Abstract
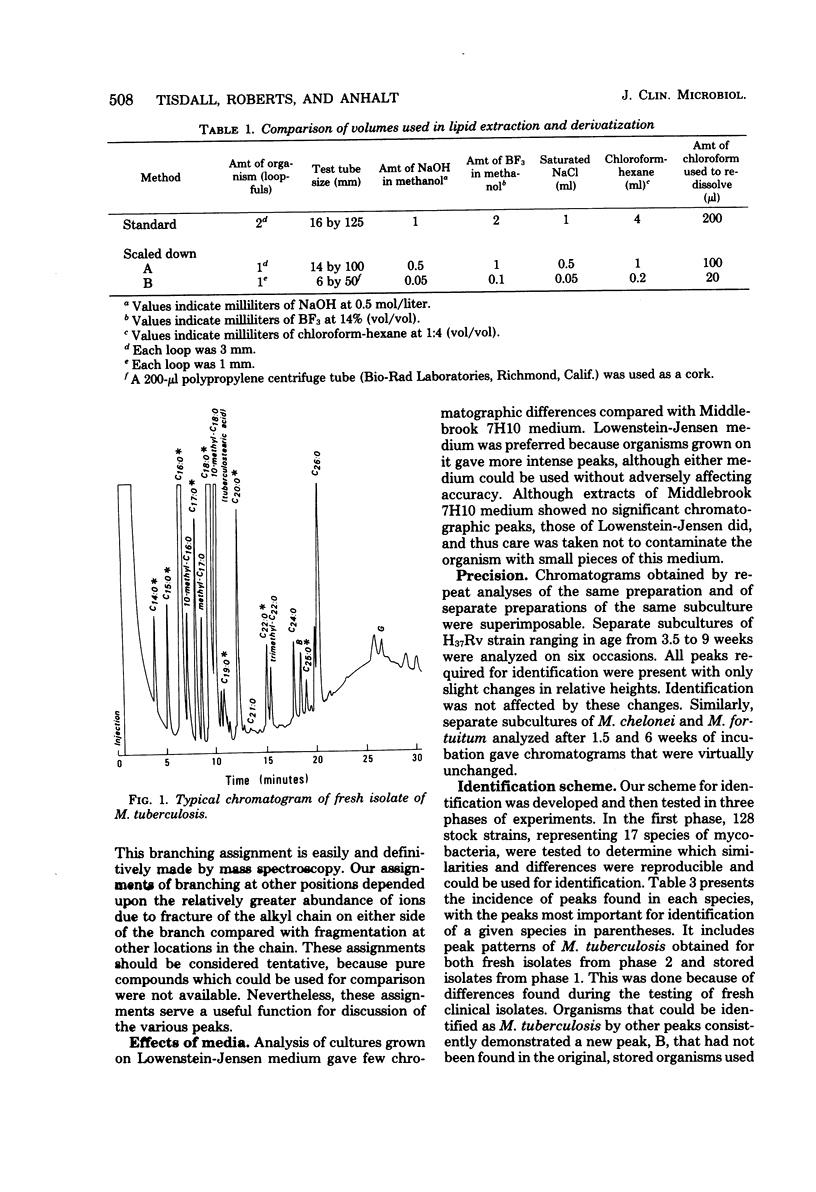
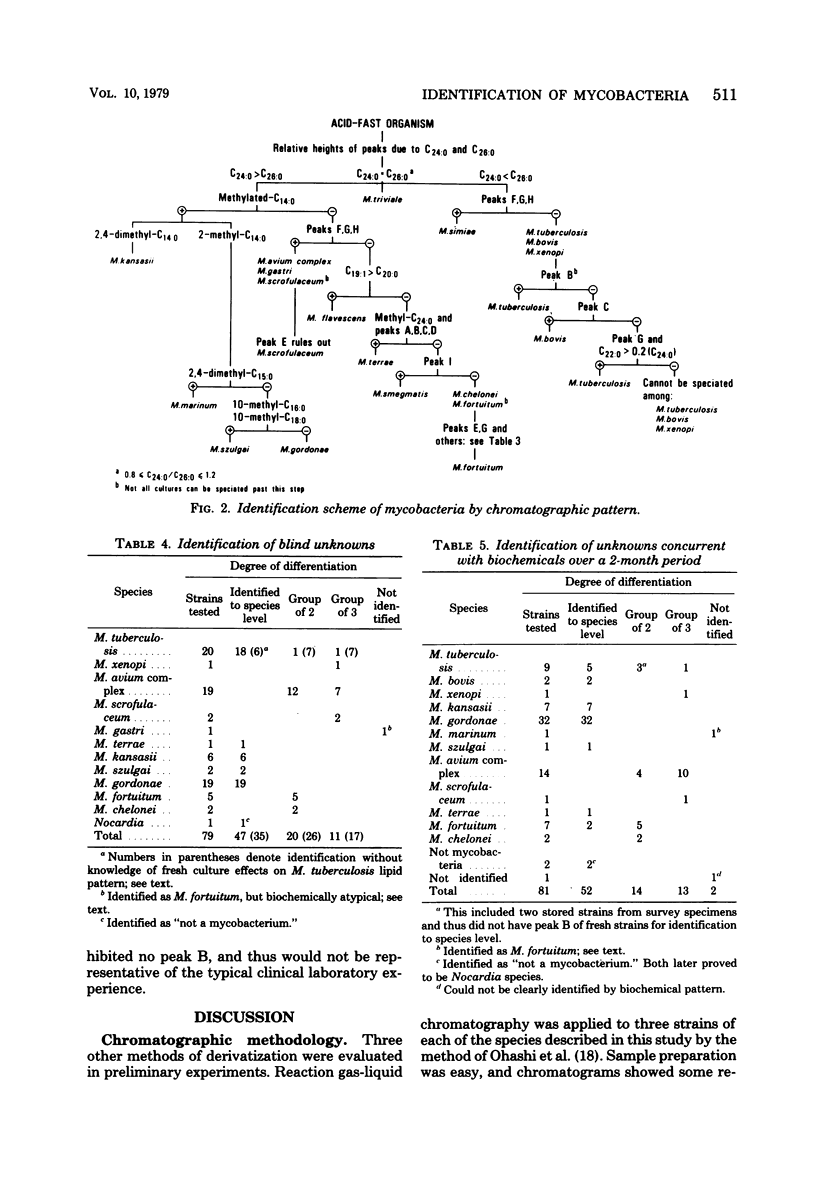
Identification of 18 mycobacterial species was performed by analysis of profiles obtained by using gas-liquid chromatography. Organisms were saponified in methanolic NaOH, and the reaction mixture was treated with BF3 in methanol and extracted with a hexane-chloroform mixture. An identification scheme was developed from 128 stock strains and tested against a collection of 79 clinical isolates. By using gas-liquid chromatographic profiles alone, 58% of specimens were correctly identified to species level, and an additional 41% were correctly identified to a group of two or three organisms. Use in a clinical laboratory over a 2-month period proved chromatography to be as accurate as and more rapid than concurrent biochemical testing. Of 81 isolates tested, 64% were identified to species level by chromatography alone. An additional 35% were differentiated to the same groups of two or three organisms as found in our analysis of stock strains. These groups consisted of: Mycobacterium tuberculosis, M. bovis, and M. xenopi; M. avium complex, M. gastri, and M. scrofulaceum; or M. fortuitum and M. chelonei. Identification to species level from these groups could usually be done by colonial morphology alone and could always be done by the addition of one selected biochemical test. This study demonstrated the practical application of gas-liquid chromatography in the identification of mycobacteria in a clinical laboratory. In particular, all strains of M. gordonae and M. kansasii were identified to species level. M. tuberculosis was definitively identified in 85% of cases. When it could not be definitely identified, the only alternatives were M. bovis and M. xenopi, both of which are rare causes of infection.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBIER M., LEDERER E. Sur l'isolement et la constitution chimique des acides mycoliques de Mycobacterium phlei et de Mycobacterium smegmatis. Biochim Biophys Acta. 1954 Jun;14(2):246–258. doi: 10.1016/0006-3002(54)90165-1. [DOI] [PubMed] [Google Scholar]
- Birn K. J., Schaefer W. B., Jenkins P. A., Szulga T., Marks J. Classification of Mycobacterium avium and related opportunist mycobacteria met in England and Wales. J Hyg (Lond) 1967 Dec;65(4):575–589. doi: 10.1017/s0022172400046106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. M., Naworal J. Composition of the saturated and monounsaturated fatty acids of Mycobacterium phlei. J Lipid Res. 1969 Sep;10(5):593–598. [PubMed] [Google Scholar]
- Campbell I. M., Naworal J. Mass spectral discrimination between monoenoic and cyclopropanoid, and between normal, iso, and anteiso fatty acid methyl esters. J Lipid Res. 1969 Sep;10(5):589–592. [PubMed] [Google Scholar]
- Jenkins P. A., Marks J., Schaefer W. B. Lipid chromatography and seroagglutination in the classification of rapidly growing mycobacteria. Am Rev Respir Dis. 1971 Feb;103(2):179–187. doi: 10.1164/arrd.1971.103.2.179. [DOI] [PubMed] [Google Scholar]
- Jenkins P. A., Marks J., Schaefer W. B. Thin-layer chromatography of mycobacterial lipids as an aid to classification: the scotochromogenic mycobacteria, including Mycobacterium scrofulaceum, M. xenopi, M. aquae, M. gordonae, M. flavescens. Tubercle. 1972 Jun;53(2):118–127. doi: 10.1016/0041-3879(72)90028-1. [DOI] [PubMed] [Google Scholar]
- Kubica G. P. Differential identification of mycobacteria. VII. Key features for identification of clinically significant mycobacteria. Am Rev Respir Dis. 1973 Jan;107(1):9–21. doi: 10.1164/arrd.1973.107.1.9. [DOI] [PubMed] [Google Scholar]
- Larsson L., Mårdh P. A. Gas chromatographic characterization of mycobacteria: analysis of fatty acids and trifluoroacetylated whole-cell methanolysates. J Clin Microbiol. 1976 Feb;3(2):81–85. doi: 10.1128/jcm.3.2.81-85.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi M., Cattaneo C., De Ritis G. C. The chromatographic separation of fatty acids (C11-C2O) from mycobacteria. Bull Int Union Tuberc. 1967 Jun;39(1):65–70. [PubMed] [Google Scholar]
- Marks J., Jenkins P. A., Schaefer W. B. Thin-layer chromatography of mycobacterial lipids as an aid to classification: technical improvements: mycobacterium avium, M. intracellulare (Battey bacilli). Tubercle. 1971 Sep;52(3):219–225. doi: 10.1016/0041-3879(71)90044-4. [DOI] [PubMed] [Google Scholar]
- Marks J., Jenkins P. A., Tsukamura M. Mycobacterium szulgai--a new pathogen. Tubercle. 1972 Sep;53(3):210–214. doi: 10.1016/0041-3879(72)90018-9. [DOI] [PubMed] [Google Scholar]
- Ohashi D. K., Wade T. J., Mandle R. J. Characterization of ten species of mycobacteria by reaction-gas-liquid chromatography. J Clin Microbiol. 1977 Nov;6(5):469–473. doi: 10.1128/jcm.6.5.469-473.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaels F. Difficulties encountered in identification of M. avium-M. intracellulare, M. scrofulaceum, and related strains. Am Rev Respir Dis. 1978 Nov;118(5):969–969. doi: 10.1164/arrd.1978.118.5.969. [DOI] [PubMed] [Google Scholar]
- Ratledge C. The physiology of the mycobacteria. Adv Microb Physiol. 1976;13:115–244. doi: 10.1016/s0065-2911(08)60039-9. [DOI] [PubMed] [Google Scholar]
- Reiner E., Beam R. E., Kubica G. P. A rapid chemotaxonomic method for distinguishing mycobacterial strains. J Chromatogr. 1967 Apr;27(2):495–496. doi: 10.1016/s0021-9673(01)85910-0. [DOI] [PubMed] [Google Scholar]
- Reiner E., Beam R. E., Kubica G. P. Pyrolysis-gas-liquid chromatography studies for the classification of mycobacteria. Am Rev Respir Dis. 1969 May;99(5):750–759. doi: 10.1164/arrd.1969.99.5.750. [DOI] [PubMed] [Google Scholar]
- Reiner E., Hicks J. J., Beam R. E., David H. L. Recent studies on mycobacterial differentiation by means of pyrolysis-gas-liquid chromatography. Am Rev Respir Dis. 1971 Nov;104(5):656–660. doi: 10.1164/arrd.1971.104.5.656. [DOI] [PubMed] [Google Scholar]
- Reiner E. Identification of bacterial strains by pyrolysis-gas-liquid chromatography. Nature. 1965 Jun 19;206(990):1272–1273. doi: 10.1038/2061272b0. [DOI] [PubMed] [Google Scholar]
- SMITH D. W., HARRELL W. K., RANDALL H. M. Correlation of biologic properties of strains of Mycobacterium with their infrared spectrums. III. Differentiation of bovine and human varieties of M. tuberculosis by means of their infrared spectrums. Am Rev Tuberc. 1954 Apr;69(4):505–510. doi: 10.1164/art.1954.69.4.505. [DOI] [PubMed] [Google Scholar]
- SMITH D. W., RANDALL H. M., GASTAMBIDE-ODIER M. M., KOEVOET A. L. The characterization of mycobacterial strains by the composition of their lipide extracts. Ann N Y Acad Sci. 1957 Sep 7;69(1):145–157. doi: 10.1111/j.1749-6632.1957.tb49655.x. [DOI] [PubMed] [Google Scholar]
- SMITH D. W., RANDALL H. M., MACLENNAN A. P., PUTNEY R. K., RAO S. V. Detection of specific lipids in mycobacteria by infrared spectroscopy. J Bacteriol. 1960 Feb;79:217–229. doi: 10.1128/jb.79.2.217-229.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulga T., Jenkins P. A., Marks J. Thin-layer chromatography of mycobacterial lipids as an aid to classification; Mycobacterium kansasii; and Mycobacterium marinum (balnei). Tubercle. 1966 Mar;47(1):130–136. doi: 10.1016/s0041-3879(66)80055-7. [DOI] [PubMed] [Google Scholar]
- Thoen C. O., Karlson A. G., Ellefson R. D. Comparison by gas-liquid chromatography of the fatty acids acids of Mycobacterium avium and some other nonphotochromogenic mycobacteria. Appl Microbiol. 1971 Oct;22(4):560–563. doi: 10.1128/am.22.4.560-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen C. O., Karlson A. G., Ellefson R. D. Differentiation between Mycobacterium kansasii and Mycobacterium marinum by gas-liquid chromatographic analysis of cellular fatty acids. Appl Microbiol. 1972 Dec;24(6):1009–1010. doi: 10.1128/am.24.6.1009-1010.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen C. O., Karlson A. G., Ellefson R. D. Fatty acids of Mycobacterium kansasii. Appl Microbiol. 1971 Apr;21(4):628–632. doi: 10.1128/am.21.4.628-632.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamura M. Differentiation between Mycobacterium abscessus and Mycobacterium borstelense. Am Rev Respir Dis. 1970 Mar;101(3):426–428. doi: 10.1164/arrd.1970.101.3.426. [DOI] [PubMed] [Google Scholar]
- Tsukamura M., Mizuno S., Tsukamura S. Numerical classification of slowly growing mycobacteria. Am Rev Respir Dis. 1969 Feb;99(2):299–303. doi: 10.1164/arrd.1969.99.2.299. [DOI] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]


