Abstract
The striatum is a vital substrate for performance, procedural memory, and learning. The ventral and medial striatum are thought to be critical for acquisition of tasks while the dorsolateral striatum is important for performance and habitual enactment of skills. Evidence based on cortical, thalamic, and amygdaloid inputs to the striatum suggests a medio-lateral zonation imposed on the classical dorso-ventral distinction. We therefore investigated the functional significance of dopaminergic signaling in cognitive tasks by studying dopamine-deficient (DD) mice and mice with dopamine signaling restored to only the dorsolateral (DL) striatum by viral rescue (vrDD-DL mice). Whereas DD mice failed in all of the tasks examined here, vrDD-DL mice displayed intact discriminatory learning, object recognition, visuospatial learning and spatial memory. Acquisition of operant behavior for food rewards was delayed in vrDD-DL mice and their motivation in a progressive ratio experiments was reduced. Therefore, dopaminergic signaling in the dorsolateral striatum is sufficient for mice to learn several different cognitive tasks although the rate of learning some of them was reduced. These results indicate that dopaminergic signaling in the ventromedial striatum is not absolutely necessary for mastery of these behaviors, but may facilitate them.
Keywords: knockout, learning, memory, tyrosine hydroxylase, viral gene transfer
Midbrain dopamine (DA) neurons mediate not only motor control, but also play a crucial role in many emotional and cognitive functions (1). DA projections from the ventral tegmental area (VTA) to the ventral striatum play a key role in reward, motivation, formation of Pavlovian associations and Pavlovian to instrumental transfer (2–5). DA projections from the substantia nigra pars compacta (SNc) to the dorsomedial striatum contribute to goal-directed operant learning and projections to the dorsolateral striatum contribute to stimulus-response learning (6–8). It is widely accepted that the ventral striatum is essential for the acquisition of learned behavior, whereas the dorsolateral striatum is thought to be important for general performance, rather than acquisition of learning (9–11). Within this theoretical framework, the transition from goal-directed actions during early learning to stimulus-response controlled habits reflects a reallocation from ventral to dorsal striatal control over behavior (12, 13). Recently, a spiraling mechanism has been proposed through which a cascade of serial connectivity linking the ventral striatum with progressively more dorsal regions of the striatum allows the ventral striatum to exert control, mediated by DA neurotransmission, over dorsal striatal processes (14, 15).
Based on the associations of cognitive and psychiatric symptoms with Parkinson's disease (PD) (16–18), the striatum is also thought to contribute to memory and visuospatial learning (19–21). In accordance with these observations, data from rodent models of PD indicate that the dorsal striatum contributes to various forms of learning and memory (22–26).
DA regulates the activity of medium spiny neurons (MSNs), which are the major output neurons of the striatum. Most studies that examine the function of specific striatal regions measure either where DA signaling is necessary or where MSN activity is necessary to facilitate normal behavior. The DA-deficient (DD) mice generated in our laboratory provide a different model to assess the contributions of DA to behavior. These mice lack the critical biosynthetic enzyme, tyrosine hydroxylase (TH), selectively in DA neurons (27). DD mice have severe deficits in motivation and locomotion and will not perform tasks that require intentional movement (28). DD mice require daily injections with L-Dopa for feeding and survival (29). These mice have conditional Th alleles that can be reactivated in a region-specific manner by the action of a Cre recombinase-expressing virus (CAV2-Cre). Through retrograde transport from the site of injection to DA cell bodies the CAV2-Cre reactivates Th alleles and restores normal DA signaling to the brain region that was injected with virus (30). With this technique we can explore region-specific contributions of DA projections to the motivational, locomotor, and cognitive abilities of the virally treated mice and hence examine where DA signaling is sufficient for normal function. Because of the medio-lateral zonation imposed on the classical dorso-ventral distinction (31), in this study we assess the cognitive abilities of mice in which DA signaling has been selectively restored to only the dorsolateral striatum. We identify some cognitive abilities that are completely rescued and conclude that their acquisition does not depend on DA signaling in the ventral striatum.
Results
Experimental Paradigm.
Groups of DD mice were tested sequentially in several behavioral paradigms to ascertain which behaviors they could not perform. Because DD mice are hypoactive and will not engage in some behaviors at all, we trained groups of DD mice after injection with caffeine (15 mg/kg), which restores locomotor activity independently of DA signaling (32). Caffeine-treated DD mice were injected with L-Dopa (30 mg/kg) before behavioral testing to determine whether learning had occurred but could not be expressed without DA signaling. The results of these experiments are shown in Fig. 1 A–D. Then groups of DD or control mice were injected with CAV2-Cre virus in the dorsolateral (DL) striatum. DD mice that were rescued, as assessed by their ability to feed and maintain body weight, were referred to as virally rescued vrDD-DL mice. These control and vrDD-DL mice were tested in the same battery of behavioral experiments as those mice that were not treated with virus to determine if restoring DA signaling to the DL striatum improved the performance of the mice (Fig. 1 E–H).
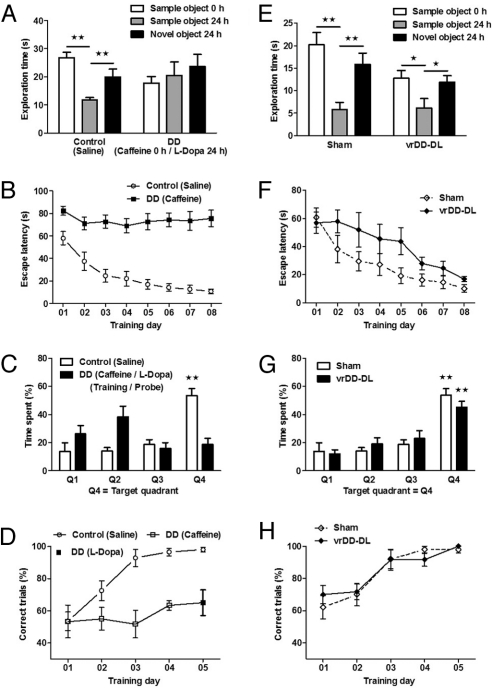
Fig. 1.
Learning and memory tasks that depend on DA signaling and that are restored in vrDD-DL mice.) Shown are experiments with DD mice (A–D) and with vrDD-DL mice (E–H). (A) Exploration time of a sample object upon first exposure (white bars) and presented 24 h later (gray bars) together with a novel object (black bars). Control mice (n = 9) received saline injections before exposure on both days. DD mice (n = 7) were stimulated with caffeine on the first and with L-Dopa on the second day. (B) Latency to escape to a hidden platform in a Morris water maze by saline treated control (n = 9) and caffeine treated DD mice (n = 7). (C) Time spent searching in the quadrants of the Morris water maze after 8 days of training. Control mice (n = 9) were injected with saline and DD mice (n = 7) with L-Dopa. (D) Percentage of correct choices of control (n = 8) and caffeine treated DD mice (n = 6) during a discrimination task in a water-based U-maze. DD mice were trained 4 days with caffeine stimulation and received L-Dopa on the fifth day. (E) Object exploration time by sham control (n = 8) and vrDD-DL mice (n = 6). (F) Latency to escape to a hidden platform in a Morris water maze by sham control (n = 8) and vrDD-DL mice (n = 6). (G) Time spent searching in the quadrants of the Morris water maze after 8 days of training by sham control (n = 8) and vrDD-DL mice (n = 6). (H) Percentage of correct choices of sham control (n = 8) and vrDD-DL mice (n = 6) in the water-based U-maze. Data are expressed as mean ± SEM. *, P < 0.05; **, P < 0.01.
Dopaminergic Signaling Is Necessary for Object Memory, Visuospatial, and Discriminatory Learning.
Without any treatment, DD mice did not explore any of the objects in an object recognition task. Stimulation of DD mice with caffeine restored object exploration to control levels (Fig. 1A). However, when DD mice were tested the next day when DA signaling was restored, they did not manifest object recognition. Control animals habituated to the sample object (t test, P < 0.01) and spent significantly more time with the novel object (t test, P < 0.05), DD mice explored the sample and the novel object equally (Fig. 1A).
To assess visuospatial function and spatial memory, DD mice were tested in a Morris water maze. While untreated DD mice just float in the water, caffeine-treated DD mice increased their swim speed and explored the maze (Fig. S1 A and B). Control animals rapidly learned to find the hidden platform and decreased their escape latencies over the course of training (ANOVA, F7,71 = 6.58; P < 0.01) (Fig. 1B); however, caffeine-stimulated DD did not learn the location of the platform or decrease their escape latencies (Fig. 1B). When these DD mice were tested the next day on a probe trial when DA signaling was restored, they did not manifest spatial memory. Contrary to control animals, which sought the area in the maze where the platform was located during training (quadrant 04) and spent more time there than in any other quadrant (ANOVA, F3,35 = 18.79, P < 0.01; Bonferroni post hoc test, P < 0.01), the L-Dopa-treated DD mice explored all quadrants of the maze equally (Fig. 1C).
We also tested DD mice in a cue-dependent, discriminatory water-based U-maze. In this maze, mice swim to a choice point where they can see a cue indicating which of the 2 bent arms contains an escape platform. The location of the correct arm (left vs. right) was varied randomly and mice were given 10 trials per day for 5 days. DD mice are hypoactive and did not explore the maze. Caffeine-treated DD mice did not improve their performance and had approximately 60% correct trials after 4 days of training, whereas control mice had nearly 100% correct trials after 4 days of training (ANOVA, F3,31 = 23.49, P < 0.01) (Fig. 1D). DD mice did not improve their performance when they were given L-Dopa on day 5, indicating that they had not learned the task in the absence of DA. The same dose of caffeine had no effect on control mice (Fig. S2 A–D). Comparing caffeine-treated control with caffeine-treated DD mice gave the same results in all of the behavioral tests as comparing control and DD mice that were not treated with caffeine. We conclude that DA signaling is necessary for object and spatial memory, visuospatial and discriminatory learning; hence, these are suitable tests for evaluating mice with DA signaling restored to specific brain regions.
Restoration of Dopaminergic Signaling to the Dorsolateral Striatum.
To evaluate the function of the dorsal striatum in memory and learning, we selectively restored DA signaling in DD mice by bilaterally injecting CAV2-Cre into the DL striatum. Based on our previous work showing that feeding can be restored to DD mice with viral injections into the dorsal striatum (30, 33), only those mice in which feeding was restored were used for the following experiments; they are referred to as virally rescued or vrDD-DL mice. The control mice for these experiments (sham controls) were also injected with CAV2-Cre at the same coordinates.
TH expression was restored to DA neurons in the SNc in vrDD-DL mice, with no TH expression in the VTA of the midbrain (Fig. 2 A–D). In the striatum, TH immunostaining was restored specifically to the DL striatum with occasional staining in the dorsomedial striatum (Fig. 2 E–H). The ventral striatum was completely devoid of TH staining (Fig. 2 E–H). Quantitative analysis of DA and its metabolites by HPLC-electrochemical detection revealed that DA in the dorsal striatum was restored to approximately 30% of control levels (Table 1). DA content in the ventral striatum of vrDD-DL mice was approximately 2% of control levels, similar to levels found in DD mice (33). These results indicate that we achieved selective restoration of DA signaling to the DL striatum.
Fig. 2.
Restoration of DA signaling in midbrain and striatum of vrDD-DL mice. TH (green) and dopamine transporter (red) immunostaining were visualized in coronal brain sections. There is no TH staining in SNc or striatum of DD mice (30). (A–D) TH expression pattern is revealed in sections through the SNc and VTA of vrDD-DL (A and B) and control mice (C and D). Expression of TH was specifically restored to DA neurons (DAT-positive cells) in the SNc of vrDD-DL mice. TH-positive DA neurons were not present in the VTA of vrDD-DL mice. (E–H) Sections through the striatum reveal projections of midbrain DA neurons in control (G and H) and vrDD-DL mice (E and F). TH staining was primarily present in the DL striatum of vrDD-DL mice. Ventral parts of the striatum were devoid of TH signal in vrDD-DL mice.
Table 1.
Tissue concentrations of dopamine and its metabolites in the striatum
| Group | n | Region | DA, % control | DA, ng/mg | HVA, ng/mg | DOPAC, ng/mg | 3-MT, ng/mg |
|---|---|---|---|---|---|---|---|
| vrDD-DL | 4 | Dorsal | 32.3 | 40.33 ± 5.67 | 3.76 ± 0.48 | 2.58 ± 0.69 | 7.52 ± 1.08 |
| Sham | 4 | Dorsal | 100 | 124.85 ± 16.2 | 10.15 ± 1.01 | 8.53 ± 0.77 | 11.81 ± 0.96 |
| vrDD-DL | 4 | Ventral | 2.03 | 1.43 ± 0.29 | 1.30 ± 0.16 | 0.37 ± 0.02 | n.d. |
| Sham | 4 | Ventral | 100 | 70.54 ± 14.87 | 8.81 ± 1.58 | 8.31 ± 1.28 | 7.95 ± 0.93 |
DA, dopamine; HVA, homovanillic acid; DOPAC, 3.4-dihydroxyphenylacetic acid; 3-MT, 3-methoxytyramine content. Data are presented as mean ± SEM (n.d. = none detected).
Feeding and Locomotion Are Restored in vrDD-DL Mice.
After surgery, vrDD-DL mice were maintained on L-Dopa for 14 days to allow viral reactivation of the conditionally inactive Th genes. The vrDD-DL mice were no longer dependent on L-Dopa for survival and were able to maintain their body weight (Fig. S3). Locomotor activity by the vrDD-DL mice over a 24-h period was the same as sham controls mice (Fig. S4).
Object Memory and Visuospatial Learning Are Partially Restored in vrDD-DL Mice.
In the object-recognition test, vrDD-DL mice displayed less explorative behavior than sham control animals, but they habituated to the sample object and spent significantly more time with the novel object on the test day (t test, each P < 0.05) (Fig. 1E).
In the Morris water maze test, both vrDD-DL and sham control mice located the hidden platform and decreased their escape latencies over the course of training [ANOVA (vrDD-DL), F7,47 = 2.92, P < 0.05; ANOVA (Sham), F7,71 = 6.58, P < 0.01 (Fig. 1F)]. Although performance was greatly improved, visuospatial learning was delayed in vrDD-DL mice, as indicated by higher escape latencies (two-way ANOVA, F1,111 = 6.51, P < 0.05) (Fig. 1F) and lower swim speed (two-way ANOVA, F1,111 = 45.46, P < 0.01) (Fig. S5) compared with sham controls. Both groups had an equal spatial reference memory on the probe trial after 8 days of training [ANOVA (vrDD-DL), F3,23 = 14.74, P < 0.01; ANOVA (Sham), F3,35 = 18.84, P < 0.01; Bonferroni post hoc test (each), P < 0.01 (Fig. 1G)].
Cue-Dependent Learning Is Restored in vrDD-DL Mice.
Sham control and vrDD-DL mice increased their performance in the discriminatory U-maze over the course of training [ANOVA (vrDD-DL), F4,29 = 13.23; P < 0.01; ANOVA (Sham), F4,39 = 18.63; P < 0.01] and had nearly 100% correct trials after 5 days of training, indicating intact discriminatory learning (Fig. 1H). There were no differences in learning between sham control and vrDD-DL mice.
Instrumental Reward Learning Is Delayed in vrDD-DL Mice.
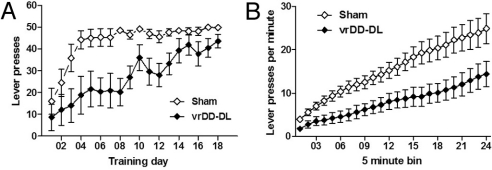
Previous experiments have shown that DA is necessary for operant conditioning in a simple lever-pressing paradigm for food rewards (34); hence, operant conditioning experiments with DD mice were not repeated here. We used a similar conditioning paradigm to examine learning and motivation by vrDD-DL mice. The overall pattern of results showed that vrDD-DL mice learned the instrumental lever-pressing response (Fig. 3A). Like sham control mice they improved their responses significantly over the course of training [ANOVA (vrDD-DL), F17,143 = 3.05; P < 0.01; ANOVA (Sham), F17,215 = 7.28; P < 0.01], but still retained an acquisition deficit when compared with sham control mice (two-way ANOVA, F1,359 = 16.86, P < 0.01; Fig. 3A). To assess their motivation to work for rewards, we tested vrDD-DL mice under a 2-h progressive-ratio training schedule. Although rescued mice increased their efforts to attain rewards during this schedule, their performance was not equal to that of sham control mice (two-way ANOVA, F1,414 = 7.65; P < 0.05; Fig. 3B), suggesting that motivation to work for food rewards is not completely restored in vrDD-DL mice. To ascertain whether lever-press responses by vrDD-DL mice resulted from reduced activity in the operant chambers, we also measured the number of magazine entries during all training sessions; no significant difference between vrDD-DL and sham control mice were noted (Fig. S6).
Fig. 3.
Rescue of instrumental lever pressing by vrDD-DL mice. Operant conditioning was assessed in sham control (n = 12) and vrDD-DL mice (n = 8) using a simple lever pressing task. (A) Total number of lever presses for food per training day over a period of 18 days. (B) Lever press ratio during a 2-h progressive ratio training schedule. Data are expressed as mean ± SEM.
Discussion
DD mice have severe deficits in motivation and locomotion and will die of starvation without daily L-Dopa injections (27). Here, we restore Th gene expression to DA neurons that project to the DL striatum and examine the motivational, locomotor and cognitive abilities of these virally rescued mice. This strategy is fundamentally different from killing DA neurons that project to specific brain regions or using pharmacological blockade of DA receptors (35–38). Those approaches are aimed at preventing DA signaling in specific brain regions to assess where it is necessary to facilitate normal behavior. With our strategy, we evaluate the behavior of mice with DA signaling restricted to specific brain regions and hence can examine where it is sufficient for specific functions.
Testing the cognitive abilities of DD mice is challenging because they are very inactive and unmotivated (28). To discriminate between failure of DD mice to learn or to perform tasks, they can be tested after training when DA-signaling is restored with L-Dopa. If they have learned the task without DA, but failed to demonstrate their learning, then they should perform well after DA signaling is restored. We have shown that DD mice can learn conditioned place preference for morphine (39) and cocaine (40). They can also learn a simple water-escape (41) and a spatial T-maze task (42), but not operant lever pressing for food rewards (34). Here, we show that DD mice cannot master the novel object, Morris water maze or cue-dependent U-maze tests; thus, these more demanding cognitive behaviors are appropriate tests for virally rescued mice. Because we evaluated memory function when DA signaling was restored with L-Dopa, the observed deficits in DD mice are not attributed to impaired retrieval of memory, but rather to the lack of memory formation in the absence of DA.
Immunocytochemical examination of the extent of TH expression within the midbrain and striatum of vrDD-DL mice confirmed the presence of TH-positive cell bodies in the SNc with complete absence of TH-positive cell bodies in the VTA. Consistently, we observed TH-positive fibers mainly in the DL striatum with some encroachment on dorsomedial areas, which probably reflects the huge arborization of individual DA neurons (43). DA content was restored specifically to the dorsal striatum, but not to the ventral striatum. All of these assays affirm that DA-signaling was restricted to the DL striatum in our model.
Our findings that object recognition and visuospatial function are blunted in DD mice is strong evidence that DA is essential for spatial learning, spatial and object memory. Restoration of DA to the DL striatum was sufficient to rescue these behaviors. These tasks are also dependent on the hippocampus and prefrontal cortex (44); however, they project to the ventral and medial striatum (31), whereas the thalamus, sensorimotor, and anterior cingulate cortex project to the DL striatum (31). Our findings imply an involvement of these latter structures in memory, visuospatial function, and learning. These findings expand upon models and experimental data that propose an interaction between hippocampus-based and procedural memory systems (44–46). We could not find any correlations between DA content and behavioral performance of individual vrDD-DL mice.
Our conclusions are consistent with observations from rodent models of PD, where lesioning of DA neurons in the SNc results in deficits of visuospatial function (24–26). However, these models make no distinction between loss of DA signaling versus loss of DA neurons and do not separate performance from cognitive deficits. They have also been difficult to interpret because rodents with complete lesions are very hypoactive and can only be kept alive by forced feeding (38, 47); the animals that eat on their own have incomplete lesions (48). Because restoring DA to 5% of normal levels is sufficient for mice to eat adequately (29), we argue that most experiments performed with DA lesions in the dorsal striatum were done on animals with incomplete lesions.
Although it is difficult to distinguish effects of DA receptor antagonists and neurotoxins on behavior from their motor-inhibitory actions (49), several studies report similar effects for blockade of DA signaling and blockade of MSN function in the striatum (4, 50–53). The modulatory function of DA in the striatum involves regulation of glutamatergic input to MSNs (54). Experiments in which MSNs were either ablated or shut down temporarily with GABAA agonists provide evidence that the ventral and dorsomedial striatum are crucial for instrumental learning, whereas the DL striatum is critical for performance of those tasks (4, 11, 55, 56). Based on this distinction, some learning models suggest a spiraling mechanism through which neuronal signaling from the ventromedial striatum exerts control over processes in DL striatal regions, promoting the transition from goal-directed to habit-driven behaviors (14). In contrast, our observation that vrDD-DL mice were able to master cue-based discriminatory tasks and learned both the visuospatial and the reward-mediated instrumental task, indicates that DA signaling to the ventral striatum is not a prerequisite for acquisition of these tasks; therefore, spiraling from ventral to dorsal striatum is not required for those tasks. However, the delayed acquisition of both the visuospatial and the lever-pressing task by vrDD-DL mice indicates that fully functional spatial and reward-mediated, instrumental learning is facilitated by DA signaling in regions outside the DL striatum. We also observed that vrDD-DL mice displayed increased latency to initiate action in almost all of the tests. For example, their lever-press ratio in the instrumental task was ≈20% that of control animals. It is unlikely that this was due to a general motor deficit because locomotor activity was restored to the level of control animals. However, their motivation in the instrumental lever pressing task was reduced, leaving open the possibility that vrDD-DL mice are not sufficiently motivated to initiate actions as quickly as control animals. While lever pressing was impaired, head entries (which reflect a Pavlovian approach to the reward) were normal in vrDD-DL mice, suggesting that DA signaling outside the DL striatum is necessary for operant learning and motivation for operant actions rather than for Pavlovian approach. Mice with viral rescue of DA signaling to the entire dorsal striatum learned to lever press for food rewards as quickly as control mice and had completely restored motivation to work for food (34); thus, restoring DA signaling to the entire dorsal striatum appears to be sufficient for learning this task.
Instead of a spiral of DA signaling that promotes action-outcome processes in the ventral striatum early in learning that subsequently supports habitual responses in the dorsal striatum during extensive training, we hypothesize that parallel DA signaling to ventral and dorsal striatum occurs during learning. This idea is supported by studies reporting neuronal activity in the dorsal striatum early in the learning process (57–59) and by reports of prediction error signals, which are thought to drive learning in midbrain DA neurons that project to the dorsal striatum (60, 61). Our hypothesis does not preclude the possibility that mesocortical or mesolimbic DA signaling contributes to learning these tasks in normal animals; it only indicates that such signaling is not absolutely necessary.
Together, the results of the present study demonstrate that DA signaling in the DL striatum is sufficient for visuospatial function, memory and discriminatory learning. Hence, DA-mediated spiraling from the ventral to the dorsal striatum is not necessary for learning these tasks, but it may facilitate the rate of learning in some of them. In contrast, other learned activities such as reward-mediated lever pressing, depend on, or are facilitated by, DA signaling outside of the DL striatum—presumably in the dorsomedial striatum or mesolimbic circuits.
Materials and Methods
Drugs.
L-Dopa (Sigma) was dissolved in saline solution containing 0.25% ascorbic acid and then filtered. Caffeine (Sigma) was dissolved in saline solution. All drugs were administered i.p.
Animals.
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Washington. The DD mice used here were generated as described (30). Mice were maintained on a mixed C57BL/6 × 129/SvEv genetic background. Mice were housed under a 12-h, light–dark cycle in a temperature-controlled environment with food and water available ad libitum unless noted otherwise. CAV2-Cre virus was generated and titered as described (62). The virus preparation had a titer of 6 × 1012 particles per mL. Bilateral injections of 0.5 μL CAV2-Cre into the DL region of the striatum (0.9 mm anterior to Bregma, ± 2.0 mm lateral to midline, 2.6 mm ventral from the skull surface) were performed on anesthetized (isoflurane) 2- to 3-month-old male and female DD or control mice (referred to as sham controls). Virally injected DD mice were removed from L-Dopa treatment 2 weeks after viral injection, and those mice that maintained body weight after 1 week without L-Dopa treatment were designated as vrDD-DL mice and allowed 1 more week of recovery before behavioral testing began. DD mice (whether treated with caffeine or L-Dopa) received daily L-Dopa injections at least 6 h after completion of the experimental sessions.
Behavioral Studies.
To allow DD mice to perform the explorative aspects of the tests in a DA-depleted state they received 15 mg/kg caffeine 10 min before habituation or training sessions in all tests. DD mice were tested 15 min after injection with L-Dopa (30 mg/kg). The same group of vrDD-DL and sham mice was used for all experiments. Behavioral experiments with DD mice and vrDD-DL mice were performed in the order listed below with at least 14 days between experiments.
Locomotor activity was assessed in locomotor chambers equipped with photobeams (Columbus Instruments) and monitored for 4 days after an initial acclimatisation phase of 12 h.
Object recognition was measured during a 6-min test in a circular open field arena (45-cm diameter) as time spent exploring a concomitantly presented novel and sample object. Exploration was scored by measuring the time when mice made direct contact (mouth, nose or paw) with an object that was not accidental or in a way as to explore other aspects of the arena. The scoring was done in a blindfolded manner. One day prior to the testing, mice were habituated to a sample object during three consecutive 6-min trials. A hemispherical and an oblong plastic object, each approximately 7 cm high were used as sample and novel object.
The Morris water maze was used to evaluate visuospatial learning and spatial reference memory. Mice were videotaped while being trained to locate a hidden platform over a period of 8 days with four 90-s trials per day. On each trial, mice were released into the water from a different location. Video data were analyzed with Ethovision software (Noldus). The circular pool was 84 cm in diameter and filled with opaque water at 22 °C. No visual cues were present within the pool. Extra-maze cues were provided through the wall decoration of the room. Visuospatial learning was measured as latency to reach the hidden platform. Swim speed was also calculated. One day after the last training session, mice were subjected to one 90-s test trial with no platform present in the maze. Spatial reference memory was scored as the amount of time the mouse spent in the quadrant of the pool where the platform was located during training.
Discrimination learning was measured as percentage of correct trials per day in a water-based U-shaped maze. Mice were released into a gray stem (45 cm) that ended in two choice arms (50 cm), one black and one white, which bent back toward the stem so that the mouse could not see the escape platform at the end of the positive arm (63). For half of the mice, the escape arm was black and for the other half it was white. Mice were trained for 5 days with 10 trials per day. Each day a different pseudorandom sequence of left-right locations for the positive arm was used for all animals tested on that day. DD mice were stimulated with caffeine during the first 4 days and injected with L-Dopa before testing on day 5.
For instrumental learning, mouse operant chambers (Med Associates) were used. Mice were maintained at 85% of ad libitum body weight for the duration of this experiment. They received 18 days of training with one session per day. During each session, two levers were available to the mice with the depression of either one resulting in the delivery of one 20-mg food pellet (BioServe). Each session lasted until 50 rewards were earned or 2 h passed. For the breakpoint analysis the number of lever presses required for food delivery increased as described (34). The breakpoint session lasted 2 h with no limit to the number of rewards that could be earned.
Immunohistochemistry and DA Measurement.
Proteins were detected on 30-μm brain sections by using the following primary antibodies: rabbit anti-TH (1:2000; Chemicon) and rat anti-DA-transporter (1:1,000; Chemicon). Immunofluorescence was revealed by using CY2- and/or CY3- labeled IgG secondary antibodies (1:200; Jackson ImmunoResearch). For determination of striatal DA content, brain regions of interest were dissected, immediately frozen in liquid nitrogen and stored at −80°C until analysis. HPLC coupled with electrochemical detection was used to measure DA content by the Neurochemistry Core Laboratory at Vanderbilt University's Center for Molecular Neuroscience Research.
Supplementary Material
Acknowledgments.
We thank Dr. Miguel Chillon (Vector Production Unit of Centre de Biotecnologia Animal i Teràpia Gènica at Universitat Autonoma Barcelona) for preparing the CAV2-Cre virus, Glenda Froelick for sectioning, and Devi Nitiutomo for assistance with the behavioral experiments.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907299106/DCSupplemental.
References
- 1.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 2.Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: Intracranial self-administration studies. J Neurosci. 2003;23:9305–9311. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFarland K, Ettenberg A. Haloperidol differentially affects reinforcement and motivational processes in rats running an alley for intravenous heroin. Psychopharmacology. 1995;122:346–350. doi: 10.1007/BF02246264. [DOI] [PubMed] [Google Scholar]
- 4.Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci. 2000;20:7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolterink G, et al. Relative roles of ventral striatal D1 and D2 dopamine receptors in responding with conditioned reinforcement. Psychopharmacology. 1993;110:355–364. doi: 10.1007/BF02251293. [DOI] [PubMed] [Google Scholar]
- 6.Featherstone RE, McDonald RJ. Dorsal striatum and stimulus-response learning: Lesions of the dorsolateral, but not dorsomedial, striatum impair acquisition of a simple discrimination task. Behav Brain Res. 2004;150:15–23. doi: 10.1016/S0166-4328(03)00218-3. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura K, Hikosaka O. Role of dopamine in the primate caudate nucleus in reward modulation of saccades. J Neurosci. 2006;26:5360–5369. doi: 10.1523/JNEUROSCI.4853-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Doherty J, et al. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 9.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 10.Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23:6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atallah HE, Lopez-Paniagua D, Rudy JW, O'Reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nat Neurosci. 2007;10:126–131. doi: 10.1038/nn1817. [DOI] [PubMed] [Google Scholar]
- 12.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 13.Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain. 2004;127:550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- 17.Levin BE, Katzen HL. Early cognitive changes and nondementing behavioral abnormalities in Parkinson's disease. Adv Neurol. 2005;96:84–94. [PubMed] [Google Scholar]
- 18.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130:1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 19.Dubois B, Pillon B. Cognitive deficits in Parkinson's disease. Neurology. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 20.Owen AM, et al. Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain. 1992;115:1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- 21.Taylor AE, Saint-Cyr JA, Lang AE. Frontal lobe dysfunction in Parkinson's disease. The cortical focus of neostriatal outflow. Brain. 1986;109:845–883. doi: 10.1093/brain/109.5.845. [DOI] [PubMed] [Google Scholar]
- 22.Baunez C, Robbins TW. Effects of dopamine depletion of the dorsal striatum and further interaction with subthalamic nucleus lesions in an attentional task in the rat. Neuroscience. 1999;92:1343–1356. doi: 10.1016/s0306-4522(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 23.Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Da Cunha C, Angelucci ME, Canteras NS, Wonnacott S, Takahashi RN. The lesion of the rat substantia nigra pars compacta dopaminergic neurons as a model for Parkinson's disease memory disabilities. Cell Mol Neurobiol. 2002;22:227–237. doi: 10.1023/a:1020736131907. [DOI] [PubMed] [Google Scholar]
- 25.De Leonibus E, et al. Spatial deficits in a mouse model of Parkinson disease. Psychopharmacology. 2007;94:517–525. doi: 10.1007/s00213-007-0862-4. [DOI] [PubMed] [Google Scholar]
- 26.Mura A, Feldon J. Spatial learning in rats is impaired after degeneration of the nigrostriatal dopaminergic system. Movement Disorders. 2003;18:860–871. doi: 10.1002/mds.10472. [DOI] [PubMed] [Google Scholar]
- 27.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 28.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: Lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szczypka MS, et al. Feeding behavior in dopamine-deficient mice. Proc Natl Acad Sci USA. 1999;96:12138–12143. doi: 10.1073/pnas.96.21.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hnasko TS, et al. Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proc Natl Acad Sci USA. 2006;103:8858–8863. doi: 10.1073/pnas.0603081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Kim DS, Palmiter RD. Adenosine receptor blockade reverses hypophagia and enhances locomotor activity of dopamine-deficient mice. Proc Natl Acad Sci USA. 2003;100:1346–1351. doi: 10.1073/pnas.252753799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szczypka MS, et al. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 34.Robinson S, Rainwater AJ, Hnasko TS, Palmiter RD. Viral restoration of dopamine signaling to the dorsal striatum restores instrumental conditioning to dopamine-deficient mice. Psychopharmacology. 2007;191:567–578. doi: 10.1007/s00213-006-0579-9. [DOI] [PubMed] [Google Scholar]
- 35.Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- 36.Dickinson A, Smith J, Mirenowicz J. Dissociation of Pavlovian and instrumental incentive learning under dopamine antagonists. Behav Neurosci. 2000;114:468–483. doi: 10.1037//0735-7044.114.3.468. [DOI] [PubMed] [Google Scholar]
- 37.Sokolowski JD, Salamone JD. The role of accumbens dopamine in lever pressing and response allocation: Effects of 6-OHDA injected into core and dorsomedial shell. Pharmacol Biochem Behav. 1998;59:557–566. doi: 10.1016/s0091-3057(97)00544-3. [DOI] [PubMed] [Google Scholar]
- 38.Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- 39.Hnasko TS, Sotak BN, Palmiter RD. Morphine reward in dopamine-deficient mice. Nature. 2005;438:854–857. doi: 10.1038/nature04172. [DOI] [PubMed] [Google Scholar]
- 40.Hnasko TS, Sotak BN, Palmiter RD. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci. 2007;27:12484–12488. doi: 10.1523/JNEUROSCI.3133-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denenberg VH, Kim DS, Palmiter RD. The role of dopamine in learning, memory, and performance of a water escape task. Behav Brain Res. 2004;148:73–78. doi: 10.1016/s0166-4328(03)00183-9. [DOI] [PubMed] [Google Scholar]
- 42.Robinson S, Sandstrom SM, Denenberg VH, Palmiter RD. Distinguishing whether dopamine regulates liking, wanting, and/or learning about rewards. Behav Neurosci. 2005;119:5–15. doi: 10.1037/0735-7044.119.1.5. [DOI] [PubMed] [Google Scholar]
- 43.Matsuda W, et al. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devan BD, White NM. Parallel information processing in the dorsal striatum: Relation to hippocampal function. J Neurosci. 1999;19:2789–2798. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hazy TE, Frank MJ, O'Reilly RC. Banishing the homunculus: Making working memory work. Neuroscience. 2006;139:105–118. doi: 10.1016/j.neuroscience.2005.04.067. [DOI] [PubMed] [Google Scholar]
- 46.Yin HH, Knowlton BJ. Contributions of striatal subregions to place and response learning. Learn Mem. 2004;11:459–463. doi: 10.1101/lm.81004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zigmond MJ, Stricker EM. Recovery of feeding and drinking by rats after intraventricular 6-hydroxydopamine or lateral hypothalamic lesions. Science. 1973;182:717–720. doi: 10.1126/science.182.4113.717. [DOI] [PubMed] [Google Scholar]
- 48.Brot MD, et al. Neonatal 6-hydroxydopamine administration to mice is fatal. Dev Neurosci. 2002;24:531–538. doi: 10.1159/000069364. [DOI] [PubMed] [Google Scholar]
- 49.Salamone JD. The actions of neuroleptic drugs on appetitive instrumental behaviors. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of Psychopharmacology. Vol 19. New York: Plenum; 1987. pp. 575–608. [Google Scholar]
- 50.Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135:959–968. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 51.Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faure A, Haberland U, Conde F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci. 2005;25:2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishiwari K, Weber SM, Mingote S, Correa M, Salamone JD. Accumbens dopamine and the regulation of effort in food-seeking behavior: Modulation of work output by different ratio or force requirements. Behav Brain Res. 2004;151:83–91. doi: 10.1016/j.bbr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: Evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- 55.Hernandez PJ, Sadeghian K, Kelley AE. Early consolidation of instrumental learning requires protein synthesis in the nucleus accumbens. Nat Neurosci. 2002;5:1327–1331. doi: 10.1038/nn973. [DOI] [PubMed] [Google Scholar]
- 56.Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- 57.Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- 58.Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- 59.Yin HH, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 61.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 62.Kremer EJ, Boutin S, Chillon M, Danos O. Canine adenovirus vectors: An alternative for adenovirus-mediated gene transfer. J Virol. 2000;74:505–512. doi: 10.1128/jvi.74.1.505-512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gorski JA, Balogh SA, Wehner JM, Jones KR. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121:341–354. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.