Abstract
Steroid hormone synthesis is a vital function of the adrenal cortex, serves a critical role in gonadal function, and maintains pregnancy if normally executed in the placenta. The substrate for the synthesis of all steroid hormones is cholesterol, and its conversion to the first steroid, pregnenolone, by the cholesterol side-chain cleavage cytochrome P450 (CYP11A1) enzyme complex takes place in the inner mitochondrial membranes. Steroidogenic acute regulatory protein (STAR) facilitates the rate-limiting transfer of cholesterol from the outer mitochondrial membrane to CYP11A1 located in the inner organelle membranes. The current study explored the mechanisms controlling transcription of the Star gene in primary cell cultures of mouse placental trophoblast giant cells and rat ovarian granulosa cells examined throughout the course of their functional differentiation. Our findings show that the cis-elements required for Star transcription in the rodent placenta and the ovary are centered in a relatively small proximal region of the promoter. In placental trophoblast giant cells, cAMP is required for activation of the Star promoter, and the cis-elements mediating a maximal response were defined as cAMP response element 2 and GATA. EMSA studies show that placental cAMP-responsive element binding protein (CREB)-1 and activating transcription factor-2 (ATF2) bind to a −81/−78 sequence, whereas GATA-2 binds to a −66/−61 sequence. In comparison, patterns of Star regulation in the ovary suggested tissue-specific and developmental controlled modes of Star transcription. During the follicular phase, FSH/cAMP induced CREB-1 dependent activity, whereas upon luteinization STAR expression becomes cAMP and CREB independent, a functional shift conferred by FOS-related antigen-2 displacement of CREB-1 binding, and the appearance of a new requirement for CCAAT enhancer-binding protein β and steroidogenic factor 1 that bind to upstream elements (−117/−95). These findings suggest that during evolution, the promoters of the Star gene acquired nonconsensus sequence elements enabling expression of a single gene in different organs, or allowing dynamic temporal changes corresponding to progressing phases of differentiation in a given cell type.
Proximal cis-elements in the steroidogenic acute regulatory protein (Star) promoter allow versatile transcriptional regulation in tissue-specific manners and differentiation-dependent patterns of STAR expression in rodent ovary and placenta.
Steroid hormone synthesis is a vital function of the adrenal cortex, serves a critical role in gonadal development and fertility (1), and maintains pregnancy if normally produced in the human placenta (2). The substrate for the synthesis of all steroid hormones is cholesterol, and the conversion of this substrate to pregnenolone, the first steroid formed, by cholesterol side-chain cleavage cytochrome P450 (P450scc) takes place in the inner mitochondrial membranes (3). Steroidogenic acute regulatory protein (STAR) facilitates the transfer of cholesterol from the outer mitochondrial membrane to P450scc located in the inner organelle membranes (4,5). Thus, STAR is a critical, rate-limiting regulator of steroid production in steroidogenic tissues (6).
Previous findings reported by us and others showed that in rodents, STAR is expressed in placental giant trophoblast cells during a limited time at midpregnancy (2,7). The expression of STAR is correlated with a local increase in placental progesterone synthesis, which unlike the human placenta, is not essential to maintain pregnancy as can be learned by the Cyp11a1 knockout mice that die only after birth (8). In the present study, we aimed to explore the expression and transcriptional regulation of the Star gene in the rodent placenta, although the primary signaling pathways generating STAR expression are not known at the moment. In the gonads, STAR expression is generally thought to be controlled by gonadotropins (FSH, LH) and cAMP signaling (9). These hormones also control the progress of the ovarian follicle toward ovulation, a process that requires steroid hormone production and STAR activity in the follicular phase (10). Shortly before ovulation and during corpus luteum formation thereafter in the luteal phase, steroidogenesis becomes a cAMP-independent process (11), and the ramifications of such a molecular switch on STAR regulation are examined in the present study.
Exploring the patterns of transcriptional regulation of the Star gene in the placenta and the ovary implies the use of two tissues of different embryonic cell origin: the granulosa cells that are derived from the ovarian surface epithelium (12) that is of mesodermal origin, and the blastocyst polar trophectoderm cell lineage that contributes to the steroidogenic placental giant cells we used herein (13). After a detailed characterization of Star transcription in primary cultures from the ovary (14) and the placenta (15,16), it became clear that similar or identical cis-regulatory sequences in the Star promoter can recruit different organ-specific trans-factors to activate the gene throughout different phases of cell differentiation.
Materials and Methods
Materials
Acetyl-coenzyme A, deoxyribonuclease I, poly (dI-dC), protease inhibitor cocktail, sodium orthovanadate, 8-bromo-cAMP (8-br-cAMP), NaF, DMEM, and peroxidase-conjugated goat antirabbit serum were obtained from Sigma-Aldrich Corp. (St. Louis, MO). BSA was purchased from ICN Biochemicals, Inc. (Cleveland, OH). Heat-inactivated fetal calf serum, trypsin-EDTA (0.25% trypsin, 1:2000 EDTA), and penicillin (10,000 μm/ml)/streptomycin (10 mg/ml) solution were from Kibbutz Beit Haemek, Israel. Lipofectamine and PLUS transfection reagents were from Invitrogen (Paisley, UK). Ovine FSH (National Institute of Diabetes and Digestive and Kidney Diseases oFSH-20) was kindly provided by National Institute of Diabetes and Digestive and Kidney Diseases’ National Hormone and Pituitary Program and A. F. Parlow (Harbor-University of California Los Angeles Medical Center, Torrance, CA). Equine chorionic gonadotropin (eCG) (pregnant mare serum gonadotrophin) was obtained from Vetimex (Bladel, The Netherlands) and human chorionic gonadotropin (hCG) from Organon (Oss, The Netherlands). Restriction enzymes were obtained from New England Biolabs, Inc. (Beverly, MA).
Polyclonal antisera to GATA-2 (sc-9008X), GATA-3 (sc-268X), GATA-4 (sc-1237X), CCAAT enhancer-binding protein β (C/EBPβ) (sc-150X), LRH-1 (sc-5997X), cFOS (sc-8047), FOSB (sc-48), FOS-related antigen (FRA)-1 (sc-183), FRA-2 (sc-171X), c-JUN (sc-1694), JUNB (sc-8051), JUND (sc-74), activation transcription factor (ATF)-2 (sc-187X), and ATF-1 (sc-243X) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Steroidogenic factor 1 (SF-1) antibody was purchased from Upstate Biotechnology Inc. (Lake Placid, NY). Antibody against cAMP-responsive element binding protein (CREB)-1 (AHP337) for EMSA was from Serotec (Oxford, UK), and antibody against phospho-CREB and CREB for Western were from Cell Signaling Technology, Inc. (Danvers, MA). Oligonucleotides and PCR primers were synthesized by Sigma-Genosys (Cambridgeshire, UK).
Animals
Timed pregnant female CB6F1/OlaHsd mice and female Sprague Dawley rats (21 d old) were obtained from Harlan Laboratories (Jerusalem, Israel) and maintained under a schedule of 16 h light, 8 h dark with food and water. Noontime on the day after mating was considered d 0.5 postcoitus, or embryonic d 0.5 (E0.5). Animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols had the approval of the Institutional Committee on Animal Care and Use, The Alexander Silberman Institute of Life Sciences, The Hebrew University of Jerusalem. Preovulatory eCG/hCG-treated ovaries were prepared by administration of 15 IU eCG to 24-d-old rats, which were further treated with 4 IU hCG administered (sc) 50 h later. The animals were killed either before treatment, at 10 h after eCG treatment, or at 8 h after hCG. Ovaries were retrieved for protein extraction for either EMSA or Western blot, as described below.
Promoter constructs and expression plasmids
The −1002/+6, −254/+6, −152/+6, −123/+6, −96/+6, −51/+6 region of the murine Star promoter was cloned by a PCR-based approach as previously described (14). Mutations (lowercase nucleotides) of either the GATA, cAMP response element (CRE), C/EBPβ, or SF-1 elements were obtained using a variant forward 5′-primer: −96mutGATA, ggccaagcttTGACCCTCTGCACAATGACTGATGACTTTTTTAagT-CAAGTG; −96mutTGA, ggccaagcttTGACCCTCTTGCACAATGA-CctgTGACTT; −96mutCRE2, ggccaagcttTGACCCTCTTGCACAAT-agaTGATGACTT; −96mutCRE3, ggccaagcttTGACCCTCTTGCACAATGACTGAgatCTT; −123mutSF-1, ggccaagcttTGCAGGATGAGGCAATCATTCCATtaTTGACCCT; and −123mutCEBP/β-1, ggccaagcttTGCAGGATGAGtcccaCATTCCAT. PCR consisted of 30 cycles at 94 C (1 min), 58 C (2 min), and 72 C (3 min). The PCR products were digested with HindIII and XbaI before ligation (T4-DNA ligase; Roche Molecular Biochemicals, Mannheim, Germany) into promoterless pCAT-Basic vector (Promega Corp., Madison, WI) restricted by the same enzymes. All plasmids generated were verified by PCR, restriction analysis, and sequencing.
Expression vector for GATA-4 was kindly provided by R. Schwarts (Baylor College of Medicine, Houston, TX). The dominant-negative expression vector GATA-DN was kindly provided by Professor Robert Viger (Chul Research Center and Department of Obstetrics and Gynecology, Laval University, Quebec, Canada) (17), ACREB by Dr. Richard H. Goodman (Oregon Health Science University, Portland, OR), and A-FOS by Eugene Tulchinsky of University of Leicester (Leicester, UK) (18).
Cell cultures
Trophoblast giant (TG) cells
TG cells were prepared from E9.5 mouse uteri as previously described (15) using trypsin dissociation medium (1.7 mg/ml trypsin and 80 μg/ml deoxyribonuclease I) (19), and seeded in 12-well plates (approximately two implantation sites per well) in DMEM supplemented with 10% fetal calf serum.
Granulosa cell cultures
Cells were prepared from ovaries of either estradiol (E2)-primed or eCG/hCG-treated animals (20,21) and plated onto serum-coated 12-well plates (∼0.8 ovary per well).
In situ hybridization
On d 9.5 postcoitus, mice were killed, and uterine horns were surgically removed into PBS. Implantation sites were separated from the myometrial layers and placed in buffered formalin. In situ hybridization of formalin-fixed, paraffin-embedded tissue sections with 35S-labeled antisense riboprobe was performed as previously described (22). The Star hybridization probe was cloned using the following primers: forward (749), 5′-TGCCGAAGACAATCATCAAC, and reverse, (1456), 5′-TTCTGCTGAAGCTGTGATTC, as previously described (23). It should be noted that other primers tested did not result in hybridization: forward (7), 5′-GGCCTCTAGAGCAGGACTCAGGACCTTGAAAGGCT and reverse (808), 5′-GGCGCCACCCCTTCAGGTCAATA. Cyp11a1 hybridization probe was cloned as previously described (24).
Transfection and reporter gene assays
Transfection of TG cells by Lipofectamine
On the morning after seeding, TG cells in each well were transfected with 700 ng DNA and Lipofectamine PLUS reagent (2 μl Lipofectamine, 5 μl PLUS reagent) in serum-free medium according to the basic protocol provided by the manufacturer. Three hours after onset of transfection, the medium was changed to serum-containing medium. Where indicated, 0.5 mm 8-Br-cAMP was added 24 h after transfection. Extracts for chloramphenicol acetyltransferase (CAT) analysis were performed 48 h after transfection.
Transfection of granulosa cells by electroporation
To this aim, electroporation was performed in suspension (4 × 105 cells/ 0.8 ml electroporation cuvette) containing CAT constructs (25 μg reporter plus 25 μg empty/expression vector) as previously described (14,25). The cell content from each cuvette was seeded into two wells (12-well plate), and 4 h after seeding, the E2 primed granulosa cells (defined as predifferentiated) were treated for 6 h with FSH (100 ng/ml), and extracts for CAT were made. The eCG and eCG/hCG granulosa cells (defined as graafian and luteal cells, respectively) were kept in culture for 10 h before a 6-h treatment with high-dose LH (500 ng/ml).
EMSA
Tissue extracts
Cell and tissue extracts for EMSA were obtained as described previously (14). Briefly, cells from whole tissue were homogenized using a Dounce homogenizer in three to four volumes of buffer A [400 mm KCl, 10 mm NaH2PO4 (pH 7.4), 10% glycerol, 1 mm EDTA, 1 mm dithiothreitol, 5 mm NaF, 1 mm sodium orthovanadate, and 1% vol/vol protease inhibitor cocktail], and the protein slurry was freeze thawed three times in liquid nitrogen and a 37 C bath. Finally, the cell lysates were centrifuged for 3 min at 14,000 × g, and the protein content in the supernatants was determined by a modified Bradford assay (26). Extracts were kept at −70 C until use.
EMSA
Protein extract (10–15 μg) was incubated with 2 ng double-stranded DNA previously labeled by a fill-in reaction using Klenow fragment (Promega) and [α-32P]-dCTP (Amersham Pharmacia Biotech, Little Chalfont, UK). Binding assay was performed using a final volume of 30 μl containing 100 mm KCl, 15 mm Tris-HCl (pH 7.5), 10 mm dithiothreitol, 1 mm EDTA, and 12% glycerol. When EMSA was performed on ovarian extract, 5 mm MgCl2 was added and 4.5 μg poly(dI-dC). The binding buffer for TG cells did not contain any MgCl2, and only 0.75 μg poly(dI-dC) was used. After incubation for 35 min at room temperature, the binding products were resolved on a native pre-run polyacrylamide gel (5%) using 0.5× Tris-borate, EDTA running buffer [50 mm Tris, 50 mm boric acid, and 10 mm EDTA (pH 8.3)]. When competition experiments were conducted, the protein extract was added last to the reaction mixture. When antibodies were used for detection of a given protein-DNA complex, the reaction cocktail without the probe was preincubated with 4 μg of the antibody for 20 min at room temperature before addition of the labeled DNA. The dried gels were analyzed using a FLA-3000 Bio-Imaging analyzer (Fuji Photo Film Co., Ltd., Tokyo, Japan). Gels were also exposed to Super RX medical x-ray film (Fuji Photo Film) for 2–24 h at −70 C and developed by an X-Omat processor (Curix 60; Agfa, Munchen, Germany).
Oligonucleotide probes
Probes used for EMSA were: wild-type (WT) −87/−66 (5′-ggccaagcttGCACAATGACTGATGACTTTTT); −73/−48 (5′-ggccaagcttGACTTTTTTATCTCAAGTGATGATGC); −73/−48mutGATA (5′-ggccaagcttGACTTTTTTAagTCAAGTGATGATGC); −87/−66mutCRE2 (5′-ggccaagcttGCACAATagaTGATGACTTTTT); and −60/−34scc1 (5′-GATCGCTCCTCTCTTAGCCTTGAGCTAGTTACCTA).
Synthetic oligonucleotides were annealed at a concentration of 1 μg/μl in annealing buffer [50 mm Tris-HCl (pH 7.5), 250 mm NaCl, and 0.5 mm EDTA] at 85 C for 5 min, and then cooled slowly to room temperature overnight and stored at −20 C. The annealed oligonucleotides were diluted to 50 ng/μl in double-distilled water.
Western blot
Total protein from giant trophoblast cells or ovaries (fresh or cultured, prepared as described previously) was extracted by lysis buffer containing protease-inhibitor cocktail and 10 mm Na-vanadate, and analyzed by SDS-PAGE and electroblotting as previously described (10). Specific signals were detected by chemiluminescence using the LumiGlo substrate (New England Biolabs) and signals recorded on x-ray film.
CAT assay
CAT activity was analyzed as previously described (14). Quantitation of the CAT assay was performed using a Fuji Bio-Imaging analyzer (BAS-1000; Fuji Photo Film). CAT activity was either presented as percentage of 14C-chloramphenicol converted to its acetylated products (per protein and time of assay), or shown as percentage of maximal arbitrary units over a given reference activity. Data are presented as the mean ± sd of several independent transfections (n ≥ 3).
Statistical analyses
Data are presented as the average ± sd of multiple (n ≥ 3) independent transfections. The Student’s unpaired two-tailed t test was performed using Microsoft Excel statistical analysis functions (Microsoft Corp., Redmond, WA). Differences were considered statistically significant at P < 0.05.
Results
Promoter analyses in TG cells
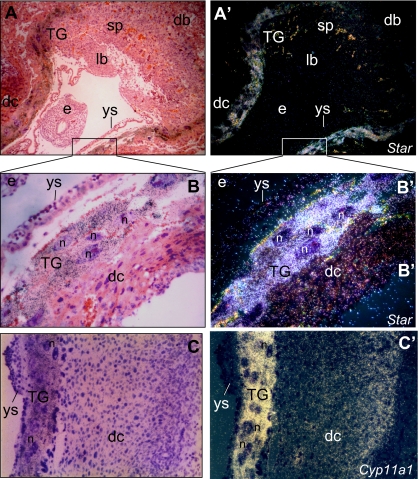
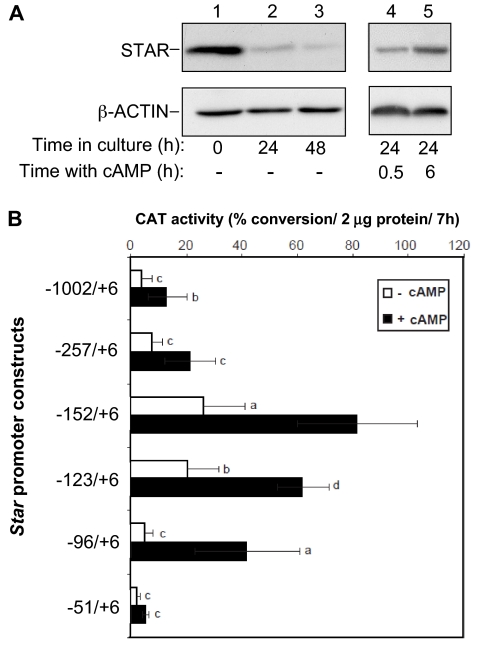
Unlike the apparent lack of STAR expression in the steroidogenic cells of the human placenta (27), one trophoblast cell subtype of the rodent placenta, the TG cells, exhibit a midpregnancy burst of STAR expression that is well synchronized with expression of P450scc and 3β-hydroxysteroid dehydrogenase type VI (7,24,28,29,30). Yet, the regulation of the Star gene in the rodent placenta has not been described. The predominant localization of STAR and P450scc transcript expression in TG cells is demonstrated in Fig. 1, B′ and C′, respectively, showing strong in situ hybridization signals in E9.5 implantation sites in the mouse uterus. In addition, a low level of Star expression was also noticed in the cells of the decidua capsularis (Fig. 1B′), which is in accordance with a low expression of Cyp11a1 (Fig. 1C′), as shown in previous studies (24). When the TG cells were isolated to be examined in vitro, Western blot analyses showed high levels of STAR content at cell preparation (Fig. 2A, time zero), which declined with time in culture (lanes 2 and 3). Treating the cells with 8-Br-cAMP restored the protein levels within a few hours (lanes 4 and 5).
Figure 1.
Localization of Star and Cyp11a1 mRNAs in E9.5 mouse placenta. By d 9.5 mouse pregnancy, implantation sites were prepared for in situ hybridization as described in Materials and Methods. Shown are low and high-power bright-field micrographs depicting a sagittal-to-embryo/transverse-to-uterus section stained with hematoxylin and eosin (A and C) and dark-field micrographs of the same microscope field (A′ and C′). A and A′ note a strong hybridization signal of Star transcript in the TG cells, but no labeling signals in the decidua basalis (db), the placental layers of spongiotrophoblasts (sp) and labyrinth (lb), the embryo (e) and the yolk sac (ys). B and B′, Bright- and dark-field micrographs, respectively, of the boxed area in A and A′ (magnification, ×40). Note a strong labeling of the TG cells, in contrast to lack of signal in the embryo and the yolk sac cells. A weak signal is observed in the decidua capsularis (dc). C and C′, Bright- and dark-field micrographs, respectively, of a decidua capsularis (dc) section processed for Cyp11a1 in situ hybridization, similar to that shown in B/B′ (magnification, ×33). Note a strong labeling of the TG cells, in contrast to the lack of signal in the yolk sac cells (ys). A weak signal is observed in the decidua capsularis (dc), as described before (24). n, Nucleus.
Figure 2.
Effect of cAMP on STAR levels and promoter activity in TG cells. A, TG cells were extracted for Western blot analysis either immediately after isolation (time zero), or after 24 and 48 h in culture. Twenty-four h after plating, the TG cells were treated with 0.5 mm 8-Br-cAMP, and the levels of STAR were examined (15 μg protein/lane) at time 0.5 and 6 h after the addition of cAMP. β-Actin served as loading marker. B, A series of promoter truncations subcloned into a CAT reporter gene (14) were used to transfect E9.5 mouse TG cells (see Materials and Methods). Twenty-four hours after transfection, cells were treated with 8-Br-cAMP (0.5 mm), and 24 h later all monolayers were harvested for CAT assays. CAT activity was determined using 2 μg protein for a 7-h assay. Promoter activity is presented as the mean ± sd of 14C-chloramphenicol converted to the acetylated products. Activity levels were statistically significant when compared with the respective values obtained for the −152/+6 construct treated with cAMP: a, P < 0.05; b, P < 0.005; c, P < 0.001; d, P > 0.1.
To identify the regulatory elements potentially involved in Star transcription, a series of 5′ deletion constructs of the murine Star promoter were placed upstream of a CAT reporter gene and transiently expressed in miniature cultures of TG cells (16). Figure 2B shows that maximal activities were obtained using the −152/+6 to −96/+6 region of the promoter; a maximal cAMP responsive region is located between −51 and −96; a high basal activity between −123 and −152; and sequences containing inhibitory elements are probably located between −152 and −257. The latter results are consistent with previous studies in MA-10 mouse Leydig cells suggesting that a negative regulatory region is localized between −180 and −150 bp of the murine Star promoter (31).
Tissue-specific GATA isoforms necessary for Star transcription
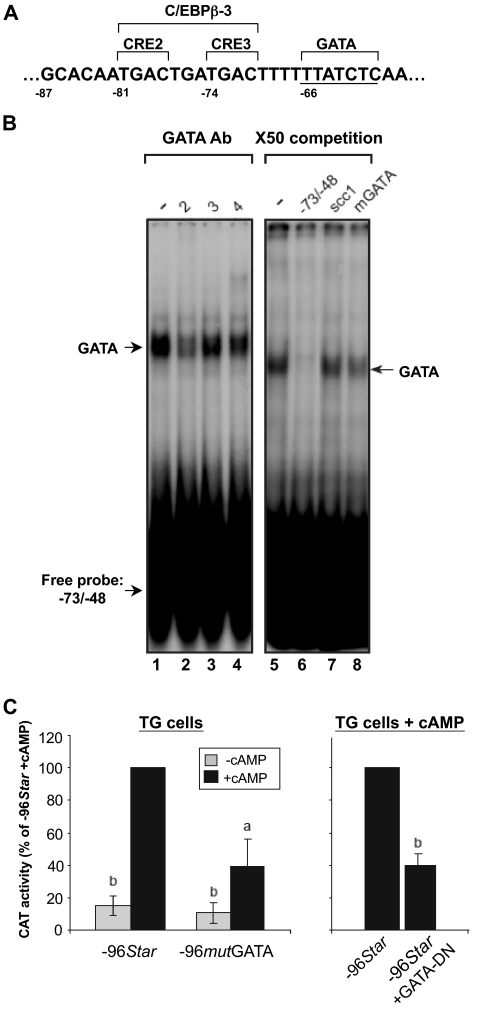
In general the results presented in Fig. 2 were consistent with findings previously described in ovarian granulosa cells (14) and in MA-10 mouse Leydig tumor cells (32,33,34); both cell models demonstrated the importance of the first 150 nucleotides of the promoter for the transcription of the Star gene and its induction by trophic hormones or cAMP analog. Within this proximal promoter region, several putative regulatory elements were found (35). In our previous work, we showed that in the ovary, GATA-4 and C/EBPβ bound to a GATA consensus element at −66/−61 and a nonconsensus C/EBPβ site, named C/EBPβ-3 at −81/−71 (Fig. 3A), respectively (14). The latter site also contains two CRE half-sites designated as CRE2 and CRE3, and it was shown that the CRE2 site is important for the cAMP response of the Star promoter in mouse Leydig cells, probably through binding of a member of the CREM/CREB family (33). Binding of CREB and phospho-CREB to the proximal Star promoter was also demonstrated in MA-10 mouse Leydig cells (36). To examine whether GATA proteins may also be relevant for Star transcription in the TG cells, we performed EMSAs using a −73/−48 probe (14,37). Figure 3B shows that a substantial DNA-protein complex was formed in the presence of TG cell extract (lane 1) and that this complex was ablated using a GATA-2 antiserum (lane 2). Antibodies against GATA-3 (lane 3) and GATA-4 (lane 4) did not affect the complex. This notion was consistent with Western blot assays published elsewhere (16), showing that GATA-2 and GATA-3 are the predominant isoforms expressed in the mouse placenta. In addition, the DNA-protein complex from the TG cells was readily competed with × 50 molar excess of self-oligonucleotide (lane 6), but not by a nonrelevant SF-1 binding sequence from the Cyp11a1 promoter (scc1, lane 7) (15). Interestingly, a mutated GATA oligonucleotide (TTATCT to TTAagT) partially competed with the radiolabeled WT oligonucleotide (lane 8), suggesting that an additional placental protein other than GATA was also capable of binding the probe sequence. This possibility is supported by the incomplete ablation observed with the anti-GATA-2 serum mentioned previously (lane 2).
Figure 3.
The −66/−61 GATA motif binds placental GATA-2 and is functionally important for cAMP inducibility of the Star promoter. A, Positioning of the −66/−61 (label in A) GATA site relative to a sequence previously defined as nonconsensus C/EBPβ-3 binding site (14), which overlaps two CRE half-sites originally described by Manna et al. (33). B, EMSAs were performed using 32P-labeled probes corresponding to the WT −73/−48 sequence of the Star gene. Extracts were prepared from E9.5 TG cells (15 μg) and, where indicated, preincubated with specific antisera to the GATA proteins 2–4. Arrows denote the DNA-GATA complexes. Competition with ×50 excess of nonlabeled −73/−48 oligonucleotides included the WT sequence and a mutated GATA element mGATA (TTATCT to TTAagT, mGATA), as well as an AP-2/SF-1 binding element from the P450scc promoter [scc1 (15)]. C, PCR-based mutations in the −66/−61 GATA element (TTATCT to TTAagT; −96mutGATA) were created within the context of the −96/+6 sequence of the Star promoter and ligated to pCAT-Basic (14). The activities of the WT and mutated promoters were assayed in TG cells in the absence or presence of cAMP, as indicated. In addition, the −96/+6 WT promoter was coexpressed in the presence of a dominant-negative mutant protein of GATA (GATA DN). Transient transfection and CAT assays were conducted as described in Fig. 2. The results are presented as activity values relative to the activity of −96Star (mean ± sd). Activity levels were statistically significant when compared with the respective values obtained for the −96Star construct treated with cAMP: a, P < 0.05; b, P < 0.005.
To examine whether the binding of GATA-2 to the −66/−61 GATA element is functionally relevant in the TG cells, we analyzed the effect of mutation of this element in the context of the −96/+6 promoter. As can be seen in Fig. 3C, 60% of the cAMP induced activity was lost when compared with the WT promoter; no significant effect was observed in basal activity. Figure 3C shows that a similar loss of activity was observed in TG cells coexpressing a dominant-negative form of the GATA proteins (17) and the promoter construct.
A −81/−78 CRE is necessary for placental Star transcription
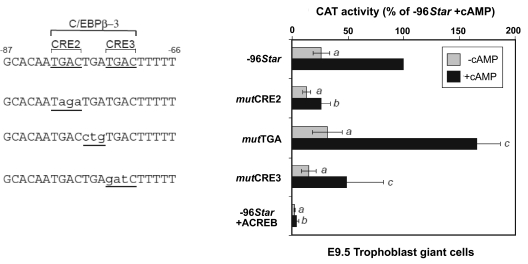
The robust response of the TG cells to cAMP indicated the likelihood of the involvement of CREs located between −96 and −51 in the Star promoter (Fig. 2). Although no consensus CRE exists in this region of the Star promoter, two CRE half-sites, CRE2 and CRE3 (TGAC, Fig. 3A), were previously identified (33) within the region defined before as a nonconsensus C/EBPβ-3 binding site (14,37). The importance of the CREs in the placenta was functionally corroborated by cloning three different mutations, as shown in Fig. 4, within the context of the −96/+6 Star CAT plasmid. In TG cells, substantial reduction of the basal and cAMP dependent activities was observed when the CRE2 half-site was mutated (mutCRE2). A barely significant effect was observed after mutation of CRE3, and mutating the intervening TGA sequence (mutTGA) that disables potential binding of C/EBPβ (data not shown) resulted in a somewhat higher promoter activity. Importantly, a dominant-negative mutant of CREB, “acidic CREB” (ACREB), which associates with the endogenous CREB and hinders its DNA binding, practically abolished both basal and cAMP-induced promoter activity, suggesting a critical role for CREB-1 in the regulation of placental Star (Fig. 4).
Figure 4.
Role of CRE2, CRE3, and C/EBPβ sites for Star transcription in TG cells. CAT constructs controlled by WT −96Star promoter, or its mutations, were examined in TG cells treated with 8-Br-cAMP as described in Fig. 2. Note that the mutTGA construct specifically targets the C/EBPβ-3 binding site. In addition, WT 96Star was coexpressed in the presence of the dominant-negative mutant protein CREB, ACREB. The activity values (mean ± sd) are presented relative to the activity of −96Star in cAMP treated cells. Activity levels were statistically significant when compared with the respective values obtained for the −96Star construct: a, P < 0.05; b, P < 0.001; c, P > 0.05.
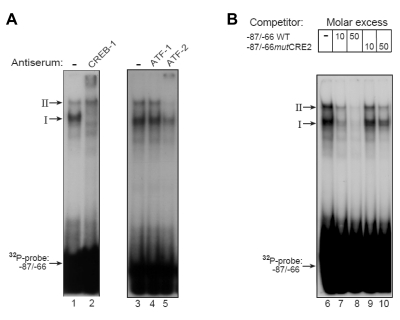
To examine which TG proteins bind to the various cis-elements, we performed EMSAs using a −87/−66 oligonucleotide previously used by Manna et al. (33) to study STAR expression in testicular MA-10 cells. When the −87/−66 oligonucleotide probe was incubated with protein extract obtained from the TG cells, two DNA-protein complexes were observed (I and II, Fig. 5A). Specific antibodies showed that complex I was completely supershifted by anti-CREB-1 (lane 2), whereas this antibody did not affect complex II. A specific ATF-2 antibody, but not anti-ATF-1 serum, supershifted complex II, and partially ablated complex I (lanes 4 and 5). These results suggest that the −87/−66 region of the Star promoter can bind placental CREB-1 homodimer, ATF-2 homodimer, and ATF-2/CREB-1 heterodimer. Competition experiments suggested that oligonucleotide mutated in the CRE2 markedly lost its ability to bind and displace the radiolabeled oligonucleotide (lanes 9 and 10) when compared with the WT sequence (lanes 7 and 8). Collectively, the functional tests and binding assays suggested that placental GATA and CREBs bind to the Star promoter and markedly activate it.
Figure 5.
Placental CREB-1 and ATF-2 proteins bind to the −87/−66 region of Star. A, Extracts from TG cells were incubated in the absence or presence of specific antibodies against members of the CREB/ATF protein family and analyzed by EMSAs using the −87/−66 32P-oligonucleotide probe. Arrows denote two predominant DNA-protein complexes, I and II. B, EMSAs using 32P-labeled −87/−66 oligonucleotide probe and TG cell extracts were performed in the presence of competing × 10 and × 50-fold molar excess of nonlabeled oligonucleotides, including a WT −87/−66 sequence (−87/−66 WT) and −87/−66mutCRE2 (TGACTGATGAC to TagaTGATGAC), also shown in Fig. 4.
Roles for CREB, C/EBPβ, and FRA-2 during follicular development
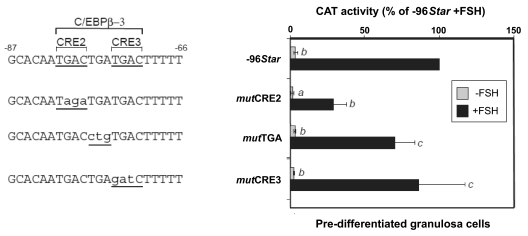
Because a potential role for CREB was never examined in gonadotropin-regulated ovarian cells, we assessed the functional relevance of CRE2, CRE3, and the intervening TGA sequences for the activity of the Star promoter in predifferentiated rat granulosa cells. That was of interest because we previously defined the −81/−71 element as a C/EBPβ-3 site by showing the capacity for the −87/−70 oligonucleotide to bind ovarian C/EBPβ (14,37) and increase promoter activity (37). Figure 6 shows that FSH treatment resulted in an over 20-fold increase in −96Star activity over characteristically low basal activities. Mutating the CRE2 half-site (mutCRE2) decreased both basal and FSH-induced activity, whereas mutCRE3 and mutTGA had no profound effect on the promoter activity in ovarian cells. These precise mutations were never examined in our previous studies, in which we used a compound mutation that altered the intervening TGA and additional C of the CRE2 sequence (TGACTGATGAC to TGAtctgTGAC). Consequently, this change resulted in the loss of the promoter activity (14), similar to the disabled activity of mutCRE2 shown in the present study.
Figure 6.
Role of CRE2, CRE3, and C/EBPβ sites in predifferentiated granulosa cells. CAT constructs controlled by WT −96Star promoter or its mutations shown in Fig. 4, were examined in E2-primed granulosa cells, with or without 6 h 100 ng/ml FSH treatment. The activity values (mean ± sd) are presented relative to the activity of −96Star in FSH treated cells. Activity levels were statistically significant when compared with the respective values obtained for the −96Star construct: a, P < 0.001; b, P < 0.05; c, P > 0.1.
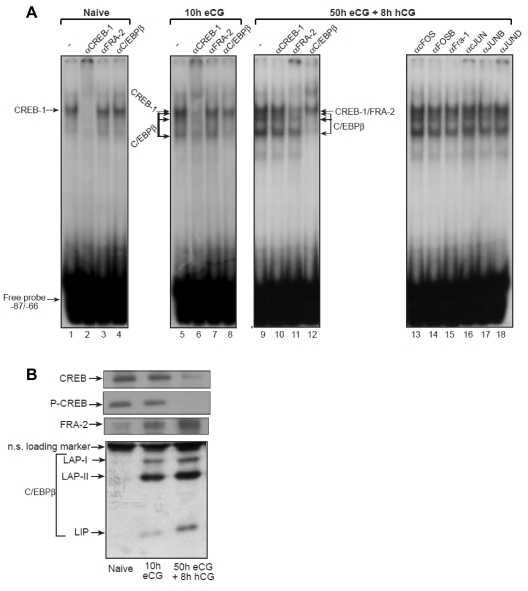
In view of the pivotal role CREB seemed to have on Star promoter activity in the naive predifferentiated granulosa cells, we aimed to test the involvement of CREB in the regulation of Star during the entire sequence of follicular development in the ovary, a process known to progress from cAMP-dependent to cAMP-independent gene expression patterns (11,16). Initially, we conducted EMSAs using the −87/−66 oligonucleotide probe and protein extracts from naive prepubertal animals (hereby defined as predifferentiated cells), eCG treated animals (10 h eCG) representing granulosa cells from growing follicles, and terminally differentiated cells from eCG/hCG treated rats (50 h eCG plus 8 h with hCG). Specific antisera showed that the −87/−66 oligonucleotide binds CREB-1 when incubated with protein extracts from the naive rat ovaries (Fig. 7A, lane 2). Antisera to FRA-2 and C/EBPβ were not effective in these ovaries (Fig. 7, lanes 3 and 4). Ten hours after the onset of follicular development, the major complex binding the promoter is still CREB-1 (Fig. 7, lane 6), but anti-C/EBPβ serum ablated two lower complexes previously identified as containing C/EBPβ proteins (Fig. 7, lane 8; and Refs. 14 and 37). A substantial switch in promoter binding was observed upon luteinization that occurred 8 h after hCG administration to the eCG primed animals: 1) the signal intensity of the C/EBPβ band shifts further increased (compare lane 9 with lanes 1 and 5), which could be ablated and supershifted by the C/EBPβ antiserum (lane 12); and 2) the CREB-1 binding to the DNA markedly reduced, and a pronounced binding of FRA-2 replaced it in the DNA complex (lane 11). Antibodies against other members of the AP-1 protein family, i.e. cFOS, FOSB, FRA-1, and JUNB, had no clear affect on the complex patterns (lanes 13–15 and 17), whereas it is likely that low levels of cJUN may pair with FRA-2 in such AP-1 complexes (lanes 16 and 18). These results suggested that dynamic changes take place in the follicular content of the trans-factors controlling Star transcription as the follicle progresses toward cAMP-independent gene expression. Western analyses shown in Fig. 7B supported this notion, as CREB and phospho-CREB levels decreased upon follicular differentiation, and the level of FRA-2 and C/EBPβ greatly increased in luteinizing follicles. The increase in C/EBPβ proteins after gonadotropin treatment was previously described by our group (37).
Figure 7.
Ovarian CREB-1, C/EBPβ, and FRA-2 bind to the −87/−66 region of the Star promoter. Binding to cis-elements in a 32P-labeled −87/−66 oligonucleotide probe was examined using three types of ovarian extracts prepared as described in Materials and Methods: naive rats, 24 d of age, 10 h after administration of eCG, and 8 h after administration of an ovulating dose of hCG to females treated 50 h earlier with eCG. A, EMSAs. The ovarian extracts were preincubated with the indicated antisera before addition of the 32P-probe and gel analysis. Arrows denote the band shift location of DNA complex with CREB-1, FRA-2, or C/EBPβ proteins. B, Western blot. CREB, phospho-CREB (P-CREB), FRA-2, and C/EBPβ protein levels were determined by Western blot analysis (10 μg protein per lane). The ovarian extracts were prepared from animals as described previously. The proteins defined as C/EBPβ (LAP-I, LAP-II, and LIP) were previously described (14,37).
CREB-1 to FRA-2 switch regulates STAR during luteinization
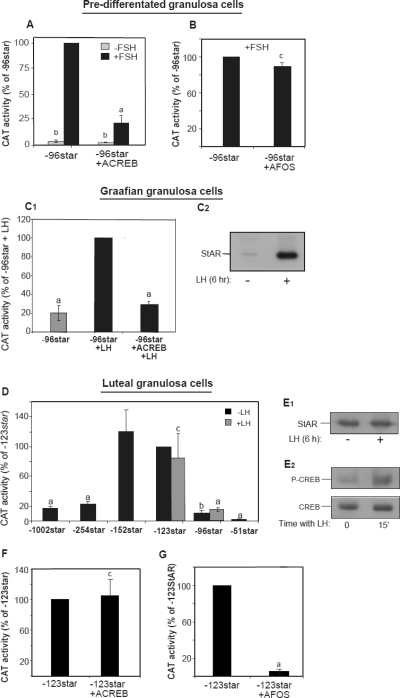
Because a follicular-to-luteal differentiation switch in the ovary depends on the LH surge, we further characterized the functional roles of CREB-1 and FRA-2 in granulosa cells isolated from naive prepubertal animals before hormonal induction of the first follicular cycle (predifferentiated), as well as 48 h after eCG administration (graafian granulosa cells), or upon their cellular luteinization in eCG/hCG induced animals. First, a predominant role of CREB-1 in predifferentiated granulosa cells was readily demonstrable by the fact that FSH induced a marked promoter activity that was strongly reduced in the presence of overexpressed ACREB (Fig. 8A). A similar effect of ACREB was observed when tested in the Graafian granulosa cells responding to a simulated LH surge in vitro (Fig. 8C1); by contrast, a dominant-negative version of FRA/FOS, AFOS, did not have any effect in the predifferentiated cells (Fig. 8B), suggesting that the AP-1 protein had no functional role during this phase of the cells’ function. Additional promoter trimming analyses examined in granulosa luteal cells (Fig. 8D) then revealed several novel insights: 1) Star promoter activity ceased to be hormone responsive, 2) the hormone-independent (basal) expression elevated 7-fold when tested with the −123Star construct over that observed with −96Star shown to exhibit the best hormonal responsive in the predifferentiated cells (14), and 3) the latter basal activity substantially reduced when the proximal promoter was extended to −254. These luteal cell-specific characteristics suggested that additional cis-elements located between −96 and −123 of the promoter are critical for the hormone-independent activity of Star expression, and inhibitory elements (−152/−254) can cause attenuation of the basal activity as the cells advanced toward ovulation and luteinization.
Figure 8.
Effects of dominant-negative CREB and FOS/FRA-2 proteins on Star promoter activity in predifferentiated, graafian, and luteal granulosa cells. Granulosa cells were collected from ovaries at three phases of follicular development: prepubertal animals treated with E2 (A and B), known to origin from tertiary follicles and represent steroidogenically predifferentiated cells (57), granulosa cells from animals treated with eCG for 48 h (C, graafian cells), and luteinizing granulosa cells (D–G) expressed from animals treated with eCG and hCG, as described in Materials and Methods. The cells were electroporation transfected with the indicated promoter-CAT constructs and expression plasmids, followed by 6 h incubations without or with FSH (100 ng/ml). When indicated the cells received high-dose LH (500 ng/ml). A and B, Effect of FSH and dominant-negative mutant proteins ACREB or AFOS on expression of −96/+6Star-CAT (−96Star) in predifferentiated cells. CAT activity was determined using 5 μg protein for a 1-h assay. The activity values (mean ± sd) are presented relative to the activity of −96Star in FSH treated cells. Activity levels were statistically significant when compared with the respective values obtained for the −96Star construct: a, P < 0.005; b, P < 0.001; c, P > 0.1. C1, Effect of LH and ACREB on −96/+6Star-CAT expression in graafian granulosa cells. C2, Western blot analyses of graafian granulosa cells exposed to 500 ng/ml LH for 6 h. Antisera to STAR was used to determine the antigen level in the treated cells (10 μg/lane). D, Star promoter trimming and lack of LH effect on activity of −123Star and −96Star CAT constructs in luteinizing granulosa cells; 10 h after transfection, the cells were treated with 500 ng/ml LH for 6 h. CAT activity was determined using 20 μg protein for a 7-h assay. The activity values (mean ± sd) are presented relative to the activity of −123Star in untreated cells. a, P < 0.005; b, P < 0.01; c, P > 0.1. E1 and E2, Western blot analyses of luteinizing granulosa cells exposed to LH for either 6 h (E1) or 15 min (E2). Antisera to STAR, CREB, and phospho-CREB (P-CREB) were used to determine the antigen levels in the treated cells (10 μg/lane). F and G, FOS-related protein, but not CREB, is required for activation of the −123Star construct in luteinizing granulosa cells. The latter cells were transfected with the promoter gene and plasmids carrying dominant-negative mutant proteins ACREB (F) or AFOS (G). CAT activity was determined using 20 μg protein for a 7-h assay. Activity values (mean ± sd) are presented relative to the activity of −123Star. Activity levels were statistically significant when compared with the respective values obtained for the −123Star construct. a, P < 0.005; c, P > 0.1.
Consistent with the aforementioned observation, LH failed to elevate the level of STAR in the luteal cells, which was already found to be high after preparation of the cells from eCG/hCG treated animals (Fig. 8E1). However, in culture of the LH-tolerant cells, CREB was reasonably high, and its phosphorylation was readily observed after addition of LH to the cells (Fig. 8E2). Neutralizing the functional activity of CREB by ACREB expression did not change the promoter activity in the luteal cells (Fig. 8F), and, instead, expression of a dominant-negative AFOS protein in the luteinizing cells caused a nearly complete ablation of the basal promoter activity (Fig. 8G), suggesting that AP-1 protein(s) replaced the CREB activation of the gene in the differentiated cells.
C/EBPβ and SF-1 are involved in cAMP-independent Star transcription
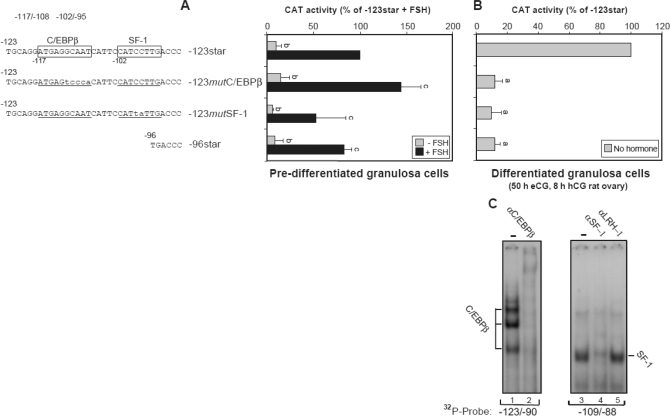
The observation suggesting that a longer promoter sequence is required for maximal manifestation of basal Star activity in the luteinizing granulosa cell raised the possibility that cis-element(s) other than those previously studied within the −81/−61 “cassette,” is involved in Star transcription during this phase of follicular differentiation. Two potential binding sites are nested within the −123/−96 sequence: one is the consensus C/EBPβ-1 site, and the other is a nonconsensus “SF-1” element. In this regard we considered an earlier observation by Sugawara et al. (38) suggesting that SF-1 is important for cAMP independent transcriptional regulation of Star in the human adrenocortical carcinoma cell line, NCI-H295R. To examine the relevance of these two cis-elements, we compared the activity of the −96Star promoter to that of −123Star that contained mutations within the C/EBPβ-1 (−117/−108) and SF-1 (−102/−95) recognition sites, as shown in Fig. 9 (−123mutC/EBPβ-1, −123mutSF-1). When expressed in luteinizing granulosa cells, removal or mutation of the C/EBPβ-1 or SF-1 binding site resulted in a 90% ablation of the promoter activity (Fig. 9B). The requirement for the two cis-elements was specific for the luteinizing granulosa cells because manipulating this region of the promoter did not affect the hormone-dependent regulation of Star promoter in predifferentiated granulosa cells incubated with or without FSH (Fig. 9A); FSH strongly induced the promoter constructs 10-fold or more, an observation that was also evident for the −123mutC/EBPβ-1 and −123mutSF-1 constructs. To determine if C/EBPβ and SF-1 bind to their respective sites, we performed EMSAs using the −123/−90 and −109/−88 oligonucleotides. Use of the respective antisera demonstrates specific binding of the four typical forms of C/EBPβ proteins existing in the ovary (14) (Fig. 9C). In addition, SF-1, and not LRH-1, binds to the −102/−95 Star DNA. LRH-1 was previously highly expressed in granulosa cells at all stages of follicular development (39), whereas both LRH-1 and SF-1 are capable of activating the human Star promoter in a heterologous cell model (40). Therefore, at least in the eCG/hCG treated superovulating rat model we show herein that SF-1 seems to bind predominantly to the −102/−95 element and up-regulate transcription. Together, these results support the notion that binding of C/EBPβ and SF-1 proteins to their upstream elements is required for Star transcription after the onset of luteinization in preovulatory follicles. Data shown in Fig. 2 and the apparent lack of SF-1 (15) and C/EBPβ expression in the placenta (data not shown) suggest that these distal sites are not necessarily required for transcription of Star in placental cells.
Figure 9.
Involvement of C/EBPβ and SF-1 in activation of the Star promoter in luteinizing granulosa cells, through binding to their upstream C/EBPβ-1 and SF-1 elements. A, Predifferentiated granulosa cells were transfected by electroporation with CAT plasmids controlled by −123Star, −96Star, or a −123Star promoter mutated in the C/EBPβ-1 (−123mutC/EBPβ-1) or SF-1 site (−123mutSF1). Four hours after seeding, the cells were treated with 100 ng/ml FSH for 6 h, and cell extracts were prepared. CAT activity was determined using 5 μg protein for a 1-h assay. Activity values (mean ± sd) are presented relative to the activity of −123Star treated with FSH and were statistically significant when compared with the −123Star activity; b, P < 0.05; c, P > 0.1. B, Luteinizing granulosa cells from eCG/hCG treated rats were transfected by electroporation with −123Star-CAT, −123mutC/EBPβ-1, −123mutSF1a, or the −96Star plasmid. CAT activity was determined using 20 μg protein for a 7-h assay. Activity values (mean ± sd) are presented relative to the activity of −123Star and were statistically significant when compared with the −123Star activity; a, P < 0.001. C, EMSA was performed using ovarian extracts from eCG/hCG treated rats. Antiserum to C/EBPβ was preincubated with the protein extract before assay with radiolabeled −123/−90 probe, and antibodies against SF-1 or LRH-1 were incubated with the protein extract before assay with −109/−88 DNA probe.
Discussion
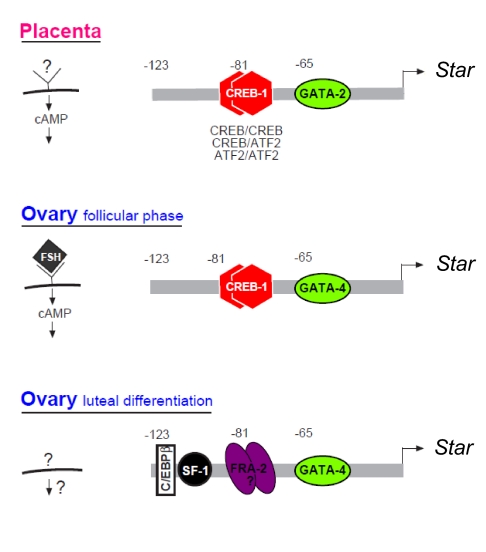
The present study revealed temporal dynamic changes of Star transcription confined to steroidogenic cells of the female reproductive organs, e.g. in ovarian follicular cells proceeding toward ovulation, and the rodent placental TG cells that transiently synthesize progesterone and androgens during midpregnancy. Figure 10 illustrates our current understanding of STAR regulation in these tissues. The functional regulatory mode of STAR expression in the placental TG cells appears to be relatively straightforward, and requires GATA and CREB binding to proximal cis-sites (Fig. 10). The role of CREB in placental STAR regulation is not necessarily expected because patterns of hormonal activation coupled to cAMP signaling in the TG cells have not yet been elucidated. Thus, our results predict that a yet to be identified cAMP dependent pathway conferring transcriptional up-regulation on this gene could exist. CREB and GATA were also found to be important for Star regulation throughout the phase of follicular growth in the ovary (Fig. 10), known to be controlled by FSH, cAMP, and the cognate PKA (21). Consequently, a loss in promoter activity was observed after the introduction of disabling mutations in the conserved GATA binding site at −66/−61 or the predominant CRE half-site, CRE2, positioned at −81/−78.
Figure 10.
Tissue-specific modes of Star regulation in the placenta and the ovary schematic representation. Upper panel, In placental cells, cAMP up-regulates transcription requiring binding of GATA-2 (−66/−61), as well trans-factor dimers that bind a CRE half-site defined as CRE2 (−81/−78). CRE2 binds CREB homodimers, ATF-2 homodimers, and CREB-ATF-2 heterodimers. Middle and lower panels, The ovarian mode of Star regulation distinguishes between FSH/cAMP responsive granulosa cells in the follicular phase (i.e. before LH-induced luteinization), and after LH surge terminally differentiated luteal cells when Star expression becomes cAMP independent. As shown, regulation of Star in FSH/cAMP responsive cells is similar to the placental pattern, but for GATA-4 that is predominantly expressed in the ovary, instead of the placental GATA-2 isoform. Bottom panel, In hormone-independent luteal cells, Star transcription requires additional factors interacting at the promoter, including GATA-4, FRA-2 (a dimmer with a yet unknown partner) that substitutes CREB binding to the −81/−78 site, and upstream binding of C/EBPβ (−117/−108) and SF-1 (−102/−95).
GATA factors are abundantly expressed in the gonads of vertebrates (16,41), and have activated transcription of other steroidogenic genes in the reproductive tract (16,17,42,43,44). Therefore, our previous evidence (14) coupled with the present results lend strong support to the concept that STAR joins a growing list of GATA-regulated genes controlling reproductive functions, differentiation, and development. In this regard we have recently identified a novel important GATA binding site required for transcription of the Cyp11a1 gene (P450scc) in the ovary and placenta (16). Moreover, for both Cyp11a1 and Star genes, the GATA site accommodates binding of GATA proteins in a tissue-specific manner, so that GATA-2 predominantly regulates Cyp11a1 and Star in the placenta, whereas GATA-4 plays this role in ovarian steroidogenic cells. These results are consistent with the fact that GATA-2 and GATA-3 are known to be expressed in rodent TG cells (16,45,46), whereas GATA-4/6 are present in rodent ovarian granulosa cells (16). We previously showed that the protein levels of GATA-2 and 4 are not elevated in response to cAMP or gonadotropin treatment (14,16), but earlier work with chromatin immunoprecipitation assays has shown that GATA-4 interacts with the Star promoter within minutes after the addition of cAMP analog in mouse Leydig cells, or in hCG treated granulosa cells (47).
An additional common feature of the Star and Cyp11a1 genes is the apparent lack of consensus CREs, which could be expected to be found in promoters strongly dependent on cAMP/CREB activation. Instead, two CRE1/2 elements were identified at −81/−78 and −74/−71 in the Star promoter (33), and serve unique roles in the ovary because the predominant role of CREB-1 observed in prepubertal follicles and early stages of gonadotropin-induced development was progressively replaced by FRA-2 binding to the same CRE1/2 elements in follicles approaching terminal luteinization (Fig. 10). In fact, the intriguing switch of CREB to AP-1 binding was made possible due to the nonconserved nature of the CRE half-site, in the form of which allowing the replacement of CREB by FRA-2 binding when the levels of the latter elevate during luteinization (48,49) (Fig. 7B). It is tempting to suggest that during evolution, the use of a CRE half-site element conferred a functional advantage over the classical CRE palindrome by allowing promiscuous binding of different bZIP superfamily members expressed in different organs or tissues resulting in similar, yet distinct differentiation programs, such as occurs in follicular ovarian cells. The functional relevance of FRA-2 binding to Star was supported by the loss of promoter activation in the presence of dominant-negative FOS protein, suggesting that the importance of protein members of the AP-1 family in rodent Star and Cyp11a1 regulation has been over looked until now.
The progressive loss of CREB-1 binding predominance during follicular growth may constitute a mechanism for the well-documented regulatory switch of P450scc expression in the rodent granulosa cells, turning from a cAMP-dependent mode in early developing follicles, to a cAMP-independent pattern that progresses after the onset of the ovulatory LH surge (11,50,51,52,53). Thus, this study suggests that switching from CREB-1 to FRA-2 usage constitutes at least one aspect of terminal differentiation occurring during each cycle of ovarian activity, when the dominant follicle turns to become a secretory gland. In this respect it is interesting to note that functional pairing of FRA-2 and phospho-CREB action is also known to regulate circadian rhythm by exhibiting circadian fluctuations in the melatonin-secreting cells of the pineal gland (54,55). Therefore, it is not unlikely that pairing of CREB-1 and FRA-2 may represent a general mechanism to regulate genes in organs with a cyclical pattern of functionality.
Expanding the present study to distinguish between predifferentiated granulosa cells and those undergoing terminal differentiation revealed a consensus C/EBPβ cis-site whose function was not previously understood. Previous studies exploring the potential role of the −117/−108 C/EBPβ (C/EBPβ-1) consensus cis-element did not attribute any functional relevance to this distal site (14,56). The present study shows a novel differentiation-dependent role for C/EBPβ binding to this distal site, which becomes essential for Star promoter activation (6-fold) when the granulosa cells terminally differentiate. In addition, flanking the C/EBPβ-1 site resides a novel SF-1 cis-element that is important for promoter activation in differentiated cells. These novel findings address expectations for an effect of SF-1 in the ovary, especially in the highly steroidogenic cells of the secretory corpus luteum gland that expresses ample amounts of SF-1 concomitantly with STAR (32).
As a result of the present studies, it may be necessary to rethink the potential role of the proximal nonconsensus cis-element shown to bind C/EBPβ to the −81/−71 sequence. Unlike the mutations introduced at this site previously (14,37), in the present study, we were careful to avoid any potential interference with the binding of CREB to the CRE2 site (TGACTGATGAC). Consequently, and in contrast to previous studies, the present C/EBPβ specific site-directed mutation of the CRE/FRA/CEBPβ-3 element (TGACTGATGAC to TGACctgTGAC) did not have any significant negative effect on the promoter activity in the predifferentiated cells that totally depend on CREB binding to the overlapping recognition element. Moreover, in the TG cells, a TGA to ctg mutation in the context of the −96Star promoter always improved the promoter activity (Fig. 4).
In summary, the present studies of Star expression in steroidogenic cells of the reproductive tissues, in conjunction with earlier observation, suggest that 56 bp of the proximal promoter control transcription under different physiological scenarios. Such instances include the typical cyclical changes in ovarian cell differentiation, or the chronic expression pattern of STAR in the testicular Leydig cells, or the need for STAR in the rodent placental TG cells that produce steroid hormones during a limited time window at midgestation. The present insights suggest that the regulatory sequences of the Star gene evolved by gaining enough transcriptional plasticity capable of responding to dynamic changes in multiple cell types, cellular signaling cues, and versatile differentiation programs. Toward this aim, the −117/−61 sequence of the Star promoter can respond to no less than five families of transcription factors, i.e. GATAs, CREB/CREM, AP-1 proteins, SF-1, and C/EBPβ. Interestingly, the latter two factors bind to a region upstream of CREB binding (−117/−95), which turns operational only when the cells become cAMP independent. In future studies it will be important to determine the mechanism that perpetuates Star expression in the apparent absence of a cAMP stimulus.
Acknowledgments
We thank Dr. Eli Keshet and Ahuva Itin from The Hebrew University-Hadassah University Hospital, Jerusalem, for critical help with in situ hybridization; Robert S. Viger of CHUL Research Centre, Quebec City for the GATA_DN plasmid; Eugene Tulchinsky of University of Leicester for providing the A-FOS plasmid; Richard Goodman of Vollum Institute, Oregon Health and Science University, Portland for the A-CREB plasmid.
Footnotes
This work was supported by the United States-Israel Binational Foundation 2003/398 and the Israel Science Foundation 1558/07 (to J.O.), and funds from National Institutes of Health Grant HD-17481 and Grant B1-0028 from the Robert A Welch Foundation (to D.M.S.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 9, 2008
Abbreviations: ACREB, Acidic cAMP-responsive element binding protein; ATF, activating transcription factor; 8-Br-cAMP, 8-bromo-cAMP; CAT, chloramphenicol acetyltransferase; C/EBPβ, CCAAT enhancer-binding protein β; CRE, cAMP response element; CREB, cAMP-responsive element binding protein; dI-dC, deoxyinosine-deoxycytosine; E0.5, embryonic d 0.5; eCG, equine chorionic gonadotropin; E2, estradiol; FRA, FOS-related antigen; hCG, human chorionic gonadotropin; P450scc, cholesterol side-chain cleavage cytochrome P450; SF-1, steroidogenic factor 1; STAR, steroidogenic acute regulatory protein; TG, trophoblast giant; WT, wild type.
References
- Richards JS, Hedin L 1988 Molecular aspects of hormone action in ovarian follicular development, ovulation, and luteinization. Annu Rev Physiol 50:441–463 [DOI] [PubMed] [Google Scholar]
- Strauss 3rd JF, Martinez F, Kiriakidou M 1996 Placental steroid hormone synthesis: unique features and unanswered questions. Biol Reprod 54:303–311 [DOI] [PubMed] [Google Scholar]
- Miller WL 1988 Molecular biology of steroid hormone synthesis. Endocr Rev 9:295–318 [DOI] [PubMed] [Google Scholar]
- Clark BJ, Wells J, King SR, Stocco DM 1994 The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269:28314–28322 [PubMed] [Google Scholar]
- Stocco DM, Sodeman TC 1991 The 30-kDa mitochondrial proteins induced by hormone stimulation in MA-10 mouse Leydig tumor cells are processed from larger precursors. J Biol Chem 266:19731–19738 [PubMed] [Google Scholar]
- Stocco DM, Clark BJ 1996 Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev 17:221–244 [DOI] [PubMed] [Google Scholar]
- Arensburg J, Payne AH, Orly J 1999 Expression of steroidogenic genes in maternal and extraembryonic cells during early pregnancy in mice. Endocrinology 140:5220–5232 [DOI] [PubMed] [Google Scholar]
- Hu MC, Hsu NC, El Hadj NB, Pai CI, Chu HP, Wang CK, Chung BC 2002 Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1. Mol Endocrinol 16:1943–1950 [DOI] [PubMed] [Google Scholar]
- Pon LA, Orme-Johnson NR 1988 Acute stimulation of corpus luteum cells by gonadotrophin or adenosine 3′,5′-monophosphate causes accumulation of a phosphoprotein concurrent with acceleration of steroid synthesis. Endocrinology 123:1942–1948 [DOI] [PubMed] [Google Scholar]
- Ronen-Fuhrmann T, Timberg R, King SR, Hales KH, Hales DB, Stocco DM, Orly J 1998 Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology 139:303–315 [DOI] [PubMed] [Google Scholar]
- Oonk RB, Krasnow JS, Beattie WG, Richards JS 1989 Cyclic AMP-dependent and -independent regulation of cholesterol side chain cleavage cytochrome P-450 (P-450scc) in rat ovarian granulosa cells and corpora lutea. cDNA and deduced amino acid sequence of rat P-450scc. J Biol Chem 264:21934–21942 [PubMed] [Google Scholar]
- Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP 2002 Formation of ovarian follicles during fetal development in sheep. Biol Reprod 66:1134–1150 [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J 1998 Promotion of trophoblast stem cell proliferation by FGF4. Science 282:2072–2075 [DOI] [PubMed] [Google Scholar]
- Silverman E, Eimerl S, Orly J 1999 CCAAT enhancer-binding protein β and GATA-4 binding regions within the promoter of the steroidogenic acute regulatory protein (StAR) gene are required for transcription in rat ovarian cells. J Biol Chem 274:17987–17996 [DOI] [PubMed] [Google Scholar]
- Ben-Zimra M, Koler M, Orly J 2002 Transcription of cholesterol side-chain cleavage cytochrome P450 in the placenta: activating protein-2 assumes the role of steroidogenic factor-1 by binding to an overlapping promoter element. Mol Endocrinol 16:1864–1880 [DOI] [PubMed] [Google Scholar]
- Sher N, Yivgi-Ohana N, Orly J 2007 Transcriptional regulation of the cholesterol side chain cleavage cytochrome P450 gene (CYP11A1) revisited: binding of GATA, cyclic adenosine 3′,5′-monophosphate response element-binding protein and activating protein (AP)-1 proteins to a distal novel cluster of cis-regulatory elements potentiates AP-2 and steroidogenic factor-1-dependent gene expression in the rodent placenta and ovary. Mol Endocrinol 21:948–962 [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Viger RS 2001 GATA factors differentially activate multiple gonadal promoters through conserved GATA regulatory elements. Endocrinology 142:977–986 [DOI] [PubMed] [Google Scholar]
- Tkach V, Tulchinsky E, Lukanidin E, Vinson C, Bock E, Berezin V 2003 Role of the Fos family members, c-Fos, Fra-1 and Fra-2, in the regulation of cell motility. Oncogene 22:5045–5054 [DOI] [PubMed] [Google Scholar]
- Sher N, Orly J 2006 Analysis of trophoblast giant cell steroidogenesis in primary cultures. Methods Mol Med 122:301–319 [DOI] [PubMed] [Google Scholar]
- Eimerl S, Orly J 2002 Regulation of steroidogenic genes by insulin-like growth factor-1 and follicle-stimulating hormone: differential responses of cytochrome P450 side-chain cleavage, steroidogenic acute regulatory protein, and 3β-hydroxysteroid dehydrogenase/isomerase in rat granulosa cells. Biol Reprod 67:900–910 [DOI] [PubMed] [Google Scholar]
- Orly J, Clemens JW, Singer O, Richards JS 1996 Effects of hormones and protein kinase inhibitors on expression of steroidogenic enzyme promoters in electroporated primary rat granulosa cells. Biol Reprod 54:208–218 [DOI] [PubMed] [Google Scholar]
- Motro B, Itin A, Sachs L, Keshet E 1990 Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci USA 87:3092–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T, Sonoda Y, Minegishi T, Wakabayashi K, Miyamoto K 1997 Molecular cloning, characterization and cellular distribution of rat steroidogenic acute regulatory protein (StAR) in the ovary. Life Sci 61:1497–1506 [DOI] [PubMed] [Google Scholar]
- Schiff R, Arensburg J, Itin A, Keshet E, Orly J 1993 Expression and cellular localization of uterine side-chain cleavage cytochrome P450 messenger ribonucleic acid during early pregnancy in mice. Endocrinology 133:529–537 [DOI] [PubMed] [Google Scholar]
- Orly J, Stocco DM 1999 The role of the steroidogenic acute regulatory (StAR) protein in female reproductive tissues. Horm Metab Res 31:389–398 [DOI] [PubMed] [Google Scholar]
- Zor T, Selinger Z 1996 Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem 236:302–308 [DOI] [PubMed] [Google Scholar]
- Sugawara T, Holt JA, Driscoll D, Strauss 3rd JF, Lin D, Miller WL, Patterson D, Clancy KP, Hart IM, Clark BJ 1995 Human steroidogenic acute regulatory protein: functional activity in COS-1 cells, tissue-specific expression, and mapping of the structural gene to 8p11.2 and a pseudogene to chromosome 13. Proc Natl Acad Sci USA 92:4778–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbaszade IG, Arensburg J, Park CH, Kasa-Vubu JZ, Orly J, Payne AH 1997 Isolation of a new mouse 3β-hydroxysteroid dehydrogenase isoform, 3β-HSD VI, expressed during early pregnancy. Endocrinology 138:1392–1399 [DOI] [PubMed] [Google Scholar]
- Durkee TJ, McLean MP, Hales DB, Payne AH, Waterman MR, Khan I, Gibori G 1992 P450(17α) and P450SCC gene expression and regulation in the rat placenta. Endocrinology 130:1309–1317 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Chapman BM, Johnson DC, Givens CR, Mellon SH, Soares MJ 1996 Cytochrome P450 17α-hydroxylase gene expression in differentiating rat trophoblast cells. J Endocrinol 150:161–168 [DOI] [PubMed] [Google Scholar]
- Clem BF, Clark BJ 2006 Association of the mSin3A-histone deacetylase 1/2 corepressor complex with the mouse steroidogenic acute regulatory protein gene. Mol Endocrinol 20:100–113 [DOI] [PubMed] [Google Scholar]
- Caron KM, Ikeda Y, Soo SC, Stocco DM, Parker KL, Clark BJ 1997 Characterization of the promoter region of the mouse gene encoding the steroidogenic acute regulatory protein. Mol Endocrinol 11:138–147 [DOI] [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, Zeleznik AJ, Stocco DM 2002 Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol Endocrinol 16:184–199 [DOI] [PubMed] [Google Scholar]
- Sugawara T, Kiriakidou M, McAllister JM, Kallen CB, Strauss 3rd JF 1997 Multiple steroidogenic factor 1 binding elements in the human steroidogenic acute regulatory protein gene 5′-flanking region are required for maximal promoter activity and cyclic AMP responsiveness. Biochemistry 36:7249–7255 [DOI] [PubMed] [Google Scholar]
- Manna PR, Wang XJ, Stocco DM 2003 Involvement of multiple transcription factors in the regulation of steroidogenic acute regulatory protein gene expression. Steroids 68:1125–1134 [DOI] [PubMed] [Google Scholar]
- Clem BF, Hudson EA, Clark BJ 2005 Cyclic adenosine 3′,5′-monophosphate (cAMP) enhances cAMP-responsive element binding (CREB) protein phosphorylation and phospho-CREB interaction with the mouse steroidogenic acute regulatory protein gene promoter. Endocrinology 146:1348–1356 [DOI] [PubMed] [Google Scholar]
- Silverman E, Yivgi-Ohana N, Sher N, Bell M, Eimerl S, Orly J 2006 Transcriptional activation of the steroidogenic acute regulatory protein (StAR) gene: GATA-4 and CCAAT/enhancer-binding protein β confer synergistic responsiveness in hormone-treated rat granulosa and HEK293 cell models. Mol Cell Endocrinol 252:92–101 [DOI] [PubMed] [Google Scholar]
- Sugawara T, Sakuragi N, Minakami H 2006 CREM confers cAMP responsiveness in human steroidogenic acute regulatory protein expression in NCI-H295R cells rather than SF-1/Ad4BP. J Endocrinol 191:327–337 [DOI] [PubMed] [Google Scholar]
- Falender AE, Lanz R, Malenfant D, Belanger L, Richards JS 2003 Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology 144:3598–3610 [DOI] [PubMed] [Google Scholar]
- Sirianni R, Seely JB, Attia G, Stocco DM, Carr BR, Pezzi V, Rainey WE 2002 Liver receptor homologue-1 is expressed in human steroidogenic tissues and activates transcription of genes encoding steroidogenic enzymes. J Endocrinol 174:R13–R17 [DOI] [PubMed] [Google Scholar]
- Heikinheimo M, Ermolaeva M, Bielinska M, Rahman NA, Narita N, Huhtaniemi IT, Tapanainen JS, Wilson DB 1997 Expression and hormonal regulation of transcription factors GATA-4 and GATA-6 in the mouse ovary. Endocrinology 138:3505–3514 [DOI] [PubMed] [Google Scholar]
- Fluck CE, Miller WL 2004 GATA-4 and GATA-6 modulate tissue-specific transcription of the human gene for P450c17 by direct interaction with Sp1. Mol Endocrinol 18:1144–1157 [DOI] [PubMed] [Google Scholar]
- Huang N, Dardis A, Miller WL 2005 Regulation of cytochrome b5 gene transcription by Sp3, GATA-6, and steroidogenic factor 1 in human adrenal NCI-H295A cells. Mol Endocrinol 19:2020–2034 [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Viger RS 2003 Novel roles for GATA transcription factors in the regulation of steroidogenesis. J Steroid Biochem Mol Biol 85:291–298 [DOI] [PubMed] [Google Scholar]
- LaVoie HA 2003 The role of GATA in mammalian reproduction. Exp Biol Med (Maywood) 228:1282–1290 [DOI] [PubMed] [Google Scholar]
- Ma GT, Roth ME, Groskopf JC, Tsai FY, Orkin SH, Grosveld F, Engel JD, Linzer DI 1997 GATA-2 and GATA-3 regulate trophoblast-specific gene expression in vivo. Development 124:907–914 [DOI] [PubMed] [Google Scholar]
- Hiroi H, Christenson LK, Chang L, Sammel MD, Berger SL, Strauss 3rd JF 2004 Temporal and spatial changes in transcription factor binding and histone modifications at the steroidogenic acute regulatory protein (stAR) locus associated with stAR transcription. Mol Endocrinol 18:791–806 [DOI] [PubMed] [Google Scholar]
- Rusovici R, LaVoie HA 2003 Expression and distribution of AP-1 transcription factors in the porcine ovary. Biol Reprod 69:64–74 [DOI] [PubMed] [Google Scholar]
- Sharma SC, Richards JS 2000 Regulation of AP1 (Jun/Fos) factor expression and activation in ovarian granulosa cells. Relation of JunD and Fra2 to terminal differentiation. J Biol Chem 275:33718–33728 [DOI] [PubMed] [Google Scholar]
- Carlone DL, Richards JS 1997 Functional interactions, phosphorylation, and levels of 3′,5′-cyclic adenosine monophosphate-regulatory element binding protein and steroidogenic factor-1 mediate hormone-regulated and constitutive expression of aromatase in gonadal cells. Mol Endocrinol 11:292–304 [DOI] [PubMed] [Google Scholar]
- Goldring NB, Durica JM, Lifka J, Hedin L, Ratoosh SL, Miller WL, Orly J, Richards JS 1987 Cholesterol side-chain cleavage P450 messenger ribonucleic acid: evidence for hormonal regulation in rat ovarian follicles and constitutive expression in corpora lutea. Endocrinology 120:1942–1950 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Alliston TN, Buse P, Firestone GL, Richards JS 1999 Functional and subcellular changes in the A-kinase-signaling pathway: relation to aromatase and Sgk expression during the transition of granulosa cells to luteal cells. Mol Endocrinol 13:1318–1337 [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Park-Sarge OK, Mayo KE 1996 Gonadotropins induce rapid phosphorylation of the 3′,5′-cyclic adenosine monophosphate response element binding protein in ovarian granulosa cells. Endocrinology 137:3234–3245 [DOI] [PubMed] [Google Scholar]
- Guillaumond F, Becquet D, Bosler O, Francois-Bellan AM 2002 Adrenergic inducibility of AP-1 binding in the rat pineal gland depends on prior photoperiod. J Neurochem 83:157–166 [DOI] [PubMed] [Google Scholar]
- Maronde E, Schomerus C, Stehle JH, Korf HW 1997 Control of CREB phosphorylation and its role for induction of melatonin synthesis in rat pinealocytes. Biol Cell 89:505–511 [DOI] [PubMed] [Google Scholar]
- Reinhart AJ, Williams SC, Clark BJ, Stocco DM 1999 SF-1 (steroidogenic factor-1) and C/EBP β (CCAAT/enhancer binding protein-β) cooperate to regulate the murine StAR (steroidogenic acute regulatory) promoter. Mol Endocrinol 13:729–741 [DOI] [PubMed] [Google Scholar]
- Richards JS, Ireland JJ, Rao MC, Bernath GA, Midgley Jr AR, Reichert Jr LE 1976 Ovarian follicular development in the rat: hormone receptor regulation by estradiol, follicle stimulating hormone and luteinizing hormone. Endocrinology 99:1562–1570 [DOI] [PubMed] [Google Scholar]