Liu G, Xiang Y, et al. alpha1-Antitrypsin Portland, a bioengineered serpin highly selective for furin: application as an antipathogenic agent. Proc. Natl. Acad. Sci. USA. 1998;95:7293–7298. doi: 10.1073/pnas.95.13.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Villemure M, Fournier A, Gauthier D, Rabah N, Wilkes BC, Lazure C. Barley serine proteinase inhibitor 2-derived cyclic peptides as potent and selective inhibitors of convertases PC1/3 and furin. Biochemistry. 2003;42:9659–9668. doi: 10.1021/bi034418w. [DOI] [PubMed] [Google Scholar]
- 90.Han J, Zhang L, Shao X, Shi J, Chi C. The potent inhibitory activity of histone H1.2 C-terminal fragments on furin. FEBS J. 2006;273:4459–4469. doi: 10.1111/j.1742-4658.2006.05451.x. [DOI] [PubMed] [Google Scholar]
- 91.Tsuji A, Kikuchi Y, Sato Y, Koide S, Yuasa K, et al. A proteomic approach reveals transient association of reticulocalbin-3, a novel member of the CREC family, with the precursor of subtilisin-like proprotein convertase, PACE4. Biochem. J. 2006;396:51–59. doi: 10.1042/BJ20051524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cornwall GA, Cameron A, Lindberg I, Hardy DM, Cormier N, Hsia N. The cystatin-related epididymal spermatogenic protein inhibits the serine protease prohormone convertase 2. Endocrinology. 2003;144:901–908. doi: 10.1210/en.2002-220997. [DOI] [PubMed] [Google Scholar]
- 93.Richer MJ, Keays CA, Waterhouse J, Minhas J, Hashimoto C, Jean F. The Spn4 gene of Drosophila encodes a potent furin-directed secretory pathway serpin. Proc. Natl. Acad. Sci. USA. 2004;101:10560–10565. doi: 10.1073/pnas.0401406101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bentele C, Kruger O, Todtmann U, Oley M, Ragg H. A proprotein convertase-inhibiting serpin with an endoplasmic reticulum targeting signal from Branchiostoma lanceolatum, a close relative of vertebrates. Biochem. J. 2006;395:449–456. doi: 10.1042/BJ20051947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dahlen JR, Jean F, Thomas G, Foster DC, Kisiel W. Inhibition of soluble recombinant furin by human proteinase inhibitor 8. J. Biol. Chem. 1998;273:1851–1854. doi: 10.1074/jbc.273.4.1851. [DOI] [PubMed] [Google Scholar]
- 96.Leblond J, Laprise MH, Gaudreau S, Grondin F, Kisiel W, Dubois CM. The serpin proteinase inhibitor 8: an endogenous furin inhibitor released from human platelets. Thromb. Haemost. 2006;95:243–252. doi: 10.1160/TH05-08-0561. [DOI] [PubMed] [Google Scholar]
- 97.Henrich S, Cameron A, Bourenkov GP, Kiefersauer R, Huber R, Lindberg I, Bode W, Than ME. The crystal structure of the proprotei processing proteinase furin explains its stringent specificity. Nat. Struct. Biol. 2003;10:520–526. doi: 10.1038/nsb941. [DOI] [PubMed] [Google Scholar]
- 98.Henrich S, Lindberg I, Bode W, Than ME. Proprotein convertase models ased on the crystal structures of furin and kexin: explanation of their specificity. J. Mol. Biol. 2005;345:211–227. doi: 10.1016/j.jmb.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 99.Coulombe R, Grochulski P, Sivaraman J, Menard R, Mort JS, Cygler M. Structure of human procathepsin L reveals the molecular bais of inhibition by the prosegment. EMBO J. 1996;15:5492–5503. [PMC free article] [PubMed] [Google Scholar]
- 100.Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001;20:4629–4633. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fujishima A, Imai Y, Nomura T, Fujisawa Y, Yamamoto Y, Sugawara T. The crystal structure of human cathepsin L complexed with E-64. FEBS Lett. 1997;407:47–50. doi: 10.1016/s0014-5793(97)00216-0. [DOI] [PubMed] [Google Scholar]
- 102.Scapin G. Structural biology and drug discovery. Curr. Pharm. Des. 2006;12:1087–1097. doi: 10.2174/138161206777585201. [DOI] [PubMed] [Google Scholar]
- 103.Yee AA, Savchenko A, Ignachenko A, Lukin J, Xu X, et al. NMR and X-ray crystallography, complementary tools in structural proteomics of small proteins. J. Amer. Chem. Soc. 2005;127:16512–16517. doi: 10.1021/ja053565+. [DOI] [PubMed] [Google Scholar]
- 104.Sreerama N, Woody RW. Computation and analysis of protein circular dichroism spectra. Methods in Enzymology. 2004;383:318–351. doi: 10.1016/S0076-6879(04)83013-1. [DOI] [PubMed] [Google Scholar]
- 105.Kelly SM, Jess TJ, Price NC. How to study proteins by circular dichroism. Biochim. Biophys. Acta. 2005;175:119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 106.Englander JJ, Del Mar C, Li W, Englander SW, Kim JS, Stranz DD, Hamuro Y, Woods VL. Protein structure change studied by hydrogen-deuterium exchange, functional labeling, and mass spectrometry. Proc. Natl. Acad. Sci. USA. 2003;100:7057–7062. doi: 10.1073/pnas.1232301100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mandell JG, Falack AM, Komives EA. Identification of protein-protein interfaces by decreased amide proton solvent accessibility. Proc. Natl. Acad. Sci. USA. 1998;95:14705–14710. doi: 10.1073/pnas.95.25.14705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- 109.Hughes J, Kosterlitz HW, Smith TW. The distribution of methionine-enkephalin and leucine-enkephalin in the brain and peripheral tissues. Br. J. Pharmacol. 1977;61:639–647. doi: 10.1111/j.1476-5381.1977.tb07557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- 111.Jonas V, Lin CR, Kawashima E, Semon D, Swanson LW, et al. Alternative RNA processing events in human calcitonin/calcitonin gene-related peptide gene expression. Proc. Natl. Acad. Sci. USA. 1985;82:1994–1998. doi: 10.1073/pnas.82.7.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hamdan M, Righetti PG. Modern strategies for protein quantification in proteome analysis: advantages and limitations. Mass Spec. Rev. 2002;21:287–302. doi: 10.1002/mas.10032. [DOI] [PubMed] [Google Scholar]
- 113.Gan CS, Chong PK, Pham TK, Wright PC. Technical, experimental, and biological variations in isobaric tags for relative and absolute quantitation (iTRAQ) Journal of Proteome Research. 2007;6:821–827. doi: 10.1021/pr060474i. [DOI] [PubMed] [Google Scholar]
- 114.Mann M. Functional and quantitative proteomics using SILAC. Nature Reviews. 2006;7:952–958. doi: 10.1038/nrm2067. [DOI] [PubMed] [Google Scholar]
- 115.Che FY, Fricker LD. Quantitative peptidomics of mouse pituitary: comparison of different stable isotopic tags. J. Mass Spectrom. 2005;40:238–249. doi: 10.1002/jms.743. [DOI] [PubMed] [Google Scholar]
- 116.Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc. National Acad. Sci. USA. 2003;100:6940–6945. doi: 10.1073/pnas.0832254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zybailov B, Mosley AL, Sardiu ME, Coleman MK, Florens L, Washburn MP. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J. Proteome Res. 2006;5:2339–2347. doi: 10.1021/pr060161n. [DOI] [PubMed] [Google Scholar]
- 118.Roth MJ, Forbes AJ, Boyne MT, Kim YB, Robinson DE, Kellehert NL. Molec. Cell. Proteomics. 2005;4:1002–1008. doi: 10.1074/mcp.M500064-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pan H, Che FY, Peng B, Steiner DF, Pintar JE, Fricker LD. The role of prohormone convertase-2 in hypothalamic neuropeptide processing: a quantitative neuropeptidomic study. J. Neurochem. 2006;98:1763–1777. doi: 10.1111/j.1471-4159.2006.04067.x. [DOI] [PubMed] [Google Scholar]
- 120.Lim J, Berezniuk I, Che FY, Parikh R, Biswas R, et al. Altered neuropeptide processing in prefrontal cortex of Cpe (fat/fat) mice: implications for neuropeptide discovery. J. Neurochem. 2006;96:1169–1181. doi: 10.1111/j.1471-4159.2005.03614.x. [DOI] [PubMed] [Google Scholar]
- 121.Wegrzyn J, Lee J, Neveu JM, Lane WS, Hook V. Proteomics of Neuroendocrine Secretory Vesicles Reveal Distinct Functional Systems for Biosynthesis and Exocytosis of Peptide Hormones and Neurotransmitters. J. Proteome Res. 2007 doi: 10.1021/pr060503p. in press. [DOI] [PubMed] [Google Scholar]
- 122.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 123.Kim T, Gondre-Lewis MC, Arnaoutova I, Loh YP. Dense-core secretory granule biogenesis. Physiology. 2006;21:124–133. doi: 10.1152/physiol.00043.2005. [DOI] [PubMed] [Google Scholar]
- 124.Kim T, Zhang CF, Sun Z, Wu H, Loh YP. Chromogranin A deficiency in transgenic mice leads to aberrant chromaffin granule biogenesis. J. Neurosci. 2005;25:6958–6961. doi: 10.1523/JNEUROSCI.1058-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim T, Loh YP. Protease nexin-1 promotes secretory granule biogenesis by preventing granule protein degradation. Mol. Biol. Cell. 2006;17:789–798. doi: 10.1091/mbc.E05-08-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kobeissy FH, Ottens AK, Zhang Z, Liu MC, Denslow ND, et al. Novel differential neuroproteomics analysis of traumatic brain injury in rats. Mol. Cell. Proteomics. 2006;5:1887–1898. doi: 10.1074/mcp.M600157-MCP200. [DOI] [PubMed] [Google Scholar]
- 127.Liao L, Pilotte J, Xu T, Wong CC, Edelman GM, et al. BDNF induces widespread changes in synaptic protein content and up-regulates components of the translation machinery: an analysis using high-throughput proteomics. J. Proteome Res. 2007;6:1059–1071. doi: 10.1021/pr060358f. [DOI] [PubMed] [Google Scholar]
- 128.Moron JA, Abul-Husn NS, Rozenfeld R, Dolios G, Wang R, Devi LA. Morphine administration alters the profile of hippocampal postsynaptic density-associated proteins: a proteomics study focusing on endocytic proteins. Mol. Cell. Proteomics. 2007;6:29–42. doi: 10.1074/mcp.M600184-MCP200. [DOI] [PubMed] [Google Scholar]
- 129.Noben JP, Dumont D, Kwasnikowska N, Verhaert P, Somers V, et al. Lumbar cerebrospinal fluid proteome in multiple sclerosis: characterization by ultrafiltration, liquid chromatography, and mass spectrometry. J. Proteome Res. 2006;5:1647–1657. doi: 10.1021/pr0504788. [DOI] [PubMed] [Google Scholar]
- 130.Svensson M, Skold K, Nilsson A, Falth M, Nydahl K, et al. Neuropeptidomics: MS applied to the discovery of novel peptides from the brain. Anal. Chem. 2007;79:15–6. 8–21. doi: 10.1021/ac071856q. [DOI] [PubMed] [Google Scholar]
- 131.Seidah NG, Khatib AM, Prat A. The proprotein convertases and their implication in sterol and/or lipid metabolism. Biol. Chem. 2006;387:871–877. doi: 10.1515/BC.2006.110. [DOI] [PubMed] [Google Scholar]
- 132.Khatib AM, Siegfried G, Chretien M, Metrakos P, Seidah NG. Proprotein convertases in tumor progression and malignancy: novel targets in cancer therapy. Am. J. Pathol. 2002;160:1921–1935. doi: 10.1016/S0002-9440(10)61140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bassi DE, Fu J, Lopez de Cicco R, Klein-Szanto AJ. Proprotein convertases: “master switches” in the regulation of tumor growth and progression. Molec. Carcinogenesis. 2005;44:151–161. doi: 10.1002/mc.20134. [DOI] [PubMed] [Google Scholar]
- 134.Stawowy P, Fleck E. Proprotein convertases furin and PC5: targeting atherosclerosis and restenosis at multiple levels. J. Molec. Med. 2005;83:865–875. doi: 10.1007/s00109-005-0723-8. [DOI] [PubMed] [Google Scholar]
- 135.Cain BM, Connolly K, Blum A, Vishnuvardhan D, Marchand JE, Beinfld MC. Distriubtion and colocalization of cholecystokinin with the prohormone convertase enzymes PC1, PC2, and PC5 in rat brain. J. Comp. Neurol. 2003;467:307–325. doi: 10.1002/cne.10924. [DOI] [PubMed] [Google Scholar]
Recommended Review Articles: Proteolytic Processing of Proneuropeptides
- Docherty K, Steiner DF. Post-translational proteolysis in polypeptide hormone biosynthesis. Ann. Rev. Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Eipper BA, Stoffers DA, Mains RE. The biosynthesis of neuropeptides: peptide alpha-amidation. Annu. Rev. Neurosci. 1992;15:57–85. doi: 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- Fricker LD. Carboxypeptidase E. Annu. Rev. Physiol. 1988;50:309–321. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- Fugere M, Day R. Cutting back on pro-protein convertases: the latest approaches to pharmacological inhibition. Trends Pharmacol. Sci. 2005;26:294–301. doi: 10.1016/j.tips.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook VY, Azaryan AV, Hwang SR, Tezapsidis N. Proteases and the emerging role of protease inhibitors in prohormone processing. FASEB J. 1994;8:1269–1278. doi: 10.1096/fasebj.8.15.8001739. [DOI] [PubMed] [Google Scholar]
- Hook V, Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Troutner K, Toneff T, Bundey R, Logvinova A, Reinheckel T, Peters C, Bogyo M. Cathepsin L and Arg/Lys aminopeptidase: a distinct prohormone processing pathway for the biosynthesis of peptide neurotransmitters and hormones. Biol. Chem. 2004;385:473–480. doi: 10.1515/BC.2004.055. [DOI] [PubMed] [Google Scholar]
- Khatib AM, Siegfried G, Chretien M, Metrakos P, Seidah NG. Proprotein convertases in tumor progression and malignancy: novel targets in cancer therapy. Am. J. Pathol. 2002;160:1921–1935. doi: 10.1016/S0002-9440(10)61140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell NC, Krysan DJ, Komiyama T, Fuller RS. Precursor processing by kex2/furin proteases. Chem. Rev. 2002;102:4525–4548. doi: 10.1021/cr010168i. [DOI] [PubMed] [Google Scholar]
- Scamuffa N, Calvo F, Chretien M, Seidah NG, Khatib AM. Proprotein convertases: lessons from knockouts. FASEB J. 2006;20:1954–1963. doi: 10.1096/fj.05-5491rev. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Khatib AM, Prat A. The proprotein convertases and their implication in sterol and/or lipid metabolism. Biol. Chem. 2006;387:871–877. doi: 10.1515/BC.2006.110. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Prat A. Precursor convertases in the secretory pathway, cytosol and extracellular milieu. Essays Biochem. 2002;38:79–94. doi: 10.1042/bse0380079. [DOI] [PubMed] [Google Scholar]
- Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nature Rev. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway. J. Biol. Chem. 1999;274:20745–20748. doi: 10.1074/jbc.274.30.20745. [DOI] [PubMed] [Google Scholar]