Abstract
Background
Dendritic cells (DC) play an important role in the induction and regulation of immune responses.
Methods
Myeloid CD11c+DC (MDC), which may have inflammatory functions, and plasmacytoid CD123+ DC (PDC), which may have tolerogenic potential, were measured by flow cytometric analysis, cross-sectionally, once, in 48 children, and longitudinally (pre-transplant, and at days 1–60, 61–200, 201–400 post transplant) in 30 children following liver transplantation (LTx). All children received 53/25 cadaveric/live donor liver allografts with rabbit anti-human thymocyte globulin (rATG) induction, and steroid-free Tacrolimus therapy. Rejectors in both groups were those children (n=35), who experienced biopsy-proven acute cellular rejection (ACR) within 60 days of DC monitoring.
Results
Among rejectors in the longitudinal and cross-sectional cohorts, the MDC: PDC ratio was higher, and was associated with decreased PDC frequencies. Logistic regression analysis, leave-one out cross-validation, and receiver operating characteristic analysis applied to 30 cross-sectional subjects revealed that an MDC:PDC ratio 1.78 was associated with rejector status with sensitivity/specificity of 76.9/88.2%. Sensitivity and specificity were replicated in the 18 remaining cross-sectional subjects (88.8 and 78.8%, respectively), but not in longitudinally-monitored subjects, during the early, 60-day period after LTx (30.76 and 62.50%, respectively). A significant negative correlation was observed between Tacrolimus whole blood concentrations and PDC frequencies (Spearman r = −0.370, p=0.005) in 48 cross-sectional subjects in whom DC subsets were monitored 1–3 years after LTx, but not during the early post-LTx period.
Conclusion
We conclude that an elevated MDC: PDC ratio associates with liver graft rejection, which occurs after first year in children induced with rATG.
Keywords: dendritic cell subsets, liver transplantation, pediatric, anti-thymocyte globulin
Introduction
Reduced cumulative exposure to immunosuppression is especially desirable in children receiving liver transplantation (LTx). In this population, delayed morbidity and mortality largely results from opportunistic infections and malignancies (1–4). Induction with rabbit anti-human thymocyte globulin (rATG, Genzyme, Cambridge, MA) reduces cumulative exposure to immunosuppression in many types of solid organ transplant recipients, in part, by enabling steroid elimination, and accelerated minimization of Tacrolimus (5–8). However, clinical drug minimization may be accompanied by rebound episodes of acute cellular rejection (ACR). These episodes can reverse gains in adaptive alloimmunity, because higher-dose immunosuppression is again required. To avoid these types of drug failures, novel diagnostic markers are needed to evaluate the risk of rejection at any given time. Most such markers remain research tools at best, and are largely based on enumerating donor-specific cytotoxic T cell function. Such assays require several days, and comparatively large blood samples (9–11).
Dendritic cells (DC) are uniquely well-equipped, professional antigen-presenting cells derived from CD34+ hematopoietic stem cells, that can be easily measured in microliter quantities of peripheral blood (12, 13). DC can induce both immunity and tolerance among alloantigen-specific T-cells (14, 15). Two major DC precursor subsets are present in the circulation. Following transplantation, host CD11c+ myeloid DC (MDC) present donor antigen indirectly to recipient T-cells, after migrating into the allograft, and facilitate pro-inflammatory T cell polarization (12). Less abundant CD123+ plasmacytoid DC (PDC) are the principal type-1 interferon-producing cells in the body, and have been implicated in both the regulation of immunity and the induction of transplant tolerance (16, 17). Previous studies have been conducted in pediatric LTx during the course of drug minimization undertaken several years after LTx, and in patients off all anti-rejection therapy (18) Several studies have extended these findings to other types of solid organ transplant (19, 20, 13). However, it is not known whether DC subsets associate with liver rejection during the first three years after rATG induction, when the most aggressive reductions in immunosuppression are usually undertaken. Also, it is not known whether DC subsets may have a predictive role, which may guide safe reduction of immunosuppression in pediatric LTx recipients.
The current study, conducted in 78 children with LTx after rATG induction, shows that an MDC: PDC ratio indicating a relative abundance of MDC, is associated with rejection after the first post-LTx year, but not during the first year after LTx, when the lymphocyte-depleting effects of rATG are most pronounced.
MATERIALS AND METHODS
Human Subjects
Research procedures were approved by the University of Pittsburgh Institutional Review Board. DC subsets were measured in ten adult normal human subjects, and in 78 non-consecutive children, who received LTx, after steroid-free Tacrolimus, and 5 mg/kg rATG (Genzyme, Cambridge, MA), as described (21). Target Tacrolimus (formerly FK506 [FK]) whole blood concentration (FKWBC) was 12–15 ng/ml in the first month, and 8–10 ng/ml after the third month. By the end of month 12, target FKWBC was 5–7 ng/ml as described (19). If ACR occurred, these targets were delayed by 3–6 months.
Assay procedure
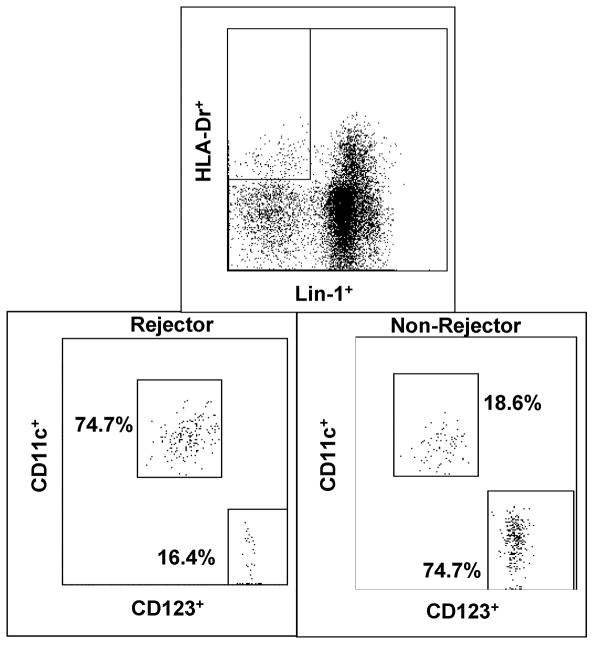
DC subsets were measured in 50 μl whole blood samples obtained for clinical surveillance of graft function. Flow-cytometry identified DC as high HLA-DR-positive (Figure 1) and lineage 1 (LIN1=CD3, CD14, CD19, CD20 CD16 and CD56) -negative peripheral blood leukocytes (PBL), using commercially-available immunofluorescent monoclonal antibodies (BD Biosciences, San Jose, CA). The frequencies of HLA-DRhighCD11c+ (MDC) and HLA-DRhighCD123+ (PDC) (Figure 1) were used to determine the MDC: PDC ratio. Absolute MDC and PDC numbers were calculated by multiplying the WBC count on the day of the experiment by the respective proportions of MDC and PDC in HLA-DRhigh, lin negative PBL.
Figure 1.
Flow cytometry gating strategy shows derivation of CD11c+ MDC and CD123+ PDC, from HLA-DRhigh Lineage negative (Lin-) PBL in a Rejector and Non-Rejector after LTx with rATG.
Single measurements were obtained in the ten normal human subjects, and in 48 of 78 children with LTx, between 1–3 yrs after LTx. In this cross-sectional cohort, 22 of 48 children were classified as Rejectors, because they exhibited ACR on biopsies conducted within 60 days of DC subset analysis. In the remaining 30 of 78 children, multiple sequential measurements were made before LTx (pre-Tx), and at 1–60, 61–200 days and 201–400 days after LTx, to evaluate longitudinal changes in DC subsets. In this longitudinal cohort, 13 of 30 children were classified as Rejectors, because they experienced biopsy-proven ACR within 60 days of LTx, and since all subsets were measured within 60 days of biopsy.
Statistical techniques
Longitudinal observations made at the 4 time points,- Pre-Tx, and at 1–60 Days, 61–200 Days, and 201–400 days post LTx, were compared between Rejectors and Non-Rejectors for MDC and PDC frequencies and absolute counts, and the MDC: PDC ratio using the Students “t” test. The cross-sectional cohort was divided into a screening cohort of 30 randomly- chosen subjects. In the screening cohort, the association between rejection outcome and each subset, as well as the ratio, was defined by logistic regression after incorporation of five co-variates: age, gender, race, time from LTx, and FKWB. Next, leave-one out cross-validation (LOO-CV) tested the model performance, and receiver operating characteristic (ROC) analysis of 30 thresholds from LOO-CV analyses was used to derive a final threshold for the DC parameter, best associated with Rejector status. Finally, model predictions were compared with known clinical outcomes or biopsy-results in the 18 remaining cross-sectional subjects, and at each time point in the longitudinal cohort, to test whether sensitivity and specificity observed in the screening cohort was replicated.
RESULTS
Rejectors (n=35) were similar to Non-Rejectors (n=43) in general demographics (Table 1). The primary diagnoses leading to LTx in the 78 children are summarized in Supplementary Table 1, and were not different between groups.
Table 1.
Summary of general demographics in Rejectors and Non-Rejectors.
| Variable | Non-Rejectors | Rejectors | p value |
|---|---|---|---|
| Age at transplant(yrs) (n=78)(NR=43, R=35)(Median±SEM) | 5.08±1.04 | 5.78±1.04 | NS |
| Gender(M:F)(n=78) | 26:17 | 17:18 | NS |
| Race (Caucasian: African-American: Others)(n=78) | 37:02:04 | 27:02:06 | NS |
| Donor (Cadaveric: Living)(n=78) | 27:16 | 26:9 | NS |
| Time in days between LTx and assay in Cross sectional cohort(n=48)(Median± SEM) | 755±92 | 665±107 | NS |
| FKWB (ng/ml) at the time of assay in cross-sectional cohort (n=48) (NR=26, R=22) (Median±SEM) | 4.40±0.40 | 9.80±1.70 | 0.00016 |
| FKWB in cross- sectional cohort in 6 R with assay Before Biopsy (NR=26, R=6)(Median±SEM) | 4.4±0.4 | 5.00±1.36 | NS |
| FKWB in cross sectional cohort in 16 R with assay After Biopsy (NR=26, R=16)(Median±SEM) | 4.4±0.4 | 11.55±1.43 | 4.7E-05 |
| FKWB longitudinal 1–60 Days(NR=16, R=13)(Median±SEM) | 8.8±0.9 | 14.6±1.6 | NS |
| FKWB longitudinal 61–200 Days(NR=13, R=8)(Median±SEM) | 5.7±1.2 | 8.5±1.2 | NS |
| FKWB longitudinal 201–400 Days(NR=10, R=9)Median±SEM | 6.2±0.4 | 5.6±1.9 | NS |
| Time in days to early rejection in Longitudinal Cohort | NA | 21±14.77 | NA |
Clinical course
Patient and graft survival was 48/48 (100%) and 46/48 (95.83%), respectively, in the cross-sectional cohort (n=48). In the longitudinal cohort (n=30), patient and graft survival was 30/30 (100%) and 28/30 (93%), respectively. Of the four graft failures in the total subject population of 78 patients, two grafts were lost due to primary non-function, and two due to vascular thrombosis. All failed grafts were re-transplanted successfully. All ACR episodes were steroid-responsive. There were no significant differences between Rejectors and Non-Rejectors in primary diagnoses leading to LTx.
Pre-LTx DC subsets and ratios were not different when Normal controls (n=10), were compared with Rejectors (n=13) and Non-Rejectors (n=17) in the longitudinal cohort
MDC frequencies were (45.85±7.53 % vs. 37.30±4.92% vs. 46.40±5.43 % respectively, p=NS) PDC frequencies were (42.25±7.77% vs. 42.90 ±5.01% vs. 30.30±3.79%, p=NS), and the MDC: PDC ratio was (1.13±1.36 vs. 0.91±1.87 vs. 1.40±0.41, p= NS).
The MDC: PDC ratio is higher in Rejectors, because of a relative excess of MDC and a significant decrease in PDC
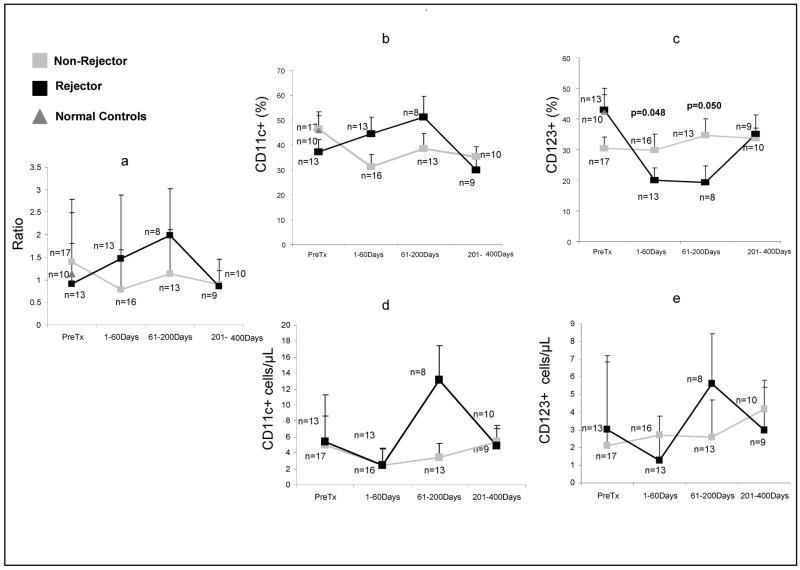
In the cross-sectional cohort, Rejectors, who were monitored within 60 days of biopsy-proven ACR, demonstrated significantly higher MDC: PDC ratio, likely due to significantly lower frequencies of PDC and higher frequencies of MDC, when compared with Non-Rejectors (Table 2). This finding is also mirrored in Rejectors in the longitudinal cohort. The MDC: PDC ratio was numerically higher during the 1–60-day time period among Rejectors, when compared with Non-Rejectors. (Supplementary Table 2, Figure 2). Significantly higher PDC frequencies and numerically higher absolute counts (Supplementary Table 3, Figure 2) were observed in Non-Rejectors in the longitudinal cohort during this time period. The 1–60-day time period also corresponds to the period of highest risk of ACR, in which ACR was diagnosed by biopsy within 60-days of DC subset monitoring. Therefore, the conditions under which Rejectors were assayed at 1–60 days in the longitudinal cohort, approximated most closely to the conditions under which Rejectors were assayed in the cross-sectional cohort, in whom outcome-specific differences in DC subsets were first observed.
Table 2.
In the cross-sectional cohort, the MDC: PDC ratio was significantly higher in Rejectors (n=22), and was acompanied by significantly lower frequencies of PDC and higher frequencies of MDC, when compared with Non-Rejectors (n=26).
| Cell/ratio | Rejector (n=22) | Non-Rejector (n=26) | p-values |
|---|---|---|---|
| CD11c % | 58.65 ± 4.31 | 35.85 ±3.33 | 0.008 |
| CD123 % | 20.00 ± 3.47 | 42.55 ± 3.00 | 0.0002 |
| Ratio | 2.87 ± 0.55 | 0.85 ± 0.20 | 0.0005 |
Figure 2.
Longitudinal changes in DC subset frequencies, absolute counts, and the MDC: PDC ratio in Rejectors and Non-Rejectors. Also shown are mean frequencies in 10 normal adult human subjects.
The MDC: PDC ratio associates with Rejector status in the cross-sectional cohort, but has reduced sensitivity in the longitudinal cohort
The most parsimonious logistic regression model was calculated using exhaustive backward and forward stepwise selection of MDC and PDC frequencies, and the MDC: PDC ratio as independent variables, and Rejector status as the dependent variable across 30 cross-sectional observations. The optimal model was built with MDC: PDC ratio alone. We then assessed five covariates, which could contribute confounding effects-age, race, gender, time after transplant, and Tacrolimus whole blood level (FKWBC). In additional stepwise model selection, only two covariates were tested with the original three independent variables at any given time to avoid model saturation. The optimal model was once again built with the MDC:PDC ratio alone. Clinical status and assay results of all subjects in the screening and replication cohorts, who were biopsied, are summarized in Table 3. The biopsied cohort included 22 Rejectors and 4 of 26 Non-rejectors. To obtain a realistic measure of model performance, LOO-CV was performed using MDC:PDC ratio as the single predictor of Rejector status on these 30 observations. Using the threshold ratio obtained after each observation drop in LOO-CV, the median was calculated for use as the threshold IR for this cell type. ROC analysis confirmed this threshold ratio of 1.78 for MDC: PDC ratio as one at or above which Rejector status is predicted (Table 4).
Table 3.
Summary of assay predictions based on the MDC: PDC ratio, and corresponding biopsy result for 26 children in the cross-sectional cohort, in whom biopsy results were available. The cohort includes 22 Rejectors (R) and 4 Non-Rejectors (NR). All biopsies were performed within 60 days of DC monitoring.
| # | Biopsy Diagnosis | Time between biopsy and sampling date | MDC:PDC ratio | Assay Prediction |
|---|---|---|---|---|
| 1 | R | −60 | 8.50 | R |
| 2 | R | −5 | 4.61 | R |
| 3 | R | 13 | 4.1 | R |
| 4 | R | 56 | 0.30 | NR |
| 5 | R | −7 | 2.66 | R |
| 6 | R | 6 | 2.00 | R |
| 7 | R | 3 | 3.99 | R |
| 8 | R | 0 | 3.28 | R |
| 9 | R | 45 | 2.73 | R |
| 10 | R | 49 | 4.41 | R |
| 11 | R | −40 | 3.49 | R |
| 12 | R | −1 | 2.67 | R |
| 13 | R | 1 | 3.40 | R |
| 14 | R | 30 | 1.77 | NR |
| 15 | R | 60 | 11.92 | R |
| 16 | R | 6 | 1.84 | R |
| 17 | R | 2 | 2.16 | R |
| 18 | R | 4 | 5.51 | R |
| 19 | R | 3 | 2.01 | R |
| 20 | R | 15 | 0.49 | NR |
| 21 | R | 3 | 1.00 | NR |
| 22 | R | −5 | 4.10 | R |
| 1 | NR | −14 | 2.10 | R |
| 2 | NR | −1 | 0.75 | NR |
| 3 | NR | 56 | 1.10 | NR |
| 4 | NR | 2 | 1.10 | NR |
Table 4.
Performance of the threshold MDC: PDC ratio ≥1.78 which predicts Rejectors status, in the screening (n=30) and validation (n=18) cross-sectional cohorts.
| Sensitivity | Specificity | Accuracy | Threshold | |
|---|---|---|---|---|
| Screening cohort (n=30) | 76.9% (10 of 13 correct) | 88.2% (15 of 17 correct) | 83.3% | 1.78 |
| Replication cohort (n=18) | 88.9% (8 of 9 correct) | 78.8% (7 of 9 correct) | 83.3% |
The threshold MDC: PDC ratio ≥1.78 was met in 10 of 13 Rejectors (sensitivity 76.9%), while the ratio was <1.78 in 15 of 17 Non-Rejectors (specificity 88.2%) in the cross-sectional screening cohort. In the cross-sectional replication cohort, the threshold MDC: PDC ratio ≥1.78 was met in 8 of 9 Rejectors (sensitivity 88.8%), while the ratio was <1.78 in 7 of 9 Non-Rejectors (specificity 78.8%) (Table 4). During the 1–60-day time period in the longitudinal cohort, the threshold MDC: PDC ratio ≥1.78 was met in 4 of 13 Rejectors (sensitivity 30.76%), while the ratio was <1.78 in 10 of 16 Non-Rejectors (specificity 62.50%).
Logistic regression models built exclusively with 1–60-day data from the longitudinal cohort, failed to show any association between rejection outcomes and DC subsets.
Tacrolimus is associated with reduced PDC frequencies during the late post-LTx period, but not early after LTx
We asked if DC subsets failed to achieve comparable sensitivity and specificity in the longitudinal cohorts due to differential effects of immunosuppressants such as Tacrolimus, on DC subsets. It must be noted that higher FKWB targets of roughly 10 ng/ml were used during days 1–60 after LTx. Also, FKWB were not significantly different between Rejectors and Non-Rejectors. FKWB were maintained within a lower range in the cross-sectional cohort, especially among Non-Rejectors. A significant negative correlation was seen between FKWB and PDC frequencies in the cross-sectional cohort (Spearman r = −0.370, p=0.005)(Supplementary Figure 1), but not in the 1–60-day time period in the longitudinal cohort.
Discussion
Our study represents the first characterization of DC subsets in children with LTx, who have been uniformly immunosuppressed with the lymphocyte depleting agent rATG, and steroid-free Tacrolimus monotherapy. Several findings are noteworthy. Firstly, we show a significant association between rejection outcomes, and an elevated MDC: PDC ratio during the later post-LTx period using screening/replication testing. This increase likely occurs due to higher absolute MDC counts among Rejectors, as seen in the longitudinal cohort. Second, we define an MDC: PDC threshold ratio of 1.78, at or above which Rejectors can be distinguished from Non-Rejectors. Previous work from our center has shown significant differences in the DC ratio between rejectors and non-rejectors among children with LTx (18). Third, we find that of the two DC subsets, PDC frequencies show larger differences between Rejectors and Non-Rejectors in either cohort, cross-sectional or longitudinal. These findings reproduce those seen previously, whereby PDC frequencies in non-rejecting children with LTx normalized toward baseline values seen in normal controls (18). These previous studies were performed an average of 4 years after LTx in children who did not receive lymphocyte-depleting induction therapy. Therefore, the relationship between DC subset subsets and transplant outcomes in rATG – induced children suggests a generic utility for DC subset analysis in different types of immunosuppressive regimens.
The lack of a clear association between DC subsets and rejection outcome during the early risk period for LTx rejection is puzzling, compared with the association with rejection, during the 1–3 year time period after LTx. We therefore sought confounding relationships between DC subsets and immunosuppressants, exemplified by Tacrolimus. We did so because Tacrolimus whole blood concentrations (FKWB) are maintained in a narrow range early after LTx, and are similar in the two outcome groups (Mean ± SD: Non-Rejector 9.35±3.8 vs Rejector 12.2±5.7 ng/ml, p=NS). During the 1–3 year time-period, significantly higher FKWB were seen among Rejectors, compared with Non-Rejectors. We find that FKWB potentially lowers PDC frequencies, but not MDC frequencies during the 1–3 year period after LTx. No such effect is observed during the early 1–60 day after LTx period. This apparent relative resistance of DC to a potential effect of Tacrolimus early after LTx may have several explanations. First, it is possible that the smaller cohort and narrow range of FKWB during the early post-LTx period may have masked the significance of any relationships between drug levels and DC subsets. Secondly, rejection-related changes in DC subsets may have been blunted by variable reconstitution kinetics of MDC and PDC after rATG-induced depletion, or due to an altered sensitivity of reconstituted DC to Tacrolimus. It is known that the polyclonal rATG has antibody specificities toward many antigens expressed by PBL, some of which are expressed on DC, e.g. HLA-DR (22). It is also known that rATG may render several types of PBL refractory to mitogen stimulation (23).
Our findings add to the relatively scant literature on DC subsets in LTx, by identifying conditions under which DC monitoring may have clinical utility after polyclonal lymphocyte depletion. Our findings also show fundamental differences with previous observations. For example, in a cardiac transplant population, MDC decreased immediately before cardiac allograft rejection, which occurred after induction with multiple doses of polyclonal anti-lymphocyte antibodies, and maintenance immunosuppression with multiple drugs including steroids (20). In our subject population, a relative excess of MDC was seen at times corresponding to early and late rejection, likely because limited doses of rATG were used for lymphocyte depletion, and because maintenance immunosuppression consisted only of Tacrolimus monotherapy. One way to reconcile these differences would be to evaluate the kinetics of DC reconstitution after varying doses of induction therapy. These studies are being initiated at our center.
Supplementary Material
Abbreviations
- ACR
Acute Cellular Rejection
- DC
Dendritic Cells
- FKWBC
Tacrolimus Whole Blood Concentration
- LOO-CV
Leave one out-Cross validation
- LTx
Liver Transplantation
- MDC
Myeloid CD11c+DC
- PDC
Plasmacytoid CD123+DC
- rATG
Rabbit anti-thymocyte globulin
- ROC
Receiver operating characteristic
Footnotes
Support: 5RO1AI49156, 5RO1AI073895, Children’s Hospital Research Foundation, and Hillman Research Foundation.
References
- 1.Fridell JA, Jain A, Reyes J, et al. Causes of mortality beyond 1 year after primary pediatric liver transplant under tacrolimus. Transplantation. 2002;27:74(12):1721–4. doi: 10.1097/00007890-200212270-00014. [DOI] [PubMed] [Google Scholar]
- 2.Soltys KA, Mazariegos GV, Squires RH, Sindhi RK, Anand R SPLIT Research Group. Late graft loss or death in pediatric liver transplantation: an analysis of the SPLIT database. Am J Transplant. 2007;7(9):2165–71. doi: 10.1111/j.1600-6143.2007.01893.x. [DOI] [PubMed] [Google Scholar]
- 3.Balistreri WF, Bucuvalas JC, Ryckman FC. The effect of immunosuppression on growth and development. Liver Transpl Surg. 1995;1 (5 Suppl 1):64–73. [PubMed] [Google Scholar]
- 4.Jain A, Nalesnik M, Reyes J, et al. Post-Transplant lymphoproliferative disorders in Liver Transplantation (LTx): A Twenty Year experience. Ann Surg. 2002;236(4):429–37. doi: 10.1097/00000658-200210000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calne R. Prope tolerance: a step in the search for tolerance in the clinic. Transplant Proc. 2000;32(7):2058–9. doi: 10.1016/s0041-1345(00)01557-8. [DOI] [PubMed] [Google Scholar]
- 6.Hale SJ, Mannon DA, Kleiner RB, et al. Kidney transplantation with rabbit antithymocyte globulin induction and sirolimus monotherapy. Lancet. 2002;360(9346):1662–4. doi: 10.1016/S0140-6736(02)11606-0. [DOI] [PubMed] [Google Scholar]
- 7.Starzl TE, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2000;361 (9638):1502–10. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eason JD, Nair S, Cohen AJ, Blazek JL, Loss GE., Jr Steroid-free liver transplantation using rabbit antithymocyte globulin and early tacrolimus monotherapy. Transplantation. 2003;75(8):1396–9. doi: 10.1097/01.TP.0000062834.30922.FE. [DOI] [PubMed] [Google Scholar]
- 9.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. Journal of Immunology. 2001;166(2):973–81. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson AC, Martin JN, Younger SR, et al. Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen-specific T cells. Journal of Immunological Methods. 2003;283(1–2):141–53. doi: 10.1016/j.jim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Newell KA, Larsen CP. Tolerance assays: measuring the unknown. Transplantation. 2006;81(11):1503–9. doi: 10.1097/01.tp.0000222912.69532.1e. [DOI] [PubMed] [Google Scholar]
- 12.Olweus J, BitMansour A, Warnke R, et al. Dendritic cell ontogeny: A human dendritic cell lineage of myeloid origin. PNAS. 1997;94:12551–556. doi: 10.1073/pnas.94.23.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solari MG, Thomson AW. Human dendritic cells and transplant outcome. Transplantation. 2008;85(11):1513–22. doi: 10.1097/TP.0b013e318173a768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 15.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7(8):610–21. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 16.Ochando JC, Homma C, Yang Y, et al. Alloantigen presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nature Immunology. 2006;7(6):652–6. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 17.Grouard G, Rissoan MC, Filgueira L, et al. The Enigmatic Plasmacytoid T Cells Develop into Dendritic Cells with Interleukin (IL)-3 and CD40-Ligand. JEM. 1997;185(6):1101–1112. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazariegos GV, Zahorchak AF, Reyes J, et al. Dendritic cell subset ratio in peripheral blood correlates with successful withdrawal of immunosuppression in liver transplant patients. Am J Transplant. 2003;3(6):689–96. doi: 10.1034/j.1600-6143.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 19.Womer KL, Peng R, Patton PR, et al. The effects of renal transplantation on peripheral dendritic cells. Clin Transplant. 2005;19:659–667. doi: 10.1111/j.1399-0012.2005.00405.x. [DOI] [PubMed] [Google Scholar]
- 20.Athanassopoulos P, Vaessen LM, Maat AP, et al. Preferential depletion of blood myeloid dendritic cells during acute cardiac allograft rejection under controlled immunosuppression. Am J Transplant. 2005;5 (4 Part 1):810–20. doi: 10.1111/j.1600-6143.2005.00777.x. [DOI] [PubMed] [Google Scholar]
- 21.Sindhi R, Magill A, Abdullah AM, et al. Enhanced Donor-specific Alloreactivity occurs independent of immunosuppression in children with early liver allograft rejection. Am J Transplant. 2005;5:96–102. doi: 10.1111/j.1600-6143.2004.00639.x. [DOI] [PubMed] [Google Scholar]
- 22.Bourdage JS, Hamlin DM. Comparative polyclonal antithymocyte globulin and antilymphocyte/antilymphoblast globulin anti-CD antigen analysis by flow cytometry. Transplantation. 1995;27:59(8):1194–200. [PubMed] [Google Scholar]
- 23.Merion RM, Howell T, Bromberg JS. Partial T-cell activation and anergy induction by polyclonal antithymocyte globulin. Transplantation. 1998;15:65(11):1481–9. doi: 10.1097/00007890-199806150-00013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.