Abstract
Plants accumulate free L-proline (Pro) in response to abiotic stresses (drought and salinity) and presence of bacterial pathogens, including the tumor-inducing bacterium Agrobacterium tumefaciens. However, the function of Pro accumulation in host-pathogen interaction is still unclear. Here, we demonstrated that Pro antagonizes plant GABA-defense in the A. tumefaciens C58-induced tumor by interfering with the import of GABA and consequently the GABA-induced degradation of the bacterial quorum-sensing signal, 3-oxo-octanoylhomoserine lactone. We identified a bacterial receptor Atu2422, which is implicated in the uptake of GABA and Pro, suggesting that Pro acts as a natural antagonist of GABA-signaling. The Atu2422 amino acid sequence contains a Venus flytrap domain that is required for trapping GABA in human GABAB receptors. A constructed atu2422 mutant was more virulent than the wild type bacterium; moreover, transgenic plants with a low level of Pro exhibited less severe tumor symptoms than did their wild-type parents, revealing a crucial role for Venus flytrap GABA-receptor and relative abundance of GABA and Pro in host-pathogen interaction.
Keywords: plant defense, virulence, plant tumor, lactonase
The bacterial pathogen Agrobacterium tumefaciens genetically engineers the plant host by transferring a piece of DNA (the T-DNA) from its tumor-inducing (Ti) plasmid to the nuclear genome of plants. Proliferation of the transformed plant cells results in formation of a tumor (crown gall disease), which is colonized by the pathogen (a review in ref. 1). In addition, expression of the T-DNA redirects the metabolism of plant cells toward the production of compounds called opines, which are used by the pathogen as nutrients or signals to control the expression of some virulence functions. In bacterial cells, the opines stimulate the synthesis of a quorum-sensing (QS) signal, 3-oxo-octanoylhomoserine lactone (OC8HSL) (2), which in turn activates the horizontal transfer and amplification of the copy number of the Ti plasmid (3), thereby increasing the aggressiveness of A. tumefaciens populations and contributing to the dissemination of the Ti plasmid.
In response to A. tumefaciens infection, plants activate a complex program of defense (4–7), which includes the synthesis of γ-aminobutyric acid (GABA). In A. tumefaciens, GABA promotes degradation of the OC8HSL signal by the lactonase AttM, thereby attenuating expression of the OC8HSL-dependent functions (8, 9). Paradoxically, tumors simultaneously accumulate opines and GABA which induce two opposite functions in A. tumefaciens that are the synthesis and degradation of the OC8HSL signal, respectively. Because the plant tumor is a unique ecological niche in which A. tumefaciens expresses OC8HSL-dependent functions including horizontal transfer of the Ti plasmid (2), it can be hypothesized that the bacterial pathogen has evolved mechanisms to escape plant GABA-defense.
Here, we report that Pro antagonizes the GABA-induced degradation of OC8HSL. Therefore Pro, which accumulates in the tumor (10), could be used by the pathogen to by-pass the plant GABA-defense. Moreover, we demonstrate that the mechanism for perception of GABA and Pro involves a bacterial receptor containing a Venus flytrap domain which is also required for GABA sensing by the human GABAB-receptor (11). This research highlights that the anti-GABA paradigm, which permits the development of medicines against neurologic diseases, is already exploited by a tumor-inducing pathogen to escape host GABA-defense.
Results
Free Proline and Valine Antagonize the AttM-Mediated Degradation of OC8HSL in the Presence of GABA.
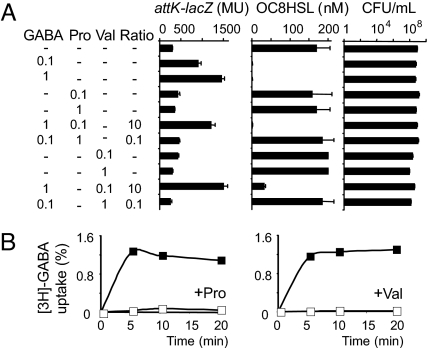
We screened amino acids and organic acids (1 mM) for their capacity to reduce expression of an attK-lacZ fusion which harbors the promoter for the attKLM operon of A. tumefaciens C58 (12), in the presence of GABA (0.1 mM). Among the molecules tested, that include opines, a 10-fold excess of proline (Pro) and valine (Val) was sufficient to antagonize the GABA-dependent expression of the AttM lactonase, as well as the degradation of the OC8HSL signal (Fig. 1A). Under those culture conditions, the addition of Pro or Val (1 mM) did not significantly affect bacterial cell density (Fig. 1A). Because the import of GABA is required for expression of attM (9), the direct effect of Pro or Val was investigated. Both Pro and Val abolished transport of GABA in A. tumefaciens C58 cells (Fig. 1B).
Fig. 1.
Effect of proline and valine on the GABA-dependent expression of AttM in A. tumefaciens. (A) Expression of attK-lacZ fusion, OC8HSL concentration (added at 200 nM at the beginning of the culture) and bacterial densities (CFU/mL) in cultures of A. tumefaciens C58 supplemented with GABA, Pro, and/or Val. (B) Uptake of radiolabeled GABA (% of total available) in A. tumefaciens C58 cells in the absence (closed symbol) or presence (open symbol) of Pro (left graph) or Val (right graph) antagonists (1 mM).
Variation of GABA/Pro Ratio in Plant Hosts.
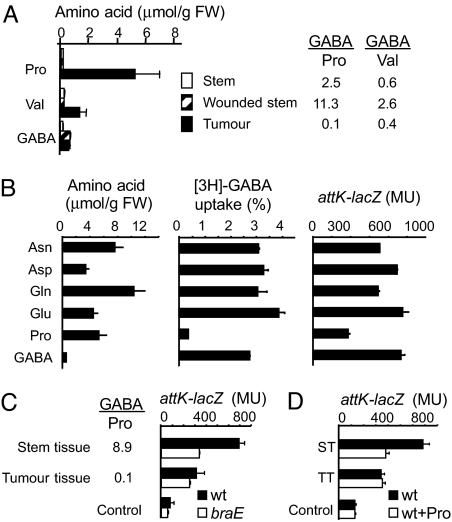
Wounding, which is a prerequisite event for the emergence of a tumor, had no effect on Val and Pro concentrations, but stimulated the accumulation of GABA (Fig. 2A). Tumor tissues also accumulated GABA as a part of the plant response to bacterial infection (7, 9). High GABA/Val ratios in healthy (0.6), wounded (2.6) and tumor (0.4) tissues disqualified Val as a bona fide anti-GABA compound in planta. In contrast, the GABA/Pro ratio in tumor tissues was 0.1, a value similar to that permitting effective anti-GABA activity in vitro (Fig. 1A). Asn, Asp, Gln, Glu, and Pro were the most abundant amino acids in the plant tumor (Fig. 2B); together, they represented 84% of the total free amino acids. None but Pro significantly affected the uptake of GABA, nor the expression of the GABA-regulated operon attKLM (Fig. 2B). Pro, which accumulates in plant tumor (Fig. 2A), would therefore be the most probable natural antagonist of GABA-signaling in the bacterial pathogen A. tumefaciens C58. To test this hypothesis, expression of the GABA-induced operon attKLM was compared in the presence of stem or tumor tissues, which release GABA (up to 0.08 mM) and Pro (up to 0.84 mM) at different ratios in the culture medium. The results indicate that high expression of the attK-lacZ fusion in the wild-type A. tumefaciens C58 was correlated with a high GABA/Pro ratio (Fig. 2C). Moreover, in the presence of stem tissues, attK-lacZ expression was reduced by addition of Pro (Fig. 2D) and in the braE mutant (Fig. 2C), which is defective for the main route of GABA uptake (9).
Fig. 2.
Variation of GABA/Pro ratio in plant host. (A) level of GABA, Pro and Val in stem and A. tumefaciens C58-induced tumor of tomato plant. (B) Level of the most abundant amino acids in the same plant tumors, as well as their effect (at 1 mM) on uptake of radiolabeled GABA (% of total available) and expression of attK-lacZ fusion (β-gal activity in Miller units) in the presence of GABA (0.1 mM). (C) Ratio of GABA and Pro released in liquid medium by stem and tumor slices of tomato (0.25 g/mL) after 20 h. (C and D) Expression of the attK-lacZ transcriptional fusion (β-gal activity in Miller units) in cultures of A. tumefaciens C58 and its braE derivative after 20 h of growth in AB-medium supplemented with slices of stem (ST) and tumor tissues (TT) of tomato plant (C), and in the absence or presence of additional Pro at 5 mM (D).
The Receptor Atu2422 Is Required for Import of GABA, and Belongs to Venus Flytrap Family.
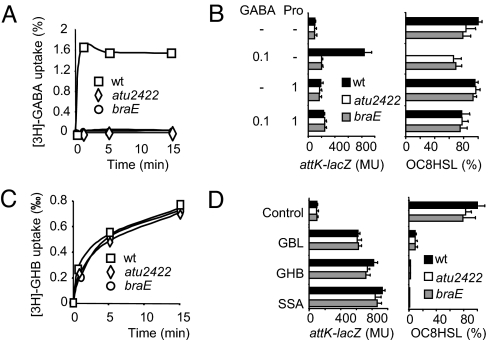
The ABC-transporter Bra belongs to a subfamily of ABC-transporters that requires a periplasmic solute binding protein (SBP) for trapping and accompanying the transported compounds to the channel (13). A mutation in the SBP-encoding gene atu2422, which is located downstream of the braDEFG operon, was constructed. Import of GABA (Fig. 3A), growth in the presence of GABA as a sole nitrogen source, GABA-dependent expression of lactonase AttM and degradation of the OC8HSL signal (Fig. 3B) were abolished in the atu2422 and braE mutants. By contrast, those phenotypes were not affected in A. tumefaciens mutants defective for other putative SBPs, namely Atu4519, Atu5531, and Atu2276, which have high amino acid sequence similarity to Atu2422 (from 56% to 79%). The receptor Atu2422 is therefore strictly required for GABA signaling pathway in A. tumefaciens C58. Atu2422 has significant sequence identity (40%) with SBPs such as the Leu-Binding Protein and the Leu-Ile-Val-Binding Protein of Escherichia coli. Their three-dimensional structure (14, 15) revealed a bilobate domain that is called the Venus flytrap by analogy with the carnivorous plants. Common residues involved in ligand binding by bacterial Venus flytrap domain (16) are conserved in Atu2422. Moreover, our preliminary X-ray data analysis of Atu2422 protein confirmed a Venus flytrap fold (17).
Fig. 3.
The Venus-Flytrap receptor Atu2422 is required for GABA-signaling pathway. (A and C) Uptake of radiolabeled GABA and GHB (% of total available) in cultures of A. tumefaciens C58, and its mutants atu2422 and braE. (B and D) Expression of the attK-lacZ fusion (β-gal activity in Miller units) and OC8HSL concentration (added at 200 nM) in cultures of A. tumefaciens C58 wild type, atu2422 and braE mutants, grown in AB-medium supplemented with GABA, GBL, GHB, SSA (0.1 mM), and/or Pro (1 mM). Experiments were done in triplicate.
By-Products of GABA Bypass Atu2422 for Stimulating Expression of attKLM Operon.
GABA may be converted by plant enzymes into γ-hydroxybutyrate (GHB) with succinic semialdehyde (SSA) as a toxic intermediate (18, 19). A nonenzymatic conversion of GHB into γ-butyrolactone (GBL) may occur at acidic pH. We observed that import of GHB (Fig. 3C) and expression of lactonase AttM in the presence of SSA, GHB, and GBL (Fig. 3D) was similar in the wild-type strain and the atu2422 and braE mutants, suggesting these compounds use other carrier(s) to enter into the bacterial cells. These alternative pathways may explain residual expression of the attKLM operon in the braE mutant when challenged with tumor and stem tissues (Fig. 2C). Moreover, addition of Pro did not affect expression of attKLM operon in the presence of by-products of GABA suggesting that Pro would specifically act on GABA-signaling pathway.
The Receptor Atu2422 and Transporter Bra Contribute to the Import of Pro.
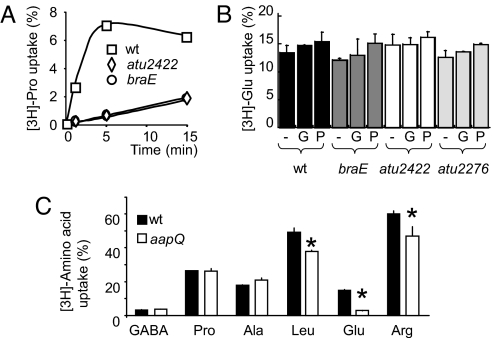
Transport of Pro is affected in the atu2422 and braE mutants (Fig. 4A), revealing that the Atu2422/Bra system may transport both GABA and Pro. Pro would therefore act as a competitive antagonist of GABA via the Atu2422/Bra system. The Bra ortholog in Rhizobium leguminosarum strain 3841 is required for uptake of a broad spectrum of amino acids including GABA, Ala, Arg, Glu, and Leu (13). In contrast, transport of Glu (Fig. 4B), Ala, Arg, or Leu was not affected in braE, atu2422 (ortholog of rl3745), and atu2276 (ortholog of rl3540) mutants of A. tumefaciens C58. Moreover, the addition of GABA and Pro at a 100-fold excess had no effect on transport of Glu (Fig. 4B), Ala, Arg, or Leu, suggesting that A. tumefaciens expresses other dedicated transporters for these amino acids. To test this hypothesis we constructed a derivative of A. tumefaciens C58 in which the operon encoding the general amino acid ABC-transporter Aap (13) is mutated. The aap mutant retained only 20% of the capacity for Glu uptake in the wild type (Fig. 4C), and 75% of that for Arg and Leu, whereas GABA, Pro and Ala uptake was unaffected. Thus, the ABC-transporter Aap of A. tumefaicens C58 is the major transporter of Glu, which is the main precursor of GABA in plants.
Fig. 4.
The receptor Atu2422 is involved in transport of Pro. Uptake (% of total available) of radiolabeled compounds, Pro (A), Glu (B), GABA, Pro, Ala, Leu, Glu, and Arg (C), in cells of A. tumefaciens C58, and its derivatives atu2422, atu2276, braE, and aapQ. In B, uptake of Glu was measured in the absence (-) or presence of GABA (G) or Pro (P) at 1 mM. In C, asterisk indicates a significant decrease in amino acid uptake by mutant aapQ (Student's t test; P < 0.01).
Involvement of the GABA-VFT-Receptor Atu2422 in Virulence.
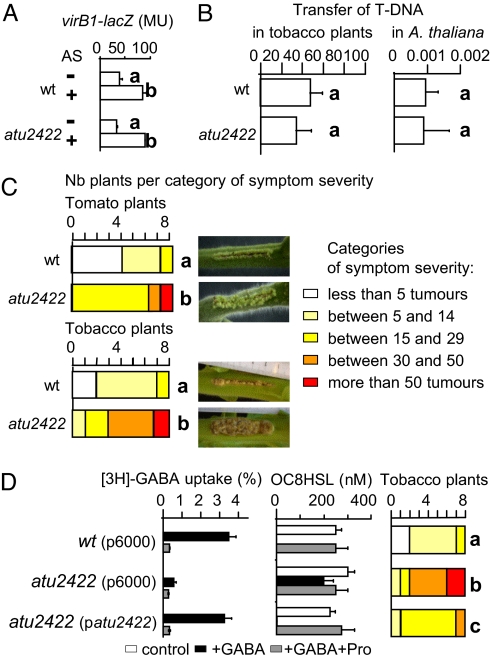
The mutation atu2422 affected neither the expression of virB-lacZ fusion in the presence of acetosyringone (Fig. 5A), nor the transfer of T-DNA in tomato and tobacco plants, nor T-DNA integration in A. thaliana under the conditions tested (Fig. 5B). In contrast, a higher number of emerging tumors was observed in incisions infected by the atu2422 mutant than in that infected by wild type strain C58 (Fig. 5C). In a complemented atu2422 mutant, the transport of GABA, the GABA-induced degradation of OC8HSL and the antagonist activity of Pro were restored (Fig. 5D). Moreover, a decrease of plant symptoms was observed in the complemented mutant compared with the atu2422 mutant harboring the empty vector pME6000 (Fig. 5D), suggesting that the assimilation of GABA and/or GABA-regulated functions can modulate the emergence of tumors.
Fig. 5.
Involvement of Atu2422 in virulence. (A) Expression of the virB1-lacZ fusion (β-gal activity in Miller units) in the presence (+) and absence (-) of acetosyringone (AS) at 100 μM. (B) T-DNA transfer in tobacco plant 2-dpi (GUS-expressing tissue in % of surface of disks collected from infiltrated leaves) and A. thaliana (number of transformed seeds/total number of infected seeds) infected by A. tumefaciens C58 and its atu2422 derivative harbouring pCAMBIA3301. (C and D) A. tumefaciens C58 and atu2422 mutant (C) harboring vector pME6000 or a pME6000-derivative expressing atu2422 gene (D) were used for measuring virulence symptoms (number of emerging tumors) on infected plant hosts. Bacterial genotypes showing statistical differences (Student or Kruskal-wallis test; P < 0.01) in their aggressiveness categories are noted by different letters. (D) Uptake of rabiolabeled GABA in the presence or absence of Pro (1 mM), and OC8HSL concentration in presence or absence of GABA (0.1 mM) and Pro (1 mM).
Involvement of GABA-Regulated Genes in Virulence.
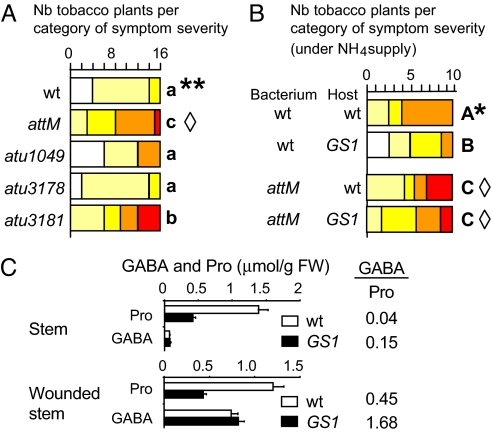
We investigated the involvement in virulence of GABA-regulated genes previously identified as up-regulated (atu5139 = attM) and down-regulated (atu3181, atu1049, and atu3178) by comparative transcriptomics (20). Appropriate mutants were used for performing the infection assay on tobacco plants. We previously reported (9) that fresh weight of the tumors induced by A. tumefaciens C58 wild-type or mutant attM on tobacco plants was similar (0.7 ± 0.4 g vs. 0.7 ± 0.3 g, respectively). In this article, the method for measuring virulence symptoms on host plant was refined (i.e., measurement of the number of emerging tumors instead of the weight of all tumors at infection site) according to a procedure published by Pappas and Winans (3). The refined method was used in all virulence assays shown in Figs. 5 and 6. Among the tested mutants attM, atu3181, atu1049, and atu3178, only attM and atu3181 derivatives were more virulent than the wild-type strain (Fig. 6A), exemplifying the involvement of GABA-regulated functions in the control of tumor emergence. This phenotype observed on tobacco plants was confirmed on tomato plants.
Fig. 6.
Involvement of GABA-regulated genes in virulence. (A) Aggressiveness of A. tumefaciens C58 and its derivatives attM, atu1049, atu3178, and atu3181 derivatives on tobacco plants. (B) aggressiveness of A. tumefaciens C58 and attM mutant on wild-type and GS1 line of tobacco plants supplied with NH4. Categories of symptom severity are the same as in Fig. 5. Bacterial genotypes showing statistical differences (Kruskal-wallis test; P < 0.01) in their aggressiveness categories are noted by different letters (a–c, A–C). In A and B, asterisks indicate a statistical difference in the severity of symptoms caused by A. tumefaciens C58 on wild-type tobacco plants supplied with water or NH4 solution; diamonds indicate no statistical difference when symptoms were caused by the attM mutant on wild-type and GS1 tobacco plants supplied with water or NH4 solution. All data were collected from two independent replicates. (C) level of GABA and Pro in wild type and GS1 line of tobacco plants under NH4 supply.
The Emergence of Tumors Is Attenuated in a Tobacco Transgenic Line with Reduced Pro Accumulation.
In all of the above plant assays (Figs. 5 and 6A), the plants were grown on compost and were automatically supplied with water. According to a previous report (21), the accumulation of free Pro in stem tissues could be stimulated by growing plants on sand with an ammonium supply (5 mM). Under this growth condition, a 10-fold increase of Pro in the tobacco stem (0.16 vs. 1.5 μmol/g FW) was observed. However, because cytosolic glutamine synthetase GS1 is required for Pro accumulation in stem (21), the level of Pro was reduced in an antisense GS1 tobacco line (Fig. 6C). Using both physiologic (with or without ammonium) and genetic (wild-type vs. GS1 lines) approaches, we were able to modulate Pro level (therefore GABA/Pro ratio) in tobacco plants and compared their virulence symptoms induced by A. tumefaciens.
Symptoms induced by A. tumefaciens C58 wild type were more severe (columns with asterisks in Fig. 6 A and B) on tobacco plants with high (with NH4 supply) levels of Pro than on those with a low level. Moreover, wild-type A. tumefaciens C58 induced fewer symptoms on the GS1 line than on the wild-type line (columns with letters A and B in Fig. 6B), suggesting the involvement of Pro level in tumor emergence. Because lactonase AttM is regulated by GABA/Pro ratio (Fig. 1A) and appears to contribute to virulence symptoms (Fig. 6A), we hypothesized the involvement of AttM in the Pro-dependent variation of the observed plant symptoms. This hypothesis predicts that varying the Pro concentration in the plant host should not affect virulence symptoms caused by an attM mutant. Comparable symptoms were indeed observed on wild type plants (with or without ammonium) and GS1 derivatives (columns with a diamond in Fig. 6 A and B) inoculated by a attM mutant, revealing the involvement of lactonase AttM in the modulation of plant symptoms under ammonium supply.
Discussion
This study reveals that GABA and Pro play opposite roles in the virulence of A. tumefaciens C58. A high GABA concentration in the plant host decreases the number of emerging tumors (9), whereas a high Pro concentration enhances tumor emergence (Fig. 6). Pro level is directly correlated to the nature of the nitrogen source assimilated by the plants (21). Therefore, an immediate agronomic implication of this work is that ammonium-containing nutrient solutions, which stimulate a decrease of the GABA/Pro ratio in the host plants, could increase their sensitivity to A. tumefaciens C58. However, Pro is proposed to participate in the defense response of the host plant challenged by bacterial pathogens: accumulation of Pro is induced by reactive oxygen species, depends upon the plant signal salicylic acid, and contributes to the hypersensitive response and cell death of infected tissues (22, 23). In particular, Pro steadily accumulates in the A. tumefaciens-induced plant tumor (7, 10), wherein it also plays a role in the protection of plant tissues from desiccation (10). One could not exclude a direct contribution of the pathogen to Pro accumulation in the plant tumor, because the Ti plasmid of A. tumefaciens C58 encodes an ornithine cyclodeaminase that releases Pro from ornithine, a by-product of the most abundant opine, nopaline (24). Other Ti plasmids encode ornithine cyclodeaminase and proline formation (25). Remarkably, in A. rhizogenes, the ornithine cyclodeaminase encoding gene, rolD, is localized in the T-DNA (26); this oncogene contributes to virulence of the pathogen by an unknown mechanism (27). Through manipulation of Pro synthesis or utilization of the stress-induced accumulation of Pro, A. tumefaciens, and A. rhizogenes pathogens should be able to by-pass a part of the plant defense response and optimize the expression of their virulence factors.
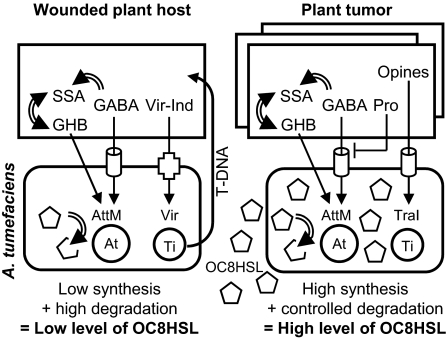
The mechanism by which Pro antagonizes GABA-signaling in A. tumefaciens C58 was discovered. Pro acts as a competitive antagonist of GABA import by the Atu2422-Bra ABC-transporter (Figs. 1, 3, and 4), thereby affecting the expression of GABA-regulated functions, such as the degradation of QS-signal OC8HSL by the lactonase AttM (Figs. 1 and 2). In A. tumefaciens C58, QS controls horizontal transfer (by conjugation) and copy number of Ti plasmid. An increase in the number of emerging tumors is correlated with overaccumulation of the QS-signal OC8HSL and amplification of Ti-plasmid copy-number (3, 9, 28), suggesting that severity of plant symptoms may in part be modulated by QS. Because GABA-receptor Atu2422 plays a critical role in the expression of the lactonase AttM, its implication in the A. tumefaciens-plant host interaction was investigated using several virulence assays. The expression of vir genes and the transfer of T-DNA to different hosts was not affected in an atu2422 mutant (Fig. 5 A and B), confirming that the GABA-signaling pathway and vir-signaling pathway are independent (9). However, the bacterial GABA receptor (Fig. 5 C and D) and consequently GABA-regulated functions, including AttM-mediated degradation of the QS signal (Fig. 6 A and B), play a role in the emergence of plant tumors. The mechanism by which QS may modulate plant symptoms remains to be investigated. Furthermore, this work offers one clue for solving the paradoxical capability of A. tumefaciens C58 to transfer its Ti plasmid in a QS-dependent manner even in the presence of the GABA-up-regulated lactonase AttM (28, 29). Indeed, the effect of AttM on the QS-dependent conjugation of Ti plasmid was observed in young tumors with low level of Pro, but the role of AttM was weak in old tumors with elevated concentration of Pro. A model summarizing the role of plant signals in regulation of the QS-pathway is proposed (Fig. 7).
Fig. 7.
Control of the AttM-mediated inactivation of QS-signal in A. tumefaciens C58. During infection (left part), phenolics (Vir-Ind) activate the expression of virulence functions (Vir) via the VirA/G two-component system, thereby permitting the transfer of T-DNA from the Ti plasmid of A. tumefaciens to the plant host. Concomitantly, plant GABA is imported via the Atu2422/Bra system, thereby stimulating the expression of lactonase AttM (encoded by At plasmid) and allowing inactivation of the QS-signal (OC8HSL). In the tumor (right part), opines stimulate the synthesis of OC8HSL by TraI, whereas Pro moderates the GABA-dependent expression of the lactonase AttM, thereby permitting expression of QS-regulated functions, including the transfer of Ti plasmid by conjugation. Residual expression of AttM is probable in the presence of signal salicylic acid (4) and GABA-derivatives such as GHB (12), and in young tumor when Pro level is weak (10). Opine-dependent regulation of the OC8HSL-lactonase AiiB (28) is not shown. Simple and stopped arrows represent regulatory pathways, whereas double-line arrows, cylinders, cross, and pentagons represent enzymatic reactions, bacterial ABC-transporters, VirA/G system, and OC8HSL, respectively.
The GABA-receptor Atu2422 of A. tumefaciens C58 exhibits a Venus Flytrap fold (17), which is also essential for perception of GABA by other bacteria, such as R. leguminosarum (13), and by several eukaryotic receptors, including GABAB in human brain and GrlE in the amoeba Dictyostelium discoideum (11, 30). Thus, the Venus Flytrap domain represents a common fold for sensing exogenous GABA in both bacteria and eukaryotes. In eukaryotic GABA-VFT receptors, sensitivity to a strict or wide range of amino acids results in crucial consequences for their biological role in vivo (11, 30). In A. tumefaciens C58, the selectivity of the Atu2422-Bra system (Fig. 4) could reveal an evolutionary innovation implicating Pro as a natural antagonist of GABA to modulate the QS-pathway and escape plant GABA-defense in the plant tumor.
Methods
Bacterial Strains and Culture Conditions.
The A. tumefaciens strains used in this work were derivatives of A. tumefaciens C58. The mutants atu1049::acc1, atu2422::acc1, atu2276::aphA, atu3178::acc1, atu3181::aphA, atu4519::aphA, atu5531::acc1, aapQ::acc1, and braE::aphA were constructed by inserting the Gm-cassette (acc1) or Km-cassette (aphA) within the amplified genes (31). The mutated alleles were introduced into Agrobacterium by electroporation as previously described (9) and verified by PCR and sequencing. A. tumefaciens C58 and its derivatives were cultivated at 30°C in Agrobacterium broth (AB) medium (32) in the presence of mannitol and ammonium chloride, except where alternative carbon and nitrogen sources are indicated.
β-Galactosidase Activity, OC8HSL, and Amino Acid Measurements.
Using the strategy described previously (12), the virB1 promoter was amplified, fused with the reporter gene lacZ, and subcloned into the broad host-range vector pME6031. The attK-lacZ (12) and virB1-lacZ fusions were introduced into A. tumefaciens strains by electroporation. Induction with acetosyringone (100 μM) was performed in IB medium (33). The β-galactosidase activity of cells was measured as previously described (12). OC8HSL detection was performed on TLC silica plates (C18-reverse phase, Whatman) using pure OC8HSL (a generous gift from Pr. P. Williams, University of Notthingham) as a standard, and the biosensor A. tumefaciens NT1(pZLR4) (34). The composition of individual amino acids (including GABA) in unwounded and wounded stems of tomato and tobacco plants, and plant tumors was determined by ion-exchange chromatography using an AminoTac JLC-500V amino acid analyzer (35). Wounded tissues were frozen 10 min after cutting 0.1-cm sections from plant stems (9). The amino acids released into the liquid medium by slices of tomato stem and tumor (0.25 g/mL) were also determined using the AminoTac JLC-500V amino acid analyzer.
Uptake Assays.
GABA uptake assays were performed on cultures of A. tumefaciens grown in AB minimal medium. Reactions were initiated by adding [3H]-GABA (γ-[2,3-3H(N)]-ABA to cell suspensions (5. 107 CFU/mL) at a final concentration of 1 μM in the presence of unlabelled amino acid at different concentrations. The reaction was terminated by rapid filtration of samples (50 μL) on ultrafiltration membrane (0.22 μm Millipore HA, Millipore), and filter washing three times with 4 mL of AB-medium (13). Filter-bound radioactivity was quantified in a Packard TriCarb 2200 CA liquid scintillation counter. L- [2,3-3H]-Pro, L-[3-3H]-Ala, L-[2,3,4-3H]-Arg, [3,4-3H]-Glu, [4,5-3H(N)]-Leu, and [2,3-3H]-GHB uptake assays were performed using the same protocol. The 3H-derivatives of GABA (94 Ci/mmol), Pro (49 Ci/mmol), Ala (65 Ci/mmol), Arg (51 Ci/mmol), Glu (50 Ci/mmol), and Leu (56 Ci/mmol) were provided by Perkin-Elmer, and GHB (50 Ci/mmol) by American Radiolabeled Chemicals (USA).
Plant Assays.
Transfer of T-DNA was performed using the floral dip method (36) with A. thaliana (n = 48) as plant host and plasmid pCAMBIA3301 as T-DNA donor conferring Basta resistance. The same plasmid pCAMBIA3301 carrying the 35S::GUS-intron transgene was used for evaluating transient expression of T-DNA in infiltrated leaves of 10-week-old tobacco plants (Nicotiana tabacum L. cv. Samson) and infected stem of 8-week-old tomato plants (Solanum lycopersicum L. cv. Dona). Before infection, bacterial cells were incubated in MES buffer (pH 5,6) supplemented with acetosyringone (150 μM) according to ref. 5. Then, 2, 4, and 6 days post-infection, GUS staining was performed (37) on sections of tomato stem and disks (1 cm in diameter) excised from tobacco leaves. We measured the number of colored spots per section of tomato stem (n = 15), and percentage of GUS-colored surface on each tobacco leaf disk (n = 10). Other virulence assays were conducted under greenhouse conditions (9) on stems of 10-week-old tobacco plants (N. tabacum L. cv. Samson) and 6-week-old tomato plants (S. lycopersicum L. cv. Dona) grown on compost and automatically supplied with water three times per day, as well as on stems of 12-week-old tobacco plants (cv. Xanthi) and its GS1 derivative grown on sand and automatically supplied with an ammonium-rich nutrient solution (5 mM NH4Cl and 1 mM KH2PO4, pH 6.5) three times daily. Under these conditions, GS1 tobacco plants exhibit lower levels of GS1 mRNA, glutamine synthetase activity, and Pro than the wild-type parent (21). Each stem was incised (4 cm) with a scalpel and infected by approximately 106 CFU of A. tumefaciens C58 or its derivatives that were picked up from 48-h cultures on LBm-agar plates. The number of tumors per incision was counted 5 weeks (tobacco) or 3 weeks (tomato) post inoculation. Each plant was categorized into five classes of severity symptoms: less than five tumors per infection site; from five to 14, 15 to 29, 30 to 50, or more than 50. Each virulence assay was performed in at least two independent experiments.
Acknowledgments.
We thank A. Raffoux and T. Huchet for technical assistance; S. Mondy for GUS protocols; Y. Dessaux (Centre National de la Recherche Scientifique, Gif-sur-Yvette) for critical reviewing of the manuscript; and S. Boutet and B. Hirel (Institut National de la Recherche Agronomique-Versailles) for amino acid measurements and the GS1 line. Financial support was provided from Centre National de la Recherche Scientifique-Projet International de Coopération Scientifique “GABA signaling across kingdoms” (D.F.) and the Natural Science and Engineering Research Council of Canada (B.J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Escobar MA, Dandekar AM. Agrobacterium tumefaciens as an agent of disease. Trends Plants Sci. 2003;8:380–386. doi: 10.1016/S1360-1385(03)00162-6. [DOI] [PubMed] [Google Scholar]
- 2.White CE, Winans SK. Cell-cell communication in the plant pathogen Agrobacterium tumefaciens. Philos Trans R Soc Lond B Biol Sci. 2007;362:1135–1148. doi: 10.1098/rstb.2007.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pappas KM, Winans SC. A LuxR-type regulator from Agrobacterium tumefaciens elevates Ti plasmid copy number by activating transcription of plasmid replication genes. Mol Microbiol. 2003;48:1059–1073. doi: 10.1046/j.1365-2958.2003.03488.x. [DOI] [PubMed] [Google Scholar]
- 4.Yuan ZC, et al. The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching genes in Agrobacterium. Proc Natl Acad Sci USA. 2007;104:11790–11795. doi: 10.1073/pnas.0704866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zipfel C, et al. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 6.Djamei A, Pitzschke A, Nakagami H, Rajh I, Hirt H. Trojan horse strategy in Agrobacterium transformation: Abusing MAPK defense signaling. Science. 2007;318:453–456. doi: 10.1126/science.1148110. [DOI] [PubMed] [Google Scholar]
- 7.Deeken R, et al. An integrated view of gene expression and solute profiles of Arabidopsis tumors: A genome-wide approach. Plant Cell. 2006;18:3617–3634. doi: 10.1105/tpc.106.044743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang HB, Wang LH, Zhang LH. Genetic control of quorum-sensing signal turnover in Agrobacterium tumefaciens. Proc Natl Acad Sci USA. 2002;99:4638–4643. doi: 10.1073/pnas.022056699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevrot R, et al. GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc Natl Acad Sci USA. 2006;103:7201–7202. doi: 10.1073/pnas.0600313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wächter R, et al. Vascularization, high-volume solution flow, and localized roles for enzymes of sucrose metabolism during tumorigenesis by Agrobacterium tumefaciens. Plant Physiol. 2003;133:1024–1037. doi: 10.1104/pp.103.028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galvez T, et al. Mutagenesis and modeling of the GABAB receptor extracellular domain support a Venus flytrap mechanism for ligand binding. J Biol Chem. 1999;274:13362–13369. doi: 10.1074/jbc.274.19.13362. [DOI] [PubMed] [Google Scholar]
- 12.Carlier A, Chevrot R, Dessaux Y, Faure D. In Agrobacterium tumefaciens strain C58, the assimilation of γ-butyrolactone interferes with the accumulation of the N-acyl-homoserine lactone signal. Mol Plant-Microbe Interact. 2004;17:951–957. doi: 10.1094/MPMI.2004.17.9.951. [DOI] [PubMed] [Google Scholar]
- 13.Hosie AH, Allaway D, Galloway CS, Dunsby HA, Poole PS. Rhizobium leguminosarum has a second general amino acid permease with unusually broad substrate specificity and high similarity to branched-chain amino acid transporters (Bra/LIV) of the ABC family. J Bacteriol. 2002;184:4071–4080. doi: 10.1128/JB.184.15.4071-4080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnusson U, Salopek-Sondi B, Luck LA, Mowbray SL. X-ray structures of the leucine-binding protein illustrate conformational changes and the basis of ligand specificity. J Biol Chem. 2004;279:8747–8752. doi: 10.1074/jbc.M311890200. [DOI] [PubMed] [Google Scholar]
- 15.Sack JS, Saper MA, Quiocho FA. Periplasmic binding protein structure and function. Refined X-ray structures of the leucine/isoleucine/valine-binding protein and its complex with leucine. J Mol Biol. 1989;206:171–191. doi: 10.1016/0022-2836(89)90531-7. [DOI] [PubMed] [Google Scholar]
- 16.Acher FC, Bertrand HO. Amino acid recognition by Venus flytrap domains is encoded in an 8-residue motif. Biopolymers. 2005;80:357–366. doi: 10.1002/bip.20229. [DOI] [PubMed] [Google Scholar]
- 17.Moréra S, Gueguen-Chaignon V, Raffoux A, Faure D. Cloning, purification, crystallization and preliminary X-ray analysis of a bacterial GABA receptor with a Venus flytrap fold. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008;64:1153–1155. doi: 10.1107/S1744309108036555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breitkreuz K, et al. A novel γ-hydroxybutyrate dehydrogenase. Identification and expression of an Arabidopsis cDNA and potential role under oxygen deficiency. J Biol Chem. 2003;278:41552–41556. doi: 10.1074/jbc.M305717200. [DOI] [PubMed] [Google Scholar]
- 19.Hoover GJ, et al. Characteristics of an Arabidopsis glyoxylate reductase: General biochemical properties and substrate specificity for the recombinant protein, and developmental expression and implications for glyoxylate and succinic semialdehyde metabolism in planta. Can J Bot. 2007;85:883–895. [Google Scholar]
- 20.Yuan ZC, Haudecoeur E, Faure D, Kerr KF, Nester EW. Comparative transcriptome analysis of Agrobacterium tumefaciens in response to plant signal salicylic acid, indole-3-acetic acid and gamma-amino butyric acid reveals signalling cross-talk and Agrobacterium–plant co-evolution. Cell Microbiol. 2008;10:2339–2354. doi: 10.1111/j.1462-5822.2008.01215.x. [DOI] [PubMed] [Google Scholar]
- 21.Brugière N, et al. Glutamine synthetase in the phloem plays a major role in controlling proline production. Plant Cell. 1999;11:1995–2011. doi: 10.1105/tpc.11.10.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deuschle K, et al. A nuclear gene encoding mitochondrial delta-pyrroline-5-carboxylate dehydrogenase and its potential role in protection from proline toxicity. Plant J. 2001;27:345–355. doi: 10.1046/j.1365-313x.2001.01101.x. [DOI] [PubMed] [Google Scholar]
- 23.Fabro G, Kovacs I, Pavet V, Szabados L, Alvarez MA. Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol Plant-Microbe Interact. 2004;17:343–350. doi: 10.1094/MPMI.2004.17.4.343. [DOI] [PubMed] [Google Scholar]
- 24.Sans N, Schindler U, Schröder J. Ornithine cyclodeaminase from Ti plasmid C58: DNA sequence, enzyme properties and regulation of activity by arginine. Eur J Biochem. 1988;173:123–130. doi: 10.1111/j.1432-1033.1988.tb13975.x. [DOI] [PubMed] [Google Scholar]
- 25.Farrand SK, Dessaux Y. Proline biosynthesis encoded by the noc and occ loci of Agrobacterium Ti plasmids. J Bacteriol. 1986;167:732–734. doi: 10.1128/jb.167.2.732-734.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trovato M, Maras B, Linhares F, Costantino P. The plant oncogene rolD encodes a functional ornithine cyclodeaminase. Proc Natl Acad Sci USA. 2001;98:13449–13453. doi: 10.1073/pnas.231320398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauro ML, et al. The plant oncogene rolD stimulates flowering in transgenic tobacco plants. Planta. 1996;215:494–501. doi: 10.1006/dbio.1996.0338. [DOI] [PubMed] [Google Scholar]
- 28.Haudecoeur E, et al. Different regulation and roles of lactonases AiiB and AttM in Agrobacterium tumefaciens C58. Mol Plant-Microbe Interact. 2009;22:529–537. doi: 10.1094/MPMI-22-5-0529. [DOI] [PubMed] [Google Scholar]
- 29.Khan SR, Farrand SK. The BlcC (AttM) Lactonase of Agrobacterium tumefaciens does not quench the quorum-sensing system that regulates Ti plasmid conjugative transfer. J Bacteriol. 2009;191:1320–1329. doi: 10.1128/JB.01304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anjard C, Loomis WF. GABA induces terminal differentiation of Dictyostelium through a GABAB receptor. Development. 2006;133:2253–2261. doi: 10.1242/dev.02399. [DOI] [PubMed] [Google Scholar]
- 31.Dennis J, Zylstra G. Plasposons: Modular self-cloning minitransposon derivatives for rapid genetic analysis of gram negative bacterial genomes. Appl Environ Microbiol. 1998;64:2710–2715. doi: 10.1128/aem.64.7.2710-2715.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chilton MD, et al. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci USA. 1974;71:3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winans SC, Kerstetter RA, Nester EW. Transcriptional regulation of the virA and virG genes of Agrobacterium tumefaciens. J Bacteriol. 1988;170:4047–4054. doi: 10.1128/jb.170.9.4047-4054.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cha C, Gao P, Chen YC, Shaw PD, Farrand SK. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol Plant-Microbe Interact. 1998;11:1119–1129. doi: 10.1094/MPMI.1998.11.11.1119. [DOI] [PubMed] [Google Scholar]
- 35.Diaz C, et al. Characterization of markers to determine the extent and variability of leaf senescence in Arabidopsis, a metabolic profiling approach. Plant Physiol. 2005;138:898–908. doi: 10.1104/pp.105.060764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clough SJ, Bent A. Floral dip: A simplified mehod for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 37.Jefferson RA, Kavanagh TA, Bevan MW. GUS fusion: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]