Abstract
Annexin V is a ubiquitous intracellular protein in humans that has a variety of intriguing characteristics, including a nanomolar affinity for the membrane-bound constitutive anionic phospholipid known as phosphatidylserine (PS). PS is selectively expressed on the surface of apoptotic or physiologically stressed cells. As such, radiolabeled forms of annexin V have been used in both animal models and human Phase I and Phase II trials to determine if this tracer can be employed as an early surrogate marker of therapeutic efficacy in NSCLC and non-Hodgkin's lymphoma. Many other pulmonary imaging applications of radiolabeled annexin V are also possible, including the detection and monitoring of active pulmonary inflammation and other pathophysiologic stressors in a variety of diseases. In this article, the salient molecular features of apoptosis (and other forms of cell death) that permits imaging with radiolabeled annexin V will be discussed. The latest results from Phase II imaging trials with NSCLC and non-Hodgkin's lymphoma will be also be detailed. Finally, the potential future application of this tracer for the imaging of other pulmonary pathologies will be outlined.
Keywords: apoptosis; SPECT; annexin V; imaging, pulmonary
Despite over a decade of intense investigation, there is still no fully validated method for the imaging of apoptosis (irreversible injury) or physiologic cellular stress (potentially reversible injury) in humans. There have been multiple tracers proposed, but none as of yet has received FDA approval. Radiolabeled annexin V is one of the few radiotracers that has been widely used in Phase II trials in NSCLC and is still under development. In this article we will first outline the signaling pathways of apoptotic cell death that form the basis of radiolabeled annexin V imaging. Then the imaging applications of radiolabeled annexin V in animal models and clinical studies of several common cardiopulmonary pathologies will be examined. Lastly, the possible future directions of radiolabeled annexin V imaging will be described.
THE INTRINSIC AND EXTRINSIC PATHWAYS OF APOPTOTIC CELL DEATH
Apoptosis, or type I cell death, is the organized, energy-dependent self disassembly of unneeded or senescent cells (1, 2). When triggered by appropriate internal and/or external signals, these cells undergo pre-programmed cytoplasmic shrinkage, membrane blebbing, and budding off of intracellular contents carefully packaged into small membrane-bound packets called “apoptotic bodies.” Apoptotic bodies are subsequently ingested by adjacent cells and phagocytes without provoking an inflammatory response.
Before these morphologic changes there is an initiation sequence called the “lag or trigger phase” (3, 4). There are many triggers of apoptosis, such as withdrawal of growth factors, antihormonal therapy, DNA damage, immune reactions, ionizing radiation, chemotherapy, and ischemic injury. The lag time between exposure to the trigger(s) and the time of observable morphologic signs of apoptosis is highly variable depending heavily on cell type, type of trigger(s), its intensity and duration, as well as the local environmental conditions of the cell. Most apoptotic pathways, however, converge on a family of cysteine aspartate–specific proteases known as the “caspases” (5). When activated, each caspase, whether as an initiator (8–10) or executioner (3, 6, 7), cross-links and cleaves specific intracellular proteins involved with apoptosis.
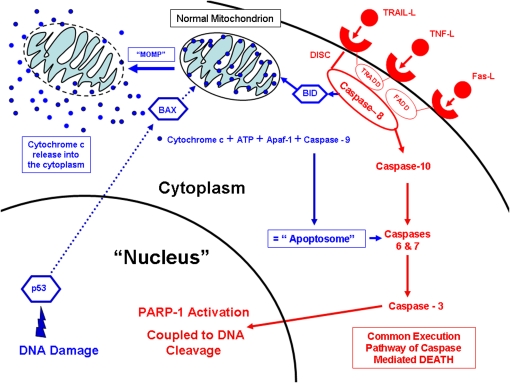
Activation of the executioner set of caspases occurs via the extrinsic (i.e., Type I Apoptosis, Figure 1 in red) and/or intrinsic (i.e., Type II Apoptosis, Figure 1 in blue) pathways of apoptosis. Type I and II caspase-dependent apoptosis are categorized more generally as Type I cell death (as opposed to Type II cell death, known as “autophagy” and defined later in this article). The extrinsic pathway is mediated by death receptors that bind specific molecules, including tumor necrosis factor (TNF) that binds to the TNF receptor (TNFR), TNF-related apoptosis-inducing ligand (TRAIL) that binds to the DR4 and DR5 death receptors, or Fas ligand (FasL) that binds to the Fas receptor. After binding to a given receptor, adapter molecules such as Fas-associated death domain (FADD) or tumor-associated death domain (TRADD) are recruited from the cytoplasm along with caspase-8 to form what is known as death-inducing signaling complex (DISC).
Figure 1.
Extrinsic and intrinsic pathways of apoptosis.
DISC propagates the “extrinsic” death signal by proteolytic activation of caspase 10 that in turns cleaves and activates the downstream executioner caspases. The final enzyme activated is caspase-3. Activated caspase-3 then travels to the nucleus and in turn activates poly-ADP-ribose polymerase (PARP-1), an enzyme that facilitates the degradation of nuclear DNA into 50- to 300-kilobase–sized pieces (i.e., “DNA ladder formation,” as seen by gel electrophoresis). Spliced or fragmented DNA can also be readily detected in situ and in vitro by the application of terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL)-based immunohistochemical or fluorescent methods. Activated caspase-3 also triggers other processes that produce the morphologic events of apoptosis. The net result is the orderly breakdown and self packaging of cellular proteins, including the cytoskeleton, and nuclear matrix (chromatin clumping and condensation that can be seen by bright light or fluorescent microscopy).
Alternatively, DISC can provoke the translocation of truncated Bcl interacting domain (BID), a pro-apoptotic Bcl 2 family protein, to mitochondria. BID induces the oligomerization of the pro-apoptotic proteins, BAK and BAX (Bcl-2 antagonist/Killer and Bcl-2–associated X protein, respectively). These proteins are required to form holes/channels in the outer mitochondrial membrane in a process known as mitochondrial outer membrane permeabilization (MOMP). These channels permit the escape of multiple proteins including cytochrome c from the mitochondrial intermembrane space to the cytoplasm. The release of cytochrome c into the cytoplasm is the hallmark of the intrinsic (mitochondrial) pathway of apoptosis. This is accompanied by the loss of the normally high negative mitochondrial membrane potential (ΔΨm). Cytochrome c interacts with apoptosis-activating factor-1 (Apaf-1), ATP, and pro-caspase 9 to form a structure known as the apoptosome. The apoptosome then cleaves and activates caspase 9, which in turn leads to the activation of caspase 3, 6, and 7. Activation of caspase 9 without involvement of the apoptosome has also been described (6).
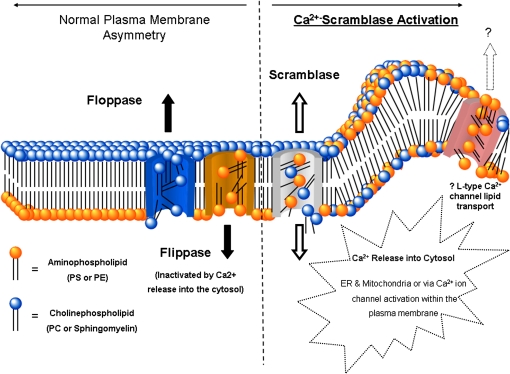
After caspase-3 activation, the terminal step of caspase-dependent apoptosis, there is a rapid redistribution and exposure of the anionic phospholipid phosphatidylserine (PS) on the cell surface (7, 8) (Figure 2). PS is normally restricted to the inner surface (inner leaflet) of the lipid bilayer by an ATP-dependent enzyme called “flippase (translocase).” Flippase, in concert with a second ATP-dependent enzyme, “floppase,” that pumps cationic phospholipids such as phosphatidylcholine (PC) and sphingomyelin to the cell surface, maintains an asymmetric distribution of different phospholipids between the inner and outer leaflets of the plasma membrane (9). The rapid redistribution of across the cell membrane (measured in minutes) is facilitated by a calcium-dependent deactivation of flippase and the activation of a third enzyme called “scramblase.” It is this selective exposure of PS that forms the basis of annexin V binding to apoptotic cells both in vitro and in vivo. In fact, the most widely used in vitro assay for apoptotic cells involves the use of fluorescent or biotinylated annexin V.
Figure 2.
Mechanisms of phosphatidylserine exposure with apoptosis. Reprinted by permission from Reference 72.
Annexin V is a ubiquitous intracellular human protein (MW ≈ 36,000, 319 amino-acid residues) that has a nanomolar affinity for membrane bound PS (10). The protein is folded into a planar cyclic arrangement of four repeats, with each repeat composed of five α-helical segments. The binding to membrane-bound PS is quite complex (beyond the scope of this article), and may involve the binding of eight PS molecules per molecule annexin V at low levels of membrane occupancy (with respect to protein) (11). The function(s) of annexin V, despite its widespread intracellular distribution are still unknown but a variety of in vitro and in vivo properties have been described (12, 13).
OTHER SIGNALING PATHWAYS THAT CAN INDUCE APOPTOTIC CELL DEATH
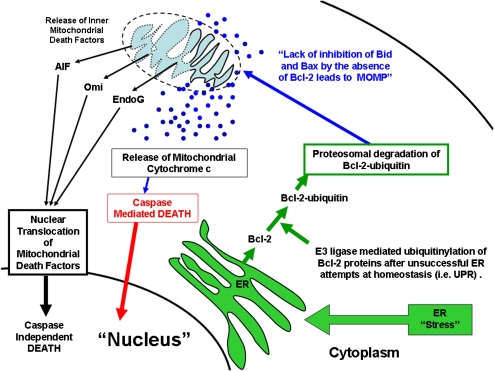
Under certain circumstances the inner mitochondrial membrane can also be permeabilized along with MOMP, releasing other proteins within the intercristal space including apoptosis-inducing factor (AIF), Omi, and EndoG (14) (Figure 3 in black). These factors are then translocated to the nucleus and, once there, induce the autodigestion of a cell's DNA, resulting in a noncaspase form of apoptosis.
Figure 3.
Alternate pathways of programmed cell death.
The endoplasmic reticulum (ER) can also trigger apoptosis (i.e., ER stress–induced cell death) (15) (Figure 3 in green). Normally, the ER is the site of secretory protein synthesis, conformational maturation, and quality control for correctly folded proteins. Proteins failing to adopt a stable conformation are dislocated into the cytosol, where they are targeted for ubiquitylation (a tag to identify a protein for elimination) and proteosomal degradation. Certain conditions and/or drugs can lead to the abnormal accumulation of unfolded proteins, resulting in ER stress. During ER stress, cells can re-achieve homeostasis by initiating a series of orchestrated events known as the unfolded protein response (UPR). If unsuccessful, ER stress can directly initiate a specific ubiquitin E3 ligase that tags anti-apoptotic Bcl-2 family members with ubiquitin. Subsequently, the proteosome degrades these anti-apoptotic molecules, thereby tipping the balance between pro- and anti-apoptotic factors toward apoptosis. Subsequently, mitochondria are activated, and pro-apoptotic factors such as cytochrome c, AIF, Omi, and others are released. As a result, both caspase-dependent and -independent signaling cascades can be activated and eventually lead to apoptotic cell death.
AUTOPHAGY AND ITS RELATIONSHIP(S) WITH APOPTOTIC CELL DEATH
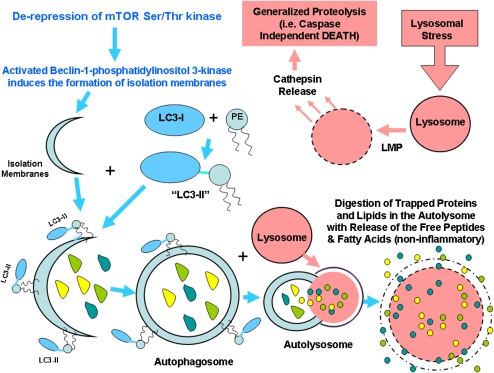
While apoptotic cells externalize PS, other forms of cell death can also demonstrate this same feature, including necrosis/oncosis, mitotic catastrophe, cell senescence, pyroptosis, PARP-1–mediated cell death, and autophagy (16). Autophagy (Type II cell death as opposed to apoptosis), otherwise known as “self-eating,” is a highly regulated form of cell death in a fashion that has considerable overlap with apoptosis (17) (Figure 4 in light blue). Autophagy is initiated by derepression of mTOR Ser/Thr kinase (mammalian target of rapamycin) quickly followed by the formation of the Beclin-1–class III phosphatidylinositol 3-kinase complex. This complex mediates the formation of isolation membranes that engulf targeted cytoplasmic material (or organelles) resulting in double-membraned vesicles called autophagosomes (autophagic vacuoles). Bcl-2 and Bcl-XL are regulators of beclin-1 and can be a link with apoptotic (Type I) cell death (18). Vesicle elongation requires the conjugation of phosphatidylethanolamine (PE) to LC3. Lipid conjugation leads to the conversion of the soluble form of LC3 (named LC3-I) to the autophagic vesicle–associated form (LC3-II). LC3-II is used as a marker of autophagy. Autophagosomes then undergo maturation by fusion with lysosomes to create autolysosomes. In the autolysosome, the inner membrane and the luminal contents of the autophagic vacuole are degraded without inciting inflammation.
Figure 4.
Major steps involved in autophagy (Type II cell death).
As opposed to apoptosis, autophagy normally serves a housekeeping function by removing unneeded, senescent, or damaged cytoplasmic contents. It also permits a cell to survive periods of cellular famine through the autodigestion of intracellular DNA/RNA, proteins, and lipids into free nucleotides, amino acids, and fatty acids, respectively. These free nucleotides, amino acids, and fatty acids can then be reused by a cell to maintain vital functions, such as macromolecular synthesis and energy production. Autophagic cell death, however, can be analternative to apoptosis if the classical apoptotic mechanisms are damaged or are inhibited. There can also be a massive induction of autophagy to such an extent that a cell can literally eat itself to death. In this circumstance PS is again exposed on the cell surface or the cell particles (autophagic vesicles) and can therefore be detected by radiolabeled annexin V.
A related form of cell death involves lysosomal stress (Figure 4 in pink) followed by LMP (lysosomal membrane permeabilization) and cathepsin releases that causes generalized proteolysis. Details of when and how this process occurs are still poorly understood, but again result in the accessibility of PS to extracellular radiolabeled annexin V.
PARP-1–MEDIATED CELL DEATH
PARP-1 normally functions as a DNA damage sensor, and its activation serves to repair low levels of DNA damage (19). With high levels of DNA damage, however, PARP-1 activation promotes cell death by consuming all available stores of NAD+, its primary substrate. As NAD+ can only be regenerated by cleavage of ATP, the cell literally runs out of energy and dies. Once the cell can no longer maintain its ATP-dependent membrane functions, it swells, resulting in necrosis/oncosis (i.e., irreversible membrane injury). With the loss of plasma membrane integrity, PS becomes accessible to relatively large impermeable molecules such annexin V and therefore detectable by radiolabeled annexin V imaging. Similarly necrotic cell death (by any noxious stimuli), a process characterized by the primary irreversible loss of membrane integrity, can also be detected by radiolabeled annexin V.
PS EXPOSURE AND THE COMMITMENT TO APOPTOSIS
While the set of events outlined above are believed to be largely correct, Balasubramanian and coworkers (20) have recently found that PS externalization can be reversibly induced in a process independent of cytochrome c release, caspase activation, or DNA fragmentation. Reversible PS externalization, however, does require a sustained elevation in cytosolic ionized calcium; an event that can be inhibited by calcium channel blockers in vitro. PS exposure, whether related to apoptosis (irreversible) or associated with reversible cellular events (physiologic stress), has also been found to be necessarily preceded by cell shrinkage and increased lipid mobility (decreased packing) (21).
Reversible PS externalization demonstrates far lower levels of PS exposure as compared with apoptosis and other forms of cell death (22, 23). The relatively low levels of PS exposure observed with reversible PS externalization can be readily counteracted by the prompt removal the offending physiologic stressor such as nitric-oxide, p53 activation, allergic mediators, or growth factor deprivation. However, if the stress remains uncorrected, a cell may undergo apoptosis.
Reversible uptake of radiolabeled annexin V has not only been observed in vitro but also in human models of forearm muscle exercised induced ischemia (24–26). The ability of annexin V to bind to cells with low but potentially reversible levels of PS exposure may also explain the uptake of radiolabeled annexin V outside regions of apoptosis (as seen histologically by TUNEL staining) in patients with hypoxic-ischemic reperfusion injury in the heart (27, 28) or brain (29–31).
In fact, even in regions with apoptosis/ischemic injury, there are far more annexin V–positive cells after the administration of radiolabeled annexin V than apoptotic nuclei as seen by TUNEL staining (28). These observations suggest that much (if not most) uptake of annexin V after ischemic reperfusion injury maybe due to large numbers of stressed cells (not necessarily committed to apoptosis) with relatively low levels of PS expression in contrast to the relatively fewer cells with high levels of PS exposure that are irreversibly committed to apoptosis.
The ability of radiolabeled annexin V to bind to “stressed” cells with relatively low levels of PS exposure also implies that annexin V imaging maybe far more sensitive than what would be expected if the tracer only localized to the relatively few apoptotic cells observed histologically. The ability of annexin V to localize cells that are stressed but not necessarily committed to apoptotic cell death suggests that this radiotracer can be used to identify tissues or organs at risk for irreversible injury, such as seen in hypoxic-ischemic injury (32) or chronic heart failure (33), or sites of active disease that are seen in infection (34–36), unstable atherosclerotic plaques (37), allograft rejection (38), or autoimmune disorders (39, 40).
Annexin V imaging could therefore be useful in the serial assessment of acute and chronic disorders in organs or tissues at risk for permanent damage in which prompt treatment with effective drug or surgical intervention may prevent irreversible cellular injury and cell death.
RADIOLABELED FORMS OF ANNEXIN V FOR ONCOLOGIC IMAGING
In an alternate clinical paradigm, annexin V imaging can also be used to noninvasively assess chemotherapeutic and radiation efficacy, treatments in which the induction of tumor cell apoptosis/necrosis is the major goal of therapy.
The first human trials with annexin V were conducted in patients with primary and metastatic lung tumors using human rh-annexin V labeled with 99mTc-pertechnetate by the penthioate radioligand (N2S2) method (41). This method begins with the chelation of 99mTc in the presence of stannous gluconate to yield 99mTc-gluconate, which is then reacted with acidified phenthioate ligand under heating to form a stable 99mTc–N2S2 complex. The 99mTc–N2S2-TFP ester complex is then randomly conjugated to the N-H groups of lysine of the protein at basic pH. Despite this cumbersome labeling method and nonspecific excretion of radiolabeled ligand into bile, Belhocine and colleagues (42) successfully conducted a study of 15 patients with cancer with pulmonary disease before and after chemotherapy. In this trial, a negative annexin V study after therapy (i.e., no change in tumor uptake of tracer from pretreatment baseline SPECT scans) correlated well with a lack of treatment response in six of eight patients. The remaining two patients (with metastatic breast cancer) actually had a clinically significant response to Taxol-based chemotherapy. All seven patients with increased tumor uptake over baseline (positive annexin V study), however, had an objective tumor response (shrinkage of tumor). Five of these patients showed increased annexin V uptake at the 40–48th hour after chemotherapy (1 NHL, 1HL, 1 SCLC, and 2 NSCLC), and two patients had increases in annexin V uptake observed at the 20–24th hour after treatment (1 NSCLC and 1 SCLC). Together, these preliminary results suggested a significant variability of the optimal timing with regard to the cancer type and therapeutic regimen (43).
An improved labeling method based on the bifunctional agent hydrazino nicotinamide (HYNIC) was selected for further clinical trials (44). Similar to the penthioate radioligand (N2S2 method, i.e., BTAP-Anx V kit), 99mTc-HYNIC–annexin V showed the greatest uptake in the kidneys, liver, and urinary bladder but demonstrated no biliary excretion (45). Multiple trials have confirmed the potential clinical utility of HYNIC–annexin V in determining the efficacy of chemotherapy (46–49). (See Figure 5.) Kartachova and coworkers (50) found that degree of tumor response to platinum-based chemotherapy (i.e., % decrease in tumor size 4 to 8 wk after therapy) in patients with non–small cell lung carcinoma directly correlated with the percentage increase in annexin V tumor uptake (as compared with pretreatment baseline) at 48 hours after the first injection of cisplatin, whereas a less successful treatment (i.e., stable disease) was associated with a slightly increased, unchanged, or even a slightly decreased annexin V tumor uptake (r2 = 0.86; P < 0.001). In patients with progressive disease (i.e., PD) a marked decrease of annexin V tumor uptake was noted as compared with baseline tumor uptake.
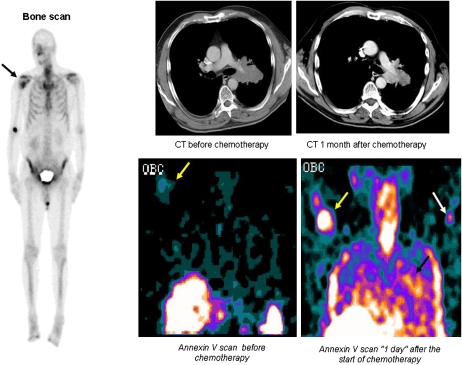
Figure 5.
Early detection of tumor response in non–small cell lung cancer (NSCLC) with radiolabeled Annexin V. A 70-year-old man with stage IV NSCLC presenting with a new right humeral metastasis as demonstrated on bone scan (black arrow). 99mTc-HYNIC-Annexin V assessment (in the coronal plane) revealed a faint uptake at the level of the bony metastasis 2 hours before the first course of chemotherapy (yellow arrows), which significantly increased “1 day” after the start of treatment; in addition, increased tracer uptake was detected within the primary lung tumor as early as 1 day after the initiation of cisplatinum-based chemotherapy (black arrow). White arrow shows intravenous injection site of left arm. Chest computed tomography (axial or transverse plane) showed a response to treatment 1 month after treatment with a decrease of the greatest tumor diameter from 7.5 cm to 6.5 cm. This patient was still alive after 126 days. This clinical case, which was part of a Phase II/III clinical trial (NAS 2021, Middelheim Hospital, Antwerp, Belgium), illustrates the capability of 99mTc-HYNIC-annexin V to localize at tumor sites undergoing spontaneous and chemotherapy-induced apoptosis. Reprinted by permission from Reference 73.
Kartachova and colleagues (51) also systematically examined how best to measure chemotherapy induced increases in annexin V uptake as seen by SPECT in a study of 38 patients with lymphoma (n = 31), non–small cell lung cancer (n = 4), and head and neck squamous cell carcinoma (n = 3). Maximal counts per pixel in the tumor volume (Cmax) were calculated for every target lesion in addition to grading on a visual four-grade score (i.e., Cmax /expressed as percentages of baseline values: grade −1, decrease > 25%; grade 0, 1–25% decrease; grade +1, 1–25% increase; grade +2, > 25% increase; visual analysis: 0 = absent, 1 = weak, 2 = moderate, 3 = intense). Both the quantitative and visual assessments of increases in annexin V uptake after treatment correlated well with therapeutic outcome as determined by RECIST criteria (r = 0.99 [P < 0.0001] and r = 0.97 [P < 0.0001], respectively]. Excellent intra-observer reproducibility, with high kappa values (0.82–0.90) and an inter-observer variability of 0.82, suggest that chemotherapy-induced increases in annexin V uptake seen at SPECT can be consistently and routinely applied to the early (24–48 h after initiation of therapy) noninvasive assessment of anticancer treatment efficacy, weeks before actual tumor shrinkage.
Another recent clinical finding is that the uptake of annexin V in normal tissues such as the spleen and bone marrow, organs that are susceptible to drug-induced injury, is not significantly changed with chemotherapy or prior administration of radiolabeled annexin V within a 48-hour period (52). Furthermore, these investigators also found that the biodistribution of radiolabeled annexin V in the kidneys, liver, and whole body remained unchanged after chemotherapy or prior administration of tracer.
MUTANT FORMS OF RADIOLABELED ANNEXIN V FOR RADIONUCLIDE IMAGING
There are alternative methods to radiolabel annexin V, including the use of self-chelating annexin V mutants; V-117 (53) and V-128 (54, 55). These proteins have an endogenous site for 99mTc chelation consisting of six amino acid tags added at the N-terminus, followed by amino acids 1–320 of wild-type annexin V, while the amino acid Cys-316 is also mutated to serine. 99mTc chelation is thought to occur via formation of an N3S structure involving the N-terminal cysteine and the immediately adjacent amino acids. The purified protein is then reduced and stored for later labeling with 99mTc using glucoheptonate as an exchange reagent.
Both V-117 and V-128 have major advantages over HYNIC–annexin V, including a 50 to 75% decrease renal uptake of 99mTc and a markedly improved in vivo localization to sites of apoptosis in animal models (56). Using related annexin mutants, it has been found that all four calcium-binding sites are needed for full in vitro and in vivo binding of annexin V. Mutation (loss of function) of any one the four calcium-binding sites decreased in vivo location of tracer by 25% and any two site mutations resulted in a 50% decline. Further work also has established that random modification of the lysine residues of annexin V with HYNIC, mercaptoacetyltriglycine (MAG3), fluorescein isothiocyanate, and biotin-labeled annexin V showed a 50% decrease in liver uptake of tracer as compared with self-chelating (site-specific) protein. The adverse effects of the random modification of annexin V have also been observed with 111In-DTPA-PEG–annexin V (57). Annexin V has also been proven to be quite heat labile and loses most of its activity even with heating at 56°C for 10 minutes (58) (while being quite stable at 37°C) precluding the use of many different types of labeling chemistries.
As compared with SPECT, PET has major advantages for quantitative imaging, and has spurred the development of several approaches to label annexin V with fluorine 18 (18F) (59). One method has used N-succinimidyl 4-fluorobenzoate to synthesize F–annexin V. The fluorine-labeled agent has lower uptake in the liver, spleen, and kidney compared with HYNIC–annexin V. Another method involves site-specific derivatization with an 18F-maleimide–labeled compound to mutant annexin V-117 or annexin V-128 (60). Both these methods however, need more preclinical study before further development as imaging markers of apoptosis.
FDG PET AND ANNEXIN V SPECT IMAGING
The relationship between 18F-FDG uptake as seen by PET as compared with annexin V SPECT imaging has not been systematically compared in clinical trials. Tumor models, however, have demonstrated that that an enhanced apoptotic reaction (increased radiolabeled annexin V uptake) correlated with suppressed tumor glucose utilization (decreased FDG uptake) 48 hours after the start of cytotoxic chemotherapy (61). In a trial of 45 patients with breast cancer receiving three cycles of neoadjuvant chemotherapy before and after therapy (before surgical removal of primary tumor), PET scans with 18F-FDG showed significant decreases in tracer uptake coupled to marked increases in TUNEL-positive (apoptotic) tumor cells (62). These data suggested that neoadjuvant chemotherapy may effectively induce apoptosis in breast tumors and decrease their glucose uptake.
Decreases in FDG uptake have also been confirmed in several studies, including: (1) human gastric tumor cells treated with epirubicin, cisplatin, and 5-fluorouracil (63); (2) effective anticancer therapy for patients with GIST (gastrointestinal stromal tumor) with the selective tyrosine kinase inhibitor, imatinib mesylate (STI571,Gleevec) (64); and (3) and EGFR kinase inhibition of non–small cell lung cancer with gefitinib (65).
However, because apoptosis must use energy, at least initially, glucose demand may increase temporarily in some clinical situations (66). One example appears to be the “metabolic flare” often observed on 18F-FDG PET images after hormonal therapy administrated for estrogen receptor–positive human breast cancer (67). The metabolic flare in this select patient population may also be a useful indicator of responsiveness to anti-estrogen therapy as opposed to nonhormonal chemotherapy.
In summary, while apoptosis is an energy requiring process, it appears that outside hormonal therapy for breast cancer, short-term tumor response is directly correlated with a significant decrease in FDG uptake.
ANNEXIN V DEVELOPMENT AND IMPLICATIONS FOR PULMONARY IMAGING
With the current lack of GMP-grade HYNIC–annexin V kits for clinical imaging trials due to the closure of the Theseus Imaging Corporation (Cambridge, MA), there is a great unmet need to complete not only the studies of patients with NSCLC and NHL but to pursue new imaging trials for other pulmonary diseases in which apoptosis and cellular stress play important pathophysiologic roles (68, 69). These diseases include acute respiratory distress syndrome (ARDS), respiratory distress syndrome (RDS) (70), chronic obstructive pulmonary disease (COPD), bronchial asthma, usual interstitial pneumonitis (UIP), idiopathic pulmonary fibrosis (IPF) (71), bronchiolitis obliterans with organizing pneumonitis (BOOP), and a variety of viral, bacterial, and fungal infections. Common to all these important disease entities is the increased rates of apoptosis of alveolar cells with or without proliferation of myofibroblasts in response to a variety of ongoing stresses. These include reactive oxygen species (ROS, NO, etc.), overexpression of Fas (death) receptors, increased presence of inflammatory cells and protease activity, and in many cases decreased levels of VEGF and VEGF receptor expression (i.e., loss of normal growth factors for the vascular endothelial cells of the lung vessels/capillaries). The basis of therapy for these pulmonary diseases, as opposed to cancer treatment, is the prevention of apoptosis (irreversible tissue injury) and the reversal of physiologic stress or neutralization of an infectious/inflammatory agent(s).
As radiolabeled annexin V has the demonstrated ability to identify both stressed and apoptotic cells in the lung and other sites in the body, it should prove to be a very helpful agent in determining presence, extent, and severity of active disease, as well as the serial monitoring of the effect(s) of therapy in the pulmonary disorders listed above.
Supported in part by NIH Grant # EB000898 (to F.G.B.).
Conflict of Interest Statement: F.G.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ameisen JC. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Differ 2002;9:367–393. [DOI] [PubMed] [Google Scholar]
- 2.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 2005;73:1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan A, Reiter R, Wiese S, Fertig G, Gold R. Plasma membrane phospholipid asymmetry precedes DNA fragmentation in different apoptotic cell models. Histochem Cell Biol 1998;110:553–558. [DOI] [PubMed] [Google Scholar]
- 4.Martin SJ, Reutelingsperger CPM, McGahon AJ. Early redistribution of plasma membrane phosphatidylserine in a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 1995;182:1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huerta S, Goulet EJ, Huerta-Yepez S, Livingston EH. Screening and detection of apoptosis. J Surg Res 2007;139:143–156. [DOI] [PubMed] [Google Scholar]
- 6.Sperandio S, de Belle I, Bredesen DE. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci USA 2000;97:14376–14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zwaal RFA, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood 1997;89:1121–1132. [PubMed] [Google Scholar]
- 8.Zwaal RFA, Comfurius P, Bevers EM. Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci 2005;62:971–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood BL, Gibson DF, Tait JF. Increased phosphatidylserine exposure in sickle cell disease: flow cytometric measurement and clinical associations. Blood 1996;88:1873–1880. [PubMed] [Google Scholar]
- 10.Boersma HH, Kietselaer BL, Stolk LM, Bennaghmouch A, Hofstra L, Narula J, Heidendal GA, Reutelingsperger CP. Past, present, and future of annexin A5: from protein discovery to clinical applications. J Nucl Med 2005;46:2035–2050. [PubMed] [Google Scholar]
- 11.Meers P, Mealy T. Calcium-dependent annexin V binding to phospholipids: stoichiometry, specificity, and the role of negative charge. Biochemistry 1993;32:11711–11721. [DOI] [PubMed] [Google Scholar]
- 12.Lahorte CMM, Vanderheyden J-L, Steinmetz N, Van de Wiele C, Dierckx RA, Slegers G. Apoptosis-detecting radioligands: current state of the art and future perspectives. Eur J Nucl Med Mol Imaging 2004;31:887–919. [DOI] [PubMed] [Google Scholar]
- 13.Munoz LE, Frey B, Pausch F, Baum W, Mueller RB, Brachvogel B, Poschl E, Rödel F, von der Mark K, Herrmann M, Gaipl US. The role of annexin A5 in the modulation of the immune response against dying and dead cells. Curr Med Chem 2007;14:271–277. [DOI] [PubMed] [Google Scholar]
- 14.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 2004;305:626–629. [DOI] [PubMed] [Google Scholar]
- 15.Egger L, Madden DT, Rheme C, Rao RV, Bredesen DE. Endoplasmic reticulum stress-induced cell death mediated by the proteasome. Cell Death Differ 2007;14:1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verheij M. Clinical biomarkers and imaging for radiotherapy-induced cell death. Cancer Metastasis Rev 2008;27:471–480. [DOI] [PubMed] [Google Scholar]
- 17.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008;132:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 2007;8:741–752. [DOI] [PubMed] [Google Scholar]
- 19.Aguilar-Quesada R, Muñoz-Gámez JA, Martín-Oliva D, Peralta-Leal A, Quiles-Pérez R. Rodríguez-Vargas JM, Ruiz de Almodóvar M, Conde C, Ruiz-Extremera A, Oliver FJ. Modulation of transcription by PARP-1: consequences in carcinogenesis and inflammation. Curr Med Chem 2007;14:1179–1187. [DOI] [PubMed] [Google Scholar]
- 20.Balasubramanian K, Mirnikjoo B, Schroit AJ. Regulated externalization of phosphatidylserine at the cell surface: implication for apoptosis. J Biol Chem 2007;282:18357–18364. [DOI] [PubMed] [Google Scholar]
- 21.Elliott JI, Sardini A, Cooper JC, Alexander DR, Davanture S, Chimini G, Higgins CF. Phosphatidylserine exposure in B lymphocytes: a role for lipid packing. Blood 2006;108:1611–1617. [DOI] [PubMed] [Google Scholar]
- 22.Hammill AK, Uhr JW, Scheuermann RH. Annexin V staining due to loss of membrane asymmetry can be reversible and precede commitment to apoptotic death. Exp Cell Res 1999;251:16–21. [DOI] [PubMed] [Google Scholar]
- 23.Geske FJ, Lieberman R, Strange R, Gerschenson LE. Early stages of p53-induced apoptosis are reversible. Cell Death Differ 2001;8:182–191. [DOI] [PubMed] [Google Scholar]
- 24.Riksen NP, Oyen WJ, Ramakers BP, Van den Broek PH, Engbersen R, Boerman OC, Smits P, Rongen GA. Oral therapy with dipyridamole limits ischemia-reperfusion injury in humans. Clin Pharmacol Ther 2005;78:52–59. [DOI] [PubMed] [Google Scholar]
- 25.Rongen GA, Oyen WJ, Ramakers BP, Riksen NP, Boerman OC, Steinmetz N, Smits P. Annexin A5 scintigraphy of forearm as a novel in vivo model of skeletal muscle preconditioning in humans. Circulation 2005;111:173–178. [DOI] [PubMed] [Google Scholar]
- 26.Riksen NP, Zhou Z, Oyen WJ, Jaspers R, Ramakers BP, Brouwer RM, Boerman OC, Steinmetz N, Smits P, Rongen GA. Caffeine prevents protection in two human models of ischemic preconditioning. J Am Coll Cardiol 2006;48:700–707. [DOI] [PubMed] [Google Scholar]
- 27.Thimister PW, Hofstra L, Liem IH, Boersma HH, Kemerink G, Reutelingsperger CP, Heidendal GA. In vivo detection of cell death in the area at risk in acute myocardial infarction. J Nucl Med 2003;44:391–396. [PubMed] [Google Scholar]
- 28.Sarda-Mantel L, Michel JB, Rouzet F, Martet G, Louedec L, Vanderheyden JL, Hervatin F, Raguin O, Vrigneaud JM, Khaw BA, et al. (99m)Tc-annexin V and (111)In-antimyosin antibody uptake in experimental myocardial infarction in rats. Eur J Nucl Med Mol Imaging 2006;33:239–245. [DOI] [PubMed] [Google Scholar]
- 29.Lorberboym M, Blankenberg FG, Sadeh M, Lampl Y. In vivo imaging of apoptosis in patients with acute stroke: correlation with blood-brain barrier permeability. Brain Res 2006;1103:13–19. [DOI] [PubMed] [Google Scholar]
- 30.Blankenberg FG, Kalinyak J, Liu L, Koike M, Cheng D, Goris ML, Green A, Vanderheyden JL, Tong DC, Yenari MA. 99mTc-HYNIC-annexin V SPECT imaging of acute stroke and its response to neuroprotective therapy with anti-Fas ligand antibody. Eur J Nucl Med Mol Imaging 2006;33:566–574. [DOI] [PubMed] [Google Scholar]
- 31.Tang X-N, Wang Q, Koike M, Cheng D, Goris ML, Blankenberg FG, Yenari MA. Monitoring the protective effects of minocycline treatment with radiolabeled annexin V in an experimental model of acute focal cortical ischemia. J Nucl Med 2007;48:1822–1828. [DOI] [PubMed] [Google Scholar]
- 32.Taki J, Higuchi T, Kawashima A, Fukuoka M, Kayano D, Tait JF, Matsunari I, Nakajima K, Kinuya S, Strauss HW. Effect of postconditioning on myocardial 99mTc-annexin-V uptake: comparison with ischemic preconditioning and caspase inhibitor treatment. J Nucl Med 2007;48:1301–1307. [DOI] [PubMed] [Google Scholar]
- 33.Kietselaer BL, Reutelingsperger CP, Boersma HH, Heidendal GA, Liem IH, Crijns HJ, Narula J, Hofstra L. Noninvasive detection of programmed cell loss with 99mTc-labeled annexin A5 in heart failure. J Nucl Med 2007;48:562–567. [DOI] [PubMed] [Google Scholar]
- 34.Lorberboym M, Feldbrin Z, Hendel D, Blankenberg FG, Schachter P. The use of 99mTc-recombinant human annexin V imaging for differential diagnosis of aseptic loosening and low-grade infection in hip and knee prostheses. J Nucl Med 2009;50:534–537. [DOI] [PubMed] [Google Scholar]
- 35.Rouzet F, Dominguez Hernandez M, Hervatin F, Sarda-Mantel L, Lefort A, Duval X, Louedec L, Fantin B, Le Guludec D, Michel JB. Technetium 99m-labeled annexin V scintigraphy of platelet activation in vegetations of experimental endocarditis. Circulation 2008;117:781–789. [DOI] [PubMed] [Google Scholar]
- 36.Kietselaer BL, Narula J, Hofstra L. The Annexin code: revealing endocarditis. Eur Heart J 2007;28:948. [DOI] [PubMed] [Google Scholar]
- 37.Tahara N, Imaizumi T, Virmani R, Narula J. Clinical feasibility of molecular imaging of plaque inflammation in atherosclerosis. J Nucl Med 2009;50:331–334. [DOI] [PubMed] [Google Scholar]
- 38.Narula J, Acio ER, Narula N, Samuels LE, Fyfe B, Wood D, Fitzpatrick JM, Raghunath PN, Tomaszewski JE, Kelly C, et al. Annexin-V imaging for noninvasive detection of cardiac allograft rejection. Nat Med 2001;7:1347–1352. [DOI] [PubMed] [Google Scholar]
- 39.Peker C, Sarda-Mantel L, Loiseau P, Rouzet F, Nazneen L, Martet G, Vrigneaud JM, Meulemans A, Saumon G, Michel JB, et al. Imaging apoptosis with (99m)Tc-annexin-V in experimental subacute myocarditis. J Nucl Med 2004;45:1081–1086. [PubMed] [Google Scholar]
- 40.Tokita N, Hasegawa S, Maruyama K, Izumi T, Blankenberg FG, Tait JF, Strauss HW, Nishimura T. 99mTc-Hynic-annexin V imaging to evaluate inflammation and apoptosis in rats with autoimmune myocarditis. Eur J Nucl Med Mol Imaging 2003;30:232–238. [DOI] [PubMed] [Google Scholar]
- 41.Boersma HH, Liem IH, Kemerink GJ, Thimister PW, Hofstra L, Stolk LM, van Heerde WL, Pakbiers MT, Janssen D, Beysens AJ, et al. Comparison between human pharmacokinetics and imaging properties of two conjugation methods for 99mTc-annexin A5. Br J Radiol 2003;76:553–560. [DOI] [PubMed] [Google Scholar]
- 42.Belhocine T, Steinmetz N, Hustinx R, Bartsch P, Jerusalem G, Seidel L, Rigo P, Green A. Increased uptake of the apoptosis-imaging agent (99m)Tc recombinant human Annexin V in human tumors after one course of chemotherapy as a predictor of tumor response and patient prognosis. Clin Cancer Res 2002;8:2766–2774. [PubMed] [Google Scholar]
- 43.Blankenberg F. To scan or not to scan, it is a question of timing: technetium-99m-annexin V radionuclide imaging assessment of treatment efficacy after one course of chemotherapy. Clin Cancer Res 2002;8:2757–2758. [PubMed] [Google Scholar]
- 44.Kemerink GJ, Liu X, Kieffer D, Ceyssens S, Mortelmans L, Verbruggen AM, Steinmetz ND, Vanderheyden J-L, Green A, Verbeke K. Safety, biodistribution, and dosimetry of 99mTc- HYNIC-annexin V, a novel human recombinant annexin V for human application. J Nucl Med 2003;44:947–952. [PubMed] [Google Scholar]
- 45.Van den Brande JM, Koehler TC, Zelinkova Z, Bennink RJ, te Velde AA, ten Cate FJ, van Deventer SJ, Peppelenbosch MP, Hommes DW. Prediction of antitumour necrosis factor clinical efficacy by real-time visualisation of apoptosis in patients with Crohn's disease. Gut 2007;56:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haas RL, de Jong D, Valdes Olmos RA, Hoefnagel CA, van den Heuvel I, Zerp SF, Bartelink H, Verheij M. In vivo imaging of radiation-induced apoptosis in follicular lymphoma patients. Int J Radiat Oncol Biol Phys 2004;59:782–787. [DOI] [PubMed] [Google Scholar]
- 47.Vermeersch H, Ham H, Rottey S, Lahorte C, Corsetti F, Dierckx R, Steinmetz N, Van de Wiele C. Intraobserver, interobserver, and day-to-day reproducibility of quantitative 99mTc-HYNIC annexin-V imaging in head and neck carcinoma. Cancer Biother Radiopharm 2004;19:205–210. [DOI] [PubMed] [Google Scholar]
- 48.Kartachova M, Haas RL, Olmos RA, Hoebers FJ, van Zandwijk N, Verheij M. In vivo imaging of apoptosis by 99mTc-Annexin V scintigraphy: visual analysis in relation to treatment response. Radiother Oncol 2004;72:333–339. [DOI] [PubMed] [Google Scholar]
- 49.Rottey S, Slegers G, Van Belle S, Goethals I, Van de Wiele C. Sequential 99mTc-hydrazinonicotinamide-annexin V imaging for predicting response to chemotherapy. J Nucl Med 2006;47:1813–1818. [PubMed] [Google Scholar]
- 50.Kartachova M, van Zandwijk N, Burgers S, van Tinteren H, Verheij M, Valdés Olmos RA. Prognostic significance of 99mTc Hynic-rh-annexin V scintigraphy during platinum-based chemotherapy in advanced lung cancer. J Clin Oncol 2007;25:2534–2539. [DOI] [PubMed] [Google Scholar]
- 51.Kartachova MS, Valdés Olmos RA, Haas RL, Hoebers FJ, van Herk M, Verheij M. 99mTc-HYNIC-rh-annexin-V scintigraphy: visual and quantitative evaluation of early treatment-induced apoptosis to predict treatment outcome. Nucl Med Commun 2008;29:39–44. [DOI] [PubMed] [Google Scholar]
- 52.Rottey S, Van den Bossche B, Slegers G, Van Belle S, van de Wiele C. Influence of chemotherapy on the biodistribution of [(99m)Tc]hydrazinonicotinamide annexin V in cancer patients. Q J Nucl Med Mol Imaging 2009;53:127–132. [PubMed] [Google Scholar]
- 53.Tait JF, Brown DS, Gibson DF, Blankenberg FG, Strauss HW. Development and characterization of annexin V mutants with endogenous chelation sites for (99m)Tc. Bioconjug Chem 2000;11:918–925. [DOI] [PubMed] [Google Scholar]
- 54.Jin M, Smith C, Hsieh HY, Gibson DF, Tait JF. Essential role of B-helix calcium binding sites in annexin V-membrane binding. J Biol Chem 2004;279:40351–40357. [DOI] [PubMed] [Google Scholar]
- 55.Tait JF, Smith C, Blankenberg FG. Structural requirements for in vivo detection of cell death with 99mTc-annexin V. J Nucl Med 2005;46:807–815. [PMC free article] [PubMed] [Google Scholar]
- 56.Tait JF, Smith C, Levashova Z, Patel B, Blankenberg FG, Vanderheyden JL. Improved detection of cell death in vivo with annexin V radiolabeled by site-specific methods. J Nucl Med 2006;47:1546–1553. [PubMed] [Google Scholar]
- 57.Ke S, Wen X, Wu QP, Wallace S, Charnsangavej C, Stachowiak AM, Stephens CL, Abbruzzese JL, Podoloff DA, Li C. Imaging taxane-induced tumor apoptosis using PEGylated, 111In-labeled annexin V. J Nucl Med 2004;45:108–115. [PubMed] [Google Scholar]
- 58.Van den Eijnde SM, Boshart L, Reutelingsperger CPM, De Zeeuw CI, Vermeij-Keers C. Phosphatidylserine plasma membrane asymetry in vivo: a pancellular phenomenon which alters during apoptosis. Cell Death Differ 1997;4:311–316. [DOI] [PubMed] [Google Scholar]
- 59.Murakami Y, Takamatsu H, Taki J, Tatsumi M, Noda A, Ichise R, Tait JF, Nishimura S. 18F-labelled annexin V: a PET tracer for apoptosis imaging. Eur J Nucl Med Mol Imaging 2004;31:469–474. [DOI] [PubMed] [Google Scholar]
- 60.Li X, Link JM, Stekhova S, Yagle KJ, Smith C, Krohn KA, Tait JF. Site-specific labeling of annexin V with F-18 for apoptosis imaging. Bioconjug Chem 2008;19:1684–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takei T, Kuge Y, Zhao S, Sato M, Strauss HW, Blankenberg FG, Tait JF, Tamaki N. Enhanced apoptotic reaction correlates with suppressed tumor glucose utilization after cytotoxic chemotherapy: use of 99mTc-Annexin V, 18F-FDG, and histologic evaluation. J Nucl Med 2005;46:794–799. [PubMed] [Google Scholar]
- 62.Li D, Yao Q, Li L, Wang L, Chen J. Correlation between hybrid 18F-FDG PET/CT and apoptosis induced by neoadjuvant chemotherapy in breast cancer. Cancer Biol Ther 2007;6:1442–1448. [DOI] [PubMed] [Google Scholar]
- 63.Suttie SA, Park KGM, Smith TAD. [18F]-2-Fluoro-2-deoxy-D-glucose incorporation by AGS gastric adenocarcinoma cells in vitro during response to epirubicin, cisplatin and 5-fluorouracil. Br J Cancer 2007;97:902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trent JC, Ramdas L, Dupart J, Hunt K, Macapinlac H, Taylor E, Hu L, Salvado A, Abbruzzese JL, Pollock R, et al. Early effects of imatinib mesylate on the expression of insulin-like growth factor binding protein-3 and positron emission tomography in patients with gastrointestinal stromal tumor. Cancer 2006;107:1898–1908. [DOI] [PubMed] [Google Scholar]
- 65.Su H, Bodenstein C, Dumont RA, Seimbille Y, Dubinett S, Phelps ME, Herschman H, Czernin J, Weber W. Monitoringtumor glucose utilization by positron emission tomography for the prediction of treatment response to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res 2006;12:5659–5667. [DOI] [PubMed] [Google Scholar]
- 66.Haberkorn U, Bellemann ME, Brix G, Kamencic H, Morr I, Traut U, Altmann A, Doll J, Blatter J, Kinscherf R. Apoptosis and changes in glucose transport early after treatment of Morris hepatoma with gemcitabine. Eur J Nucl Med 2001;28:418–425. [DOI] [PubMed] [Google Scholar]
- 67.Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol 2001;19:2797–2803. [DOI] [PubMed] [Google Scholar]
- 68.Tang PS, Mura M, Seth R, Liu M. Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol 2008;294:L632–L641. [DOI] [PubMed] [Google Scholar]
- 69.Kuwano K. Epithelial cell apoptosis and lung remodeling. Cell Mol Immunol 2007;4:419–429. [PubMed] [Google Scholar]
- 70.Bem RA, Bos AP, Matute-Bello G, van Tuyl M, van Woensel JB. Lung epithelial cell apoptosis during acute lung injury in infancy. Pediatr Crit Care Med 2007;8:132–137. [DOI] [PubMed] [Google Scholar]
- 71.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc 2006;3:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blankenberg F. In vivo imaging of apoptosis. Cancer Biol Ther 2008;7:1525–1532. [DOI] [PubMed] [Google Scholar]
- 73.Blankenberg F. In vivo detection of apoptosis. J Nucl Med 2008;49:81S–95S. [DOI] [PubMed] [Google Scholar]