Abstract
Antibody production in humans and three species of laboratory animals infected with Rickettsia rickettsii was determined with the indirect hemagglutination test. Rabbits, guinea pigs, and mice were inoculated with R. rickettsii and bled at intervals. Antibody which agglutinated both fresh and glutaraldehyde-fixed sheep erythrocytes sensitized with antigen prepared either from purified rickettsiae or from infected yolk sacs was found in rabbit sera at all intervals tested (10 to 59 days postinfection). Antibody which agglutinated fresh but not glutaraldehyde-fixed erythrocytes sensitized with either of the above antigens was detected in guinea pig sera obtained 7, 14, and 28 days postinfection. Antibody was found in mice inoculated with 5.6 x 10(6) plaque-forming units of R. rickettsii but not in mice given 5.6 x 10(2) plaque-forming units. Peak indirect hemagglutination titers occurred in nonvaccinated human Rocky Mountain spotted fever patients about 3 weeks after onset of illness, and antibody was still detectable after 1 year. Both human immunoglobulin G and human immunoglobulin M antibodies agglutinated sensitized cells, but immunoglobulin M antibodies apparently were more efficient. The indirect hemagglutination test is useful for the titration of human, rabbit, guinea pig, and mouse antibodies when the appropriate erythrocytes are used.
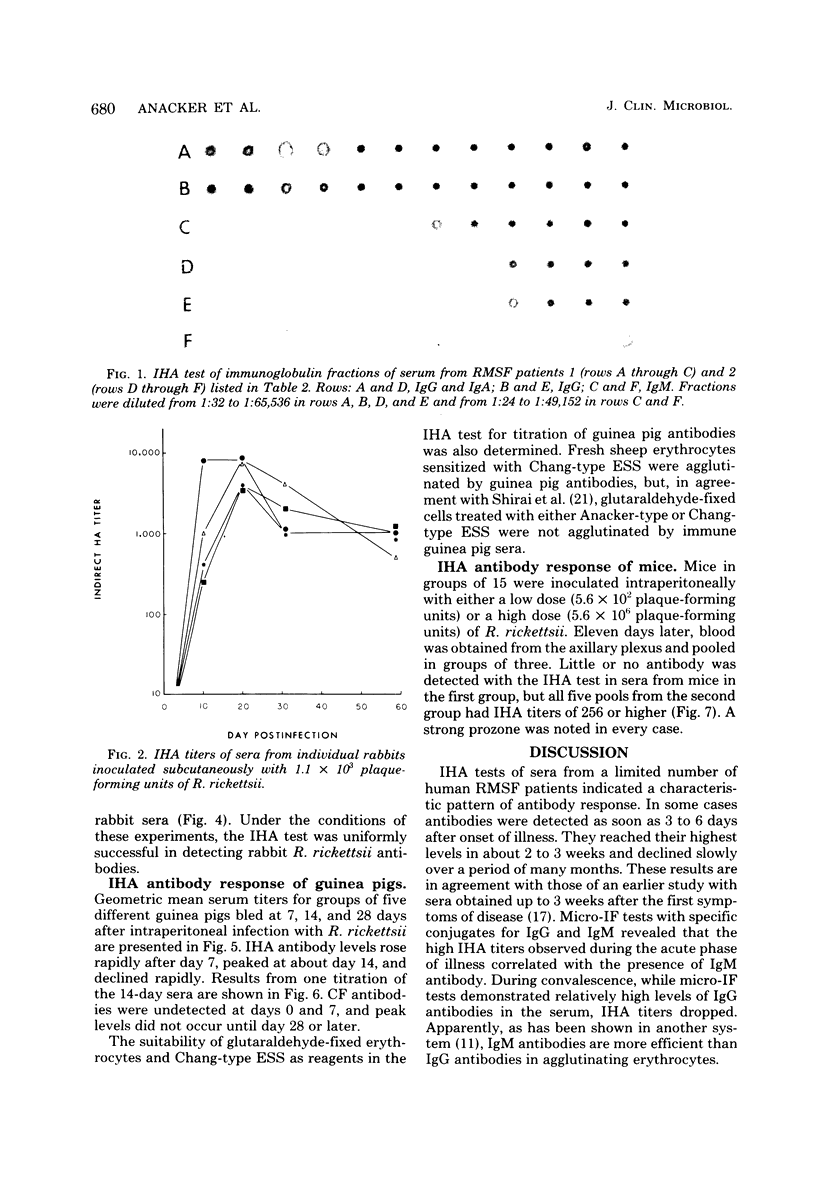
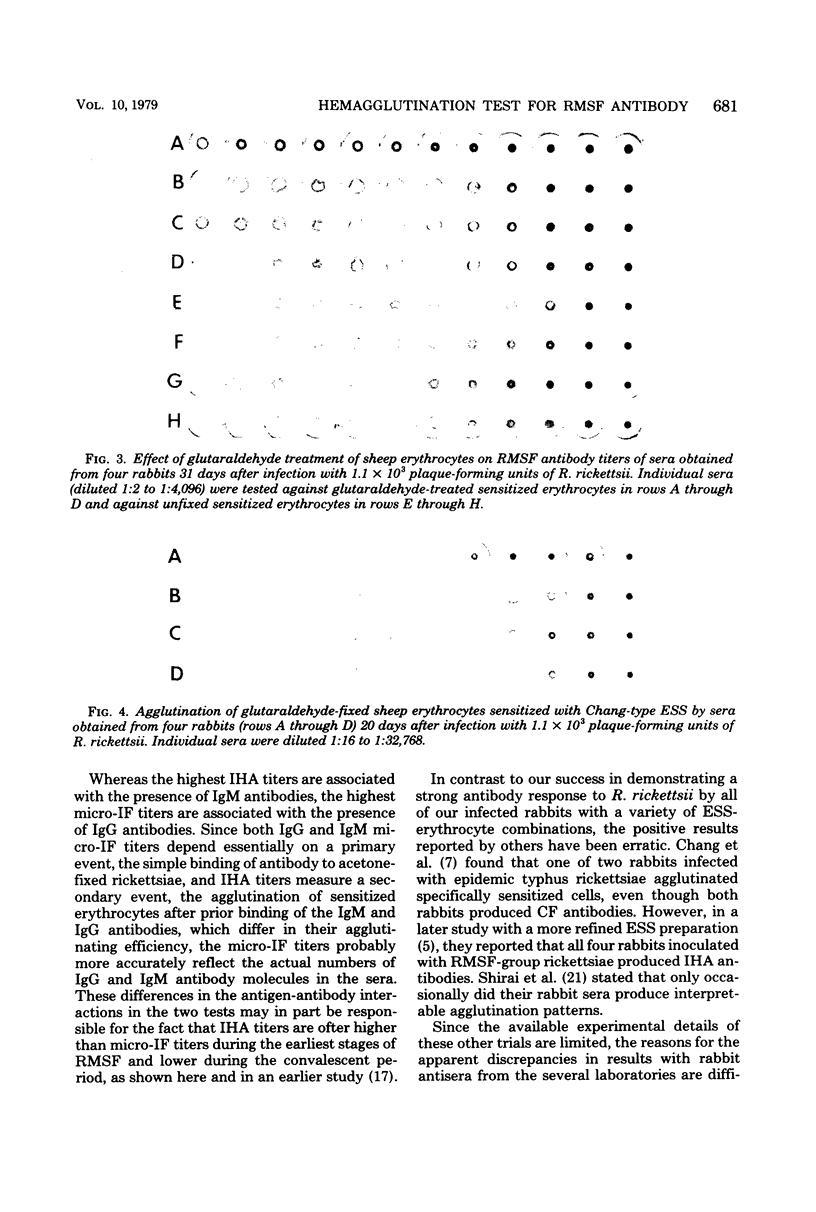
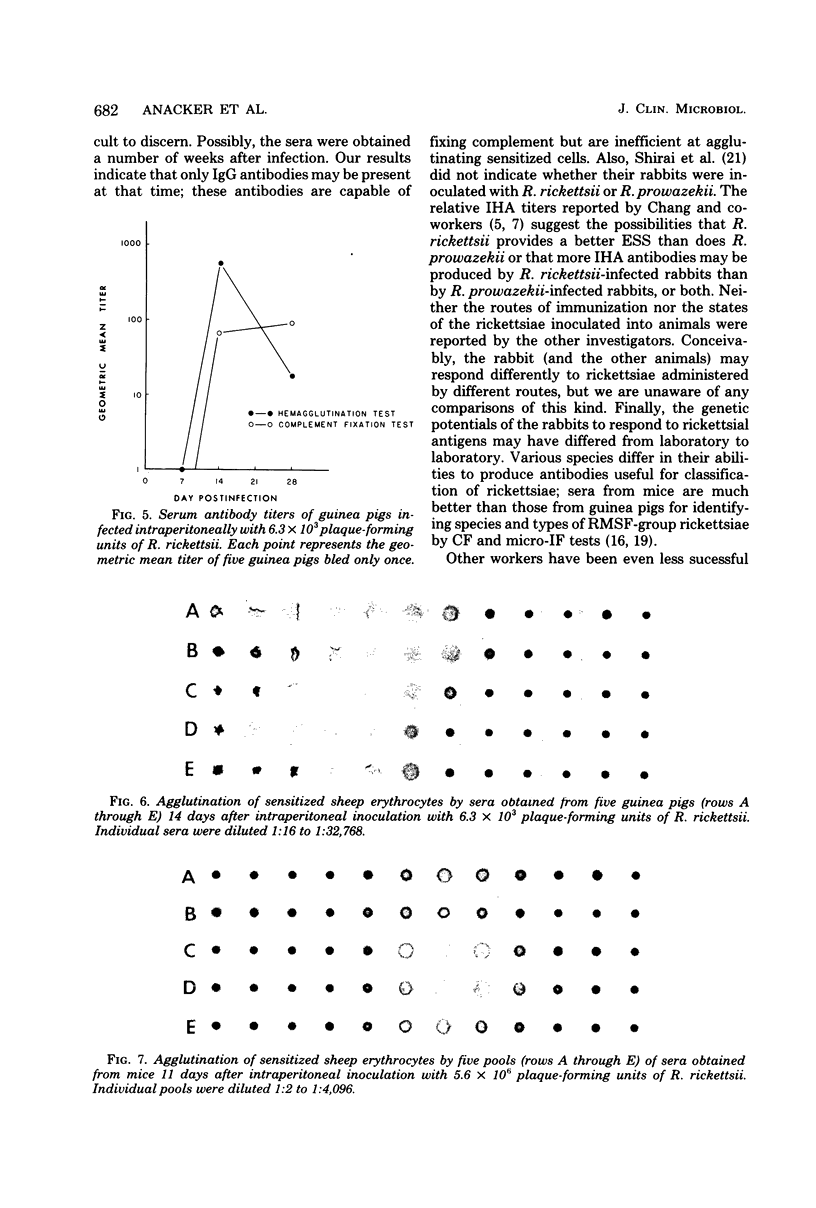
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Gerloff R. K., Thomas L. A., Mann R. E., Bickel W. D. Immunological properties of Rickettsia rickettsii purified by zonal centrifugation. Infect Immun. 1975 Jun;11(6):1203–1209. doi: 10.1128/iai.11.6.1203-1209.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Gerloff R. K., Thomas L. A., Mann R. E., Brown W. R., Bickel W. D. Purification of Rickettsia rickettsi by density-gradient zonal centrifugation. Can J Microbiol. 1974 Nov;20(11):1523–1527. doi: 10.1139/m74-238. [DOI] [PubMed] [Google Scholar]
- Bakemeier R. F. A study of phase variation in Coxiella burneti employing hemagglutination tests. J Immunol. 1965 Nov;95(5):880–886. [PubMed] [Google Scholar]
- Brezina R., Pospísil V., Schramek S. Study of the antigenic structure of Coxiella burneti. VII. Properties of phenol-extracted phase I antigenic component. Acta Virol. 1970 Jul;14(4):295–301. [PubMed] [Google Scholar]
- CHANG R. S., MURRAY E. S., SNYDER J. C. Erythrocyte-sensitizing substances from Rickettsiae of the Rocky Mountain spotted fever group. J Immunol. 1954 Jul;73(1):8–15. [PubMed] [Google Scholar]
- CHANG S. M. A serologically-active erythrocyte-sensitizing substance from typhus rickettsiae. I. Isolation and titration. J Immunol. 1953 Mar;70(3):212–214. [PubMed] [Google Scholar]
- CHANG S. M., SNYDER J. C., MURRAY E. S. A serologically active erythrocyte sensitizing substance from typhus rickettsiae. II. Serological properties. J Immunol. 1953 Mar;70(3):215–221. [PubMed] [Google Scholar]
- Cooper M. D., Hollingdale M. R., Vinson J. W., Costa J. A passive hemagglutination test for diagnosis of trench fever due to Rochalimaea quintana. J Infect Dis. 1976 Dec;134(6):605–609. doi: 10.1093/infdis/134.6.605. [DOI] [PubMed] [Google Scholar]
- Cremer N. E., Schmidt N. J., Jensen F., Hoffman M., Oshiro L. S., Lennette E. H. Complement-fixing antibody in human sera reactive with viral and soluble antigens of cytomegalovirus. J Clin Microbiol. 1975 Mar;1(3):262–267. doi: 10.1128/jcm.1.3.262-267.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLECK L., PORAT S., EVENCHIK Z., KLINGBERG M. A. The renal excretion of specific microbial substances during the course of infection with murine typhus rickettsiae. Am J Hyg. 1960 Nov;72:351–361. doi: 10.1093/oxfordjournals.aje.a120158. [DOI] [PubMed] [Google Scholar]
- GREENBURY C. L., MOORE D. H., NUNN L. A. REACTION OF 7S AND 19S COMPONENTS OF IMMUNE RABBIT ANTISERA WITH HUMAN GROUP A AND AB RED CELLS. Immunology. 1963 Sep;6:421–433. [PMC free article] [PubMed] [Google Scholar]
- HERSEY D. F., COLVIN M. C., SHEPARD C. C. Studies on the serologic diagnosis of murine typhus and Rocky Mountain spotted fever. II. Human infections. J Immunol. 1957 Nov;79(5):409–415. [PubMed] [Google Scholar]
- LACKMAN D. B., BELL E. J., STOENNER H. G., PICKENS E. G. THE ROCKY MOUNTAIN SPOTTED FEVER GROUP OF RICKETTSIAS. Health Lab Sci. 1965 Jul;2:135–141. [PubMed] [Google Scholar]
- Ormsbee R., Peacock M., Philip R., Casper E., Plorde J., Gabre-Kidan T., Wright L. Serologic diagnosis of epidemic typhus fever. Am J Epidemiol. 1977 Mar;105(3):261–271. doi: 10.1093/oxfordjournals.aje.a112382. [DOI] [PubMed] [Google Scholar]
- Osterman J. V., Eisemann C. S. Rickettsial indirect hemagglutination test: isolation of erythrocyte-sensitizing substance. J Clin Microbiol. 1978 Aug;8(2):189–196. doi: 10.1128/jcm.8.2.189-196.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICKENS E. G., BELL E. J., LACKMAN D. B., BURGDORFER W. USE OF MOUSE SERUM IN IDENTIFICATION AND SEROLOGIC CLASSIFICATION OF RICKETTSIA AKARI AND RICKETTSIA AUSTRALIS. J Immunol. 1965 Jun;94:883–889. [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Burgdorfer W., Gerloff R. K., Hughes L. E., Bell E. J. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978 Nov;121(5):1961–1968. [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., MacCormack J. N., Sexton D., Thomas L. A., Anacker R. L., Burgdorfer W., Vick S. A comparison of serologic methods for diagnosis of Rocky Mountain spotted fever. Am J Epidemiol. 1977 Jan;105(1):56–67. doi: 10.1093/oxfordjournals.aje.a112356. [DOI] [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Ormsbee R. A., Peacock M. G., Burgdorfer W. Microimmunofluorescence test for the serological study of rocky mountain spotted fever and typhus. J Clin Microbiol. 1976 Jan;3(1):51–61. doi: 10.1128/jcm.3.1.51-61.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOENNER H. G., LACKMAN D. B., BELL E. J. Factors affecting the growth of rickettsias of the spotted fever group in fertile hens' eggs. J Infect Dis. 1962 Mar-Apr;110:121–128. doi: 10.1093/infdis/110.2.121. [DOI] [PubMed] [Google Scholar]
- Sammons L. S., Kenyon R. H., Hickman R. L., Pedersen C. E., Jr Susceptibility of laboratory animals to infection by spotted fever group rickettsiae. Lab Anim Sci. 1977 Apr;27(2):229–234. [PubMed] [Google Scholar]
- Shirai A., Dietel J. W., Osterman J. V. Indirect hemagglutination test for human antibody to typhus and spotted fever group rickettsiae. J Clin Microbiol. 1975 Nov;2(5):430–437. doi: 10.1128/jcm.2.5.430-437.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urvölgyi J., Brezina R., Valková E. Erythrocyte-sensitizing substance from Rickettsia Canadia. Acta Virol. 1975 May;19(3):255–258. [PubMed] [Google Scholar]







