Abstract
In vitro ovarian follicle cultures may provide fertility-preserving options to women facing premature infertility due to cancer therapies. An encapsulated three-dimensional (3-D) culture system utilizing biomaterials to maintain cell-cell communication and support follicle development to produce a mature oocyte has been developed for the mouse. We tested whether this encapsulated 3-D system would also support development of nonhuman primate preantral follicles, for which in vitro growth has not been reported. Three questions were investigated: Does the cycle stage at which the follicles are isolated affect follicle development? Does the rigidity of the hydrogel influence follicle survival and growth? Do follicles require luteinizing hormone (LH), in addition to follicle-stimulating hormone (FSH), for steroidogenesis? Secondary follicles were isolated from adult rhesus monkeys, encapsulated within alginate hydrogels, and cultured individually for ≤30 days. Follicles isolated from the follicular phase of the menstrual cycle had a higher survival rate (P < 0.05) than those isolated from the luteal phase; however, this difference may also be attributed to differing sizes of follicles isolated during the different stages. Follicles survived and grew in two hydrogel conditions (0.5% and 0.25% alginate). Follicle diameters increased to a greater extent (P < 0.05) in the presence of FSH alone than in FSH plus LH. Regardless of gonadotropin treatment, follicles produced estradiol, androstenedione, and progesterone by 14–30 days in vitro. Thus, an alginate hydrogel maintains the 3-D structure of individual secondary macaque follicles, permits follicle growth, and supports steroidogenesis for ≤30 days in vitro. This study documents the first use of the alginate system to maintain primate tissue architecture, and findings suggest that encapsulated 3-D culture will be successful in supporting the in vitro development of human follicles.
Keywords: follicle, follicle-stimulating hormone, follicular development, luteinizing hormone, menstrual cycle
An alginate-based, three-dimensional culture system supports survival, growth, and gonadotropin-stimulated steroid production of preantral follicles in nonhuman primates.
INTRODUCTION
Improvements in cancer therapies have increased the survivorship of young patients [1]; therefore, quality-of-life issues are increasingly important at the time of cancer diagnosis. Many life-saving cancer treatments, including radiation and chemotherapy, can lead to premature infertility in women of reproductive age [2]. Thus, women are increasingly interested in taking steps to preserve fertility before cancer treatment [3]. Women have few options for fertility preservation. Traditional in vitro fertilization (IVF) that is followed by embryo cryopreservation is the only highly successful approach to preserve fertility. However, this approach requires a delay in cancer treatment to complete the IVF cycle, as well as an acceptable sperm donor, and is not appropriate for children. Several alternative approaches to fertility preservation are under development, including mature oocyte cryopreservation and ovarian tissue banking. While oocyte cryopreservation eliminates the need for a sperm donor, a delay in cancer treatment for hormonal stimulation is required. This approach has shown recent promise [4–8], but the success rate is still low [9–11].
Ovarian tissue cryobanking is a promising approach to fertility preservation that precludes the need for an immediate sperm donor or a delay in treatment for hormonal stimulation. Two approaches have been taken to support the maturation of these cryopreserved immature follicles: cortical strip transplantation and in vitro follicle growth, the latter of which describes the process of in vitro follicle culture that is followed by in vitro maturation of oocytes within follicles [12]. Transplantation of cryopreserved ovarian cortical strips has been performed in several patients and has shown success in producing viable offspring in monkeys (from fresh tissue) and humans (from cryopreserved tissue) [13–15]. While this approach has promise, it carries the risk of introducing cancer cells back into the patient following treatment [16, 17]. An alternative approach that does not carry the risk of reintroducing cancer cells is in vitro ovarian follicle culture that is followed by IVF of mature oocytes and embryo transfer. This approach has successfully produced live offspring in mice [18–20].
Roy and Treacy [21] were the first to demonstrate the potential application of the in vitro follicle culture approach to humans; however, success has been limited in several subsequent studies [22–25]. Abir and colleagues [22] successfully grew individual human preantral and early antral follicles for ≤4 wk of culture; however, estradiol (E2) production decreased after 3 wk, and sectioning revealed that few follicles contained oocytes. Studies [23, 24] on isolated primordial and primary follicles demonstrated growth within the first 24 h of culture, but no further growth was observed. Recently, Telfer and associates [25] obtained late preantral and early antral follicles when starting with primordial and primary follicles. They reported a two-step serum-free culture system, which included 6 days of ovarian cortical strip culture, followed by 4 days of isolated follicle culture in the presence of activin. Although oocyte and follicle development was achieved in their study, the culture time was very short (a total of only 10 days), and no further culture was attempted to explore follicle developmental competence. Additional studies [26–31] documented growth of primordial follicles within fresh and cryopreserved ovarian cortical strips. This approach produced secondary follicles but resulted in high levels of atresia. Based on these studies, further refinements of in vitro follicle culture approaches are needed to support human follicle development in vitro and ultimately to mature oocytes for fertility therapy.
Since Roy and Greenwald [32] pioneered isolation of ovarian follicles in the hamster, two general approaches to in vitro follicle growth have been pursued: a nonintact attached follicle approach, wherein follicles from rodents [18, 33–37] and domestic animals [38–43] are permitted to attach to the culture plate, and an unattached follicle approach, in which follicles from mice [19, 44–53] and human [22–24] maintain an intact three-dimensional (3-D) structure and remain unattached or free in medium. While both approaches have successfully yielded mature oocytes and live offspring in mice [18–20, 33, 46], the intact 3-D approach maintains the cell-cell and cell-matrix connections that are important in regulating follicle development in vivo [53]. In fact, human preantral follicles encapsulated in agar grew to the very early antral stage and produced steroids over 5 days in culture [21], but follicle survival and diameter were not assessed. Encapsulated 3-D approaches may also be beneficial in overcoming the difficulty of maintaining follicle architecture during culture for larger species [54, 55]. Xu and colleagues [19] recently reported the development of an encapsulated 3-D follicle culture system that utilizes alginate hydrogels to create a matrix for follicle development. Alginate hydrogels are widely used in tissue engineering applications [56], and the alginate follicle culture system has yielded mature oocytes that fertilized (when inseminated in vitro) and produced (following embryo transfer) live mouse offspring [19].
While the alginate culture system has proven successful in the mouse, further studies are required to translate the system to the human. For practical and ethical reasons, nonhuman primate ovarian tissue may be useful in identifying the optimal conditions for primate follicle culture before application to humans. Nonhuman primate follicles provide the opportunity to address issues relevant and specific to primate follicle development. For example, (1) primate follicles and oocytes differ in their hormonal and metabolic requirements relative to mouse follicles, (2) primate follicles grow to much larger sizes than mouse follicles [57, 58], and (3) the time from a preantral follicle to a preovulatory follicle in vivo is longer for primate follicles relative to mouse follicles [59]. While a mature mouse follicle is approximately 500 μm in diameter in vivo [60] and can reach this size in encapsulated 3-D culture in 14 days, mature rhesus monkey follicles reach 5 mm in diameter [58], and a mature human follicle reaches diameters >20 mm [57]. Gougeon [59] estimated it takes 90 days for a preantral follicle that has entered the growing pool to become a preovulatory follicle in women. Therefore, to successfully translate the system to the primate, many factors must be considered, including the isolation techniques used, the cycle stage during which follicles are collected, the physical properties of the matrix, and the contents of the culture media. An additional issue for consideration is selection of the dominant follicle and the health of the remaining growing follicles in the presence or absence of a dominant follicle or corpus luteum [61, 62]. In the early follicular phase, many growing and healthy antral follicles are present in the ovary [63]. After selection of the dominant follicle, only atretic small antral follicles are evident, with few antral follicles observed during the luteal phase [57].
In this article, we describe investigations of the survival and growth of nonhuman primate follicles in vitro using an encapsulated 3-D culture system. We examined the effects of the ovarian cycle stage at the time of follicle isolation, the physical properties of the culture matrix, and the effects of follicle-stimulating hormone (FSH) with or without luteinizing hormone (LH) on early secondary follicle development.
MATERIALS AND METHODS
Animals and Ovary Collection
The general care and housing of rhesus monkeys were provided by the Division of Animal Resources at the Oregon National Primate Research Center. Animals were pair caged in a temperature-controlled (22°C) 12L:12D room. Diet consisted of Purina monkey chow (Ralston-Purina, Richmond, IN) provided twice a day, supplemented with fresh fruit or vegetables once a day, and water ad libitum. Animals were treated in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and protocols were approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee.
Adult female rhesus monkeys (n = 12 [8–9 yr of age]) exhibiting normal menstrual cycles of approximately 28 days were evaluated daily for menstruation (first day of menses is Day 1). Ovaries were collected from anesthetized monkeys by laparoscopy as previously described [64]. At the time of ovariectomy, eight animals were in the follicular phase (mean ± SEM, Day 5 ± 1) of the menstrual cycle, and four animals were in the luteal phase (mean ± SEM, Day 20 ± 4 [characterized by the presence of a corpus luteum in one of the ovaries]). A blood sample was obtained by venipuncture at the time of ovariectomy. The mean ± SEM serum E2 level in animals during the follicular phase was 105 ± 41 pg/ml, with a progesterone (P4) level of 0.47 ± 0.35 ng/ml. The mean ± SEM serum E2 and P4 levels in animals during the luteal phase were 72 ± 30 pg/ml and 2.0 ± 1.1 ng/ml, respectively. Ovaries were immediately transported to the laboratory in dissection media containing Liebovitz L15 media supplemented with 1% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Invitrogen, Carlsbad, CA).
Alginate Hydrogel Preparation
Sodium alginate (55%–65% guluronic acid) was generously provided by FMC BioPolymers (Philadelphia, PA). Alginate was dissolved in deionized water at a concentration of 1% (w/v) and then mixed with activated charcoal (0.5 g of charcoal per gram of alginate) to remove organic impurities and improve alginate purity. The alginate solution was then sterilized by filtration through 0.22-μm filters (Millipore, Billerica, MA), lyophilized in Steriflip conical tubes (Millipore), and aliquoted. Before each encapsulation, alginate aliquots were reconstituted by mixing on a racking platform at room temperature overnight with sterile 1× PBS (137 mM NaCl, 10 mM phosphate, and 2.7 mM KCl; Invitrogen) to concentrations of 0.5% or 0.25% (w/v).
Ovary Processing, Follicle Isolation, Encapsulation, and Culture
Following collection, ovaries were cut in half and the medulla removed. Using scalpels and curved scissors, the cortex was cut into 2 × 2 × 1-mm cortical strips and transferred to holding media composed of alpha minimum essential medium (Invitrogen) supplemented with 1% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cortical strips were placed in an incubator at 37°C and 5% CO2 in air and were individually removed for follicle isolation. Individual cortical strips were transferred to dissection media (as already described), and secondary follicles (100–300 μm) were mechanically isolated using 25-gauge needles. Isolated follicles were transferred to holding media (as already described) and placed in an incubator at 37°C and 5% CO2 for 2–8 h to allow for processing of all cortical strips. Only multilayered secondary follicles (containing at least three layers of granulosa cells) that displayed the following characteristics were selected for encapsulation: 1) no clear antral cavity, 2) an intact basement membrane with attached stroma, and 3) a visible oocyte that was round and centrally located within the follicle.
Follicles were encapsulated in beads composed of 0.5% and 0.25% alginate as previously described [51]. Briefly, single follicles were transferred inside individual droplets of alginate (∼5 μl) on a polypropylene mesh (0.1-mm opening; McMaster-Carr, Atlanta, GA). The mesh was then inverted over a cross-linking solution (50 mM CaCl2 and 140 mM NaCl) and gently tapped, causing the droplets to fall into the solution. Following cross-linking for 2 min, alginate beads were removed and rinsed in holding media. They were then transferred to individual wells of a 48-well plate containing 300 μl of alpha minimum essential medium culture media supplemented with 3 mg/ml bovine serum albumin, 1 mg/ml bovine fetuin (Sigma-Aldrich, St. Louis, MO), 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml sodium selenite. Throughout isolation, encapsulation, and plating, follicles were maintained at 37°C and at pH 7.2–7.4. Encapsulated follicles were cultured in the presence of 500 mIU/ml (138 ng/ml) FSH (NV Organon, Oss, the Netherlands) with or without 10 mIU/ml (1.6 ng/ml) LH (Ares Serono, Randolph, MA) at 37°C in 5% CO2 in air atmosphere for ≤30 days. Every 2 days, half of the culture media (150 μl) was exchanged and stored at −20°C for subsequent hormonal measurements. Fresh culture media were prepared weekly.
Follicle Growth and Survival
Follicle survival and diameter were assessed using an Olympus (Tokyo, Japan) CK40 inverted microscope with transmitted light and phase objectives with an attached Olympus DP11 digital camera. Photographs of each follicle were collected, as well as photographs of a micrometer (1 mm with 0.01-mm divisions, Fisher Micromaster; Fisher Scientific, Fair Lawn, NJ) for calibration purposes. For measurement, photographs were imported into ImageJ 1.33U (National Institutes of Health, Bethesda, MD). The diameter of each follicle was measured in units of pixels and converted to micrometers based on the conversion determined by measuring the image of the calibrated micrometer. Follicles were measured from the outer layer of cells as previously described [51], and measurements included a measurement at the widest diameter of the follicle and a second measurement perpendicular to the first. The mean of these values was then calculated and reported as the follicle diameter. Follicles were considered to be degenerating if either the oocyte was no longer surrounded by a layer of granulosa cells, the oocyte was dark, the granulosa cells had become dark and fragmented, or the diameter of the follicle decreased.
Hormone Assays
Serum concentrations of E2 and P4 were determined by the Endocrine Technology and Support Core at the Oregon National Primate Research Center (http://www.ohsu.edu/xd/research/centers-institutes/onprc/research-services/research-support/endocrine-technology.cfm) using an Immulite 2000, a chemiluminescence-based automatic clinical platform (Siemens Healthcare Diagnostics, Deerfield, IL). The E2 and P4 levels in rhesus monkey serum were validated using a direct comparison of samples analyzed coordinately by the Immulite 2000 and a Roche Elecsys 2010 analyzer (also a chemiluminescence-based clinical platform; F. Hoffmann-La Roche Ltd., Basel, Switzerland), which was previously validated for macaque steroid measurements [65]. The comparison involved 105 macaque samples with E2 levels ranging from 10 to 400 pg/ml; the coefficient of correlation between the two platforms was 0.9303. The P4 values in 62 macaque samples ranged from 0.2 to 9 ng/ml; the coefficient of correlation between the two platforms was 0.9467. The sensitivity of E2 assays by the Immulite 2000 is 20 pg/ml and 0.2 ng/ml for P4. The intraassay and interassay coefficients of variation with the Immulite 2000 are <15% for both the E2 and P4 assays. Each steroid assay also includes quality control samples provided by the company (Siemens Healthcare Diagnostics).
Steroid concentrations were measured in media collected on Culture Days 7, 14, and 30 in the Endocrine Services Laboratory at the Oregon National Primate Research Center. Culture medium was subjected to column chromatography to separate androstenedione (A4), E2, and P4 [66]. In brief, 75 μl of each media sample was extracted with 5 ml of fresh or redistilled diethyl ether, and the concentrated extract was dissolved in ethanol. The samples were dried under an air stream and chromatographed on 1.0-g Sephadex LH-20 microcolumns (Sigma-Aldrich) to isolate a neutral fraction and E2. The neutral fraction was dried, concentrated, and chromatographed on 2.0-g Sephadex LH-20 microcolumns, and the P4 and A4 fractions were collected. All three steroids were reconstituted in 200 μl of ethanol and analyzed in multiple doses (equivalent to 7.5 μl and 56 μl for E2 by radioimmunoassay; 1.5 μl, 7.5 μl, and 45 μl for P4; and 7.5 μl and 56 μl for A4) as already described. Values reported were corrected for culture medium blanks (<5 pg in the E2 and A4 assays and <30 pg in the P4 assay) and for extraction and chromatography losses (trace recoveries for E2, P4, and A4 after extraction and chromatography were 89.9%, 72.5%, and 52.4%, respectively). The sensitivities for the E2, P4, and A4 assays were <5 pg, <20 pg, and <5 pg, respectively. The intraassay coefficient of variation did not exceed 15% for any of the assays.
Statistical Analysis
Statistical calculations were performed using JMP 4.0.4 software (SAS Institute, Cary, NC). Statistical significance for follicle size measurements and steroid measurements was analyzed using two-way ANOVA with repeated-measures or one-way ANOVA, followed by Tukey honestly significant difference test for single time points. Categorical data were analyzed by chi-square analysis. Differences were considered significant at P < 0.05.
RESULTS
Effect of Menstrual Cycle on Follicle Survival and Growth
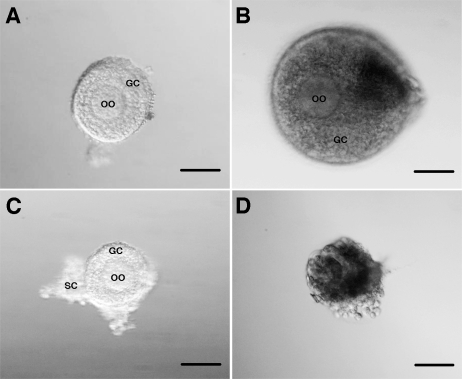
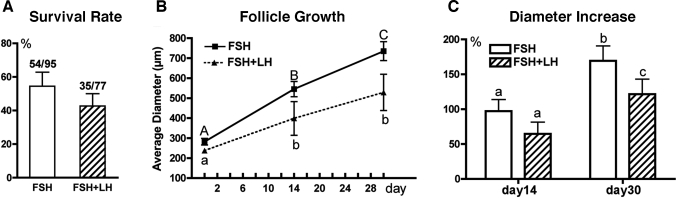
A total of 300 secondary follicles were distributed into several culture conditions. To determine the effect of stage of the menstrual cycle on follicle parameters, 110 follicles isolated during the follicular phase and 53 follicles isolated during the luteal phase were compared (Table 1). The sizes of collected secondary follicles varied between the ovarian stages; the isolated follicle pool was, on average, larger during the follicular phase (P < 0.05), resulting in different starting sizes at Day 0 in the two experimental groups. Following 14 days of culture in 0.5% alginate, follicles isolated during the follicular phase had a 78% survival rate, which was greater (P < 0.05) than the 42% survival rate of follicles isolated during the luteal phase of the cycle. In addition, follicle diameters increased in surviving follicles in both stages of the cycle (Table 1). At the time of isolation (Fig. 1, A and C), follicles from each ovarian stage have (1) a spherical and centrally located oocyte with an obvious zona pellucida, (2) an intact basement membrane with up to three layers of granulosa cells, and (3) areas of attached stromal cells. Follicles isolated during the follicular phase grew (as judged by their increasing diameter) in culture (Fig. 1B), and the oocyte remained spherical within the expanding granulosa cell layers. In contrast, more follicles isolated during the luteal phase did not grow and became atretic as demonstrated by size, dark granulosa cell layers, and a partially denuded oocyte (Fig. 1D).
TABLE 1.
Comparison of follicle survival and growth follicle isolation during the follicular phase (FP) vs. luteal phase (LP): Day 14 of culture.*
FIG. 1.
Follicle growth over 14 days of culture. Follicles from both the follicular phase (A) and the luteal phase (C) begin as secondary follicles with an intact basement membrane, spherical and centrally located oocyte, and attached stromal cells. After 2 wk, the follicles have either increased in size, while maintaining a spherical and centrally located oocyte (B), or have become atretic with dark granulosa cells and a partially denuded oocyte (D). GC, granulosa cells; OO, oocyte; SC, stromal cells; bar = 50 μm.
Effect of Alginate Rigidity on Follicle Survival and Growth
Follicles from the follicular phase were cultured in either 0.25% or 0.5% (w/v) alginate gels to determine which physical properties of the matrix best support primate follicle survival and growth. As summarized in Table 2, 60 follicles were cultured in 0.25% alginate, and 110 follicles were cultured in 0.5% alginate. Follicles used for each condition were from different animals. By 14 days of culture, follicles cultured in 0.5% alginate had a higher survival rate (78%) relative to follicles cultured in 0.25% alginate (60%) (P < 0.05). Follicles in 0.5% alginate also reached a larger size (P < 0.05), with follicles increasing 78% in diameter relative to a 20% increase in diameter in 0.25% alginate.
TABLE 2.
Comparison of follicle survival and growth when cultured in 0.25% and 0.5% alginate for 14 days.*
Effect of Gonadotropins on Follicle Growth and Steroidogenesis
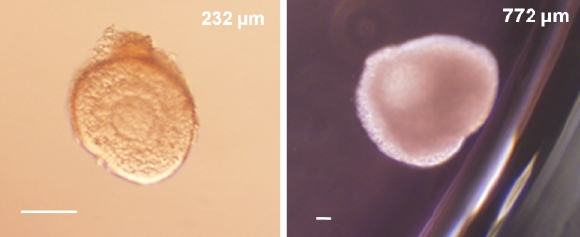
Finally, follicles from the follicular phase were cultured in the presence of FSH alone (n = 95) or in combination with LH (n = 77). Follicles from single animals were divided for culture in the two conditions. After 30 days of culture, follicles cultured in the presence of FSH alone demonstrated a mean ± SEM survival rate (55% ± 15%) similar to that of follicles cultured with LH (43% ± 13%), as shown in Figure 2A. Follicles exposed to either FSH alone or FSH plus LH demonstrated continuous growth (Fig. 2B). In addition, follicles cultured with FSH alone had a greater increase in diameter on Day 30 compared with those cultured with FSH plus LH (Fig. 2C). Preantral follicles isolated from the follicular phase could reach diameters typical of the small antral stage (≤1 mm) in rhesus monkeys by Day 30 of culture (Fig. 3).
FIG. 2.
Follicle survival (A), growth (B), and percentage increase (C) in diameter at Day 30 relative to Day 0 with or without exposure to 10 mIU/ml LH. All follicles were isolated during the follicular phase and cultured in 0.5% alginate. Statistically significant differences over time (B) with FSH alone (uppercase letters) or with FSH plus LH (lowercase letters) and between groups (FSH vs. FSH plus LH) (C) are indicated by different letters (P < 0.05). Data are given as the mean ± SEM from follicles isolated from five monkeys.
FIG. 3.
Growth of a preantral follicle isolated during the follicular phase (Day 1 [left panel]) to the small antral stage by Day 30 (right panel) of culture with FSH. Follicle diameters are shown in the upper right corner of each panel. Bar = 100 μm.
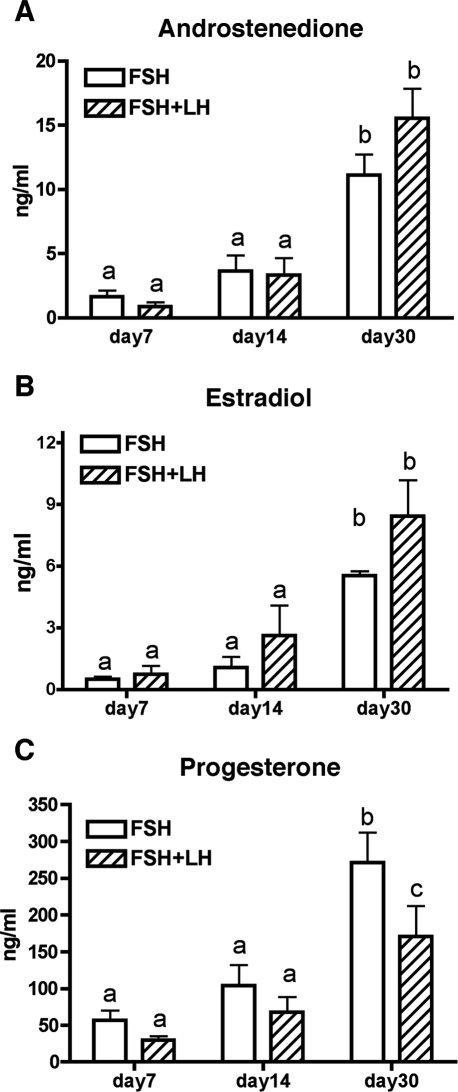
Production of A4, E2, and P4 significantly increased between Days 7 and 30 of culture (P < 0.05) in the presence of FSH alone or FSH plus LH (Fig. 4). The A4 levels in conditioned media were not different on Day 30 between treatment groups (Fig. 4A). In the presence of FSH plus LH, E2 levels tended to be greater by Day 30 vs. in FSH alone; however, the increase was not statistically significant (P = 0.07) (Fig. 4B). However, P4 levels were lower in the presence of FSH plus LH (P < 0.05) after 30 days of culture (Fig. 4C). Notably, P4 levels in conditioned media were an order of magnitude larger than levels of A4 and E2.
FIG. 4.
Steroid levels in media from secondary follicles cultured in 0.5% alginate over 30 days of culture. All steroid levels increased significantly from Day 14 to Day 30. A and B) No differences were observed between FSH vs. FSH plus LH groups for A4 and E2. C) At Day 30 of culture, the P4 concentration was significantly lower in follicles cultured in the presence of LH compared with those cultured without LH. Data are given as the mean ± SEM (n = 18). Statistically significant differences between groups are indicated by different letters (P < 0.05).
DISCUSSION
This study represents the first encapsulated 3-D culture of nonhuman primate follicles in vitro. Follicles were cultured in an encapsulated 3-D culture system, were able to survive and grow, and were functional for ≤30 days in culture based on increased steroidogenesis. Initial efforts to characterize and optimize the system examined culture of follicles from the follicular and luteal phases, the role of the physical properties of the 3-D matrix, and the role of LH in in vitro follicle development.
We began this study by examining the effect of the stage of the ovarian cycle during which follicles were isolated on in vitro follicle development and found that follicles from the follicular phase had greater survival rates than follicles from the luteal phase. The sizes of follicles isolated during the different phases differed significantly, with follicles from the luteal-phase tissue tending to be smaller (Table 1). We tried to ensure that all possible secondary follicles were isolated from the total number of ovarian pieces; therefore, isolated follicles should be representative of the secondary follicle pool at each ovarian stage, indicating that large secondary follicles may not be present in large numbers during the luteal stage. Due to this difference in starting size, however, the differences in survival and growth may be attributed to follicle size rather than follicular phase at isolation. Alternatively, the hormonal and local milieu around follicles in follicular vs. luteal phase may have differentially affected follicle potential. The dominant structures of the follicular and luteal phases differ, and paracrine-acting factors (both steroids and peptide hormones) influence the developing follicle cohort. In vivo follicle turnover is dictated by local factors and by FSH. Decreased FSH levels after selection of the dominant follicle and the presence of the corpus luteum and/or atretic follicles may alter the factors presented to adjacent preantral follicles and put them at a disadvantage at the time of isolation [58, 67]. Therefore, isolated follicles may already be progressing down an atretic pathway, resulting in their reduced survival. Little information is available regarding the effect of cycle phase on the health and persistence of preantral follicles in primates, and there is a need for more studies on follicle population dynamics in nonhuman primate ovaries.
The physical properties of the encapsulating matrix modulate mouse follicle development in encapsulated 3-D culture. Two concentrations of alginate, 0.25% and 0.5%, were shown to promote follicle growth, antrum formation, and oocyte quality [51, 68]. While 0.25% and 0.5% alginate cultures supported similar survival rates and both produced mature oocytes, only those follicles cultured in 0.25% alginate produced embryos that reached the blastocyst stage of development; hence, 0.25% alginate may be optimal for mouse follicle culture [53, 70]. Monkey follicles survived and grew in 0.5% alginate and 0.25% alginate; however, the survival and diameter increase (≤1 mm) of follicles were greater in 0.5% alginate relative to in 0.25% alginate. The ovarian stroma in the monkey and human is much more rigid than the mouse stroma; therefore, 0.5% alginate may better mimic the stromal biophysical environment in primates. However, further experiments are needed to optimize follicle isolation comparing mechanical vs. enzymatic methods, and matrix conditions may need to be modified depending on the isolation method.
The role of FSH and LH in preantral follicle development is not fully understood and is important in developing preantral follicle culture systems, specifically in the early culture period before follicles grow to reach the antral stage. Preantral follicles are generally thought to be gonadotropin responsive rather than gonadotropin dependent [59, 69–71]. While follicles in culture respond to FSH with increased oocyte quality, follicle growth, and follicle survival [45, 72], FSH and LH are generally considered not requisite until follicles reach the antral stage in vivo [73, 74]. Nonhuman ovaries exposed to a gonadotropin-deficient milieu for an interval (90 days) approximating the early growth phase were able to produce antral follicles upon stimulation with FSH or FSH plus LH [75], indicating that preantral follicles were able to grow under low circulating FSH and LH levels and maintained the ability to respond to FSH plus LH to produce antral follicles. Therefore, the precise role of FSH and LH, the doses required for follicle development, and the growth interval during which primate follicles must be exposed to these gonadotropins remain to be determined.
Follicle-stimulating hormone alone supported monkey preantral follicle survival and increases in diameter. In contrast, addition of LH during culture with FSH was detrimental to follicle survival and growth. To date, most research investigating the role of LH on follicle development has focused on LH action in late-stage follicles in the periovulatory period, and the role of LH in preantral follicle development has not been well characterized. However, it has been suggested that low LH supplementation during primary and secondary follicle culture enables follicles to respond to later LH-dependent growth [76]. In previous studies [22, 37] of follicle culture, LH supplementation did not affect follicle survival but enhanced follicle growth and antrum formation in the mouse and human, as well as the rate of oocyte maturation to the metaphase II stage in mouse. In our encapsulated 3-D mouse culture, however, addition of LH at the beginning of secondary follicle culture resulted in decreased follicle survival (Xu et al., unpublished results). The decreased follicle growth and survival in the presence of FSH plus LH in the present study may be due to the immature nature of the follicles in culture. Exposure to LH before reaching an appropriate stage of development may disrupt signaling in the follicle, thereby preventing proper follicle maturation. Whether preantral follicles benefit from LH supplementation later in development remains to be determined.
Surprisingly, in the presence of FSH alone, the encapsulated 3-D culture system enabled growing preantral follicles to produce appreciable levels of A4, E2, and P4 over 30 days. Steroid levels were not significantly different between Days 7 and 14 of culture, with or without LH exposure. By Day 30 of culture, however, follicles cultured in the presence of LH produced less P4 and tended to produce more E2. The E2 production may be attributed to increased theca cell presence, as LH has been shown to induce theca cell development during in vitro culture [37]. Theca cells are responsible for the LH-stimulated conversion of P4 to A4, which is then converted to E2 in the granulosa cells [77–79]. Thus, decreased levels of P4 in cultures containing FSH plus LH may also be due to an increased presence of theca cells, as more P4 is converted through A4 to E2. The extent to which theca-stroma cells remain attached to primate follicles isolated from ovaries or grow during 30 days of culture remains to be determined. The increasing levels of P4 in cultures with FSH alone may indicate its importance as a precursor for A4 and hence E2 production. Alternatively, elevated P4 could be due to spontaneous granulosa and/or theca cell luteinization [80] or may be a negative indicator of oocyte health.
In conclusion, this study demonstrates for the first time (to our knowledge) the feasibility of growing secondary primate follicles in vitro in a 3-D matrix. Follicles survived and continued to grow and produce increasing levels of steroid hormones for 30 days in the 3-D alginate-based culture system. The ovarian stage of follicle isolation appears to affect follicle survival and growth and should therefore be monitored and considered in future human and nonhuman primate studies. Follicle development was also regulated by the physical properties of the culture matrix, and mechanically isolated follicles cultured in 0.5% alginate had higher survival and growth rates relative to follicles cultured in 0.25% alginate, demonstrating that primate follicles may need more physical support during development relative to mouse follicles. The addition of LH to FSH was detrimental to follicle survival and reduced P4 production. Further studies are needed to optimize culture conditions for secondary follicle growth and function, with the ultimate goal of producing mature oocytes capable of fertilization and development to live offspring in primates.
Acknowledgments
The authors acknowledge Dr. Richard Yeoman and the personnel in the Endocrine Technology and Support Core, the Division of Animal Resources, and the Department of Surgery at the Oregon National Primate Research Center for their assistance in this work.
Footnotes
1Supported by the Oncofertility Consortium (NIH UL1 RRDE019587, 5RL1-HD058294, and 1PL1-EB008542) as part of the NIH Roadmap Interdisciplinary Research Consortia; NIH U54-HD18185, NIH U54-HD41857, and NCRR RR00163. M.X., R.L.S., L.D.S., T.K.W., and M.B.Z. are members of the Oncofertility Consortium.
REFERENCES
- McVie JG.Cancer treatment: the last 25 years. Cancer Treat Rev 1999; 25: 323–331.. [DOI] [PubMed] [Google Scholar]
- Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN.Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab 2003; 88: 5307–5314.. [DOI] [PubMed] [Google Scholar]
- Woodruff T, Snyder K. Oncofertility: Fertility Preservation for Cancer Survivors. New York:: Springer;; 2007. [Google Scholar]
- Fosas N, Marina F, Torres PJ, Jove I, Martin P, Perez N, Arnedo N, Marina S.The births of five Spanish babies from cryopreserved donated oocytes. Hum Reprod 2003; 18: 1417–1421.. [DOI] [PubMed] [Google Scholar]
- Borini A, Bonu MA, Coticchio G, Bianchi V, Cattoli M, Flamigni C.Pregnancies and births after oocyte cryopreservation. Fertil Steril 2004; 82: 601–605.. [DOI] [PubMed] [Google Scholar]
- Chen SU, Lien YR, Chen HF, Chang LJ, Tsai YY, Yang YS.Observational clinical follow-up of oocyte cryopreservation using a slow-freezing method with 1,2-propanediol plus sucrose followed by ICSI. Hum Reprod 2005; 20: 1975–1980.. [DOI] [PubMed] [Google Scholar]
- Porcu E, Fabbri R, Damiano G, Giunchi S, Fratto R, Ciotti PM, Venturoli S, Flamigni C.Clinical experience and applications of oocyte cryopreservation. Mol Cell Endocrinol 2000; 169: 33–37.. [DOI] [PubMed] [Google Scholar]
- Boldt J, Cline D, McLaughlin D.Human oocyte cryopreservation as an adjunct to IVF-embryo transfer cycles. Hum Reprod 2003; 18: 1250–1255.. [DOI] [PubMed] [Google Scholar]
- Gosden RG.Prospects for oocyte banking and in vitro maturation. J Natl Cancer Inst Monogr 2005; (34): 60–63.. [DOI] [PubMed] [Google Scholar]
- Yoon TK, Lee DR, Cha SK, Chung HM, Lee WS, Cha KY.Survival rate of human oocytes and pregnancy outcome after vitrification using slush nitrogen in assisted reproductive technologies. Fertil Steril 2007; 88: 952–956.. [DOI] [PubMed] [Google Scholar]
- Borini A, Sciajno R, Bianchi V, Sereni E, Flamigni C, Coticchio G.Clinical outcome of oocyte cryopreservation after slow cooling with a protocol utilizing a high sucrose concentration. Hum Reprod 2006; 21: 512–517.. [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Shea LD.The role of the extracellular matrix in ovarian follicle development. Reprod Sci 2007; 14: 6–10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J.Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med 2005; 353: 318–321.. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A.Livebirth after orthotopic transplantation of cryopreserved ovarian tissue [published correction appears in: Lancet 2004; 364:2020]. Lancet 2004; 364: 1405–1410.. [DOI] [PubMed] [Google Scholar]
- Lee DM, Yeoman RR, Battaglia DE, Stouffer RL, Zelinski-Wooten MB, Fanton JW, Wolf DP.Live birth after ovarian tissue transplant. Nature 2004; 428: 137–138.. [DOI] [PubMed] [Google Scholar]
- Meirow D, Ben Yehuda D, Prus D, Poliack A, Schenker JG, Rachmilewitz EA, Lewin A.Ovarian tissue banking in patients with Hodgkin's disease: is it safe? Fertil Steril 1998; 69: 996–998.. [DOI] [PubMed] [Google Scholar]
- Shaw J, Trounson A.Oncological implications in the replacement of ovarian tissue. Hum Reprod 1997; 12: 403–405.. [PubMed] [Google Scholar]
- O'Brien MJ, Pendola JK, Eppig JJ.A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod 2003; 68: 1682–1686.. [DOI] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK.Tissue-engineered follicles produce live, fertile offspring. Tissue Eng 2006; 12: 2739–2746.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ.Development in vitro of mouse oocytes from primordial follicles. Biol Reprod 1996; 54: 197–207.. [DOI] [PubMed] [Google Scholar]
- Roy SK, Treacy BJ.Isolation and long-term culture of human preantral follicles. Fertil Steril 1993; 59: 783–790.. [PubMed] [Google Scholar]
- Abir R, Franks S, Mobberley MA, Moore PA, Margara RA, Winston RM.Mechanical isolation and in vitro growth of preantral and small antral human follicles. Fertil Steril 1997; 68: 682–688.. [DOI] [PubMed] [Google Scholar]
- Abir R, Roizman P, Fisch B, Nitke S, Okon E, Orvieto R, Ben Rafael Z.Pilot study of isolated early human follicles cultured in collagen gels for 24 hours. Hum Reprod 1999; 14: 1299–1301.. [DOI] [PubMed] [Google Scholar]
- Abir R, Fisch B, Nitke S, Okon E, Raz A, Ben Rafael Z.Morphological study of fully and partially isolated early human follicles. Fertil Steril 2001; 75: 141–146.. [DOI] [PubMed] [Google Scholar]
- Telfer EE, McLaughlin M, Ding C, Thong KJ.A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod 2008; 23: 1151–1158.. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Wright C, Krausz T, Hardy K, Winston RM.Human primordial, primary and secondary ovarian follicles in long-term culture: effect of partial isolation. Hum Reprod 1999; 14: 2519–2524.. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Silye R, Abir R, Krausz T, Winston RM.Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod 1997; 12: 1032–1036.. [DOI] [PubMed] [Google Scholar]
- Louhio H, Hovatta O, Sjoberg J, Tuuri T.The effects of insulin, and insulin-like growth factors I and II on human ovarian follicles in long-term culture. Mol Hum Reprod 2000; 6: 694–698.. [DOI] [PubMed] [Google Scholar]
- Wright CS, Hovatta O, Margara R, Trew G, Winston RM, Franks S, Hardy K.Effects of follicle-stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Hum Reprod 1999; 14: 1555–1562.. [DOI] [PubMed] [Google Scholar]
- Scott JE, Carlsson IB, Bavister BD, Hovatta O.Human ovarian tissue cultures: extracellular matrix composition, coating density and tissue dimensions. Reprod Biomed Online 2004; 9: 287–293.. [DOI] [PubMed] [Google Scholar]
- Scott JE, Zhang P, Hovatta O.Benefits of 8-bromo-guanosine 3′,5′-cyclic monophosphate (8-br-cGMP) in human ovarian cortical tissue culture. Reprod Biomed Online 2004; 8: 319–324.. [DOI] [PubMed] [Google Scholar]
- Roy SK, Greenwald GS.An enzymatic method for dissociation of intact follicles from the hamster ovary: histological and quantitative aspects. Biol Reprod 1985; 32: 203–215.. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Schroeder AC.Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod 1989; 41: 268–276.. [DOI] [PubMed] [Google Scholar]
- Gore-Langton RE, Daniel SA.Follicle-stimulating hormone and estradiol regulate antrum-like reorganization of granulosa cells in rat preantral follicle cultures. Biol Reprod 1990; 43: 65–72.. [DOI] [PubMed] [Google Scholar]
- Li R, Phillips DM, Mather JP.Activin promotes ovarian follicle development in vitro. Endocrinology 1995; 136: 849–856.. [DOI] [PubMed] [Google Scholar]
- Cortvrindt R, Smitz J, Van Steirteghem AC.In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod 1996; 11: 2656–2666.. [DOI] [PubMed] [Google Scholar]
- Cortvrindt R, Hu Y, Smitz J.Recombinant luteinizing hormone as a survival and differentiation factor increases oocyte maturation in recombinant follicle stimulating hormone-supplemented mouse preantral follicle culture. Hum Reprod 1998; 13: 1292–1302.. [DOI] [PubMed] [Google Scholar]
- Newton H, Picton H, Gosden RG.In vitro growth of oocyte-granulosa cell complexes isolated from cryopreserved ovine tissue. J Reprod Fertil 1999; 115: 141–150.. [DOI] [PubMed] [Google Scholar]
- Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R.Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod 2000; 62: 1322–1328.. [DOI] [PubMed] [Google Scholar]
- Telfer EE, Binnie JP, McCaffery FH, Campbell BK.In vitro development of oocytes from porcine and bovine primary follicles. Mol Cell Endocrinol 2000; 163: 117–123.. [DOI] [PubMed] [Google Scholar]
- Wu J, Carrell DT, Wilcox AL.Development of in vitro-matured oocytes from porcine preantral follicles following intracytoplasmic sperm injection. Biol Reprod 2001; 65: 1579–1585.. [DOI] [PubMed] [Google Scholar]
- Picton HM, Danfour MA, Harris SE, Chambers EL, Huntriss J.Growth and maturation of oocytes in vitro. Reprod Suppl 2003; 61: 445–462.. [PubMed] [Google Scholar]
- Thomas FH, Campbell BK, Armstrong DG, Telfer EE.Effects of IGF-I bioavailability on bovine preantral follicular development in vitro. Reproduction 2007; 133: 1121–1128.. [DOI] [PubMed] [Google Scholar]
- Torrance C, Telfer E, Gosden RG.Quantitative study of the development of isolated mouse pre-antral follicles in collagen gel culture. J Reprod Fertil 1989; 87: 367–374.. [DOI] [PubMed] [Google Scholar]
- Nayudu PL, Osborn SM.Factors influencing the rate of preantral and antral growth of mouse ovarian follicles in vitro. J Reprod Fertil 1992; 95: 349–362.. [DOI] [PubMed] [Google Scholar]
- Spears N, Boland NI, Murray AA, Gosden RG.Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum Reprod 1994; 9: 527–532.. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Saudye H, Shea LD, Woodruff TK.Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng 2003; 9: 1013–1021.. [DOI] [PubMed] [Google Scholar]
- Wycherley G, Downey D, Kane MT, Hynes AC.A novel follicle culture system markedly increases follicle volume, cell number and oestradiol secretion. Reproduction 2004; 127: 669–677.. [DOI] [PubMed] [Google Scholar]
- Rowghani NM, Heise MK, McKeel D, McGee EA, Koepsel RR, Russell AJ.Maintenance of morphology and growth of ovarian follicles in suspension culture. Tissue Eng 2004; 10: 545–552.. [DOI] [PubMed] [Google Scholar]
- Heise M, Koepsel R, Russell AJ, McGee EA.Calcium alginate microencapsulation of ovarian follicles impacts FSH delivery and follicle morphology. Reprod Biol Endocrinol 2005; 3: e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, Woodruff TK.Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod 2006; 75: 916–923.. [DOI] [PubMed] [Google Scholar]
- Kreeger PK, Deck JW, Woodruff TK, Shea LD.The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials 2006; 27: 714–723.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West ER, Shea LD, Woodruff TK.Engineering the follicle microenvironment. Semin Reprod Med 2007; 25: 287–299.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abir R, Nitke S, Ben-Haroush A, Fisch B.In vitro maturation of human primordial ovarian follicles: clinical significance, progress in mammals, and methods for growth evaluation. Histol Histopathol 2006; 21: 887–898.. [DOI] [PubMed] [Google Scholar]
- Ksiazkiewicz LK.Recent achievements in in vitro culture and preservation of ovarian follicles in mammals. Biol Reprod 2006; 6: 3–16.. [PubMed] [Google Scholar]
- Lee KY, Mooney DJ.Hydrogels for tissue engineering. Chem Rev 2001; 101: 1869–1879.. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Hillier SG, van den Boogaard AM, Trimbos-Kemper TC, Reichert LE, Jr, van Hall EV.Follicular development during the luteal phase of the human menstrual cycle. J Clin Endocrinol Metab 1983; 56: 1022–1031.. [DOI] [PubMed] [Google Scholar]
- Koering MJ, Baehler EA, Goodman AL, Hodgen GD.Developing morphological asymmetry of ovarian follicular maturation in monkeys. Biol Reprod 1982; 27: 989–997.. [DOI] [PubMed] [Google Scholar]
- Gougeon A.Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod 1986; 1: 81–87.. [DOI] [PubMed] [Google Scholar]
- Woodruff TK, D'Agostino J, Schwartz NB, Mayo KE.Dynamic changes in inhibin messenger RNAs in rat ovarian follicles during the reproductive cycle. Science 1988; 239: 1296–1299.. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Bulnes A, Souza CJ, Campbell BK, Baird DT.Systemic and intraovarian effects of dominant follicles on ovine follicular growth. Anim Reprod Sci 2004; 84: 107–119.. [DOI] [PubMed] [Google Scholar]
- Contreras-Solis I, Diaz T, Lopez G, Caigua A, Lopez-Sebastian A, Gonzalez-Bulnes A.Systemic and intraovarian effects of corpus luteum on follicular dynamics during estrous cycle in hair breed sheep. Anim Reprod Sci 2008; 104: 47–55.. [DOI] [PubMed] [Google Scholar]
- Fanchin R, Schonauer LM, Cunha-Filho JS, Mendez Lozano DH, Frydman R.Coordination of antral follicle growth: basis for innovative concepts of controlled ovarian hyperstimulation. Semin Reprod Med 2005; 23: 354–362.. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL.Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum Reprod 2002; 17: 2825–2831.. [DOI] [PubMed] [Google Scholar]
- Jensen JT, Zelinski-Wooten MB, Schwinof KM, Vance JE, Stouffer RL.The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation during gonadotropin-stimulated ovarian cycles in rhesus macaques. Contraception 2005; 71: 68–73.. [DOI] [PubMed] [Google Scholar]
- Resko JA, Ellinwood WE, Pasztor LM, Huhl AE.Sex steroids in the umbilical circulation of fetal rhesus monkeys from the time of gonadal differentiation. J Clin Endocrinol Metab 1980; 50: 900–905.. [DOI] [PubMed] [Google Scholar]
- Baker SJ, Srsen V, Lapping R, Spears N.Combined effect of follicle-follicle interactions and declining follicle-stimulating hormone on murine follicle health in vitro. Biol Reprod 2001; 65: 1304–1310.. [DOI] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, Shea LD.Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 2007; 28: 4439–4448.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ.Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000; 21: 200–214.. [DOI] [PubMed] [Google Scholar]
- Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM.The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology 2000; 141: 1795–1803.. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Low MJ, Matzuk MM.Genetic rescue of follicle-stimulating hormone beta-deficient mice. Endocrinology 1998; 139: 3289–3295.. [DOI] [PubMed] [Google Scholar]
- Adriaens I, Cortvrindt R, Smitz J.Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod 2004; 19: 398–408.. [DOI] [PubMed] [Google Scholar]
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P.Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A 1998; 95: 13612–13617.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM.Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 1997; 15: 201–204.. [DOI] [PubMed] [Google Scholar]
- Zelinski-Wooten MB, Hutchison JS, Hess DL, Wolf DP, Stouffer RL.Follicle stimulating hormone alone supports follicle growth and oocyte development in gonadotrophin-releasing hormone antagonist-treated monkeys. Hum Reprod 1995; 10: 1658–1666.. [DOI] [PubMed] [Google Scholar]
- Wu J, Nayudu PL, Kiesel PS, Michelmann HW.Luteinizing hormone has a stage-limited effect on preantral follicle development in vitro. Biol Reprod 2000; 63: 320–327.. [DOI] [PubMed] [Google Scholar]
- Moon YS, Tsang BK, Simpson C, Armstrong DT.17 beta-Estradiol biosynthesis in cultured granulosa and thecal cells of human ovarian follicles: stimulation by follicle-stimulating hormone. J Clin Endocrinol Metab 1978; 47: 263–267.. [DOI] [PubMed] [Google Scholar]
- Makris A, Ryan KJ.Progesterone, androstenedione, testosterone, estrone, and estradiol synthesis in hamster ovarian follicle cells. Endocrinology 1975; 96: 694–701.. [DOI] [PubMed] [Google Scholar]
- Dorrington JH, Moon YS, Armstrong DT.Estradiol-17beta biosynthesis in cultured granulosa cells from hypophysectomized immature rats; stimulation by follicle-stimulating hormone. Endocrinology 1975; 97: 1328–1331.. [DOI] [PubMed] [Google Scholar]
- Fauser BC, Van Heusden AM.Manipulation of human ovarian function: physiological concepts and clinical consequences. Endocr Rev 1997; 18: 71–106.. [DOI] [PubMed] [Google Scholar]