Abstract
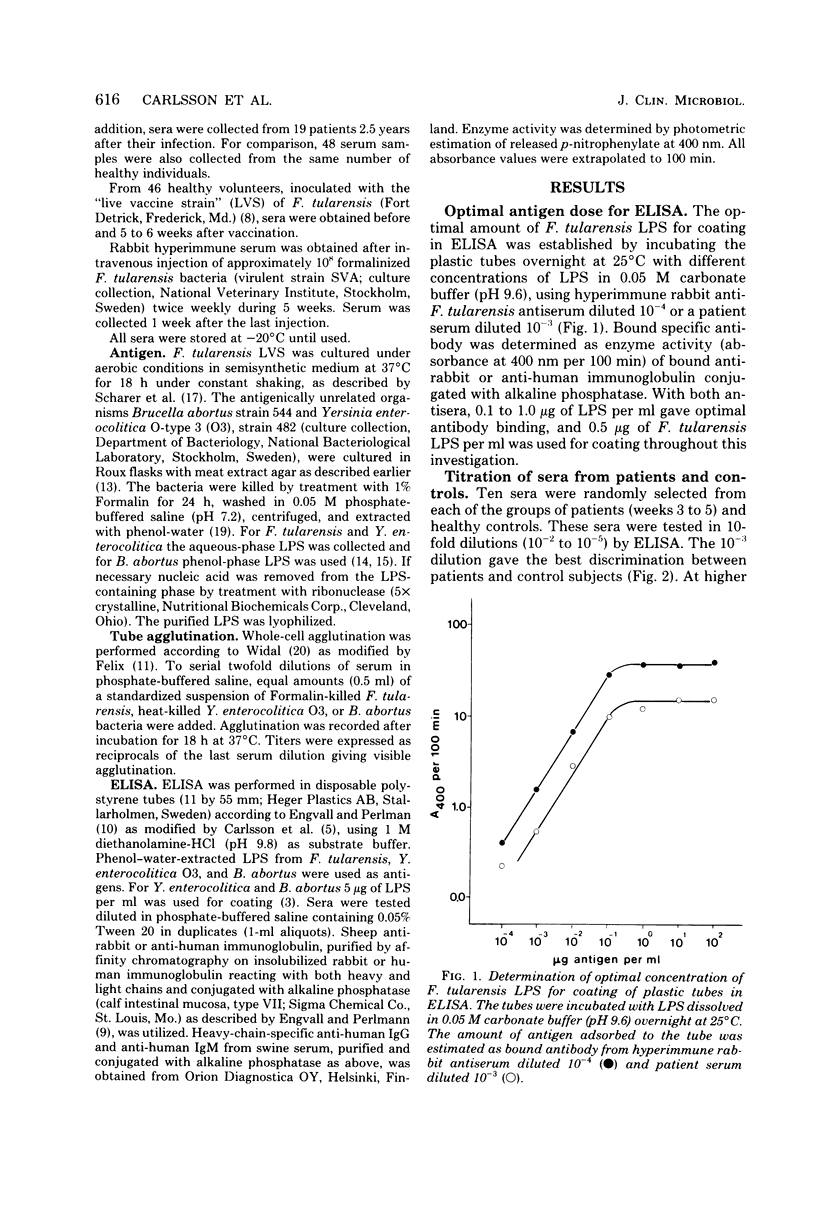
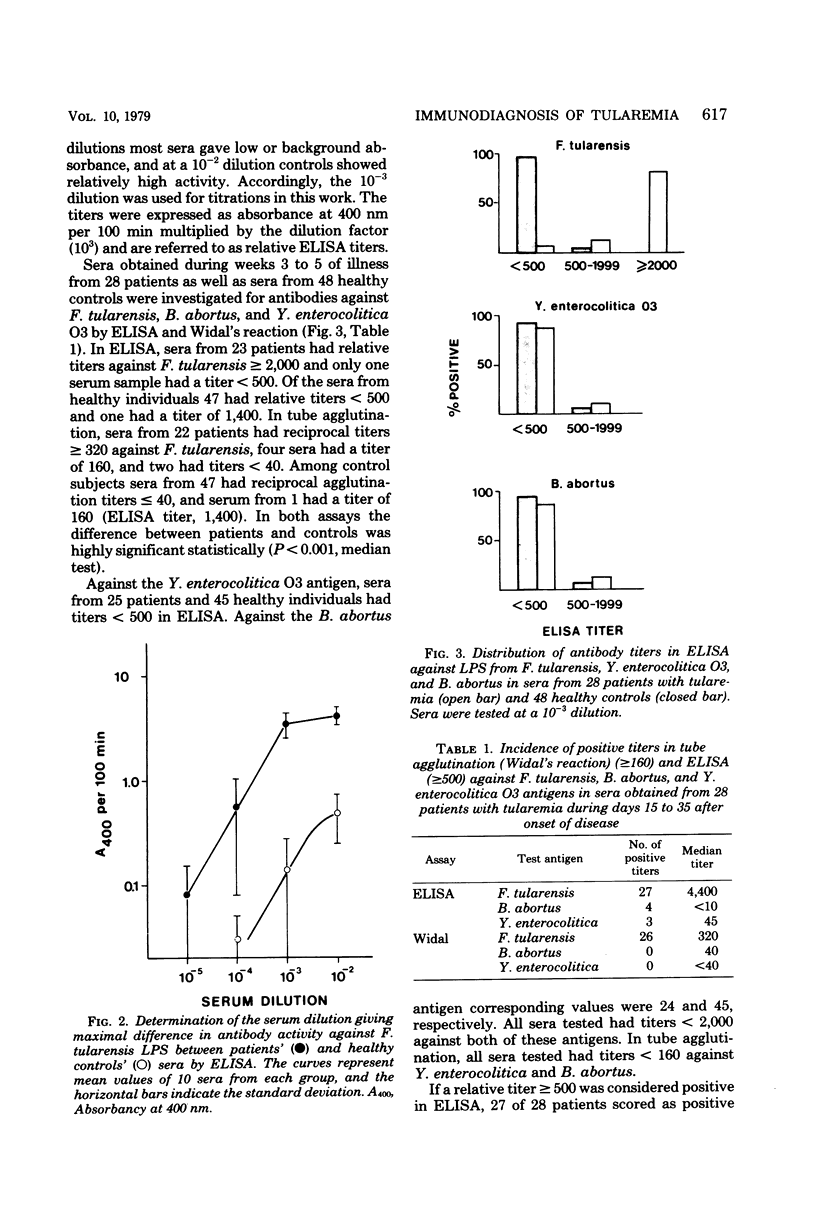
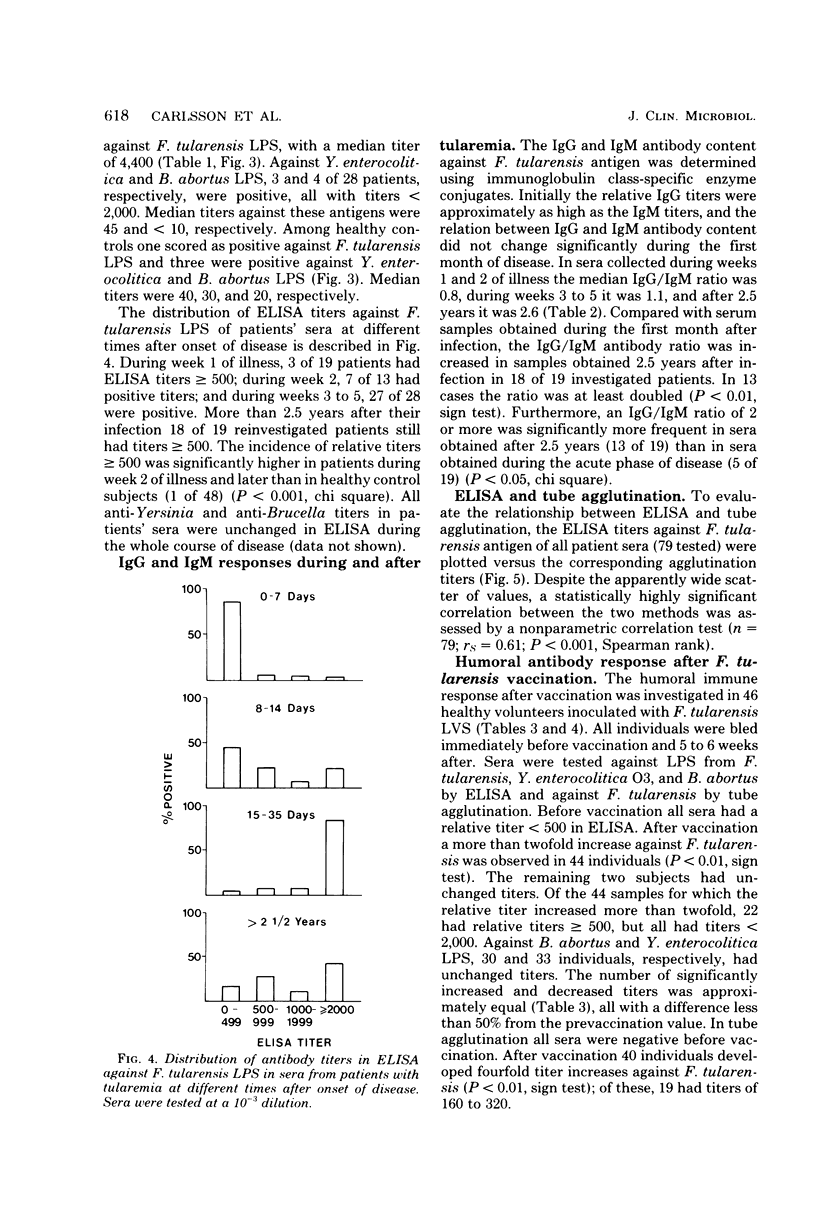
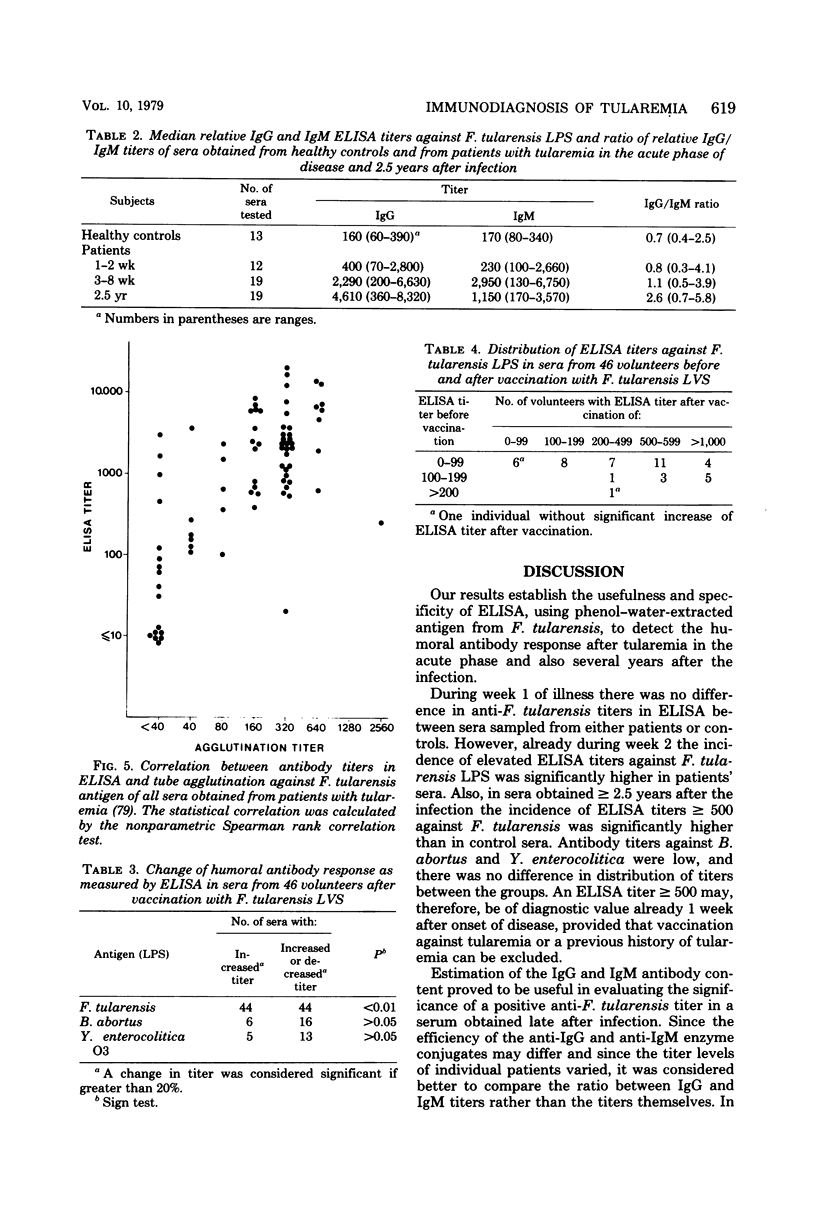
The enzyme-linked immunosorbent assay (ELISA) was applied for immunological diagnosis of human tularemia, using lipopolysaccharide from Francisella tularensis as antigen. Sera collected from patients, healthy individuals, and vaccinated volunteers were investigated for antibodies against F. tularensis by ELISA and tube agglutination. In ELISA all sera were titrated with a polyspecific anti-immunoglobulin enzyme conjugate. A limited number of consecutive sera from individual patients were also investigated for immunoglobulin G (IgG) and IgM antibodies by means of immunoglobulin class-specific conjugates. On an average ELISA was more than 10-fold as sensitive as tube agglutination. Two weeks after onset of disease, sera from patients had significantly higher titers in ELISA than sera from healthy controls. High titers persisted after more than 2 years. Significant amounts of both IgG and IgM antibodies were present within 1 to 2 weeks after infection. The antibody activity increased during the first month, without any significant change of the relation between IgG and IgM titers. After 2.5 years the IgG/IgM titer ratio of sera from patients was significantly increased. Within 6 weeks after vaccination sera from about half of the vaccinees had significantly elevated titers in ELISA. Titers observed after vaccination were generally lower than those found after infection. An elevated ELISA titer can be of diagnostic importance by the end of the first week of illness. A significant increase of titer in consecutive serum samples indicates a diagnosis of tularemia. Determination of IgG and IgM antibodies may be of value in determing whether a positive titer of a single serum sample is of longstanding or recent origin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyce J. M. Recent trends in the epidemiology of tularemia in the United States. J Infect Dis. 1975 Feb;131(2):197–199. doi: 10.1093/infdis/131.2.197. [DOI] [PubMed] [Google Scholar]
- Burke D. S. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977 Jan;135(1):55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- Carlsson H. E., Hurvell B., Lindberg A. A. Enzyme-linked immunosorbent assay (ELISA) for titration of antibodies against Brucella abortus and Yersinia enterocolitica. Acta Pathol Microbiol Scand C. 1976 Jun;84(3):168–176. doi: 10.1111/j.1699-0463.1976.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Carlsson H. E., Lindberg A. A., Hammarström S., Ljunggren A. Quantitation of Salmonella O-antibodies in human sera by enzyme-linked immunosorbent assay (ELISA). Int Arch Allergy Appl Immunol. 1975;48(4):485–494. doi: 10.1159/000231336. [DOI] [PubMed] [Google Scholar]
- Carlsson H. E., Lindberg A. A., Hammarström S. Titration of antibodies to salmonella O antigens by enzyme-linked immunosorbent assay. Infect Immun. 1972 Nov;6(5):703–708. doi: 10.1128/iai.6.5.703-708.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrand S., Ringertz O., Zetterberg B. Airborne tularemia in Sweden. Scand J Infect Dis. 1971;3(1):7–16. doi: 10.3109/inf.1971.3.issue-1.02. [DOI] [PubMed] [Google Scholar]
- EIGELSBACH H. T., DOWNS C. M. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J Immunol. 1961 Oct;87:415–425. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- FELIX A. Standardization of diagnostic agglutination tests; typhoid and paratyphoid A and B fevers. Bull World Health Organ. 1950;2(4):643–649. [PMC free article] [PubMed] [Google Scholar]
- Hurvell B. Serological cross-reactions between different Brucella species and Yersinia enterocolitica. Biological and chemical investigations of lipopolysaccharides from Brucella abortus and Yersinia enterocolitica type IX. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Feb;81(1):105–112. doi: 10.1111/j.1699-0463.1973.tb02193.x. [DOI] [PubMed] [Google Scholar]
- Hurvell B. Serological cross-reactions between different Brucella species and Yersinia enterocolitica. Immunodiffusion and immunoelectrophoresis. Acta Vet Scand. 1972;13(4):472–483. doi: 10.1186/BF03547153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In memoriam. Acta Vet Scand. 1973;14(1):1–9. [PubMed] [Google Scholar]
- Karlsson K. A., Dahlstrand S., Hanko E., Söderlind O. Demonstration of Francisella tularensis (syn. Pasteurella tularensis) in sylvan animals with the aid of fluorescent antibodies. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(5):647–651. doi: 10.1111/j.1699-0463.1970.tb04351.x. [DOI] [PubMed] [Google Scholar]
- Scharer J. M., Klein F., Lincoln R. E. Growth and metabolism of live vaccine strain of Pasteurella tularensis. Appl Microbiol. 1968 Jun;16(6):855–861. doi: 10.1128/am.16.6.855-861.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]


