Abstract
Mechanosensitive K+ channels TREK1 and TREK2 form a subclass of two P-domain K+ channels. They are potently activated by polyunsaturated fatty acids and are involved in neuroprotection, anesthesia, and pain perception. Here, we show that acidification of the extracellular medium strongly inhibits TREK1 with an apparent pK near to 7.4 corresponding to the physiological pH. The all-or-none effect of pH variation is steep and is observed within one pH unit. TREK2 is not inhibited but activated by acidification within the same range of pH, despite its close homology with TREK1. A single conserved residue, H126 in TREK1 and H151 in TREK2, is involved in proton sensing. This histidine is located in the M1P1 extracellular loop preceding the first P domain. The differential effect of acidification, that is, activation for TREK2 and inhibition for TREK1, involves other residues located in the P2M4 loop, linking the second P domain and the fourth membrane-spanning segment. Structural modeling of TREK1 and TREK2 and site-directed mutagenesis strongly suggest that attraction or repulsion between the protonated side chain of histidine and closely located negatively or positively charged residues in P2M4 control outer gating of these channels. The differential sensitivity of TREK1 and TREK2 to external pH variations discriminates between these two K+ channels that otherwise share the same regulations by physical and chemical stimuli, and by hormones and neurotransmitters.
Keywords: ion channel, mutagenesis, structural modeling
TREK1 and TREK2 are two pore-domain K+ (K2P) channels sharing 78% of sequence homology (1–3). They are expressed in large amount in the nervous system (1, 3). In mouse brain, they display both overlapping and distinct distributions (4). TREK1 is highly expressed in the striatum and the cortex and TREK2 in the cerebellar granule cell layer. The two channels are detected in the hippocampus. Unlike other K2P channels like the TASK channels, TREK1 and TREK2 are not very active at room temperature, but can be stimulated by a wide range of stimuli, including mechanical stretch (5), cell swelling (5), intracellular acidification (6, 7), heat (8, 9), lysophospholipids (10), and polyunsaturated fatty acids (PUFAs) such as arachidonic acid (AA) (5), and by pharmacological agents such as volatile anesthetics (1, 11) and riluzole (12), a drug used to protect motoneurons in amyotrophic lateral sclerosis. Both TREK1 and TREK2 are inhibited by neurotransmitters and hormones that activate protein kinase A and C pathways resulting in phosphorylation of conserved residues in their cytoplasmic C-ter (5, 13, 14). TREK1 and TREK2 also share interacting partners in the brain (15, 16). A kinase anchoring protein (AKAP) 150 is one of them. By binding to a major regulatory site of the TREK channels, AKAP150 transforms low activity outwardly rectifying channels into robust leak channels that become largely insensitive to AA, stretch, and internal acidification. Inhibition by Gs-coupled receptors is conserved but inhibition by Gq-coupled receptors is much reduced in TREK/AKAP150 complex (15). Microtubule-associated protein (Mtap) 2 is another partner of TREK1 and TREK2. It facilitates their trafficking to the plasma membrane by a mechanism that requires binding of Mtap2 to tubulin (16). TREK1 channels play an important role in the control of epileptic seizures, in the potent neuroprotection provided by PUFAs treatments (17), volatile anesthetics (17), and in all sorts of polymodal pain perception (18, 19). They control mood, and gene inactivation of TREK1 results in a depression-resistant phenotype (20). At present, no such information exists for TREK2, but an important role of TREK2 in physiological and pathophysiological processes is expected as for TREK1.
Here, we have studied the regulation of the TREK channels by external pH. TREK1 is strongly inhibited by external acidification within the physiological range. TREK2 is also sensitive to external pH variations, in the same range of pH, but is activated instead of being inhibited by acidification. Residues implicated in pH sensing and in channel response to pH variations have been identified by site-directed mutagenesis. A single conserved histidine located in the first extracellular domain forms the pH sensor. Structural modeling suggested that nonconserved residues in the extracellular domain after the second P domain were responsible for the opposite effect of pH variation on TREK1 and TREK2 activities. In agreement with this prediction, replacement of neutral and positively charged residues in TREK2 by the equivalent neutral and negatively charged residues of TREK1 was sufficient to inverse TREK2 response to acidification and to convert it into a TREK1-like response.
Results
TREK1 Channel Activity Is Inhibited by Extracellular Acidification.
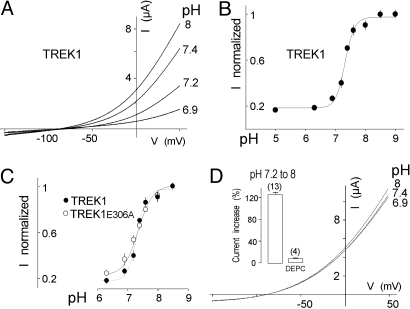
Current-potential (IV) relationships recorded from a Xenopus oocyte expressing TREK1 at acid, physiological and basic pHs are presented in Fig. 1A. At pH 6.9, a drastic current reduction was observed when compared with pH 7.4, whereas an increase was recorded at pH 8. Inhibition and stimulation were observed at all potentials and produced no modification of current kinetics. The pH dependence of TREK1 is shown in Fig. 1B. For currents recorded at 0 mV, inhibition by acidic pHs was characterized by an apparent pK of 7.35 ± 0.03 and a Hill coefficient of 1.6 ± 0.4 (n = 13). For current recorded at 50 mV, the maximum blocking effect is not statistically different with a pK of 7.32 ± 0.03 and a Hill coefficient of 1.8 ± 0.3 (n = 13). These results show that the blocking effect by external protons is not voltage-dependent between 0 and 50 mV.
Fig. 1.
TREK1 is inhibited by extracellular acidification. (A) Effect of pH on TREK1 expressed in Xenopus oocytes. Currents were elicited by voltage-ramps (from −150 to + 50 mV, 1 s in duration). (B) pH dependence of the TREK1 channel activity at 0 mV. (C) pH dependence of TREK1E306A channel activity at 0 mV. Error bars, SEM. At higher levels of expression, TREK1E306 becomes resistant to inhibition. (D) Effect of DEPC preincubation (2 mM) on pH-sensitivity of TREK1. (Inset) the histograms represent the TREK1 current increase induced by a pH shift from 8 to 7.2 at 0 mV, in the absence or in the presence of DEPC. The number of cells tested is indicated in parenthesis.
TREK1 currents are stimulated by intracellular acidification (6). A glutamate residue located on the cytoplasmic side of the channel has been associated with intracellular pH sensing (21). Replacement of this E306 residue by an alanine induces a considerable increase of the associated current. The mutant channel TREK1E306A is constitutively and maximally active, and cannot be anymore activated by PUFA or mechanostimulation. As shown in Fig. 1C, TREK1E306A is inhibited by extracellular acidification. This inhibition is similar to the inhibition of TREK1 (pK = 7.32 ± 0.03, n = 6). Binding of AKAP150 to its Cter can also switch TREK1 in a constitutively active state (15). As observed with the E306A mutation, coexpression of TREK1 with AKAP150 failed to modify its sensitivity to external pH (pK = 7.31 ± 0.03, n = 5). Taken together these results indicate that the inhibitory effect of external protons on TREK1 is largely independent of the effects of stimuli acting on its intracellular side.
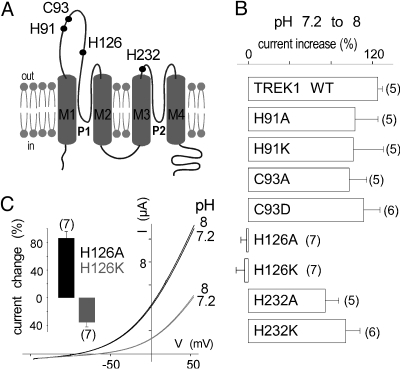
pK values ≈7.3/7.4 suggest that the imidazole side chain of one or several histidine residues are involved. Oocytes expressing TREK1 were preincubated in medium containing diethyl pyrocarbonate (DEPC, 2 mM). DEPC is a reagent that converts neutral imidazole groups into N-carbethoxyhistidyl derivatives (22). IV relationships recorded from a TREK1-expressing oocyte at pH 6.9, 7.4 and 8 after DEPC treatment are presented in Fig. 1D. In normal medium, a shift from pH 7.2 to 8 produces a 125 ± 5% increase of TREK1. After treatment with DEPC, the same shift of pH has no stimulatory effect (6 ± 2% of variation) (Fig. 1D Inset). The suppression by DEPC of the pH response indicates that histidine residues are probably involved in pH sensing. To identify this or these residues, we produced a series of point mutants in which extracellular histidines were replaced by alanine or lysine. We also mutated cysteines because DEPC is also known to react with their side chains (22). No significant alteration of the pH sensitivity was observed for TREK1 channels mutated on H91, C93, and H232 (Fig. 2 A and B). Substitution of H126 by alanine or lysine abolished the regulation of TREK1 by pH. Replacement of this particular histidine by a lysine (H126K), that in a way mimics a protonated form of the imidazole side chain histidine, decreases the basal mean amplitude of the current (36 ± 6%) whereas replacement by alanine (H126A), that replaces histidine by an uncharged residue and can be considered to mimic deprotonation, increases the mean amplitude of the current (86 ± 9%) when compared with TREK1 (Fig. 2C Inset). These results clearly indicate that (i) H126 is necessary for proton sensing and that (ii) its protonation state, as expected, is important for the regulation of TREK1 activity by external pH.
Fig. 2.
pH sensitivity of mutated TREK1 channels. (A) Membrane topology of TREK1. Extracellular cysteine and histidine residues are indicated. (B) Current increase induced by a pH shift from 7.2 to 8 at 0 mV for TREK1 and point mutants. (C) Loss of pH effect on currents produced by TREK1H126A and TREK1H126K. Currents were elicited by voltage-ramps (from −150 to + 50 mV, 1 s in duration). (Inset) TREK1H126A and TREK1H126K current amplitude changes relative to TREK1 current at 0 mV at pH 7.4. Error bars, SEM. The number of cells tested is indicated in parenthesis.
TREK2 Channel Activity Is Stimulated by External Acidification.
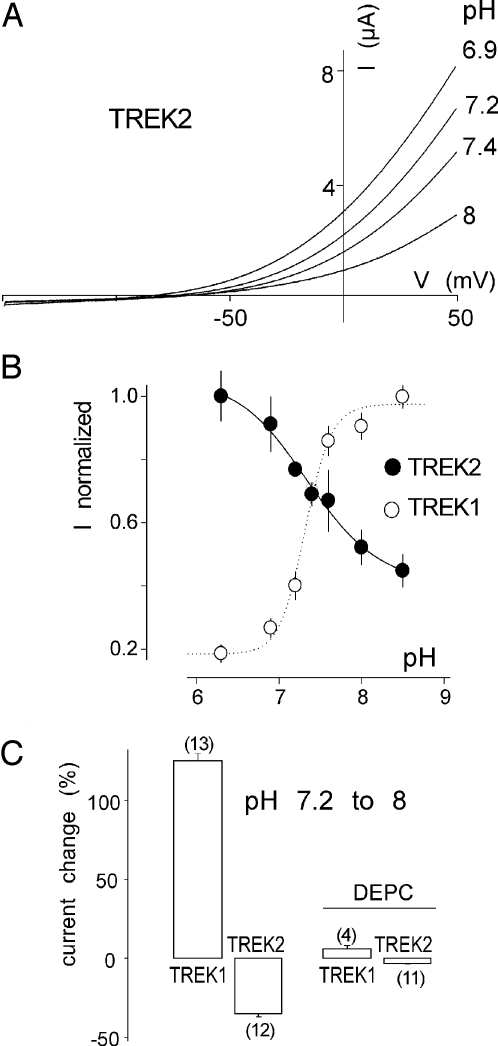
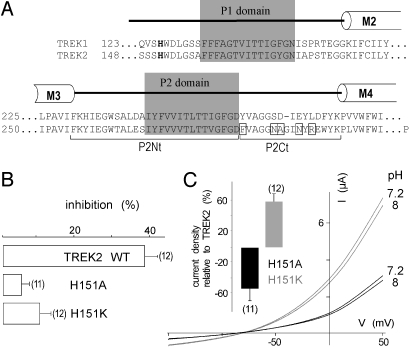
We next examined TREK2 sensitivity to acidification. Surprisingly, TREK2 is not inhibited but is activated by external protons with a pK value of 7.3 ± 0.05 (n = 8) (Fig. 3 A and B). The effects of pH on TREK2 were prevented by preincubating oocytes in a medium containing DEPC (Fig. 3C). Histidine at position 126 in TREK1 is conserved in TREK2 at position 151 (Fig. 4A). Mutation of H151 prevents the regulation of TREK2 by external pH (Fig. 4B). Mutation H151K that mimics protonation results in a 60 ± 12% increase of the mean current amplitude when compared with TREK2, whereas the H151A mutant that mimics deprotonation produces less current than TREK2 (28 ± 14%) (Fig. 4C). These results show that H151 in TREK2 plays the same role as H126 in TREK1, but with opposite effects on channel activity. H126 in TREK1 and H151 in TREK2 are the proton sensors.
Fig. 3.
pH sensitivity of TREK2. (A) Effect of pH on TREK2. Currents were elicited by voltage-ramps (from −150 to + 50 mV, 1 s in duration). (B) pH-dependence of TREK2 channel activities at 0 mV. (C) The histograms represent the current increase or decrease induced by a pH shift from 7.2 to 8 at 0 mV in the absence or in the presence of DEPC. Error bars, SEM. The number of cells tested is indicated in parenthesis.
Fig. 4.
A conserved histidine residue is the extracellular pH sensor. (A) Sequence alignment of the pore regions from TREK1 and TREK2. The pH-sensing histidine residue is in bold. Residues in TREK2 predicted to interact with the histidine pH sensor are boxed. (B) Percentage of inhibition at pH 8 relative to pH 7.2 at 0 mV for TREK2 and TREK2H151 mutants. (C) Effect of pH on representative TREK2H151A and TREK2H151K currents in Xenopus oocyte in ND96 solution at pH 7.2 and 8. Currents were elicited by voltage-ramps (from −150 to + 50 mV, 1 s in duration). (Inset) TREK2H151A and TREK2H151K current amplitude changes relative to TREK2 current at 0 mV.
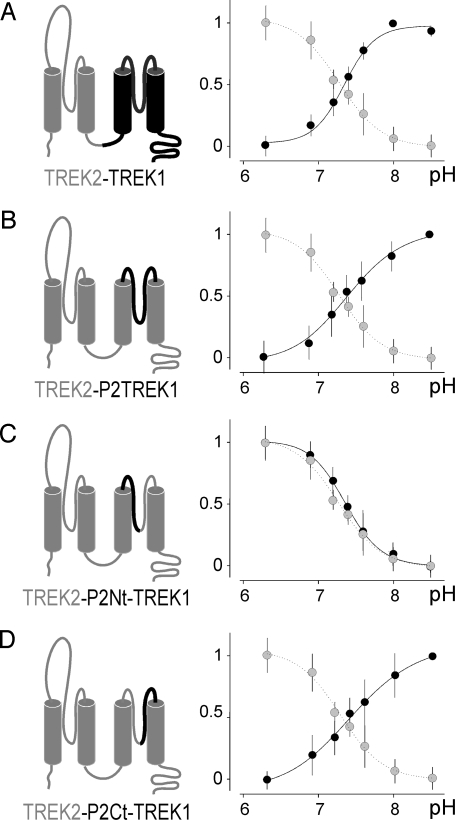
How to explain the opposite effect of external acidification on TREK1 and TREK2? If H126 and H151 are the proton sensors, then what are the other residues that confer the opposite effects of acidification on TREK1 and TREK2? To map these residues, we designed a series of chimeric channels by substituting parts of TREK2 by the corresponding segments of TREK1, and we tested systematically their sensitivity to extracellular pH variations. In the chimera designated TREK2-TREK1, the second half part of TREK2 including the M3, P2, and M4 domains was replaced by the corresponding one from TREK1 (Fig. 5A Left). Unlike TREK2, TREK2-TREK1 is not activated but inhibited by acidification with an apparent pK value of 7.35 ± 0.05 (n = 8) (Fig. 5A Right), suggesting that residues present in the second part of TREK1 are important for conferring inhibition by protons. The interdomain linking M3 to M4 in TREK1, when introduced in TREK2 to give the TREK2-P2TREK1 chimera, is sufficient to confer inhibition by acidification (pK = 7.41 ± 0.06, n = 5) (Fig. 5B). The TREK2-P2Nt-TREK1 chimera having the N-ter part of this M3M4 interdomain of TREK1 (see in Fig. 4A for a precise description of P2Nt) gave a pH response similar to TREK2 (pK = 7.37 ± 0.04, n = 5) (Fig. 5C). Finally, the chimera TREK2-P2Ct-TREK1 that contains the C-ter of the M3M4 interdomain of TREK1 (Fig. 4A) gave a pH response similar to TREK1 (pK = 7.42 ± 0.06, n = 7) (Fig. 5D). Together these results demonstrate that a short extracellular domain after the second pore domain P2 (P2Ct) is important for deciding whether proton sensing by H126/H151 will result into an inhibition or an activation.
Fig. 5.
Mapping of the region involved in the differential regulation of TREK1 and TREK2. pH dose-response curves of the variable proportion calculated from the normalized current of the current TREK2-TREK1 (A), TREK2-P2TREK1 (B), TREK2-P2Nt-TREK1 (C), and TREK2-P2Ct-TREK1 (D) chimeras, respectively. Dotted curves are the pH dose–response curve of TREK2, shown for comparison.
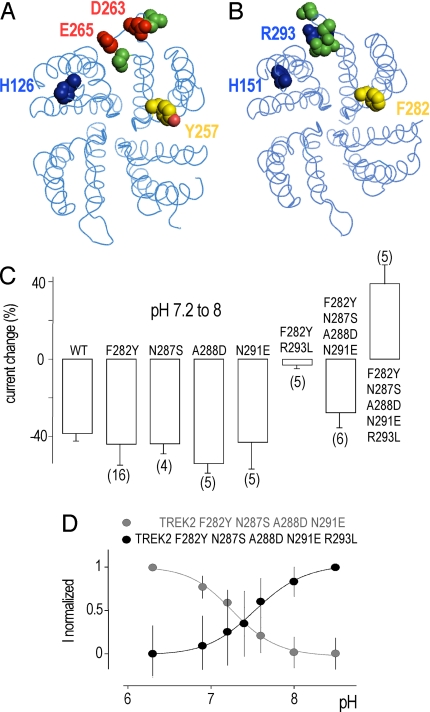
A sequence alignment of this region shows interesting differences between TREK1 and TREK2 (Fig. 4A). An obvious difference is the number of charged residues. The net charge is −2 for TREK1 and + 1 for TREK2. To get better insight into the transduction mechanism associated with extracellular pH sensing, we designed homology models of TREK1 and TREK2 based on the template structure of KcsA, a bacterial K+ channel (Fig. 6 A and B). These structural models show that in TREK1, a negatively charged loop follows the P2 domain and is localized at the interface with the P1 domain. This spatial distribution is compatible with an electrostatic attraction between protonated H126 and negatively charged residues D263 and E265. It is tempting to suggest that this attraction favors a functionally less active state of the channel. Conversely in TREK2, the equivalent loop contains R293, a positively charged residue. Protonation of H151 in TREK2 might produce an electrostatic repulsion with R293, and favors a more active state. Sequence alignment and structural modeling also suggest an important role for Y257 in TREK1 that corresponds to a phenylalanine in TREK2 (F282). This tyrosine stands at the interface between the P2 and P1 domains and is expected to stabilize the open form of the channel by interacting with charged or polar residues in the P1 domain. To evaluate the role of these different residues, we substituted each of them in TREK2 by the equivalent ones from TREK1. We also replaced N287 by the serine residue found at the corresponding position in TREK1. N287 does not carry any charge but the volume occupied by its side chain is larger than the volume occupied by the side chain of a serine, and thereby may have a direct effect on the position of its neighbor residues. As shown in Fig. 6C, when introduced individually into TREK2 mutations F282Y, N287S, A288D, and N291E did not change significantly the effects of pH variations. Mutation R293L produced a nonfunctional channel. However, the combination of R293L and F282Y mutations restored TREK2 channel function and reduced very significantly the inhibition induced by a pH shift from 7.2 to 8 at 0 mV (3 ± 2% of inhibition versus 38 ± 2% for the wild type TREK2) (Fig. 6C). This result demonstrates that R293 plays a major role in the activation of TREK2 by acidification. However, if the substitution of the positively charged R293 by a neutral residue is sufficient to decrease the pH sensitivity of TREK2, it is not enough to confer the capacity to be inhibited by protons. A combination of the following mutations F282Y, N287S, A288D, and N291E reduced slightly but significantly the inhibition (28 ± 8%). But when the four previous mutations were associated with the R293L mutation, then there was an inversion of the pH sensitivity (Fig. 6 C and D). This particular mutant of TREK2 behaves as TREK1, and is inhibited by acidic pH with a pK of 7.45 ± 0.06. This result demonstrates that to transfer to TREK2 the full capacity of TREK1 to be inhibited by protons, it is not only necessary to replace R293 by a neutral residue (R293L) but that it is also essential to introduce negatively charged residues at the same positions as in TREK1 (A288D and N291E). These experimental results are in agreement with the predictions inferred from the structural modeling of TREK1 and TREK2 and support the idea that the transduction mechanism associated with extracellular pH sensing relies on electrostatic attractions/repulsions between the protonated side chain of histidine in M1P1 and charged residues in P2M4.
Fig. 6.
Effect of single and combined mutations on pH-sensitivity to TREK2. Top view of the extracellular side of homodimeric TREK1 (A) and TREK2 (B) channels. Cytoplasmic Nter and Cter domains are not displayed. Studied residues are colored in only one monomer. pH-sensing histidines are indicated. Positively charged residues are in blue and negatively charged residues in red. Neutral residues are depicted in green and aromatics F282 and Y272 are in yellow along with the oxygen atom in red in Y272. (C) The histograms represent the percentage of inhibition or activation at pH 8 relative to pH 7.2 at 0 mV for TREK2 and TREK2 mutants. (D) pH dose-response curves of the variable proportion calculated from the normalized current of TREK2 mutants. Error bars, SEM. The number of cells tested is indicated in parenthesis.
Discussion
The pH-Sensing Properties of the TREK Channels and Their Physiological Relevance.
Here, we have shown that TREK1 and TREK2 are sensitive to extracellular pH variations within the physiological range. As reported in ref. 23, TREK1 is strongly inhibited by external acidification. It has been suggested that this effect was species specific and restricted to the human channel. However, we did not notice any significant differences in pH sensitivity between human and mouse TREK1 when both channels were expressed at the same level of ionic current expression. A surprising result is that the TREK2 channel, which shares 78% of sequence homology with TREK1 and the many regulations by stretch, PUFA, lysophospholipids, internal pH and protein kinases A and C, is activated rather than inhibited by external protons. This is an example of a mammalian K+ channel activated by external acidification in the physiological pH range.
Modulation of the TREK channels by external protons is probably important for their physiological functions. pH shifts are observed in physiological conditions, particularly in the nervous system where both TREK1 and TREK2 are well expressed. Acidifications of small amplitudes have been observed under conditions of intense stimulation. Alkalinization has been also observed. Kraig and collaborators have shown that in vivo stimulation of parallel fibers in cerebellum induced a brief alkalinization followed by a pronounced acidification lasting minutes (24). Of course, acidifications have been observed in pathological contexts corresponding to epilepsy, anoxia and ischemia. By controlling resting membrane potential and input resistance, TREK channels modulate neuronal activity. After acidosis, the closure of TREK1 is expected to lead to depolarization and to an increase of neuronal activity whereas opening of TREK2 will tend to decrease neuronal activity.
TREK channels are located both presynaptically (25) and postsynaptically (15, 16). At the presynaptic level, decrease of TREK1 activity by a local acidification would lead to an increased in neurotransmitter release and transmission efficiency. On the contrary, TREK2 activation by acidification would decrease synaptic efficiency. At the postsynaptic level, TREK1 inhibition by limited acidification would facilitate generation of electrical activity and produce a depolarization that would release Mg2+ block of NMDA receptor in glutamatergic synapses whereas TREK2 activation would inhibit activity and favor Mg2+ block. Depending of the subtype of TREK channels expressed, on levels of expression and on the specific synaptic localization, pH regulation via TREK1 and TREK2 channels could have diverse effects on neuronal activity.
Both TREK1 and TREK2 channels are present in nociceptors and TREK1 has been shown to be an important sensor of nociceptive heat and cold, and painful osmotic and mechanical stimulation (18, 19). Its acidic pH sensitivity makes it a candidate for acidic pH sensing in inflammation, ischemia, hematomas, and cancer, probably side by side with ASIC channels (26). Nociceptors also sense alkaline pH variations that also produce pain. TREK2 channels, through their inhibition at alkaline pH might be important candidates for this function, in addition to their probable role in mechano- and temperature sensing.
pH Sensing.
Mutation of a single conserved histidine in the M1P1 loop of TREK1 and TREK2 abolishes their pH sensitivity. Mimicking protonation of the imidazole side chain by replacing the histidine residue by a lysine, with a positively charged ε-amino side chain in the range of pH studied, results in larger TREK2 currents and smaller TREK1 currents. Replacing the same histidine by alanine, a residue with noncharged side chain, produced the opposite effects. These results indicate that histidine protonation is the pH sensor. There are other examples of pH-sensing histidines controlling activity of ion channels in the same pH range. The voltage-gated K+ channel Kv1.5 and inward rectifier K+ channel Kir2.1 that belong to the two other structural classes of K+ channels are also very sensitive to external pH. H452 in KV1.5 (27) and H117 in Kir2.1 (28) are necessary for proton sensing. H452 is located in the third extracellular loop of KV1.5 and H117 in the unique extracellular loop of Kir2.1, upstream the P domains at positions equivalent to H126 in TREK1. Clearly, a pH-sensing histidine at the same position has been conserved during evolution in the three different structural classes of K+ channels, with 6 (KV1.5), 2 (Kir2.1), and 4 membrane-spanning helices (TREK1 and TREK2). Regulation by pH near physiological pH at this conserved histidine is a property common to a voltage-sensitive K+ channels (KV1.5), an inward rectifier (Kir2.1), and two background K2P channels (TREK1 and TREK2). TASK1 and TASK3 that are members of the K2P channel family are also very important pH-sensing background K+ channels (29, 30). In these channels, pH sensing is associated with a histidine but this residue is located downstream the P1 domain directly adjacent to the selectivity filter (31, 32). TASK2 and TALK2, two other K2P channels, are activated by alkalinization (33, 34). However, in that case, there is no histidine residue involved (35, 36). Clearly, there is more than one possibility to introduce a pH sensor for regulating K+ channel activity.
Gating Mechanism Related to Acidification and Histidine Protonation in TREK Channels.
The opposite actions of histidine protonation in TREK1 and TREK2 that result into an acid-inhibited TREK1 and an acid-activated TREK2 are due to nonconserved residues in the P2M4 interdomain of these channels. Our structural models and data collected from mutagenesis predict that the opposite effects of pH are due to different types of electrostatic interactions between histidine and its different partners in TREK1 and TREK2. The two residue partners of histidine in TREK1 are negatively charged and therefore will be attracted by the protonated imidazole side chain. On the contrary, in TREK2 there is a basic residue at the same location that cannot be attracted when the key histidine becomes protonated and that instead probably undergoes repulsion. These changes in electrostatic attraction/repulsion certainly induce different conformational changes resulting in opposite effects on the activity of TREK1 and TREK2 channels. TRAAK is related to TREK channels. A histidine residue corresponding to H126 in TREK1 and H151 in TREK2 is conserved in TRAAK (H85). Furthermore the net charge in the P2M4 loop of TRAAK is −1. As expected, TRAAK is inhibited by external acidification and DEPC suppressed this pH sensitivity, indicating that protonation of H85 is probably requested for pH inhibition (Fig. S1). Altogether, these observations suggest that the gating mechanisms related to acidification are probably the same for the different members of this class of K2P channels.
What kind of conformational changes are involved? Activity of K+ channels is modulated by internal and external stimuli acting respectively on outer and inner gates. Protonation of H452 in KV1.5 leads a strong inhibition that has been related to the C-type inactivation (27). C-type inactivation is the mechanism by which a voltage-dependent channel undergoes a conformational change from an open state to a nonconducting state during membrane depolarization. C-type inactivation is due to a rearrangement (a collapse) of the external vestibule in the general area of the K+ channel pore (37). If TREK channels have a similar outer gate, then this one should be relatively independent of their inner gate. TREK channels can be stimulated by polyunsaturated fatty acids, volatile anesthetics and internal acidification, and are inhibited by protein kinase A (5, 11, 21). All these stimuli act on a regulatory domain located in a cytoplasmic domain after the last transmembrane segment (5, 9, 11, 21). AKAP150 is also capable to stimulate TREK channel activity by binding to this same domain (15). This regulatory domain controls the gating of TREK channels from the inside. This gating system is constitutively active in the TREK1E306A mutant (21) and in the TREK1/AKAP150 complex (15). The fact that acidification produces similar inhibitory effects on TREK1, TREK1E306A and TREK1/AKAP150 indicates that outer control of the TREK channel gating by pH is largely independent of their inner gating and could involve a collapse of their external vestibule. Another argument supporting this view is that a high external K+ concentration known to slow C-type inactivation alters pH inhibition of human (23) and mouse (Fig. S2) TREK1, and pH activation of TREK2 (Fig. S2). Taken together these results suggest that not only pH sensing but also the related conformational rearrangements of the outer gate seem to be conserved between K2P and Kv channels.
Materials and Methods
Molecular Biology and Electrophysiology.
Chimeras between TREK1 and TREK2 and point mutants were generated by PCR as described in ref. 38. All PCR were performed using the High Fidelity polymerase (Fermentas) according to the manufacture's protocol. PCR products were cloned into pEXO (39). Capped cRNAs were synthesized in vitro from the linearized plasmid by using T7 RNA polymerase (Ambion). Defolliculated Xenopus oocytes were injected with cRNA encoding mouse TREK1 (5 ng), TREK1E306A (0.1 ng), TREK2 (10 ng), or mutants (from 5 to 10 ng). They were used for electrophysiological studies 2–4 days after injection. In a 0.3-mL perfusion chamber, a single oocyte was impaled with two standard microelectrodes (1–2.5 MΩ resistance) filled with 3 M KCl and maintained under voltage clamp by using a Dagan TEV 200 amplifier, in standard ND96 solution (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 2 mM MgCl2, and 5 mM Hepes, pH 7.4, with NaOH). Stimulation of the preparation, data acquisition, and analysis were performed using pClamp software (Molecular Devices).
TREK Homology Modeling.
The amino acid sequences of human and mouse TREK1 and TREK2 were aligned with that of KcsA with the program ClustalW (40) (http://pat.cbs.cnrs.fr) and then refined manually. TREK subunits possess two repeated domains M1P1M2 and M3P2M4 and assemble as dimers. KcsA possesses only one similar domain M1P1M2 and function as a tetramer. We have aligned M1P1M2 and M3P2M4 domain of TREKs with the M1P1M2 domain of Kcsa. In the final alignment, the M1P1M2 of TREK1 displays a sequence identity of 21% with KcsA and 15% for the M3P2M4 region of TREK1. The homology modeling of the channels was carried out by using the MODELLER 9v1 package (41, 42), based on the crystallographic structure of Kcsa (PDB 1R3J chain C from residues 22 to 124). The first M1P1 extracellular loop was truncated by 10 aa (residues 89 to 100 for TREK1 and 99 to110 for TREK2) because this loop is difficult to model in the absence of relevant template. Consensus from several secondary structure predictors (VDSC, PREDATOR, PSIPRED, SIMPA96) suggests a poor folded segment. Models are available at http://bioinfo.ipmc.cnrs.fr/TREK
Supplementary Material
Acknowledgments.
We thank Dr. Amanda Patel (Centre National de la Recherche Scientifique, Sophia-Antipolis, France) for her gift of the TREK1E306A expression vector. This work was supported by the Fondation pour La Recherche Médicale (Equipe labellisée FRM to F.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906267106/DCSupplemental.
References
- 1.Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J Biol Chem. 2000;275:28398–28405. doi: 10.1074/jbc.M002822200. [DOI] [PubMed] [Google Scholar]
- 2.Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J Biol Chem. 2000;275:17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- 3.Fink M, et al. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- 4.Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel AJ, et al. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Gnatenco C, Bang H, Kim D. Localization of TREK-2 K+ channel domains that regulate channel kinetics and sensitivity to pressure, fatty acids and pHi. Pflugers Arch. 2001;442:952–960. doi: 10.1007/s004240100626. [DOI] [PubMed] [Google Scholar]
- 8.Maingret F, et al. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol. 2005;564:103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J Biol Chem. 2000;275:10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- 11.Patel AJ, et al. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- 12.Duprat F, et al. The neuroprotective agent riluzole activates the two P domain K+ channels TREK-1 and TRAAK. Mol Pharmacol. 2000;57:906–912. [PubMed] [Google Scholar]
- 13.Murbartian J, Lei Q, Sando JJ, Bayliss DA. Sequential phosphorylation mediates receptor- and kinase-induced inhibition of TREK-1 background potassium channels. J Biol Chem. 2005;280:30175–30184. doi: 10.1074/jbc.M503862200. [DOI] [PubMed] [Google Scholar]
- 14.Kang D, Han J, Kim D. Mechanism of inhibition of TREK-2 (K2P10.1) by the Gq-coupled M3 muscarinic receptor. Am J Physiol Cell Physiol. 2006;291:C649–656. doi: 10.1152/ajpcell.00047.2006. [DOI] [PubMed] [Google Scholar]
- 15.Sandoz G, et al. AKAP150, a switch to convert mechano-, pH- and arachidonic acid-sensitive TREK K+ channels into open leak channels. EMBO J. 2006;25:5864–5872. doi: 10.1038/sj.emboj.7601437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandoz G, et al. Mtap2 is a constituent of the protein network that regulates twik-related K+ channel expression and trafficking. J Neurosci. 2008;28:8545–8552. doi: 10.1523/JNEUROSCI.1962-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heurteaux C, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alloui A, et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006;25:2368–2376. doi: 10.1038/sj.emboj.7601116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noel J, et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009 doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heurteaux C, et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- 21.Honore E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miles EW. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- 23.Cohen A, Ben-Abu Y, Hen S, Zilberberg N. A novel mechanism for human K2P2.1 channel gating. Facilitation of C-type gating by protonation of extracellular histidine residues. J Biol Chem. 2008;283:19448–19455. doi: 10.1074/jbc.M801273200. [DOI] [PubMed] [Google Scholar]
- 24.Kraig RP, Ferreira-Filho CR, Nicholson C. Alkaline and acid transients in cerebellar microenvironment. J Neurophysiol. 1983;49:831–850. doi: 10.1152/jn.1983.49.3.831. [DOI] [PubMed] [Google Scholar]
- 25.Westphalen RI, Krivitski M, Amarosa A, Guy N, Hemmings HC., Jr Reduced inhibition of cortical glutamate and GABA release by halothane in mice lacking the K+ channel, TREK-1. Br J Pharmacol. 2007;152:939–945. doi: 10.1038/sj.bjp.0707450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldmann R, et al. H+-gated cation channels. Ann N Y Acad Sci. 1999;868:67–76. doi: 10.1111/j.1749-6632.1999.tb11274.x. [DOI] [PubMed] [Google Scholar]
- 27.Steidl JV, Yool AJ. Differential sensitivity of voltage-gated potassium channels Kv1.5 and Kv1.2 to acidic pH and molecular identification of pH sensor. Mol Pharmacol. 1999;55:812–820. [PubMed] [Google Scholar]
- 28.Coulter KL, Perier F, Radeke CM, Vandenberg CA. Identification and molecular localization of a pH-sensing domain for the inward rectifier potassium channel HIR. Neuron. 1995;15:1157–1168. doi: 10.1016/0896-6273(95)90103-5. [DOI] [PubMed] [Google Scholar]
- 29.Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci. 2008;29:566–575. doi: 10.1016/j.tips.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duprat F, et al. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes CM, Zilberberg N, Goldstein SA. Block of Kcnk3 by protons. Evidence that 2-P-domain potassium channel subunits function as homodimers. J Biol Chem. 2001;276:24449–24452. doi: 10.1074/jbc.C100184200. [DOI] [PubMed] [Google Scholar]
- 32.Rajan S, et al. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J Biol Chem. 2000;275:16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- 33.Reyes R, et al. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J Biol Chem. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- 34.Girard C, et al. Genomic and functional characteristics of novel human pancreatic 2P domain K+ channels. Biochem Biophys Res Commun. 2001;282:249–256. doi: 10.1006/bbrc.2001.4562. [DOI] [PubMed] [Google Scholar]
- 35.Morton MJ, Abohamed A, Sivaprasadarao A, Hunter M. pH sensing in the two-pore domain K+ channel, TASK2. Proc Natl Acad Sci USA. 2005;102:16102–16106. doi: 10.1073/pnas.0506870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niemeyer MI, et al. Neutralization of a single arginine residue gates open a two-pore domain, alkali-activated K+ channel. Proc Natl Acad Sci USA. 2007;104:666–671. doi: 10.1073/pnas.0606173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yellen G. The voltage-gated potassium channels and their relatives. Nature. 2002;419:35–42. doi: 10.1038/nature00978. [DOI] [PubMed] [Google Scholar]
- 38.Yon J, Fried M. Precise gene fusion by PCR. Nucleic Acids Res. 1989;17:4895. doi: 10.1093/nar/17.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett. 1993;318:95–99. doi: 10.1016/0014-5793(93)81336-x. [DOI] [PubMed] [Google Scholar]
- 40.Thompson JD, Higgins DG, Gibson TJ. Improved sensitivity of profile searches through the use of sequence weights and gap excision. Comput Appl Biosci. 1994;10:19–29. doi: 10.1093/bioinformatics/10.1.19. [DOI] [PubMed] [Google Scholar]
- 41.Sali A, Blundell TL. Comparative protein modeling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 42.Wallner B, Elofsson A. Can correct protein models be identified? Protein Sci. 2003;12:1073–1086. doi: 10.1110/ps.0236803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.