Abstract
Methamphetamine (METH) is a psychostimulant that causes damage to dopamine (DA) axons and to non-monoaminergic neurons in the brain. The aim of the present study was to investigate short- and long-term effects of neurotoxic METH treatment on novelty-induced locomotor activity in mice. Male BALB/c mice, 12–14 weeks old, were injected with saline or METH (i.p., 7.5 mg/kg × 4 times, every 2 hours). Behavior and neurotoxic effects were assessed at 10 days, 3 and 5 months following drug treatment. METH administration caused marked decreases in DA levels in the mouse striatum and cortex at 10 days post-drug. However, METH did not induce any changes in novelty-induced locomotor activity. At 3 and 5 months after treatment METH-exposed mice showed significant recovery of DA levels in the striatum and cortex. In contrast, these animals demonstrated significant decreases in locomotor activity at 5 months in comparison to aged-matched control mice. Further assessment of METH toxicity using TUNEL staining showed that the drug induced increased cell death in the striatum and cortex at 3 days after administration. Taken together, these data suggest that delayed deficits in novelty-induced locomotor activity observed in METH exposed animals are not due to neurodegeneration of DA terminals but to combined effects of METH and age-dependent dysfunction of non-DA intrinsic striatal and/or corticostriatal neurons.
Keywords: methamphetamine, dopamine, neurotoxicity, striatum, cortex, novelty-induced locomotor activity
1. Introduction
The abuse of the illicit psychostimulant methamphetamine (METH) has reached epidemic proportions in the USA and throughout the world (Krasnova and Cadet, 2009). This is a growing public health disaster because chronic METH abuse is associated with serious health complications that include impairments in learning, memory, decision-making (Scott et al., 2007) and motor deficits (Caligiuri and Buitenhuys, 2005) in humans. In addition to these negative neuropsychiatric consequences, neuroimaging studies have found persistent decreases in the levels of dopamine transporters (DAT) and vesicular monoamine transporters 2 in the striatum and cortex (Sekine et al., 2001, 2003; Volkow et al., 2001; Johanson et al., 2006; McCann et al., 2008), indicating dopamine (DA) axonal damage in the brains of METH addicts. Postmortem analyses have also detected decreases in tyrosine hydroxylase (TH), DAT and DA levels in the striatum of chronic METH abusers (Wilson et al., 1996; Moszczynska et al., 2004). In addition to neurodegeneration of DA axons, METH addicts show loss of gray matter in the cortex, smaller hippocampus, and hypertrophy of the white matter, suggesting cell death and gliosis secondary to neuronal damage in the brain (Thompson et al., 2004).
Evidence has accumulated to show that these drug-induced neuropathological changes might underlie deficits in cognitive behaviors in chronic METH abusers (Scott et al., 2007). These ideas are further strengthened by data from animal studies showing that METH neurotoxicity is associated with impairments in motor learning (Chapman et al., 2001; Daberkow et al., 2005), novel object recognition (Schroder et al., 2003; He et al., 2006; Belcher et al., 2008; Herring et al., 2008) and spatial memory acquisition (Friedman et al., 1998) in rodents. In addition to cognitive deficits, METH also causes disturbances in balance beam performance and increased latency in active avoidance task, suggesting appearance of motor impairments in drug-treated animals [(Walsh and Wagner, 1992); however, see (Timar et al., 2003)]. Because the majority of previous reports have focused on short-term toxic effects and related cognitive deficits, the present study was undertaken to investigate short- and long-term consequences of neurotoxic METH treatment on novelty-induced locomotor activity in mice. Thus, we examined behavioral performance in mice 10 days, 3 and 5 months after METH administration using an open field test. In addition, we studied drug-induced damage to monoaminergic and to non-monoaminergic neurons in the mouse brain.
2. Materials and Methods
2.1 Animals and METH treatment
Male BALB/cByJ mice 12–14 weeks old, weighing 30–35 g and obtained from Jackson’s Lab (Bar Harbor, ME) were used in the study. All animal procedures were performed according to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the local Animal Care Committee. Prior to injections, mice were housed one per cage to eliminate fighting and to reduce stress associated with METH treatment. The mice were injected i.p. with dl-METH hydrochloride (7.5 mg/kg × 4 times, every 2 hours) or saline. METH caused an increase in locomotor activity, which disappeared approximately one hour after the last injection of the drug. Mice were tested for behavioral performance at different time intervals after treatment.
2.2 Open field test
Mice were examined for novelty-induced locomotor activity 10 days, 3 and 5 months after METH or saline injections in an open field test (Fig. 1). This test is widely used in the assessment of locomotor activity in a novel environment in studies of vulnerability to drugs of abuse (Piazza et al., 1989), for behavioral evaluation of knockout mice (Kelly et al., 1998; Fetsko et al., 2005), and in animal models of neurodegenerative disorders (Srinivasan and Schmidt, 2004; Tadaiesky et al., 2008). On the day of the experiment, mice were transported from the animal facility to the laboratory and tested in a darkened (red light) environment. The details of the open field test and apparatus were described previously (Krasnova et al., 2007). In brief, the apparatus consisted of a small plastic pool (diameter 120 cm / 30 cm deep) (Fig. 1). The arena was divided into four quadrants with medical tape. Mice were taken out of their home cages and placed into the first quadrant of the unfamiliar open field. The number of quadrants crossed (locomotor activity) and of rearings was monitored immediately following the exposure to the novel environment. Relative time spent in the central zone was also measured. Data were collected for 6 min at the same time of day (9 am to 12 pm) for each test period. The 6 min session was chosen because a short exposure captures the locomotor activity of animals in a novel environment. After that animals become habituated to the environment and their locomotion and rearing behavior decline (Thiel et al., 1999; Antoniou et al., 2008). The open field arena was thoroughly cleaned with 75% ethanol solution before each test to eliminate possible odor cues. Mice were videotaped during the performance with an overhead camcorder and open field behaviors were assessed using CleverSys Top Scan (CleverSys, Inc., Reston, VA) software. Statistical analysis of the data was done using factorial ANOVA (GraphPad Prizm 5, GraphPad Software, Inc., San Diego, CA). Differences were considered significant at p < 0.05.
Figure 1.
A schematic diagram of the open field test. The open field arena was divided into four quadrants (Q1–Q4) with medical tape. The center zone is indicated by dashed line and the periphery indicated by the solid line.
2.3 High-performance liquid chromatography (HPLC) analysis
To examine METH-induced depletion of monoaminergic terminals in the mouse brain, animals were euthanized 10 days, 3 and 5 months after drug treatment. Cortical, striatal and hippocampal tissue samples (N=5–9 per group) were dissected from each brain, weighed and extracted with 0.01M perchloric acid, then centrifuged at 15,000g for 15 min. Concentrations of DA, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), serotonin (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA) in the tissue extracts of METH- and saline-treated mice were measured by HPLC with electrochemical detection as described earlier (Krasnova et al., 2000) and expressed as ng/mg of tissue weight. All data are presented as means ± SEM. Statistical analysis was performed using analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (PLSD) (StatView 4.02, SAS Institute, Cary, NC). Criteria for significance were set at p < 0.05.
2.4 Terminal deoxynucleotidyl transferase-mediated deoxyribonucleotide triphosphate (dNTP) nick end labeling (TUNEL) histochemistry
To test if METH causes cell death in the mouse brain, we performed TUNEL histochemistry using the protocol previously described by us (Krasnova et al., 2005). At 3 days after METH or saline injections, animals were euthanized, their brains were removed, frozen and then cut into 30µm coronal sections, and sections containing cortical, striatal and hippocampal areas were used for TUNEL staining. For statistical analyses, TUNEL-positive cells were counted in coronal sections using a Zeiss (Carl Zeiss MicroImaging, Inc., Thornwood, NY) microscope. Five sections were counted per brain region from each mouse, and 5–8 animals were used in each group. Data are presented as means ± SEM. Statistical analysis was done using analysis of variance (ANOVA) followed by Fisher’s PLSD (StatView 4.02). Differences were considered significant at p < 0.05.
3. Results
3.1 Behavioral response to a novel environment
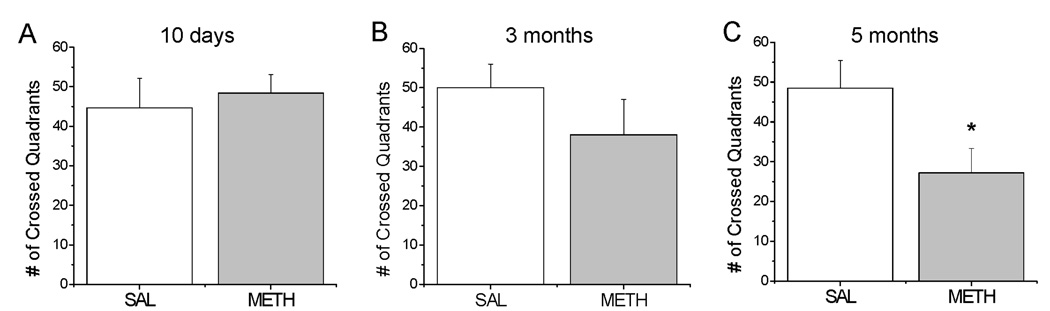
To examine METH effects on behavior, we used the open field test which allows for assessment of novelty-induced activity, including horizontal locomotion, rearing and time spent in the central versus peripheral zone in rodents. At 10 days and 3 months after treatment METH-injected mice did not show significant differences in locomotor activity in comparison to control animals when exposed to the novel environment (Fig. 2A, B). However, at 5 months after drug administration, locomotor activity was reduced (−45%) in METH-treated mice [F(1,8)=5.39, p=0.0487] (Fig. 2C). In contrast, control animals did not show any deficits in novelty-induced locomotor activity in comparison to younger saline-treated mice (compare control groups in Fig. 2C and Fig. 2A, B). Rearing and time spent in the center of the field were not significantly affected in METH-treated mice at any of the time-points studied (data not shown).
Figure 2.
Novelty-induced locomotor activity in METH- and saline-treated mice. METH administration had no effect on the number of crossed quadrants in the open field test at 10 days (A) and 3 months (B), but caused a significant decrease in the locomotion 5 months post-drug (C). N= 5–9 mice per group. * p < 0.05 versus control.
3.2 The effects of METH on monoamine levels in the brain
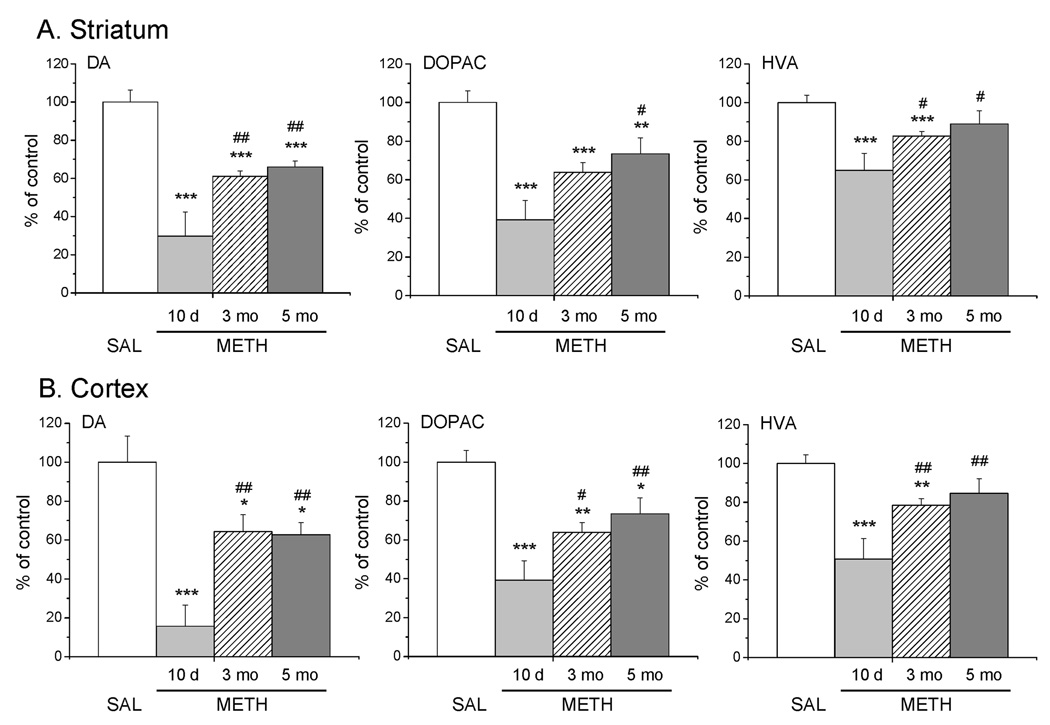
In order to assess the status of monoaminergic terminals after METH treatment, we measured levels of DA, 5-HT and their metabolites in the cortex, striatum and hippocampus using HPLC. The drug caused substantial reduction in the DA [F(3,46)=43.68, p<0.0001], DOPAC [F(3,46)=30.76, p<0.0001] and HVA [F(3,46)=13.23, p<0.0001] levels in the mouse striatum (Fig. 3A). An average of 70% DA depletion was found in the striatum of METH-treated mice euthanized at 10 days after injections (Fig. 3A). There were also 61% and 35% depletion of DOPAC and HVA concentrations, respectively, at the 10 day time-point (Fig. 3A). These decreases were partially reversible with time, with DA levels showing significant recovery to 38% and 34% depletion, respectively, at 3 and 5 months after drug injections (Fig. 3A). DOPAC concentrations also significantly recovered to 25% depletion at 5 months after treatment (Fig. 3A). HVA values had eventually normalized (Fig. 3A). METH treatment had no effect on 5-HT levels in the striatum (data not shown), however, caused significant increases in 5-HIAA [F(3,46)=3.22, p<0.05]. Post-hoc analyses showed that this was due to 20% increases in 5-HIAA concentrations at 5 months time-point.
Figure 3.
Effects of METH administration on the levels of DA and metabolites in the mouse striatum (A), and cortex (B). The METH injections caused decreases in the levels of DA, DOPAC and HVA 10 days after treatment. These values recovered significantly at 3 and 5 months post-drug. Data were calculated as percent of control values and presented as means ± SEM (N=5–9). * p < 0.05, ** p < 0.01, *** p < 0.001 in comparison with the saline group. # p < 0.05, ## p < 0.01 in comparison to METH-treated group euthanized at 10 day time-point.
In the cortex, the levels of DA [F(3,46)=7.59, p=0.0003], DOPAC [F(3,46)=22.83, p<0.0001] and HVA [F(3,46)=19.35, p<0.0001] were significantly decreased after METH treatment (Fig. 3B). The drug caused marked decreases in DA (−84%), DOPAC (−61%) and HVA (−49%) levels at 10 days following treatment (Fig. 3B). Similar to the striatum, these levels significantly recovered at 3 and 5 months, with DA showing 35% and 37% depletion, respectively, DOPAC – 36% and 27% reduction, respectively, and HVA tempering back to control values (Fig. 3B). METH treatment did not affect 5-HT levels (data not shown), but induced increases in 5-HIAA concentrations in the cortex [F(3,46)=7.65, p=0.0003]. Specifically, there were 40% increases in the 5-HIAA levels in the cortex at 5 months after treatment. METH did not cause any changes in monoamine levels in the mouse hippocampus (data not shown).
3.3 The effects of METH treatment on cell death in the brain
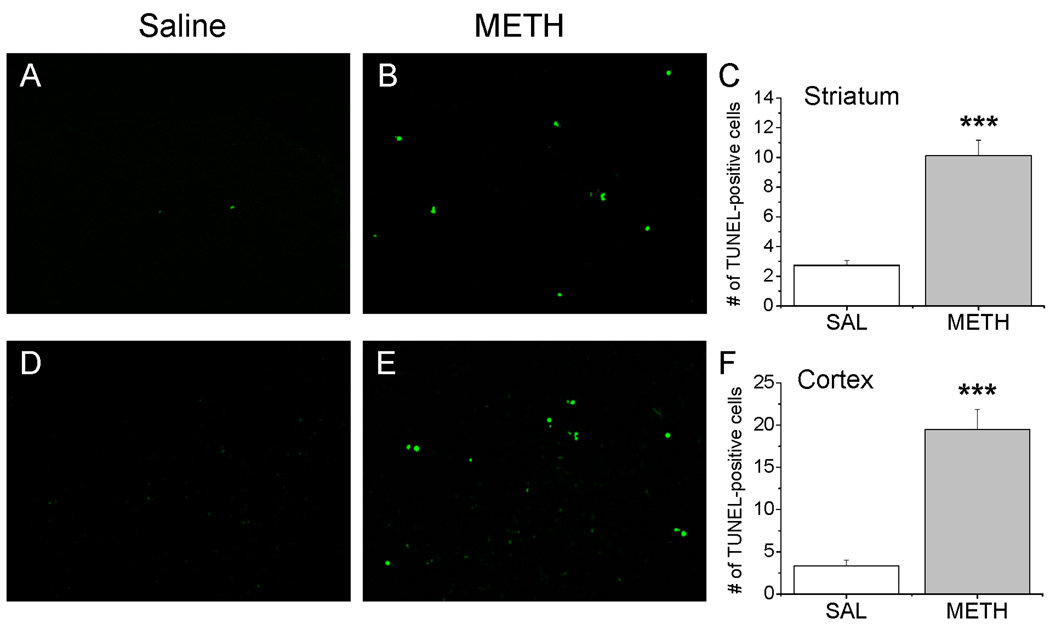
To test if METH administration caused cell death in the brains of BALB/c mice, we used TUNEL histochemistry to detect for DNA fragmentation. Previous studies from our lab have shown that treatment with neurotoxic doses of METH causes time-dependent changes in cell death in the rodent brain with significant increase at 1 – 3 days after injections and normalization to control values at 7 days post-drug (Deng et al., 2002; Jayanthi et al., 2005). Therefore, we chose 3 days as a time-point to examine METH-induced cell death in the brain in the present study. METH treatment produced significant increases in the number of TUNEL-positive cells in the striatum [F(1,26)=36.46, p<0.0001] and cortex [F(1,26)=53.21, p<0.0001] (Fig. 4). Specifically, saline-treated mice had very few TUNEL-positive cells in these brain areas (Fig. 4A, B). However, drug administration caused substantial increases in the amount of TUNEL staining in the striatum (Fig. 4B) and cortex (Fig. 4E). The quantitative and statistical data are shown in Fig. 4C, F. In contrast, METH treatment did not induce any increases in the number of TUNEL-positive cells in the hippocampus (data not shown).
Figure 4.
METH injections caused cell death in the mouse striatum and cortex. Only few apoptotic cells were observed in the striatum (A) and cortex (D) of saline-treated mice. However, METH caused marked increases in the amount of TUNEL-positive cells measured 3 days after drug injections (B, E). The quantitative and statistical data are shown in panels C and F. Values show means ± SEM (N=5–8). *** p < 0.001 in comparison with saline group.
4. Discussion
The main findings of the present study are that: (i) METH caused decreases in locomotor activity in a novel environment at 5 months but not at 10 days or 3 months after injections; (ii) METH administration resulted in selective depletion in the levels of DA and its metabolites in the cortex and striatum at 10 days, with partial recovery at 3 and 5 months following drug treatment; (iii) METH induced DNA fragmentation and cell death in the mouse striatum and cortex.
It is interesting that treatment with neurotoxic doses of METH in our study caused more profound DA depletion in the cortex than in the striatum of BALB/c mice. Previous studies by our laboratory (Ladenheim et al., 2000) and by others (Achat-Mendes et al., 2005; Anderson and Itzhak, 2006) also showed greater magnitude of decreases in DA levels in the cortex than in the striatum of METH-treated C57BL/6 and in Swiss Webster mice. Thus, these effects are probably species-specific because METH induces opposite effects in rats, with DA levels in the striatum being more affected than DA concentrations in the cortex (Ricaurte et al., 1980; Friedman et al., 1998; Danaceau et al., 2007). Our findings are consistent with previous observations showing time-dependent recovery of depleted DA levels in the brain caused by neurotoxic doses of METH in rats (Friedman et al., 1998) and in non-human primates (Melega et al., 1997). The results of our study are different from those obtained by Walsh and Wagner (1992) who reported that Long-Evans rats treated with neurotoxic doses of METH are impaired on a balance beam and show increases in response latency during active avoidance performance, suggesting appearance of motor deficits at seven days after drug administration. The differences between our studies might be due to the different species used. There is also a possibility that these motor deficits are strain-specific, since toxic doses of METH induced no changes in locomotor activity in a novel environment and no deficits in active avoidance performance in Wistar rats up to four weeks after injections (Timar et al., 2003). Our data are in agreement with those of recent studies by Boger et al. (2007, 2009) who did not find any influence of METH on locomotor activity at two weeks and three months after treatment but reported a 40% reduction in total distance traveled by the mice at six months after drug administration. Finally, our results are consistent with findings of clinical studies showing deficits in motor skills, including slower gait, in abstinent METH abusers (Volkow et al., 2001; Toomey et al., 2003).
It is interesting to discuss our data in a view of the accumulated evidence which has documented the dependence of locomotor functions on DA neurotransmission in the striatum (Smith and Villalba, 2008). For example, motor deficits in patients who suffer from Parkinson’s disease and age-related decline in locomotion in humans are linked with decreases in striatal DA markers (Reeves et al., 2002; Brooks and Piccini, 2006; Smith and Villalba, 2008). Nevertheless, a profound (80–95%) dopaminergic denervation in the striatum is required for manifestation of motor deficits (Fornaguera et al., 1993; Srinivasan and Schmidt, 2004; Grant et al., 2009), because of compensatory mechanisms and presynaptic adaptations which occur secondary to a loss of DA terminals (Zigmond, 1997; Perez et al., 2008). This compensation has been documented by microdialysis studies in 6-hydroxydopamine- and METH-treated rats, which have revealed that only 20% of normal DA input to the striatum is enough to maintain normal extracellular DA concentrations (Robinson et al., 1990). Therefore, these data suggest that delayed locomotor deficits that coincide with the recovery of DA levels in our study are not likely related to METH-induced damage to striatal DA axons because there were no decreases in motor activity at the time of the most intense DA depletion (70 %) in the striatum at 10 days post-drug. This idea is supported by findings of previous studies showing that partial (59%–75%) decreases in DA levels in the striatum induced by 6-hydroxydopamine treatment do not cause changes in locomotor activity in rats (Tadaiesky et al., 2008) and mice (De Leonibus et al., 2007).
These observations suggest the potential involvement of non-DA striatal and/or cortical systems, working in concert with aging process in the delayed decrease in novelty-induced locomotor activity found in METH-treated in mice. These systems might include intrinsic striatal neurons as well as corticostriatal projections since, in addition to damage to DA axons (Ricaurte et al., 1980), METH also causes death of striatal and cortical neurons as reported above and elsewhere (Deng et al., 2001; Jayanthi et al., 2005; Zhu et al., 2006) for review, see (Cadet et al., 2007; Krasnova and Cadet, 2009). This line of reasoning is also supported by numerous studies showing that normal aging is associated with decline in locomotor functions in rodents (Fetsko et al., 2005), non-human primates (Ingram et al., 2001) and humans (Volkow et al., 1998). Research into mechanisms that underlie such behavioral deficits has shown no overt neuronal loss in the cortex and striatum during normal aging, however, accumulation of DNA damage, shrinkage in soma size, loss of dendrites and dendritic spines and alterations in neurotransmitter receptors have been shown in individual neurons (Dickstein et al., 2007; Brasnjevic et al., 2008). These changes include a progressive decrease in the density and activity of striatal D2 DA receptors (Volkow et al., 1996; Ishibashi et al., 2009), which correlate with decline in motor functions during aging (Roth and Joseph, 1994). Because postsynaptic D2 DA receptors are localized on striatal medium spiny neurons (Surmeier et al., 1996) and because METH also causes death of medium spiny striatal neurons (Jayanthi et al., 2005; Zhu et al., 2006) it is logical to suggest that delayed motor deficits in METH-treated mice might be related to a reduction in D2 DA receptors caused by a combination of METH treatment and aging in mice. This idea is supported by findings of previous studies demonstrating that D2 DA receptor knockout mice show reduced locomotor activity, which is not associated with changes in DA or 5-HT levels in the striatum (Kelly et al., 1998). This proposition is further strengthened by reports that young mice selectively deficient in postsynaptic form of D2 DA receptor demonstrate decrease in locomotor activity similar to aged wild-type mice (Fetsko et al., 2005). Finally, this idea is also supported by a study showing that progressive loss of striatal medium spiny neurons in DAT knockout mice is associated with motor dysfunctions, such as decrease in step length and gait width, in these animals (Cyr et al., 2003).
In conclusion, although precise mechanisms were not identified, our observations suggest that METH treatment causes delayed reduction of the locomotor activity in the novel environment in a manner independent of the drug-related decreases in DA levels in the striatum and cortex. Because these declines in novelty-induced locomotor activity become evident only months after METH treatment, it is likely that degeneration of non-DA striatal and cortical cells accompanied by aging might account, in part, for such time-dependent behavioral deficits.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse of the NIH/DHHS (to I.N.K., B.L., A.C., E.A. and J.L.C.), and by grants 3SO6 GM051971 and U54 MH066417 (to C.F.H.) and R25 GM058904 (to R.R. and C.G.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achat-Mendes C, Ali SF, Itzhak Y. Differential effects of amphetamines-induced neurotoxicity on appetitive and aversive Pavlovian conditioning in mice. Neuropsychopharmacology. 2005;30:1128–1137. doi: 10.1038/sj.npp.1300675. [DOI] [PubMed] [Google Scholar]
- Anderson KL, Itzhak Y. Methamphetamine-induced selective dopaminergic neurotoxicity is accompanied by an increase in striatal nitrate in the mouse. Ann. N. Y. Acad. Sci. 2006;1074:225–233. doi: 10.1196/annals.1369.021. [DOI] [PubMed] [Google Scholar]
- Antoniou K, Papathanasiou G, Papalexi E, Hyphantis T, Nomikos GG, Spyraki C, Papadopoulou-Daifoti Z. Individual responses to novelty are associated with differences in behavioral and neurochemical profiles. Behav. Brain Res. 2008;187:462–472. doi: 10.1016/j.bbr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Belcher AM, Feinstein EM, O'Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: Comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33:1453–1463. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Granholm AC, McGinty JF. Minocycline restores striatal tyrosine hydroxylase in GDNF heterozygous mice but not in methamphetamine-treated mice. Neurobiol. Dis. 2009;33:459–466. doi: 10.1016/j.nbd.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Patrick KS, Ramamoorthy S, Denehy ED, Zhu H, Pacchioni AM, Granholm AC, McGinty JF. Long-term consequences of methamphetamine exposure in young adults are exacerbated in glial cell line-derived neurotrophic factor heterozygous mice. J. Neurosci. 2007;27:8816–8825. doi: 10.1523/JNEUROSCI.1067-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasnjevic I, Hof PR, Steinbusch HW, Schmitz C. Accumulation of nuclear DNA damage or neuron loss: molecular basis for a new approach to understanding selective neuronal vulnerability in neurodegenerative diseases. DNA Repair. 2008;7:1087–1097. doi: 10.1016/j.dnarep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DJ, Piccini P. Imaging in Parkinson's disease: the role of monoamines in behavior. Biol. Psychiatry. 2006;59:908–918. doi: 10.1016/j.biopsych.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox. Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Buitenhuys C. Do preclinical findings of methamphetamine-induced motor abnormalities translate to an observable clinical phenotype? Neuropsychopharmacology. 2005;30:2125–2134. doi: 10.1038/sj.npp.1300859. [DOI] [PubMed] [Google Scholar]
- Chapman DE, Hanson GR, Kesner RP, Keefe KA. Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J. Pharmacol. Exp. Ther. 2001;296:520–527. [PubMed] [Google Scholar]
- Cyr M, Beaulieu JM, Laakso A, Sotnikova TD, Yao WD, Bohn LM, Gainetdinov RR, Caron MG. Sustained elevation of extracellular dopamine causes motor dysfunction and selective degeneration of striatal GABAergic neurons. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11035–11040. doi: 10.1073/pnas.1831768100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daberkow DP, Kesner RP, Keefe KA. Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol. Biochem. Behav. 2005;81:198–204. doi: 10.1016/j.pbb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Danaceau JP, Deering CE, Day JE, Smeal SJ, Johnson-Davis KL, Fleckenstein AE, Wilkins DG. Persistence of tolerance to methamphetamine-induced monoamine deficits. Eur. J. Pharmacol. 2007;559:46–54. doi: 10.1016/j.ejphar.2006.11.045. [DOI] [PubMed] [Google Scholar]
- De Leonibus E, Pascucci T, Lopez S, Oliverio A, Amalric M, Mele A. Spatial deficits in a mouse model of Parkinson disease. Psychopharmacology (Berl.) 2007;194:517–525. doi: 10.1007/s00213-007-0862-4. [DOI] [PubMed] [Google Scholar]
- Deng X, Jayanthi S, Ladenheim B, Krasnova IN, Cadet JL. Mice with partial deficiency of c-Jun show attenuation of methamphetamine-induced neuronal apoptosis. Mol. Pharmacol. 2002;62:993–1000. doi: 10.1124/mol.62.5.993. [DOI] [PubMed] [Google Scholar]
- Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res. Mol. Brain Res. 2001;93:64–69. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007;6:275–284. doi: 10.1111/j.1474-9726.2007.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetsko LA, Xu R, Wang Y. Effects of age and dopamine D2L receptor-deficiency on motor and learning functions. Neurobiol. Aging. 2005;26:521–530. doi: 10.1016/j.neurobiolaging.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Fornaguera J, Schwarting RK, Boix F, Huston JP. Behavioral indices of moderate nigrostriatal 6-hydroxydopamine lesion: a preclinical Parkinson's model. Synapse. 1993;13:179–185. doi: 10.1002/syn.890130209. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Castaneda E, Hodge GK. Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol. Biochem. Behav. 1998;61:35–44. doi: 10.1016/s0091-3057(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Grant RJ, Sellings LH, Crocker SJ, Melloni E, Park DS, Clarke PB. Effects of calpain inhibition on dopaminergic markers and motor function following intrastriatal 6-hydroxydopamine administration in rats. Neuroscience. 2009;158:558–569. doi: 10.1016/j.neuroscience.2008.10.023. [DOI] [PubMed] [Google Scholar]
- He J, Yang Y, Yu Y, Li X, Li XM. The effects of chronic administration of quetiapine on the methamphetamine-induced recognition memory impairment and dopaminergic terminal deficit in rats. Behav. Brain Res. 2006;172:39–45. doi: 10.1016/j.bbr.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Effect of +methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology (Berl.) 2008;199:637–650. doi: 10.1007/s00213-008-1183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK, Chefer S, Matochik J, Moscrip TD, Weed J, Roth GS, London ED, Lane MA. Aging and caloric restriction in nonhuman primates: behavioral and in vivo brain imaging studies. Ann. N.Y. Acad. Sci. 2001;928:316–326. doi: 10.1111/j.1749-6632.2001.tb05661.x. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Ishii K, Oda K, Kawasaki K, Mizusawa H, Ishiwata K. Regional analysis of age-related decline in dopamine transporters and dopamine D2-like receptors in human striatum. Synapse. 2009;63:282–290. doi: 10.1002/syn.20603. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc. Natl. Acad. Sci. U.S.A. 2005;102:868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, Schuster CR. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl.) 2006;185:327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, Bunzow JR, Fang Y, Gerhardt GA, Grandy DK, Low MJ. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J. Neurosci. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Betts ES, Dada A, Jefferson A, Ladenheim B, Becker KG, Cadet JL, Hohmann CF. Neonatal dopamine depletion induces changes in morphogenesis and gene expression in the developing cortex. Neurotox. Res. 2007;11:107–130. doi: 10.1007/BF03033390. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Bychkov ER, Lioudyno VI, Zubareva OE, Dambinova SA. Intracerebroventricular administration of substance P increases dopamine content in the brain of 6-hydroxydopamine-lesioned rats. Neuroscience. 2000;95:113–117. doi: 10.1016/s0306-4522(99)00400-5. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res. Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Ladenheim B, Cadet JL. Amphetamine induces apoptosis of medium spiny striatal projection neurons via the mitochondria-dependent pathway. Faseb J. 2005;19:851–853. doi: 10.1096/fj.04-2881fje. [DOI] [PubMed] [Google Scholar]
- Ladenheim B, Krasnova IN, Deng X, Oyler JM, Polettini A, Moran TH, Huestis MA, Cadet JL. Methamphetamine-induced neurotoxicity is attenuated in transgenic mice with a null mutation for interleukin-6. Mol. Pharmacol. 2000;58:1247–1256. doi: 10.1124/mol.58.6.1247. [DOI] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- Melega WP, Raleigh MJ, Stout DB, Lacan G, Huang SC, Phelps ME. Recovery of striatal dopamine function after acute amphetamine- and methamphetamine-induced neurotoxicity in the vervet monkey. Brain Res. 1997;766:113–120. doi: 10.1016/s0006-8993(97)00548-9. [DOI] [PubMed] [Google Scholar]
- Moszczynska A, Fitzmaurice P, Ang L, Kalasinsky KS, Schmunk GA, Peretti FJ, Aiken SS, Wickham DJ, Kish SJ. Why is parkinsonism not a feature of human methamphetamine users? Brain. 2004;127:363–370. doi: 10.1093/brain/awh046. [DOI] [PubMed] [Google Scholar]
- Perez XA, Parameswaran N, Huang LZ, O'Leary KT, Quik M. Pre-synaptic dopaminergic compensation after moderate nigrostriatal damage in non-human primates. J. Neurochem. 2008;105:1861–1872. doi: 10.1111/j.1471-4159.2008.05268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Reeves S, Bench C, Howard R. Ageing and the nigrostriatal dopaminergic system. Int. J. Geriatr. Psychiatry. 2002;17:359–370. doi: 10.1002/gps.606. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Castaneda E, Whishaw IQ. Compensatory changes in striatal dopamine neurons following recovery from injury induced by 6-OHDA or methamphetamine: a review of evidence from microdialysis studies. Can. J. Psychol. 1990;44:253–275. doi: 10.1037/h0084241. [DOI] [PubMed] [Google Scholar]
- Roth GS, Joseph JA. Cellular and molecular mechanisms of impaired dopaminergic function during aging. Ann. N.Y. Acad. Sci. 1994;719:129–135. doi: 10.1111/j.1749-6632.1994.tb56824.x. [DOI] [PubMed] [Google Scholar]
- Schroder N, O'Dell SJ, Marshall JF. Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse. 2003;49:89–96. doi: 10.1002/syn.10210. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol. Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am. J. Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Minabe Y, Ouchi Y, Takei N, Iyo M, Nakamura K, Suzuki K, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Mori N. Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. Am. J. Psychiatry. 2003;160:1699–1701. doi: 10.1176/appi.ajp.160.9.1699. [DOI] [PubMed] [Google Scholar]
- Smith Y, Villalba R. Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains. Mov. Disord. 2008;23:S534–S547. doi: 10.1002/mds.22027. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Schmidt WJ. The effect of the alpha2-adrenoreceptor antagonist idazoxan against 6-hydroxydopamine-induced Parkinsonism in rats: multiple facets of action? Naunyn Schmiedebergs Arch. Pharmacol. 2004;369:629–638. doi: 10.1007/s00210-004-0929-2. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J. Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadaiesky MT, Dombrowski PA, Figueiredo CP, Cargnin-Ferreira E, Da Cunha C, Takahashi RN. Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson's disease. Neuroscience. 2008;156:830–840. doi: 10.1016/j.neuroscience.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Muller CP, Huston JP, Schwarting RK. High versus low reactivity to a novel environment: behavioural, pharmacological and neurochemical assessments. Neuroscience. 1999;93:243–251. doi: 10.1016/s0306-4522(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J. Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timar J, Gyarmati S, Szabo A, Furst S. Behavioural changes in rats treated with a neurotoxic dose regimen of dextrorotatory amphetamine derivatives. Behav. Pharmacol. 2003;14:199–206. doi: 10.1097/00008877-200305000-00003. [DOI] [PubMed] [Google Scholar]
- Toomey R, Lyons MJ, Eisen SA, Xian H, Chantarujikapong S, Seidman LJ, Faraone SV, Tsuang MT. A twin study of the neuropsychological consequences of stimulant abuse. Arch. Gen. Psychiatry. 2003;60:303–310. doi: 10.1001/archpsyc.60.3.303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J. Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am. J. Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, MacGregor RR, Schlyer DJ, Hitzemann R, Wolf AP. Measuring age-related changes in dopamine D2 receptors with 11C-raclopride and 18F-N-methylspiroperidol. Psychiatry Res. 1996;67:11–16. doi: 10.1016/0925-4927(96)02809-0. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Wagner GC. Motor impairments after methamphetamine-induced neurotoxicity in the rat. J. Pharmacol. Exp. Ther. 1992;263:617–626. [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat. Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Zhu JP, Xu W, Angulo JA. Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum in mice. Neuroscience. 2006;140:607–622. doi: 10.1016/j.neuroscience.2006.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond MJ. Do compensatory processes underlie the preclinical phase of neurodegenerative disease? Insights from an animal model of parkinsonism. Neurobiol. Dis. 1997;4:247–253. doi: 10.1006/nbdi.1997.0157. [DOI] [PubMed] [Google Scholar]