Abstract
Pneumonic tularemia caused by inhalation of the type A strains of Francisella tularensis is associated with high morbidity and mortality in humans. The only vaccine known to protect humans against this disease is the attenuated live vaccine strain (LVS), but it is not currently registered for human use. To develop a new generation of vaccines, multiple animal models are needed that reproduce the human response to F. tularensis infection and vaccination. We examined the potential use of Fischer 344 rat as such a model. Fischer 344 rats were very sensitive to intratracheal infection with the virulent type A strain SCHU S4 and generally succumbed less than 2 weeks after infection. Similar to humans and non-human primates, Fischer 344 rats vaccinated with LVS by subcutaneous or intradermal routes were protected against a greater range of respiratory SCHU S4 challenge doses than has been reported for LVS-vaccinated mice. Intratracheal LVS vaccination also induced effective immunity, but it was less protective when the challenge dose exceeded 105 SCHU S4. LVS vaccination did not prevent SCHU S4 infection but rather controlled bacterial growth and pathology, leading to the eventual clearance of the bacteria. Our results suggest that the Fischer 344 rat may be a good model for studying pneumonic tularemia and evaluating potential vaccine candidates.
Introduction
Pneumonic tularemia is a zoonotic disease with an estimated mortality rate as high as 30-60% [1]. It can be treated successfully with antibiotics, but under some circumstances, such as biodefense, vaccination is preferred over post-exposure therapy. The Live Vaccine Strain (LVS) of Francisella tularensis is the only vaccine shown to protect humans against infection by virulent type A strains but it is not currently registered as a tularemia vaccine in the United States. Thus, a major effort is being undertaken to develop a new generation of safe and effective vaccines against this disease. One of the primary obstacles for developing vaccines against diseases such as tularemia that have a naturally low incidence but high consequence is that a direct human challenge to confirm vaccine efficacy is not likely to be performed; instead, we will have to rely heavily on animal models for vaccine discovery, characterization and development. The ideal animal model should recapitulate the human disease and be predictive of the human response to vaccination and challenge as much as possible. Murine models have been the foundation for most of our understanding of tularemia pathogenesis and immunity, but certain characteristics of these models make them less than optimal for vaccine discovery. For example, LVS vaccination of mice using techniques similar to the scarification method commonly used to vaccinate humans in the historical vaccine literature [2-4] and in the ongoing Special Immunization Program at the US Army Medical Research Institute of Infectious Diseases only increased survival by a few days after respiratory challenge with virulent type A strains [5,6]. Although alternative LVS vaccination strategies with demonstrated efficacy in humans such oral and respiratory vaccination [4,7] induced long-term survival in mice, the protection was limited to very small challenge doses [5,6,8-10]. Since live attenuated vaccines such as LVS are usually superior than killed or subunit vaccines [2,3], the limited protection generated by LVS vaccination in the murine models suggest that additional small animal models with a greater degree of protection are needed to support and complement the existing murine models in identifying and characterizing the broadest spectrum of potential vaccine candidates.
Historically, a variety of rat strains have been examined as potential models for human tularemia and most studies before 1975 utilized “white rats” without providing specific information on their strain or breeding. The white rats were found to be naturally more resistant than mice, guinea pigs and rabbits to virulent F. tularensis infection, being most susceptible to intraperitoneal (i.p.) infection followed by subcutaneous (s.c.), intradermal (i.d.), and intranasal (i.n.) infections, respectively [11,12]. Due to considerable variations in the susceptibility of individual rats to s.c. and i.p. infection, very large groups were required to determine the LD50 values with sufficient statistical power [12,13]. Nevertheless, white rats appeared to be a good model for testing vaccines because rats that recovered from a sub-lethal infection with the highly virulent type A strain Schu developed solid, long-lasting immunity to subsequent i.p. challenge with ten thousand to one million LD50 doses [14-16]. Interestingly, vaccination with killed bacteria or bacterial extracts also protected the rats against i.d., s.c. and i.p. Schu challenges, although these vaccinated rats succumbed to smaller Schu challenge doses, had higher bacterial burden and longer bacterial persistence than rats that had recovered from a Schu challenge [15,16]. The susceptibility of other rat strains including gray rats [17] cotton rats [12], Tumblebrook rats [12], Wistar rats [12] and Sprague Dawley rats [18] had also been studied but little or no protection data is available.
Two separate rat models have been used to examine the efficacy of LVS as a vaccine against SCHU S4 infection. Fisher-Dunning rats were found to be sensitive to an i.p. SCHU S4 infection dose as small as 100 SCHU S4, but after i.p. LVS vaccination, they became resistant to 108 SCHU S4 and exhibited limited clinical signs, bacterial burden and histopathology [19,20]. Fischer 344 rats were shown to be protected against an aerosol challenge of 2 × 105 SCHU S4 by LVS vaccination through a variety of routes [18]. However, critical information including their natural sensitivity to pulmonary SCHU S4 infection and the potency of vaccine-induced protection was not reported. These results suggested to us that a thorough study of the Fischer 344 rats was warranted.
In this study, we expanded on the initial characterization of the Fischer 344 rat vaccine model. We show that Fischer 344 rats are susceptible to low doses of SCHU S4 infection and that LVS vaccination via multiple routes increased their resistance to SCHU S4 by at least 5-6 orders of magnitude. Their response to LVS vaccination and SCHU S4 challenge are similar to those reported for humans and non-human primates and therefore may be a useful model for studying pneumonic tularemia and for developing new vaccines against this disease.
Materials and Methods
Rats
Female Fischer 344 rats were purchased from The National Cancer Institute at Frederick (Frederick, MD) or Harlan Sprague Dawley (Indianapolis, IN). The rats were housed in ventilated cages in a specific pathogen-free facility at The University of New Mexico. All protocols have been approved by the Institutional Animal Care and Use Committee and the Biosafety Committee at the University of New Mexico.
Bacteria
The F. tularensis strains LVS (DVC Lot #703-0303-016) and SCHU S4 (DVC Project # 110419.2, Sub lot 1) were obtained from DynPort Vaccine Company LLC (Frederick, MD). Working stocks of LVS and SCHU S4 were prepared by expanding the original stock in Chamberlain's broth (Teknova; Hollister, CA) for 48 h at 37°C with gentle shaking and then freezing the culture in aliquots without any preservative at −80°C. Chamberlain's broth was chosen for its defined composition, clarity, superior growth property and low frequency of grey colonies; LVS cultures grown under these conditions contained 7.9 ± 3.0% grey colonies as determined by flow cytometry using antibodies against F. tularensis LPS. The concentration of viable bacteria in the working stocks after freezing was 2.91 × 109 CFU/ml for LVS and 1.26 × 109 CFU/ml for SCHU S4.
Immunization and infection
Rats were lightly anesthetized with isoflurane (Abbott Laboratories; Chicago, IL) for all procedures. The non-surgical method of intratracheal inoculation was adapted from Costa et al [21]. Briefly, anesthetized rats were immobilized on an inclined platform (Alpha Lab Supply; Albuquerque, NM) and then intubated with a 20 gauge i.v. catheter (Terumo Medical Products; Somerset, NJ) with the help of a small animal laryngoscope with a fiber optic light source (Penn-Century, Inc; Philadelphia, PA). A blunt-ended needle was then inserted to deliver 100 μl of inoculum followed by 300 μl of air to ensure complete delivery of the inoculum. In most experiments, lung delivery was confirmed by adding self-illuminating quantum dots (Zymera; San Jose, CA) into the inoculum and imaging the infected rats in vivo using the IVIS 100 Optical Imaging System (Caliper Life Sciences; Hopkinton, MA). To determine the deposition of bacteria in the lungs, entire lungs were removed aseptically 1 h after i.t. infection and homogenized in 5 ml PBS using a BeadBeater (Biospec Products; Bartlesville, OK) or a hand-held homogenizer fitted with disposable plastic homogenizing probes (Omni International; Marietta, GA). Serial dilutions of the lung homogenates were plated onto selective cystine heart agar plates with 5% rabbit blood, 100 U/ml polymyxin B and 100 U/ml penicillin G (Remel; Lexena, KS) and bacterial colonies were quantified 4-5 days later using Qcount (Spiral Biotech; Bethesda, MD). A similar procedure was followed to measure the bacterial burden in the lungs, spleens and livers over the course of infection. When no bacteria was found, a value equal to the limit of detection was used to calculate the mean bacterial burden. For intradermal and subcutaneous inoculations, anesthetized rats were injected with 100 μl of inoculum on the right flank and between the shoulder blades, respectively. The inoculation dose was determined by plating the inoculum at the end of each experiment and the deposition of bacteria at the inoculation site in the skin was not determined.
Tissue processing and histopathological assessment
Unvaccinated and LVS vaccinated rats were challenged by intratracheal instillation with approximately 300 SCHU S4. Rats were sacrificed by CO2 overexposure on day 0 (unchallenged), and day 3, 6 and 9 post-challenge (3 rats per exposure per time point). Necropsies were performed on all rats and lungs, liver and spleen were collected for microscopic examination. Lungs were removed without perfusion from the thorax en bloc and inflated with 10% neutral buffered formalin (NBF) via a tracheal cannula. Lungs and other tissues were fixed in 10% NBF for 24-72 hours and subsequently trimmed for paraffin embedding. Lungs were trimmed along the edges of the left mainstem bronchus, and the right cranial, middle and caudal lobar bronchi. Paraffin-embedded tissues were sectioned at 5 μm and stained with hematoxylin and eosin for histological analysis by a board certified veterinary pathologist. Lesions were graded in a blinded manner on a semi-quantitative scale based upon the severity and the distribution of lesions (minimal=1, mild=2, moderate=3, and marked=4).
Statistics
Survival times were modeled in R [22] using Cox Proportional Hazards Regression [23]. Power analysis was performed in PASS 2008 [24]. Details of statistical analyses are described in the Results section.
Results
Sensitivity to intratracheal and subcutaneous SCHU S4 challenge
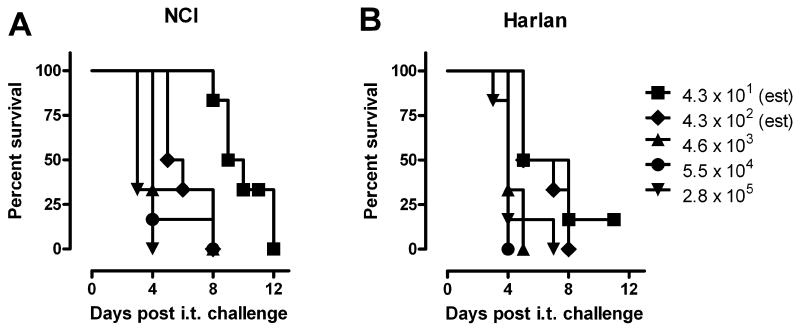
Since the historical literature suggested that rats were relatively resistant to intranasal and aerosol infection by virulent type A strains of F. tularensis [12,18], our initial studies aimed to determine the sensitivity of Fischer 344 rats to intratracheal (i.t.) SCHU S4 challenge. Fischer 344 rats obtained from two different vendors, NCI and Harlan Sprague Dawley, were challenged i.t. with SCHU S4 doses ranging from 43 to 2.8 × 105 CFU. In contrast to the relatively high natural resistance reported for other rat strains [12,18], almost all Fischer 344 rats died within 4 to 10 days of infection with an estimated LD99 < 100 CFU (Fig. 1). Since Fischer 344 rats from both sources responded similarly to i.t. SCHU S4 infection (P = 0.58), we decided to use the rats from NCI for all of the following experiments.
Figure 1.
Sensitivity of Fischer 344 rats to i.t. SCHU S4 challenge. Fischer 344 rats from (A) NCI and (B) Harlan Sprague Dawley (n = 6/group) were challenged i.t. with SCHU S4 and monitored daily for mortality. The indicated challenge doses represent actual CFU recovered from the lungs 1 h after inoculation.
Based on the clinical signs exhibited by lethally infected Fischer 344 rats, a scale of disease severity was developed (Table 1). Weight loss was the earliest measureable effect of infection, but it was not a reliable predictor of morbidity or mortality. As disease progressed, infected rats gradually became less active and responsive to stimulation and developed increasingly severe eyelids ptosis and ruffled fur. At the end, infected rats had lost up to 15% of their body weight, were weak and/or ataxic and were completely unresponsive to stimulation; their breathing was labored and their eyes were fully closed surrounded by a crust of accumulated prophyrin.
Table 1.
Rating of disease severity based on clinical signs of illness
| Disease severity | Clinical Signs |
|---|---|
| Healthy | Bright, alert and active |
| Mild | Slight weight loss, reduced alertness and activity, piloerection and eyelid ptosis covering no more than 1/4 of the eyes |
| Moderate | Pronounced weight loss, decrease in activity and responsiveness to stimulation, ruffled coat, eyelid ptosis covering ½ of the eyes and may have porphyrin secretion around the eyes |
| Severe | Severe weight loss, inactive and unresponsive, very ruffled coat, eyes completely closed with definite porphyrin secretion, labored breathing, weakness and/or ataxic |
Experiments were also performed to determine the susceptibility of Fischer 344 rats to s.c. infection with 101, 103, and 105 SCHU S4. Based on weight loss after infection, the minimum s.c. infectious dose was ≤ 10 CFU (Fig. 2). Fischer 344 rats exhibited large variations in their individual susceptibility to s.c. infection and their responses could be broadly divided into three different patterns. At least 80% of the rats at each dose lost weight following infection. For some of these, weight loss was very rapid and severe and they died at random times between 3-14 days of infection. For the others, weight loss was more gradual and sustained for 10-15 days but they recovered completely from the infection. A few rats from each dose never lost weight or showed any clinical signs of infection. There was no correlation between the SCHU S4 challenge dose and the amount of weight loss, the number of rats that died, and the time to death. The cumulative results from two separate experiments with 12 rats at each challenge dose indicated that the s.c. LD50 is greater than 105 CFU.
Figure 2.
Sensitivity of Fischer 344 rats to s.c. SCHU S4 challenge. Fischer 344 rats (n = 6) were challenged s.c. with the indicated doses of SCHU S4 and monitored daily for changes in body weight. The actual infection doses were determined by plating the inocula after infection. Each curve represents the percent change in body weight over the observation period relative to the starting weight for an individual animal. Points above and below 0 represent weight gain and weight loss, respectively. The (*) indicates animal death. The experiment was performed twice to show reproducibility
Protection of Fischer 344 rats by LVS vaccination
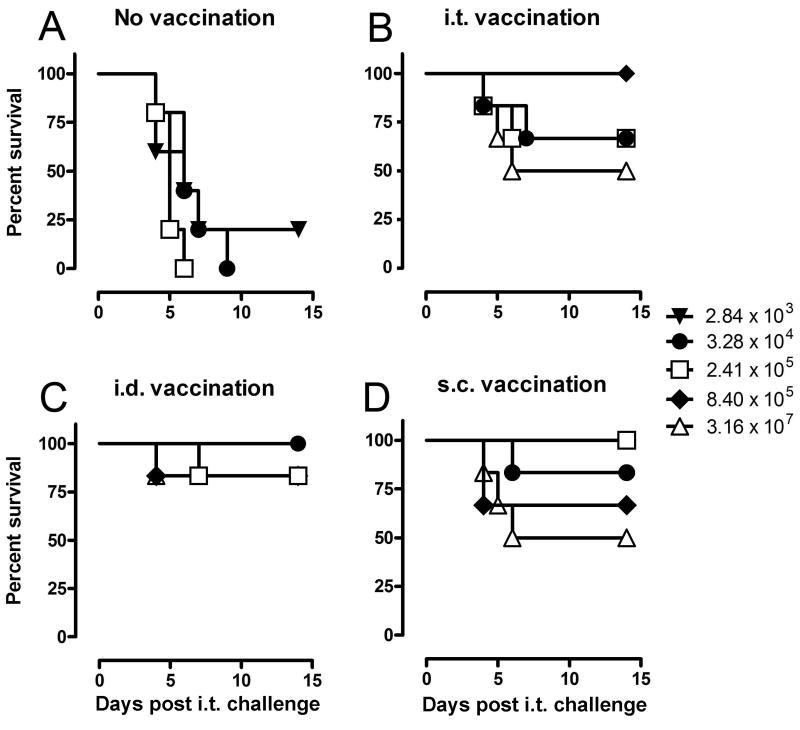
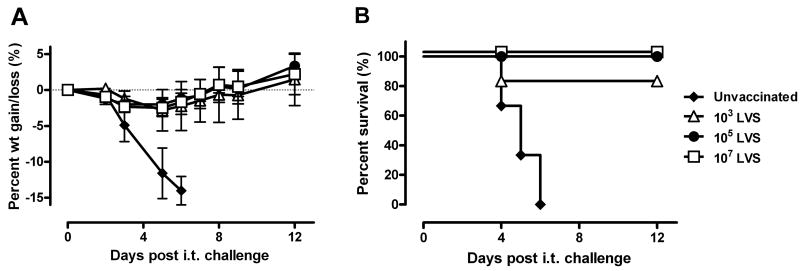
Since i.t. SCHU S4 infection resulted in consistently high mortality in Fischer 344 rats, additional studies were performed to determine whether it would be an appropriate vaccine model for pneumonic tularemia. It was previously reported that LVS vaccination by a variety of routes protected Fischer 344 rats against an aerosol challenge of 2 × 105 SCHU S4 [18]. To further characterize the potency of LVS vaccination in this model, rats were vaccinated s.c, i.d., or i.t. with 2-4 × 107 LVS and then challenged i.t. with SCHU S4 doses ranging from 101 to 107 CFU. In two separate experiments, at least 4 of 6 rats vaccinated s.c. or i.d. survived every challenge dose up to 106 SCHU S4 and some of the vaccinated rats even survived a challenge of 3 × 107 SCHU S4 (Fig. 3). LVS vaccination by the i.t. route also protected Fischer 344 rats against i.t. SCHU S4 challenge, but the survival rate fluctuated and did not always correlate with the challenge dose.
Figure 3.
Protection of Fischer 344 rats against pulmonary SCHU S4 challenge by LVS vaccination. Fischer 344 rats (n = 6) were either left unvaccinated (A) or vaccinated with 2-4 × 107 LVS by i.t. (B), i.d. (C) or s.c. (D) routes. 7 weeks after vaccination, rats were challenged i.t. with SCHU S4 and monitored daily for mortality. The indicated challenge doses represent actual CFU recovered from the lungs 1 h after inoculation. The doses represented by (Δ) and (◆) were not used for the unvaccinated rats and (▼) was not used for the vaccinated rats.
Since the statistical power for any isolated small experiment is low, we used a regression-based method for modeling survival times that combines information from many experiments in a manner that allows for comparing experiments, challenge doses, and vaccination routes simultaneously. Combining several small experiments in this framework increases the statistical power and is routinely done for clinical studies with a limited data set. The survival times of 237 rats challenged with SCHU S4 from seven independent experiments (Table 2) were modeled using Cox Proportional Hazards Regression [23]. The independent variables were log dose, vaccination route, and experiments; there was no significant difference between the experiments (Wald chi-square = 7.65, df = 7, p = 0.36) and this variable was dropped from the analyses. Figure 4 shows the predicted survival distributions for unvaccinated and vaccinated Fischer 344 rats challenged i.t. with hypothetical challenge doses of 102, 104, 106, and 108 SCHU S4. The survival curves generated using this statistical method accurately recapitulated the actual survival data from the seven experiments, including the experiment shown in Fig. 3. LVS vaccination by any of the three routes induced significant protection against i.t. SCHU S4 challenge (Wald chi-square = 112, df = 3, p < 0.0001). For example, an i.t. challenge dose of 104 SCHU S4 was lethal for 92% of the unvaccinated rats but only 8% of the s.c. or i.d. vaccinated rats and 15% of the i.t. vaccinated rats within 12 days of challenge. Statistical analyses further indicated that i.d. (HR = 0.028, 95% CI 0.011 to 0.067) and s.c. (HR = 0.029, 95% CI 0.012 to 0.069) vaccination were not significantly different (z = -0.09, p = 0.93) and the averaged effect of these two vaccination methods was only slightly better (z = -2.02, p = 0.043) than i.t. vaccination (HR = 0.061, 95% CI = 0.030 to 0.126). Based on the challenge dose predicted to achieve 50% lethality (LD50), the LVS vaccinated rats were 5-6 logs more resistant than naïve rats and LVS vaccinated BALB/c mice, currently the most widely used tularemia vaccine model.
Table 2.
Summary of animals used in statistical analyses
| Exp | Sex | Age at vaccination (d) | Vaccination route | Age at challenge [interval between vaccination and challenge](d) | Challenge dose deposited in lungs (CFU/lung) | No. per group |
|---|---|---|---|---|---|---|
| 1 | F | - | - | 69 | 56 | 6 |
| 2 | F | - | - | 52 | 102 | 3 |
| 3 | F | - | - | 66 | 344 | 5 |
| 4 | F | - | - | 77 | 1871 | 7 |
| 5 | F | - | - | 76 | 43, 430, 46001, 55000, 280000 | 6 |
| - | - | 94 | 43, 430, 4600, 55000, 280000 | 6 | ||
| 6 | F | - | - | 115 | 2840, 32800, 241000 | 5 |
| 64 | i.d., s.c., i.t.2 | 115 [51] | 32800, 241000, 840000, 31600000 | 6 | ||
| 7 | F | - | - | 129 | 5.7, 57, 600 | 6 |
| 73 | i.d., s.c., i.t.3 | 129 [56] | 8700, 28000, 2600000 | 6 |
3 rats in group
Vaccination dose: 4.7 × 107 (s.c. / i.d.), 2.2 × 107 (i.t.)
Vaccination dose: 4.0 × 107 (s.c. / i.d.), 4.4 × 107 (i.t.)
Figure 4.
Survival distributions in unvaccinated and vaccinated Fischer 344 rats challenged i.t. with escalating doses of SCHU S4. Survival times from seven independent experiments and 237 rats were modeled using Cox Proportional Hazards Regression. The estimated mortality rates for challenge doses ranging from 102 to 108 SCHU S4 for unvaccinated, and i.t., i.d. and s.c. vaccinated rats are shown. The 108 dose was not tested experimentally.
To determine the group size required for future evaluation of potential vaccine candidates, a power analysis was performed. Power analysis for the censoring pattern observed with these data (approx. 50%) and effect size (HR =0.03) for a comparison of unvaccinated to i.d. or s.c. vaccinated rats indicated that n = 6 per group should be sufficient for 80% power with a significance level of 0.05 with Cox Proportional Hazards models. For an effect size (HR = 0.06) as observed in the comparison of unvaccinated to i.t. vaccinated rats, with other parameters unchanged, the required sample size would be 8 per group.
Post-vaccination LVS kinetics and effect of LVS dose on protection
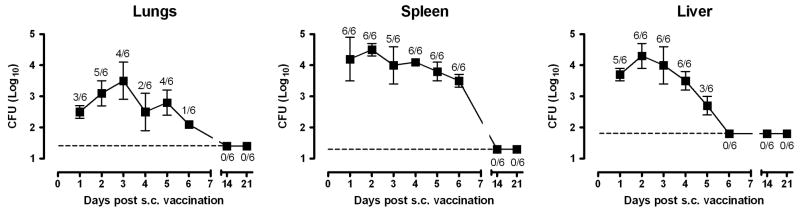
Subcutaneous LVS vaccination is similar in nature to the scarification method used to immunize humans but, unlike scarification, precise vaccination doses can be delivered. To further develop the s.c. vaccination model, studies were performed to determine the fate of LVS after s.c. administration. Within 24 hours of s.c. inoculation with ∼5 × 107 LVS, the bacteria had disseminated systemically to the lungs, liver, and spleen (Fig. 5). The total number of LVS recovered from these tissues combined was one to ten thousand-fold lower than the inoculum, suggesting that the majority of LVS either failed to escape from the inoculation site or was rapidly cleared. Over the next day or two, LVS replicated no more than 10-fold in any of the organs examined and was gradually cleared until no bacteria could be recovered 14 days after vaccination. No skin lesion at the inoculation site was detected at any time.
Figure 5.
Kinetics of LVS growth and clearance after s.c. vaccination. Fischer 344 rats were vaccinated s.c. with 3.7 × 107 LVS. The bacterial burden in the lungs, spleen and liver was determined daily for the first 6 days and on days 14 and 21 post vaccination. The ratio above each data point indicates the number of organs sampled containing bacteria over the total number of organs examined. The dashed lines show the limit of detection of each tissue and each data point shows the mean bacterial burden ± SD.
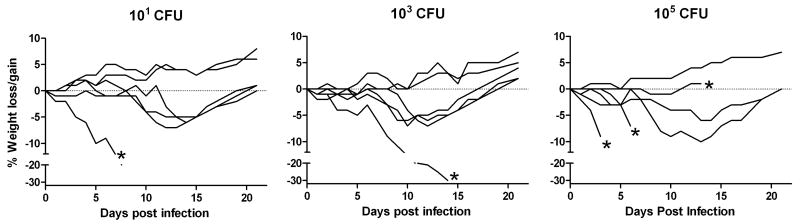
To determine whether the resistance of s.c. vaccinated rats was dependent on the vaccination dose, the survival of Fischer 344 rats vaccinated with a range of LVS doses was monitored after i.t. SCHU S4 challenge. As observed previously, rats vaccinated s.c. with 107 LVS lost weight after infection but was able to recover completely (Fig. 6). A similar pattern of weight loss and survival was observed when Fischer 344 rats were vaccinated s.c. with 103 or 105 LVS. These results suggest that LVS is a potent vaccine in Fischer 344 rats and a small vaccine dose is sufficient to induce protection against i.t. SCHU S4 challenge.
Figure 6.
Titration of s.c. LVS vaccination dose for protection against i.t. SCHU S4 challenge. Naïve Fischer 344 rats (n = 6) were either vaccinated s.c. with the indicated LVS doses or left unvaccinated. 42 days after vaccination, the rats were challenged i.t. with 3.3 × 103 SCHU S4 and monitored daily for changes in body weight (A) and survival (B). In panel A, the curves show the mean percent weight change ± SD for all surviving rats in each group relative to their starting weight. Points above and below 0 represent weight gain and weight loss, respectively. The experiment was performed twice to show reproducibility
LVS vaccinated rats control SCHU S4 growth and dissemination
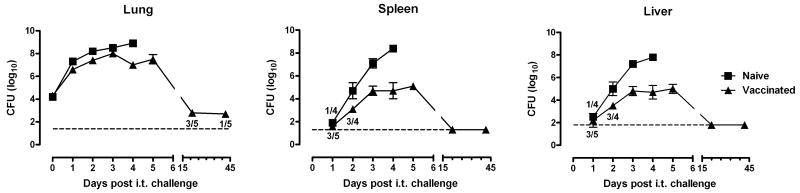
We next determined the effect of s.c. LVS vaccination on the growth and dissemination of SCHU S4 after i.t. challenge. LVS vaccination did not prevent SCHU S4 infection or dissemination from the lungs to the spleen or liver, but it limited the rate of bacterial proliferation in these organs. During the first 72 hours, the number of SCHU S4 recovered from the lungs of naïve and LVS vaccinated rats was almost identical, both reaching 108 CFU by day 3 of infection (Fig. 7). In the unvaccinated rats, the lung burden continued to increase until the rats died. In the vaccinated rats, the lung burden stabilized and was maintained at a plateau of approximately 107 CFU for at least two days before it started to decline. 7 of 10 rats examined on day 21 and 1 of 9 examined on day 42 still harbored bacteria in the lungs, reflecting a protracted clearance process.
Figure 7.
LVS vaccinated Fischer 344 rats controlled SCHU S4 growth. Fischer 344 rats were either left unvaccinated or vaccinated s.c. with 2.9 × 107 LVS and then challenged i.t. 35 days later with 2 × 104 SCHU S4. On the indicated days, 3-5 rats were killed to determine the number of SCHU S4 in the lungs, liver and spleen. Where indicated, the ratios represent the number of organs sampled containing bacteria over the total number of organs examined; otherwise, bacteria was found in all organs examined for that time point. When no bacteria were found, a value equal to the limit of detection was used to calculate the mean bacterial burden ± SD. The dashed lines show the limit of detection of each tissue.
The number of SCHU S4 seeding the spleen and liver was similar in unvaccinated and vaccinated rats based on the CFU recovered at 24 h. By 48 h, the bacterial burden in the unvaccinated rats was 10-fold higher compared to the LVS vaccinated rats. This difference increased over time as the bacterial burden climbed to greater than 108 CFU in the unvaccinated rats but never exceeded 105 CFU in the LVS vaccinated rats. No bacteria could be detected in the spleen and liver of vaccinated rats by day 14 of infection.
Histopathologic changes in rats challenged with SCHU S4
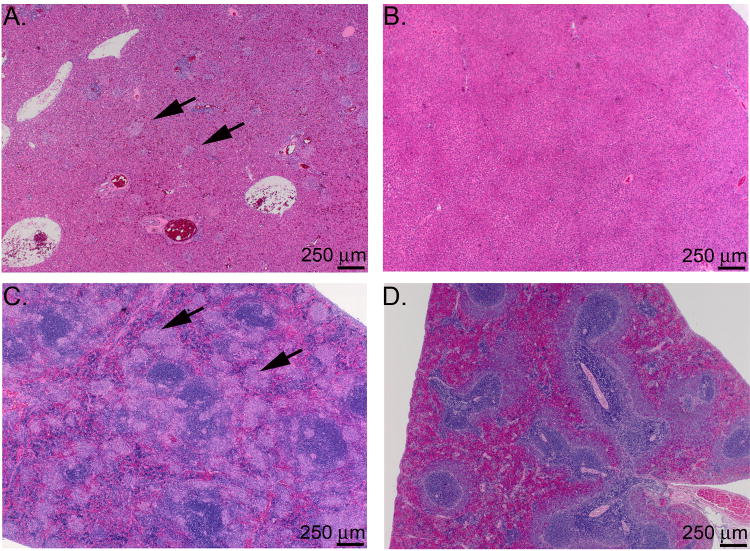
The histopathology of the lungs, liver and spleen from unvaccinated and LVS vaccinated rats after i.t. SCHU S4 challenge is shown in Figs. 8 and 9. Lesions in unvaccinated, challenged rats at day 3 post-infection consisted of mild, multifocal, pyogranulomatous bronchopneumonia (Fig 8A), and minimal to mild, multifocal, random pyogranulomatous hepatitis and splenitis (data not shown). By day 6, the pyogranulomatous lesions in the lung (Fig. 8C) increased in extent and exhibited progressively more necrosis. The lungs also exhibited hyperplasia of peribronchial and peribronchiolar lymphocytes with plasmacytosis. In the liver and spleen of the unvaccinated rats at day 6, there was moderate to marked pyogranulomatous inflammation with necrosis (Fig. 9A and C). Scattered periportal lymphocytic and histiocytic infiltrates appeared in the liver by day 6, and sinusoidal plasmacytosis and histiocytosis were evident in the spleen. In the animals surviving to day 9, the lung lesions were marked and consisted primarily of necrotizing pneumonia (Fig. 8E), while the hepatic and splenic lesions appeared more granulomatous and less necrotizing (data not shown), with a similar extent and distribution as in day 6.
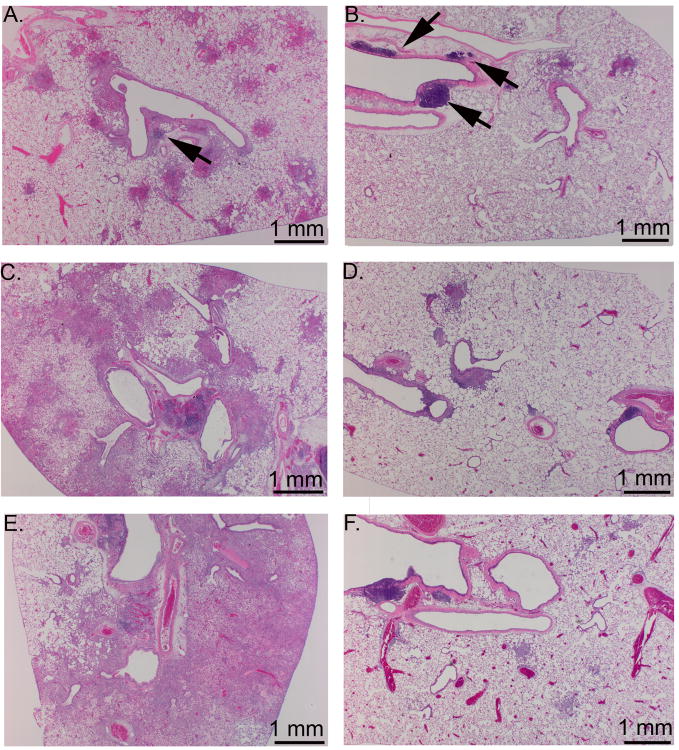
Figure 8.
Histological appearance of the lungs of unvaccinated and vaccinated Fischer 344 rats at 3, 6, and 9 days after intratracheal SCHU S4 infection. Fischer 344 rats were either left unvaccinated or vaccinated s.c. with 2.7 × 107 LVS and challenged i.t. 45 days later with 3-400 CFU SCHU S4. A) unvaccinated, day 3; B) vaccinated, day 3; C) unvaccinated, day 6; D) vaccinated, day 6; E) unvaccinated, day 9, F) vaccinated, day 9. Note the increase in severity and distribution of the bronchopneumonia in the unvaccinated rat (A, C, and E) compared to the vaccinated rat. Arrows point to airway associated lymphoid tissues, which were greater in the vaccinated rats than the unvaccinated rats at the early timepoints.
Figure 9.
Histological appearance of the liver and spleen of unvaccinated and vaccinated Fischer 344 rats at day 6 after intratracheal SCHU S4 infection. Fischer 344 rats were either left unvaccinated or vaccinated s.c. with 2.7 × 107 LVS and challenged i.t. 45 days later with 3-400 CFU SCHU S4. A) and C) liver and spleen from unvaccinated rat; B) and D) liver and spleen from vaccinated rat. Arrows point to foci of pyogranulomatous inflammation in the liver and spleen of the unvaccinated rat. Note that the liver and spleen from the vaccinated rat are histologically normal.
The lungs of vaccinated rats immediately prior to challenge exhibited slightly greater peribronchial and peribronchiolar lymphocytes than the unvaccinated rats prior to challenge (data not shown). By day 3 post-challenge, the vaccinated rats developed mild, multifocal pyogranulomatous bronchopneumonia, with moderate hyperplasia of the peribronchial and peribronchiolar lymphocytes with plasmacytosis (Fig. 8B). Only one of the three rats examined at day 3 exhibited minimal, multifocal pyogranulomatous hepatitis, and none of the rats examined at day 3 developed splenic lesions (data not shown). On day 6 post-challenge, the lung lesions were similar to those observed at day 3 (Fig. 8D), and only one of the three animals examined at day 6 developed mild, multifocal, pyogranulomatous hepatitis and splenitis (Fig. 9B and D). By day 9 post-challenge, the pyogranulomatous pneumonia had decreased in severity (Fig. 8F), and only one of the three animals examined exhibited minimal pyogranulomatous hepatitis.
Discussion
A small animal model for developing vaccines against human tularemia should reproduce the human disease and response to vaccination and challenge as closely as possible. Similar to the human situation, the animal should be very sensitive to infection by virulent type A strains, but develop more severe morbidity following respiratory infection than cutaneous infection [25]. The animal should also tolerate vaccination with high doses of LVS by either the respiratory or the dermal route to develop solid immunity against virulent respiratory challenges [2,4]. Murine models have been used for almost all of the vaccine studies to date and they have provided valuable insights into the requirements for successful vaccination. For example, murine vaccine studies showed that mucosal LVS vaccination through oral [8,9], aerosol [5] or intranasal [6] routes is the most consistent and effective method for generating protection against respiratory challenge with type A strains. Vaccination does not prevent infection but limits bacterial replication through a mechanism involving T cells and IFNγ. Murine models have also been used effectively to identify novel vaccines and vaccination strategies [26-29]. However, there are also many vaccine candidates that provided no protection at all [30-34]; while this is not surprising, the high sensitivity of vaccinated mice to type A strains makes it difficult to distinguish between failures due to the nature of the vaccine and the limitations of the animal model. In this respect, the higher level of protection induced in Fischer 344 rat by LVS vaccination may increase the sensitivity to detect sub-optimal vaccines that would otherwise fail to protect mice. Vaccines that protect both mice and rats may also be more likely to protect NHPs and humans.
The use of rats as a model to study pneumonic tularemia was historically discouraged because they were reported to have a relatively high baseline resistance to respiratory infection with virulent strains of F. tularensis [12,18]. However, our results showed that Fischer 344 rats are extremely sensitive to i.t. SCHU S4 infection. Our results also confirmed the findings reported by Jemski over 25 years ago that LVS vaccination by multiple routes protected rats fully against an aerosol challenge of 2 × 105 SCHU S4 [18]. Although it is difficult to compare our results directly with the historical data from human vaccine studies because different experimental parameters and endpoints (survival in rats vs. fever > 100° F over 24 h in humans) were used, the resistance observed in Fischer 344 rats and humans under similar vaccination and challenge conditions was comparable [2-4]. In fact, no person (0 of 46) vaccinated by respiration with 106 or 108 LVS and challenged with 2.5 × 104 SCHU S4 aerosol at 4 or 6 months of vaccination developed disease severe enough to require therapy [4]. Over half of the people vaccinated by acupuncture were also protected against this challenge [3,4]; the actual level of resistance may be even higher because those who were treated with antibiotics due to fever > 100°F and were not considered to be protected could have potentially recovered without any intervention. It is important to point out that, unlike the human studies, the endpoint for NHPs was survival and thus may offer a better comparison for the vaccinated Fischer 344 rats in our studies. The majority of rhesus macaques (Macaca mulatta) vaccinated by respiration (15 of 16 or 94%) or by acupuncture (13 of 16 or 81%) survived aerosol challenges of 104 SCHU S4 [4]. Similarly, the regression analyses shown in Figure 4 predicted that 92% of Fischer 344 rats vaccinated by s.c. or i.d. routes and 85% after i.t. vaccination will survive an i.t. challenge of 104 SCHU S4 within 2 months of vaccination. Another striking similarity between Fischer 344 rats and NHPs is their response to subcutaneous or intradermal SCHU S4 infection. Rhesus macaques were found to be very susceptible to infection and the estimated ID50 was ≤ 10 organisms [35]. Interestingly, one or more deaths occurred in each experimental group challenged with 10 to 2600 SCHU S4, but the number and time of death were not dependent on the challenge dose, similar to our observations in the Fischer 344 rats. In humans as well, the ulceroglandular disease is associated with a high rate of infection [36], but death is uncommon [25].
The protection resulting from respiratory vaccination in humans and NHPs appeared to be dependent on the vaccination dose [4]. Although a similar study of respiratory vaccination has not been performed in Fischer 344 rats, we showed that effective s.c. vaccination was independent of the vaccination dose and required very few LVS organisms. This may explain the effectiveness of human vaccination by acupuncture in which the number of LVS actually inoculated into the skin is small and detectable only by PCR but not by culture [37]. Among the important issues remaining to be addressed is whether the vaccine-induced protection wanes when interval between vaccination and challenge is extended beyond the 1.5 to 2 months period used in this study. We are also exploring the differences between mice, rats, NHPs and humans, including macrophage and T cell responses to SCHU S4 infection, which may explain the differential protection induced by LVS vaccination.
Our observation that i.t. LVS vaccination was less protective than s.c. or i.d. vaccination differs from previous studies showing that respiratory LVS vaccination was as good, if not better, than dermal vaccination in mice [5,6], guinea pigs [38], non-human primates [38,39], and humans [4]. More importantly, no such difference was observed in the earlier aerosol SCHU S4 challenge experiments in Fischer 344 rats [18]. The difference between the two studies cannot be explained by the method of pulmonary vaccination because the earlier study showed that all methods of vaccination, including i.n. and aerosol, generated similar protection against aerosolized SCHU S4 [18]. It is possible that we observed the difference between the vaccination routes because we used higher challenge doses than the 2 × 105 CFU dose used in Jemski's study. This is illustrated by the proportional hazards regression analyses of our results which showed that the difference in the estimated survival between s.c./i.d. and i.t. vaccinated rats increases in proportion to the SCHU S4 challenge dose, particularly with doses greater than 105 CFU. In fact, when we excluded challenge doses greater than 105 from the analyses, there was no significant difference among the vaccination routes (data not shown).
The number of animals required to detect a significant protective effect elicited by a vaccine candidate is an important consideration for selecting animal vaccine models. A power analysis based on the results of these studies indicated that only 6 Fischer 344 rats per group would be needed to detect a significant protective effect associated with a vaccine as potent as LVS and 8 rats per group would be required for less potent vaccines. Of note, our analyses included vaccination route, survival time, and challenge dose as the only covariates. Thus, we fully expect that the power of this test to increase when future characterization of this rat model identifies additional covariates.
Fischer 344 rats appear to be an excellent model for human respiratory tularemia. With the abundance of information about the rat as an experimental animal and the growing number of analytical tools available to study them, this rat model will help greatly to advance our current understanding of the pathogenesis of respiratory tularemia and the host mechanisms required to protect against this disease.
Acknowledgments
This project was funded with federal funds from the National Institute of Allergies and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN266200500040C and Public Health Service Grant PO1 AI056295
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stuart BM, Pullen RL. Tularemic pneumonia:Review of American literature and report of 15 additional cases. Am J Med Sci. 1945;210:223–236. [Google Scholar]
- 2.Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med. 1961;107:702–714. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- 3.McCrumb FR. Aerosol Infection of Man with Pasteurella Tularensis. Bacteriol Rev. 1961;25(3):262–267. doi: 10.1128/br.25.3.262-267.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hornick RB, Eigelsbach HT. Aerogenic immunization of man with live Tularemia vaccine. Bacteriol Rev. 1966;30(3):532–538. doi: 10.1128/br.30.3.532-538.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlan JW, Shen H, Kuolee R, Zhao X, Chen W. Aerosol-, but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an alphabeta T cell- and interferon gamma- dependent mechanism. Vaccine. 2005;23(19):2477–2485. doi: 10.1016/j.vaccine.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Wu TH, Hutt JA, Garrison KA, Berliba LS, Zhou Y, Lyons CR. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun. 2005;73(5):2644–2654. doi: 10.1128/IAI.73.5.2644-2654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornick RB, Dawkins AT, Eigelsbach HT, Tulis JJ. Oral tularemia vaccine in man. Antimicrob Agents Chemother (Bethesda) 1966;6:11–14. doi: 10.1128/AAC.6.1.11. [DOI] [PubMed] [Google Scholar]
- 8.Ray HJ, Cong Y, Murthy AK, et al. Oral Live Vaccine Strain Induced Protective Immunity Against Pulmonary Francisella tularensis Challenge is Mediated by CD4+ T cells and Antibodies Including IgA. Clin Vaccine Immunol. 2009 doi: 10.1128/CVI.00405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.KuoLee R, Harris G, Conlan JW, Chen W. Oral immunization of mice with the live vaccine strain (LVS) of Francisella tularensis protects mice against respiratory challenge with virulent type A F. tularensis Vaccine. 2007;25(19):3781–3791. doi: 10.1016/j.vaccine.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen H, Chen W, Conlan JW. Susceptibility of various mouse strains to systemically- or aerosol-initiated tularemia by virulent type A Francisella tularensis before and after immunization with the attenuated live vaccine strain of the pathogen. Vaccine. 2004;22(1718):2116–2121. doi: 10.1016/j.vaccine.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Olsufiev NG, Emelyanova OS, Dunayeva TN. Comparative study of strains of B. tularense in the old and new world and their taxonomy. J Hyg Epidemiol Microbiol Immunol. 1959;3:138–149. [PubMed] [Google Scholar]
- 12.Downs CM, Coriell LL, Pinchot GB, et al. Studies on Tularemia: I. The Comparative Susceptibility of Various Laboratory Animals. J Immunol. 1947;56(3):217–228. [PubMed] [Google Scholar]
- 13.Foshay L, Ruchman I, Nicholes PS. Antitularense Serum: Correlation between Protective Capacity for White Rats and Precipitable Antibody Content. J Clin Invest. 1947;26(4):756–760. doi: 10.1172/JCI101858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson CL. Immunization of white rats against infections with Pasteurella tularensis. Pub Health Bull. 1945;60:725–743. [Google Scholar]
- 15.Downs CM, Coriell LL, Eigelsbach HT, Plitt KF, Pinchot GB, Owen BJ. Studies on Tularemia: II. Immunization of White Rats. J Immunol. 1947;56(3):229–243. [PubMed] [Google Scholar]
- 16.Buchele L, Downs CM. Studies on pathogenesis and immunity in tularemia; immune response of the white rat to Bacterium tularense. J Immunol. 1949;63(2):135–145. [PubMed] [Google Scholar]
- 17.McCoy G. A plague-like disease of rodents. Pub Health Bull. 1911;43:53. [Google Scholar]
- 18.Jemski JV. Respiratory tularemia: comparison of selected routes of vaccination in Fischer 344 rats. Infect Immun. 1981;34(3):766–772. doi: 10.1128/iai.34.3.766-772.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canonico PG, Powanda MC, Cockerell GL, Moe JB. Relationship of serum beta-glucuronidase and lysozyme to pathogenesis of tularemia in immune and nonimmune rats. Infect Immun. 1975;12(1):42–47. doi: 10.1128/iai.12.1.42-47.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moe JB, Canonico PG, Stookey JL, Powanda MC, Cockerell GL. Pathogenesis of tularemia in immune and nonimmune rats. Am J Vet Res. 1975;36(10):1505–1510. [PubMed] [Google Scholar]
- 21.Costa DL, Lehmann JR, Harold WM, Drew RT. Transoral tracheal intubation of rodents using a fiberoptic laryngoscope. Lab Anim Sci. 1986;36(3):256–261. [PubMed] [Google Scholar]
- 22.Computing, R.F.f.S., editor. R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: 2008. [Google Scholar]
- 23.Hosmer DW, Jr, Lemeshow S. Applied survival analysis: Regression modeling of time to event data. Wiley; New York: 1999. [Google Scholar]
- 24.Hintze J. PASS 2008. NCSS, LLC; Kaysville, Utah: 2008. [Google Scholar]
- 25.Evans ME, Gregory DW, Schaffner W, McGee ZA. Tularemia: a 30-year experience with 88 cases. Medicine (Baltimore) 1985;64(4):251–269. [PubMed] [Google Scholar]
- 26.Jia Q, Lee BY, Clemens DL, Bowen RA, Horwitz MA. Recombinant attenuated Listeria monocytogenes vaccine expressing Francisella tularensis IglC induces protection in mice against aerosolized Type A F. tularensis. Vaccine. 2009;27(8):1216–1229. doi: 10.1016/j.vaccine.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakshi CS, Malik M, Mahawar M, et al. An improved vaccine for prevention of respiratory tularemia caused by Francisella tularensis SchuS4 strain. Vaccine. 2008;26(41):5276–5288. doi: 10.1016/j.vaccine.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huntley JF, Conley PG, Rasko DA, Hagman KE, Apicella MA, Norgard MV. Native outer membrane proteins protect mice against pulmonary challenge with virulent type A Francisella tularensis. Infect Immun. 2008;76(8):3664–3671. doi: 10.1128/IAI.00374-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin A, Scott DW, Thompson JA, Mann BJ. Identification of an essential Francisella tularensis subsp. tularensis virulence factor. Infect Immun. 2009;77(1):152–161. doi: 10.1128/IAI.01113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sebastian S, Pinkham JT, Lynch JG, et al. Cellular and humoral immunity are synergistic in protection against types A and B Francisella tularensis. Vaccine. 2009;27(4):597–605. doi: 10.1016/j.vaccine.2008.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Twine S, Bystrom M, Chen W, et al. A mutant of Francisella tularensis strain SCHU S4 lacking the ability to express a 58-kilodalton protein is attenuated for virulence and is an effective live vaccine. Infect Immun. 2005;73(12):8345–8352. doi: 10.1128/IAI.73.12.8345-8352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pechous RD, McCarthy TR, Mohapatra NP, et al. A Francisella tularensis Schu S4 purine auxotroph is highly attenuated in mice but offers limited protection against homologous intranasal challenge. PLoS ONE. 2008;3(6):e2487. doi: 10.1371/journal.pone.0002487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eyles JE, Hartley MG, Laws TR, Oyston PC, Griffin KF, Titball RW. Protection afforded against aerosol challenge by systemic immunisation with inactivated Francisella tularensis live vaccine strain (LVS) Microb Pathog. 2008;44(2):164–168. doi: 10.1016/j.micpath.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Qin A, Scott DW, Mann BJ. Francisella tularensis subsp. tularensis Schu S4 disulfide bond formation protein B, but not an RND-type efflux pump, is required for virulence. Infect Immun. 2008;76(7):3086–3092. doi: 10.1128/IAI.00363-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eigelsbach HT, Saslaw S, Tulis JJ, Hornick RB. Tularemia: the monkey as a model for man. In: Vagtborg H, editor. Use of nonhuman primates in drug evaluation, a symposium. Southwestern Foundation for Research and Education; San Antonio, Texas: 1968. pp. 230–248. [Google Scholar]
- 36.Saslaw S, Eigelsbach HT, Wilson HE, Prior JA, Carhart S. Tularemia vaccine study. I. Intracutaneous challenge. Arch Intern Med. 1961;107:689–701. doi: 10.1001/archinte.1961.03620050055006. [DOI] [PubMed] [Google Scholar]
- 37.Hepburn MJ, Purcell BK, Lawler JV, et al. Live vaccine strain Francisella tularensis is detectable at the inoculation site but not in blood after vaccination against tularemia. Clin Infect Dis. 2006;43(6):711–716. doi: 10.1086/506348. [DOI] [PubMed] [Google Scholar]
- 38.Eigelsbach HT, Tulis JJ, Overholt EL, Griffith WR. Aerogenic immunization of the monkey and guinea pig with live tularemia vaccine. Proc Soc Exp Biol Med. 1961;108:732–734. doi: 10.3181/00379727-108-27049. [DOI] [PubMed] [Google Scholar]
- 39.Hodge FA, Leif WR, Silverman MS. Susceptibility to infection with Pasteurella tularensis and the immune response of mice exposed to continuous low dose rate of gamma radiation. Naval Radiol Def Lab Res Dev Tech Rep. 1968;68-85:1–28. [PubMed] [Google Scholar]