Abstract
Objective
To determine the rates of, and risk factors for, meningococcal carriage and acquisition among university students.
Design
Repeated cross sectional study.
Participants
2507 students in their first year at university.
Main outcome measures
Prevalence of carriage of meningococci and risk factors for carriage and acquisition of meningococci.
Results
Carriage rates for meningoccoci increased rapidly in the first week of term from 6.9% on day 1, to 11.2% on day 2, to 19.0% on day 3, and to 23.1% on day 4. The average carriage rate during the first week of term in October among students living in catered halls was 13.9%. By November this had risen to 31.0% and in December it had reached 34.2%. Independent associations for acquisition of meningococci in the autumn term were frequency of visits to a hall bar (5-7 visits: odds ratio 2.7, 95% confidence interval 1.5 to 4.8), active smoking (1.6, 1.0 to 2.6), being male (1.6, 1.2 to 2.2), visits to night clubs (1.3, 1.0 to 1.6), and intimate kissing (1.4, 1.0 to 1.8). Lower rates of acquisition were found in female only halls (0.5, 0.3 to 0.9). The most commonly acquired meningococcal strain was C2a P1.5 (P1.2), which has been implicated in clusters of invasive meningococcal disease at other UK universities.
Conclusions
Carriage rates of meningococci among university students increase rapidly in the first week of term, with further increases during the term. The rapid rate of acquisition may explain the increased risk of invasive meningococcal disease and the timing of cases and outbreaks in university students.
Introduction
During the 1990s there have been major increases in the incidence of invasive meningococcal disease in many developed countries,1–3 with serogroup C disease most noticeable, especially among teenagers and young adults. It has also been shown that university undergraduates have higher rates of invasive meningococcal disease than young adults of the same age who are not attending university.4 The provision of places in catered halls seems to be an important factor in differences in rates of invasive meningococcal disease between universities.4 In the United Kingdom, several clusters of invasive meningococcal disease have been reported; a large outbreak occurred in November 1996 at the University of Wales in Cardiff5 and another in October 1997 at the University of Southampton.6
No studies have been published on the epidemiology of meningococcal carriage or acquisition among university students in situations where there are no outbreaks.7 We therefore performed a longitudinal study in first year university students to determine rates of carriage and acquisition of Neisseria meningitidis, together with risk factors for both.
Participants and methods
Recruitment of students
Nottingham University is a large campus based institution. As part of routine induction, all new students (mainly first year undergraduates) are asked to attend the health centre on campus during their first week at university. The order of attendance, evenly distributed across the four days, is set by degree course and not by faculty or hall of residence. During this week in October 1997, we recruited students to the study after they had registered with the health centre and undergone health screening. Each student was given an information sheet and consent form. Those agreeing to take part completed a questionnaire covering: personal characteristics, place of residence, faculty, recent symptoms of upper respiratory tract infection, medical history including meningococcal vaccination, current and recent drugs, travel abroad and to other universities in the past month; active and passive smoking, visits to bars and night clubs, amount and type of alcohol consumed, number of people kissed, and the sharing of glasses and cigarettes in the preceding week. After each student had completed the questionnaire, a trained operator took a posterior pharyngeal swab, which was plated immediately on to selective medium and handled using standard techniques (see website). The same processing methods were used throughout.
Follow up
All participants in catered halls were selected for a further pharyngeal swab in either the first week of November 1997 or the first week of December 1997 on the basis of odd or even study numbers. Pharyngeal swabs were taken from students in the only self catered hall in the study in December. At the time of reswabbing the questionnaire was repeated. We therefore had paired data available for these students for October and either November or December.
One case of serogroup C disease occurred in a catered hall in late October, and one third of the students in this hall were therefore given ciprofloxacin to eradicate meningococcal carriage. Although students in this hall were reswabbed according to the prearranged schedule, we excluded them from the main analysis. Two other cases of invasive meningococcal disease (different serogroup B infections) occurred in the study population but these were in the spring term.
Statistical analysis
Questionnaire data were scanned with Formic, an electronic scanning package8 and stored in Microsoft Access (version 2.0). We used Epi-Info (version 6.04) for χ2 and Fisher's exact tests and spss for Windows (version 8) for multiple logistic regression analysis. Data collected at the time of the first pharyngeal swab were used to determine risk factors for initial carriage through multiple logistic regression. Subsequently, further analyses of risk factors for acquisition during the first term were performed with data from the repeat questionnaires and included only those students whose pharyngeal swab was negative in October.
Results
Overall, 2507 first year students attended the university's health centre in the first week of the first term, of whom 2453 (97.8%) agreed to participate. A rapid increase in carriage of N meningitidis occurred during the first week (table 1). Date of swabbing, type of hall, active and passive smoking, and intimate kissing were all independent risk factors for meningococcal carriage during the first week (table 2).
Table 1.
Carriage rate of Neisseria meningitidis during first week of term, 1997
| Date | No of students
|
% carriage rate (95% CI) | |
|---|---|---|---|
| Swabbed | Positive for N meningitidis | ||
| 30 September | 825 | 57 | 6.9 (5.3 to 8.9) |
| 1 October | 669 | 75 | 11.2 (8.9 to 13.6) |
| 2 October | 691 | 131 | 19.0 (16.0 to 21.9) |
| 3 October | 268 | 62 | 23.1 (18.1 to 28.2) |
χ2 for linear trend = 74.
P<0.0001.
Table 2.
Risk factors for carriage of Neisseria meningitidis during first week of term
| Exposure | Odds ratio (95% CI) | P value |
|---|---|---|
| Day of throat swab | ||
| Tuesday | Reference | |
| Wednesday | 1.74 (1.2 to 2.5) | 0.003 |
| Thursday | 2.99 (2.1 to 4.2) | 0.0001 |
| Friday | 4.05 (2.7 to 6.1) | 0.0001 |
| Type of hall | ||
| Mixed sex | Reference | |
| Male only | 1.16 (0.8 to 1.6) | 0.3 |
| Female only | 0.77 (0.5 to 1.3) | 0.4 |
| Off campus | 0.64 (0.5 to 0.9) | 0.01 |
| Passive smoking (days) | ||
| 0-2 | Reference | |
| 3-4 | 1.26 (0.8 to 1.9) | 0.3 |
| 5-6 | 1.60 (1.1 to 2.4) | 0.2 |
| 7 | 2.03 (1.3 to 3.1) | 0.001 |
| Smoker | 2.40 (1.6 to 3.7) | 0.0001 |
| No of people kissed | ||
| 0 | Reference | |
| 1 | 1.41 (1.1 to 1.8) | 0.01 |
| ⩾2 | 1.19 (0.7 to 2.0) | 0.5 |
Each risk adjusted for all other variables in table and antibiotic use in previous month.
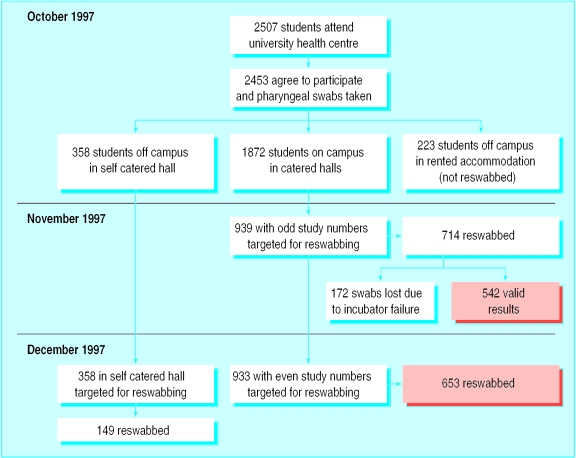
In November, 714 of the 939 eligible students (76.0%) were reinvestigated for meningococcal carriage and social behaviour. We could not process 172 swabs owing to a problem with an incubator, leaving 542 students (57.7%). In December, 653 of 933 students (70.0%) in catered halls who had participated in the first round were reinvestigated along with 149 of 358 (42%) students in the self catered hall. The figure shows the schedule of swabbing from October to December.
The carriage rate of N meningitidis increased during the first term (table 3). Six weeks after widespread treatment with ciprofloxacin the carriage rate in the excluded hall was 40 of 142 (28.2%, 95% confidence interval 20.8 to 35.6). The carriage rate of group C meningococci among students resident in catered halls was 0.5% (0.2 to 1.0) in October, 1.9% (0.9 to 3.9) in November, and 3.1% (2.0 to 4.4) in December.
Table 3.
Carriage of Neisseria meningitidis by study month and hall status among first year undergraduate university students, 1997
| Month | Type of hall | No of students
|
% carriage rate (95% CI) | |
|---|---|---|---|---|
| Swabbed | Positive for N meningitidis | |||
| October | Catered | 1872 | 261 | 13.9 (12.4 to 15.1) |
| October | Self catered | 358 | 37 | 10.3 (7.2 to 13.5) |
| November | Catered* | 542 | 168 | 31.0 (27.1 to 34.9) |
| December | Catered* | 653 | 223 | 34.2 (30.5 to 37.8) |
| December | Catered† | 142 | 40 | 28.2 (20.8 to 35.6) |
| December | Self catered | 149 | 36 | 24.2 (17.3 to 31.0) |
Excludes results where 30% of students received ciprofloxacin.
30% of students given ciprofloxacin in late October.
At some point during the first term, 349 students with initially negative pharyngeal swabs in October acquired meningococci. Table 4 shows the independently significant factors associated with meningococcal acquisition during this period. Overall, 300 of the 325 index strains isolated in October (92%) and 333 of the 349 strains acquired by students during the autumn term (95%) were fully typed. Table 5 shows the full typing of the commonest isolates in the index round and the acquired strains. Although non-C strains predominated on October 1997 (mainly B and non-groupable meningococci), C:2a:P1.5 (P1.2) was the commonest strain acquired (21 of 333) during the first term (χ2 10.8, P=0.001.)
Table 4.
Risk factors for acquisition of Neisseria meningitidis during first term
| Exposure | Odds ratio (95% CI) | P value |
|---|---|---|
| Sex | ||
| Female | Reference | |
| Male | 1.61 (1.2 to 2.2) | 0.003 |
| Passive smoking (days) | ||
| 0-2 | Reference | |
| 3-7 | 1.21 (0.9 to 1.7) | 0.2 |
| Smoker | 1.6 (1.0 to 2.6) | 0.05 |
| Weekly visits to hall bar | ||
| 0 | Reference | |
| 1-4 | 1.74 (1.1 to 2.8) | 0.03 |
| ⩾5 | 2.71 (1.5 to 4.8) | 0.0005 |
| Type of hall | ||
| Mixed sex | Reference | |
| Male only | 0.71 (0.5 to 1.0) | 0.05 |
| Female only | 0.52 (0.3 to 0.9) | 0.03 |
| Self catered | 0.73 (0.5 to 1.2) | 0.2 |
| Visited night club | ||
| No | Reference | |
| Yes | 1.25 (1.0 to 1.6) | 0.05 |
| No of people kissed | ||
| 0 | Reference | |
| 1 | 1.37 (1.0 to 1.8) | 0.04 |
| ⩾2 | 1.37 (0.9 to 2.1) | 0.1 |
Each risk adjusted for all other variables in table and antibiotic use in previous month.
Table 5.
Typing data from 300 carriers in October 1997 and 333 strains acquired in first term in students previously negative for N meningitidis
| Full typing details | No (%) of carriers | No (%) of newly acquired strains |
|---|---|---|
| C:2a:P1.5,2 (P1.5) | 3 (1.0) | 21 (6.3) |
| W135:NT:P1.3,6 | 8 (2.7) | 19 (5.7) |
| NG:NT:P1.16 | 7 (2.3) | 17 (5.1) |
| NG:NT:NT | 16 (5.3) | 11 (3.3) |
| NG:NT:P1.3,6 | 7 (2.3) | 10 (3.0) |
| NG:NT:P1.5 | 16 (5.3) | 5 (1.5) |
| B:NT:P1.15 | 10 (3.3) | 6 (1.8) |
| B:NT:NT | 9 (3.0) | 1 (0.3) |
| B:NT:P1.9 | 9 (3.0) | 0 (0) |
| NG:NT:P1.15 | 9 (3.0) | 5 (1.5) |
| 29E:NT:P1.5,2 | 8 (2.7) | 6 (1.8) |
| NG:15:P1.6 | 8 (2.7) | 5 (1.5) |
| NG:4:NT | 7 (2.3) | 3 (0.9) |
| X:21:P1.16 | 0 (0) | 9 (2.7) |
| Y:NT:P1.5,2 | 6 (2.0) | 9 (2.7) |
| B:1:P1.13 | 0 (0) | 7 (2.1) |
NG=not grouped; NT=not typed. Group C versus other strains χ2=10.8 (1df). P=0.001.
Discussion
Our results show that meningococcal carriage increases rapidly among university students in the first month of the academic year and that much of this increase probably occurs during the first week. Rapid acquisition rates have previously been found among military recruits; however, these studies were generally smaller and fundamental differences in sleeping arrangements existed compared with students.9–11
Several explanations for the rapid increase we observed can probably be discounted. The first was an improvement in swabbing techniques over the first week of the study. Although we were unable to identify the person who took each swab, most were taken by one person (KRN) with considerable experience.12 KRN also supervised the technique of the other operators. Furthermore, on each day during the first week, different operators assisted with swabbing in the morning and afternoon sessions yet there were no differences between morning and afternoon carriage rates on any day. We therefore believe that reliability was high between operators taking the swabs. The alternative explanation is that students who were more likely to be carrying meningococci on arrival at university were recruited later in the week. This seems unlikely as over 99% of students attended at their allotted time, and it seems unlikely that any systematic bias would have been introduced by choice of degree course. Furthermore, students are not allocated to halls of residence by course or faculty groups. The association of carriage with markers of social mixing also supports a causal link with acquisition after arrival at university.
Our main finding was a rapid increase in meningococcal carriage from 8% to 23% during the first week. Although the initial carriage rate was surprisingly low (8%), this finding has now been replicated by a subsequent study performed in October 1999 with a different population of students, who had pharyngeal swabs taken both on arrival and one week later (data available on request). Therefore, we do not believe the initially low carriage rate to be artefactual and speculate that clearance of meningococci occurs during the summer holiday between leaving school and starting university. This may arise from dispersal of the sixth form group, resulting in lower rates of recolonisation.
We also noted that during the first week carriage was higher in catered halls. This agrees with a previous study, which identified an increased risk of invasive meningococcal disease at universities offering comparatively more accommodation in catered halls. We speculate that this may be due to fundamental differences in the physical structure and pattern of social interaction between catered and self catered halls at Nottingham University.
Risk factors for carriage
In our regression analysis, we identified active and passive smoking and intimate kissing as risk factors for carriage. These have been previously shown by other investigators.13 In addition, we noted that students living off campus were less likely to be carriers, which is also consistent with the theory of social mixing.
Risk factors for acquisition
Although all of the students in this analysis had an initial negative pharyngeal swab result, it is possible that some students were incorrectly identified as non-carriers during the first week of the study. This type of misclassification bias, inevitable in this type of study, will have had the effect of weakening any associations detected. For the same reason, our estimates of the prevalence of carriage should also be regarded as conservative. Nevertheless, we identified male sex, active smoking, visits to hall bars and nightclubs, intimate kissing, and mixed sex halls as risk factors for acquisition. Most of these factors have been previously identified in carriage and outbreak studies,14–16 but few have been addressed the risk of acquiring carriage.11 The lower rate of acquisition seen in female only halls probably reflects different patterns of social behaviour.
What is already known on this topic
University students have been shown to be at greater risk of invasive meningococcal disease than other people of the same age
Meningococci have been shown to spread rapidly among military recruits and this is associated with increased rates of invasive disease
What this study adds
Meningococci spread rapidly among university students, probably due to social mixing
This explains the higher rates of invasive disease found among students each autumn during the first term of university and supports the recent introduction of meningococcal vaccination
As expected, NG (not grouped) and serogroup B strains predominated in all swabs. Whereas carriage rates of serogroup C meningococci are significantly lower than for serogroup B or non-groupable meningococci, and typically less than 1% even in outbreaks,12,14,17 our study found serogroup carriage rates of 3% by December. This level increases the risk of outbreaks. Serogroup C disease is of particular importance as it is preventable by vaccine and has previously been linked to large clusters of disease among university students.5,6
The large comparative increase in the C:2a:P1.5 (P1.2) strains is noteworthy. This strain is known to be virulent and has been implicated in several major outbreaks3,5,6; it represented 6.3% of all acquired strains in our study but only 1% of index strains identified in October. This illustrates the ability of highly virulent clones to transmit readily among students. Indeed the preferential transmission of this strain from a low baseline carriage rate may explain the 3-5 week delay usually observed between the start of university term and the peak incidence of cases and outbreaks.
During the beginning of university terms there is a rapid spread of meningococci in first year students, which is probably associated with social mixing, especially in catered halls. Our findings support the recent introduction of meningococcal vaccination for university students.
Supplementary Material
Figure.
Schedule for pharyngeal swabbing, October to December 1997
Acknowledgments
We thank Dr Jim Pearson for his advice on methods and statistics, Dr Angela White and her colleagues in the Cripps Health Centre for use of facilities and general support, Keith Ashford for culturing the meningococci, and the Public Health Laboratory Service's meningococcal reference unit in Manchester for serogrouping, serotyping, and serosubtyping data.
Footnotes
Funding: Meningitis Research Foundation, Bristol.
Competing interests: None declared.
website extra: Techniques for processing the pharyngeal swabs appear on the BMJ's website www.bmj.com
References
- 1.Hubert B, Caugant DA. Recent changes in meningococcal disease in Europe. Euro Surveillance. 1997;2:69–71. doi: 10.2807/esm.02.10.00145-en. [DOI] [PubMed] [Google Scholar]
- 2.Whalen CM, Hockin JC, Ryan A, Ashton F. The changing epidemiology of invasive meningococcal disease in Canada, 1985 through 1992. Emergence of a virulent clone of Neisseria meningitidis. JAMA. 1995;273:390–394. [PubMed] [Google Scholar]
- 3.Jackson LA, Schuchat A, Reeves MW, Wenger JD. Serogroup C meningococcal outbreaks in the United States. An emerging threat. JAMA. 1995;273:383–389. [PubMed] [Google Scholar]
- 4.Neal KR, Nguyen-Van-Tam JS, Monk P, O'Brien SJ, Stuart J, Ramsay M. Invasive meningococcal disease among university undergraduates: association with catered halls of residence. Epidemiol Infect. 1999;122:351–358. doi: 10.1017/s0950268899002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anon. Outbreak of meningococcal disease in students in Cardiff. Commun Disease Rep. 1996;6:425. [PubMed] [Google Scholar]
- 6.Anon. Clusters of meningococcal disease in university students. Commun Disease Rep. 1997;7:393. [PubMed] [Google Scholar]
- 7.Jackson LA, Schuchat A, Gorsky RD, Wenger JD. Should college students be vaccinated against meningococcal disease? A cost-benefit analysis. Am J Public Health. 1995;85:843–845. doi: 10.2105/ajph.85.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Formic, an integrated questionnaire design and scanning package. www.formic.co.uk. (Accessed 7 September 1999.)
- 9.Anderson J, Berthelson L, Jensen BB, Lind I. Dynamics of the meningococcal carrier state and characteristics of the carrier strains: a longitudinal study within three cohorts of military recruits. Epidemiol Infect. 1998;121:85–94. doi: 10.1017/s0950268898008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devine LF, Pierce WE, Floyd TM, Rhode SL, Edwards EA, Siess EE, et al. Evaluation of group C meningococcal polysaccharide vaccine in marine recruits, San Diego, California. Am J Epidemiol. 1970;92:25–32. doi: 10.1093/oxfordjournals.aje.a121176. [DOI] [PubMed] [Google Scholar]
- 11.Melton LJ, Edwards EA, Devine EA, Devine LF. Differences between the sexes in the nasopharyngeal carriage of Neisseria meningitidis. Am J Epidemiol. 1977;106:215–221. doi: 10.1093/oxfordjournals.aje.a112456. [DOI] [PubMed] [Google Scholar]
- 12.Neal KR, Irwin DJ, Davies S, Kaczmarski EB, Wale MCJ. Sustained reduction in the carriage of Neisseria meningitidis as a result of a community meningococcal disease control programme. Epidemiol Infect. 1998;121:487–493. doi: 10.1017/s0950268898001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuart JM, Cartwright KAV, Robinson PM, Noah ND. Effect of smoking on meningococcal carriage. Lancet. 1989;ii:723–725. doi: 10.1016/s0140-6736(89)90781-2. [DOI] [PubMed] [Google Scholar]
- 14.Rønne T, Berthelesen L, Buhl LH, Lind I. Comparative studies on pharnygeal carriage of Neisseria meningitidis during a localized outbreak of serogroup C meningococcal disease. Scand J Infect Dis. 1993;25:331–339. doi: 10.3109/00365549309008507. [DOI] [PubMed] [Google Scholar]
- 15.Imrey PB, Jackson LA, Ludwinski PH, England AC, III, Fella GA, Fox BC, et al. Outbreak of serogroup C meningococcal disease associated with campus bar patronage. Am J Epidemiol. 1996;143:624–630. doi: 10.1093/oxfordjournals.aje.a008792. [DOI] [PubMed] [Google Scholar]
- 16.Jelfs J, Jaluludin B, Munro R, Patel R, Kerr M, Daley D, et al. A cluster of meningococcal disease in western Sydney, Australia initially associated with a nightclub. Epidemiol Infect. 1998;120:263–270. doi: 10.1017/s0950268898008681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Saux N, Ashton F, Rahman F, Ryan A, Ellis E, Tamblyn S, et al. Carriage of Neisseria species in communities with different rates of meningococcal disease. Can J Infect Dis. 1992;3:60–64. doi: 10.1155/1992/928727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.