Abstract
PURPOSE
To determine which proton magnetic resonance (MR) spectroscopic imaging measures are best for lateralizing the seizure focus in patients who have temporal lobe epilepsy with and in those without hippocampal atrophy on MR images, the extent of contralateral abnormalities, and whether there is a correlation between MR spectroscopic imaging findings and surgical outcome.
MATERIALS AND METHODS
MR spectroscopic imaging was performed in 16 adult patients with temporal lobe epilepsy and unilateral electroencephalographic findings and in 16 adult control subjects. Eleven patients underwent surgery; all patients underwent MR imaging.
RESULTS
Nine patients had hippocampal atrophy on MR images. An ipsilateral decrease in the N-acetylaspartate concentration or the ratio of N-acetylaspartate to the sum of creatine and choline (N-acetylaspartate/[creatine + choline]) was found in all patients. Decreased contralateral N-acetylaspartate concentration, N-acetylaspartate/(creatine + choline), or N-acetylaspartate concentration and N-acetylaspartate/(creatine + choline) were detected in eight patients (50%), which suggests bilateral abnormalities not detected with MR imaging. In the five patients who underwent surgery and did not show hippocampal atrophy on MR images, successful and unsuccessful outcomes were correctly predicted with N-acetylaspartate concentration.
CONCLUSION
Decreased N-acetylaspartate concentration is not due solely to hippocampal atrophy. Contralateral abnormalities are much more frequent than expected. MR spectroscopic imaging is valuable in the presurgical evaluation of epilepsy.
Index terms: Brain, abnormalities, 1341.1214, 1341.12145, 13.83; Brain, atrophy, 1341.83; Brain, MR, 1341.1214, 1341.12145, 1341.83; Epilepsy, 1341.83; Magnetic resonance (MR), spectroscopy, 1341.12145
Electroencephalography (EEG) is the standard of reference for lateralizing the seizure focus in patients with intractable temporal lobe epilepsy. Patients with nonlesional temporal lobe epilepsy diagnosed with a unilateral focus at EEG and with hippocampal atrophy ipsilateral to the seizure focus at magnetic resonance (MR) imaging have an 86%–97% chance of being free of seizures after temporal lobectomy (1–3). However, in the absence of hippocampal atrophy on MR images, the prognosis for a seizure-free outcome is only about 50%.
Recently, this laboratory (4,5) and others (6–13) showed that proton MR spectroscopy (6,8,10,11) and MR spectroscopic imaging (4,5,7,9,12,13) can be used to lateralize the seizure focus in temporal and frontal lobe epilepsy. However, different MR spectroscopic measures were used in the different studies, and, in some cases, contradictory results were obtained. Several authors found ipsilateral decreases in the N-acetylaspartate signal intensity (4,7,8), the N-acetylaspartate-creatine ratio (5,6,9,13), the N-acetylaspartate-choline ratio (12), or the N-acetylaspartate-creatine and choline ratio (N-acetylaspartate/[creatine + choline]) (10,11). Although the hippocampus is the most important region in temporal lobe epilepsy, only Hugg and colleagues (4) and Hetherington and co-workers (13) evaluated spectra predominately from the hippocampus.
The purposes of this study were, therefore, not only to confirm and extend previous findings, but also to determine which MR spectroscopic imaging measures allow optimal lateralization of the seizure focus and which allow the best prediction of surgical outcome. The specific goals of this study were to determine the pattern of metabolite changes that provides the greatest concordance with EEG findings, to determine whether reduced N-acetylaspartate is due to hippocampal atrophy, to determine the extent to which contralateral abnormalities occur in patients with EEG-defined unilateral temporal lobe epilepsy, and to determine which measures best correlate with successful surgical outcome.
MATERIALS AND METHODS
Patients and Control Subjects
Sixteen patients (nine men, seven women; aged 21–49 years; mean age ± standard deviation, 35.9 years ± 8.9) with a unilateral temporal lobe seizure focus at EEG were examined. All patients were examined at the Northern California Comprehensive Epilepsy Center (San Francisco, Calif); none of the patients had clinical or EEC evidence suggestive of a second seizure focus. The seizure focus was localized by means of scalp (including sphenoidal) and, as necessary, subdural electrode recordings. Only patients whose ictal recordings demonstrated either localized voltage attenuation or rhythmic sharp activity that preceded the onset of the clinical seizure were included.
Eleven of the 16 patients underwent seizure surgery. They were followed up for 8–20 months, and their seizure outcome was defined by using the Engel classification (14). MR spectroscopic imaging data also were obtained in 16 healthy subjects (11 men, five women; aged 23–56 years; mean age, 33.3 years ± 7.9). Informed consent was obtained from all patients and control subjects before the examination.
Diagnostic MR Imaging
All patients separately underwent MR imaging. MR imaging examinations were performed with a 1.5-T system (GE Medical Systems, Milwaukee, Wis), and the following image data sets were acquired: an initial sagittal T1-weighted spin-echo data set (with a repetition time [TR] of 600 msec and a minimal echo time [TE]) through the hippocampus and temporal lobes; an axial T2-weighted spin-echo data set (with a TR of greater than 2,000 msec and TEs of 35 and 80 msec [> 2,000/35, 80]); a three-dimensional spoiled gradient-echo data set (with a TR of 50 msec and a minimal TE, a 40° flip angle, and a 1.5-mm section thickness); a coronal T2*-weighted gradient-echo data set (with 500/15–34 and a 20° flip angle); and a coronal T2-weighted fast spin-echo data set (with a 512 × 512 matrix).
MR images were read by a neuroradiologist blinded to the seizure lateralization findings. Nine patients had unilateral hippocampal atrophy; four also had a unilateral focus of increased T2 signal intensity within the hippocampus. No abnormalities were found on the MR images obtained in the other seven patients. This high percentage of patients without hippocampal atrophy does not reflect the statistical distribution of atrophy in patients with intractable temporal lobe epilepsy in the general population.
MR Spectroscopic Imaging
In all cases, the proton MR spectroscopic imaging studies were acquired and analyzed without knowledge of the side of the seizure focus. The spectra were acquired with a 1.5-T Magnetom Vision unit (Siemens, Erlangen, Germany) by using a standard, circularly polarized head coil. To reduce motion of the head, a vacuum-molded head holder (Vac-Pac; Olympic Medical, Seattle, Wash) was used. For localization, two-dimensional fast low-angle shot (FLASH) images (200/6) were acquired in coronal, sagittal, and oblique transverse orientations.
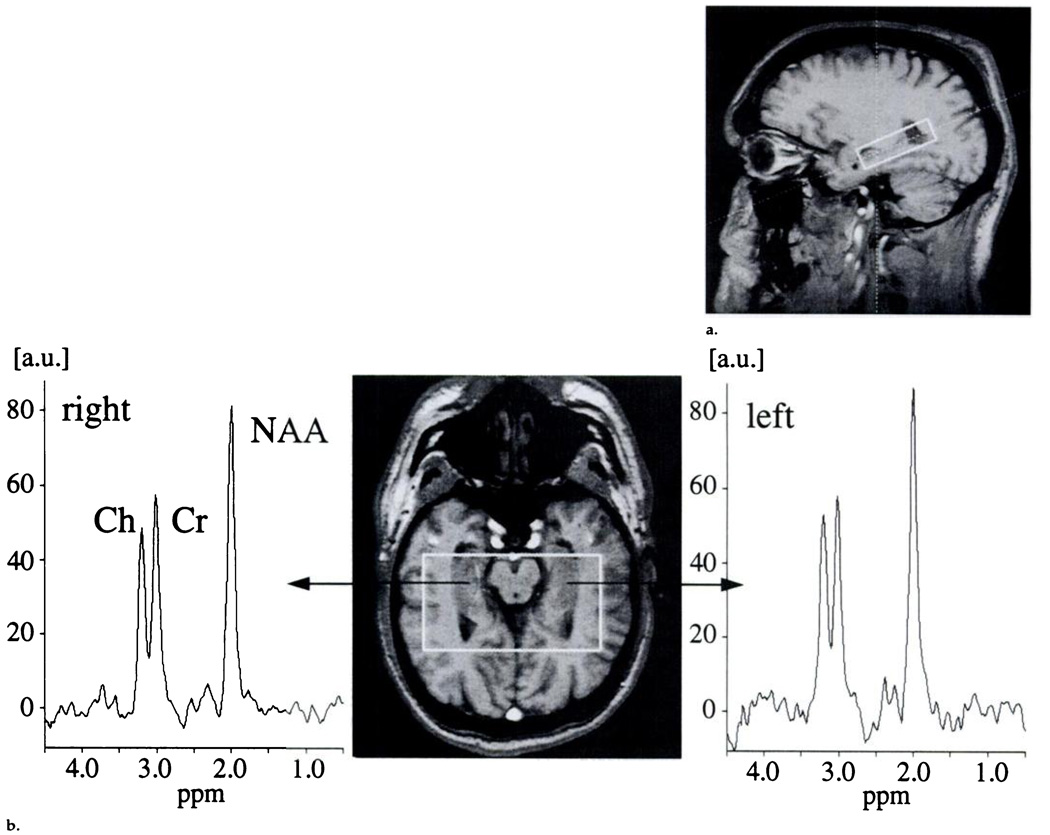
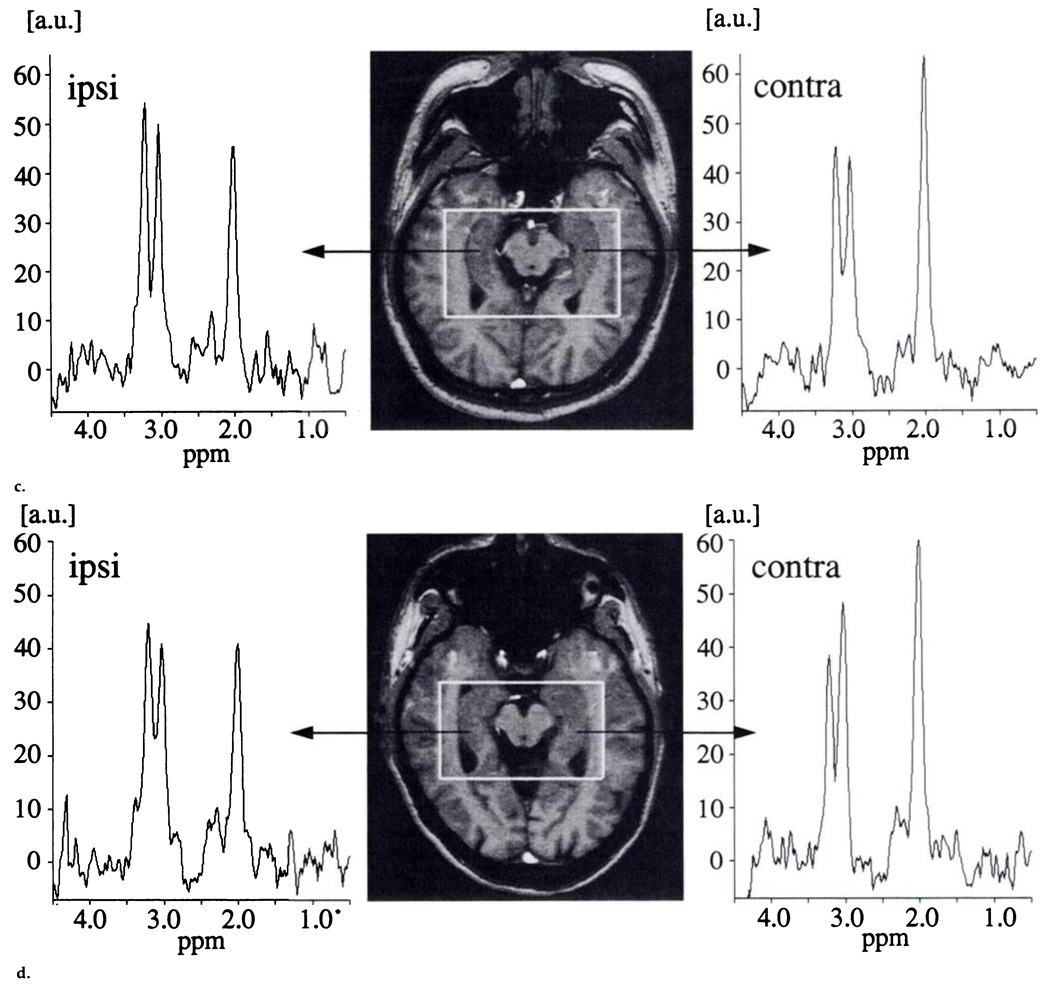
The transverse images were parallel to the long axis of the hippocampus, with the center section positioned through the center of the hippocampus. A two-dimensional MR spectroscopic imaging sequence (1.8/135) with point-resolved spectroscopic (PRESS) volume selection (average size of 72.5 × 97.7 × 15.0 mm3) was used with the volume parallel to the transverse images and including both hippocampi. An MR spectroscopic imaging field of view of 210 × 210 mm was used with circular k-space sampling equivalent to a maximum of 24 × 24 phase-encoding steps (15). Another measurement parameter was 1,800/135, which resulted in a measurement time of 13 minutes. Figure 1a shows a sagittal FLASH localizer image with the PRESS MR spectroscopic imaging box superimposed. Corresponding oblique transverse FLASH localizer images with spectra from the left and right hippocampi that were obtained in a healthy control subject, in a patient with temporal lobe epilepsy and hippocampal atrophy, and in a patient with temporal lobe epilepsy and no hippocampal atrophy are shown in Figure 1b–1d.
Figure 1.
(a) Sagittal FLASH localizer MR image (200/6) obtained in a 30-year-old male control subject shows the position of the PRESS box that covers the hippocampus, (b) Transverse FLASH localizer MR image (200/6) obtained parallel to the long axis of the hippocampus with the PRESS box in a 31-year-old female control subject as in a, with spectra from the left and right hippocampal regions added. The N-acetylaspartate signal dominates the signals from creatine and choline, and no left-versus-right differences are apparent (c) Transverse FLASH localizer MR image (200/6) obtained in a 28-year-old male patient who has ipsilateral hippocampal atrophy, with spectra from the left and right hippocampal regions added. The N-acetylaspartate signal on the same side (right anatomic side, left on MR image) is obviously decreased compared with the creatine and choline signals in the same spectrum and compared with the N-acetylaspartate signal on the opposite side. (d) Transverse FLASH localizer MR image (200/6) obtained in a 44-year-old male patient who does not have hippocampal atrophy, with spectra from the left and right hippocampal regions added. Again, the N-acetylaspartate signal on the same side is obviously decreased compared with the creatine and choline signals in the same spectrum and also compared with the N-acetylaspartate signal on the opposite side. a.u. = arbitrary units, Ch = choline, contra = opposite side, Cr = creatine, ipsi = same side, NAA = N-acetylaspartate.
Quantitation
The unsuppressed water signal intensity was used as an internal standard in a second MR spectroscopic imaging examination with otherwise identical parameters (16,17), which guaranteed that the water and metabolite signal intensities were derived under identical conditions. The quantitation method required correction of metabolite and water signals for T1 and T2 relaxation times, additional correction of the water concentration (aqueous fraction), and correction of the metabolite signals for the number of protons in the metabolite molecules. Metabolite concentrations are reported in millimoles per liter.
Arbitrarily chosen mean metabolite relaxation times from the literature (five T1 and eight T2 values) (18–24) for N-acetylaspartate (T1 = 1.31 seconds ± 0.19 [standard deviation], T2 = 0.37 seconds ± 0.07), creatine (T1 = 1.29 seconds ± 0.14, T2 = 0.20 second ± 0.03), choline (T1 = 1.31 seconds ± 0.11, T2 = 0.33 second ± 0.07), and water (T1 = 0.95 second and T2 = 0.10 second [25]) in gray matter were used. It was assumed that metabolite relaxation times were not substantially different between gray and white matter.
Metabolite concentrations in milliomoles per liter were calculated as Cmet = (CH2O[Smet × cT1(H2O) × cT2(H2O)] × 2)/(SH2O × cT1(met) × cT2(met) × n), where C is the concentration, met is metabolite. S is the peak area of the indicated resonance, c is a correction factor, and n is the number of protons in the metabolite molecule resonance. Correction factors were calculated as follows: cT1 = 1 − e−TR/T1, and cT2 = c−TE/T2.
The water signals were also corrected for the receiver gain difference between the MR spectroscopic imaging experiments performed with and those performed without water suppression. A hippocampal (gray matter) water content of 80% (ie, 44.45 mol/L) was assumed for calculation of CH2O (26). For the detection of statistically significant variations in the water signal and their influence on metabolite concentrations, metabolite and water signals were also corrected for coil loading. This was done by normalizing the metabolite signal according to the transmitter reference voltage (27–29).
MR Spectroscopic Imaging Processing
Postprocessing of the MR spectroscopic imaging data was done with software provided by Siemens (LUISE). A k-space apodization that resulted in an effective voxel size of approximately 4 cm3 and zero-filling to 32 × 32 k-space points was applied before the spatial Fourier transformation. Zero filling from 512 to 1,024 time-domain data points and Gaussian multiplication that corresponded to 0.6-Hz line broadening were performed before the time-do-main Fourier transformation. Spectral phasing and a polynomial baseline correction were also performed.
Voxels that included primarily hippocampal gray matter were selected, and the signals of N-acetylaspartate, creatine, and choline were curve fit with LUISE with the assumption of Gaussian line shapes. The profile of the 180° selective pulse in this direction was suboptimal in the first two voxels from the anterior and posterior borders of the volume of interest. This and the chemical shift displacement error of 2.4 mm between N-acetylaspartate and choline in the in-plane directions (0.8-mT/m gradient strength) lead to disturbed metabolite-metabolite and metabolite-water ratios in these voxels. Therefore, these voxels were avoided. An average of five voxels (range, two to seven voxels) were selected in each hippocampal region. Mean values of spectra from those voxels are reported, and added spectra are shown in Figure 1c and 1d.
MR Spectroscopic Imaging Lateralization
Lateralization by using MR spectroscopic imaging measures was performed in two different ways with the N-acetylaspartate/(creatine + choline) ratio and the N-acetylaspartate concentration as determined with the absolute quantitation technique (16). The first lateralization involved comparison of metabolite concentrations and metabolite peak ratios in the ipsilateral hippocampal region with those on the opposite side. This comparison is based only on intraindividual asymmetry, and no statement about an absolute decrease in metabolite concentration or metabolite peak ratio can be made. In the blinded evaluation, the side with the lower value was called the affected side.
The second lateralization involved comparison of patient hippocampal data with healthy control subject hippocampal data. Values more than 2 standard deviations below the mean of the control subject data were considered to be abnormal. Thus there were three possibilities for lateralization findings: unilateral, bilateral, or normal.
Statistical Analysis
Statistical analyses of the MR spectroscopic imaging data were performed by using analysis of covariance and repeated measures analysis of variance. The N-acetylaspartate/(creatine + choline) ratio and N-acetylaspartate concentration were analyzed with Bonferroni correction for multiple comparisons and the Duncan multiple range test (30). N-acetylaspartate-creatine and N-acetylaspartate-choline ratios and the concentrations of choline and creatine are presented in secondary analyses for descriptive purpose only and without statistical comparisons.
RESULTS
Metabolite Measures That Allow Optimal Lateralization
The concentrations of N-acetylaspartate, creatine, and choline, as well as the N-acetylaspartate/(creatine + choline), N-acetylaspartate-creatine, and N-acetylaspartate-choline metabolite peak ratios, were evaluated. In the patients, the N-acetylaspartate ratios and N-acetylaspartate concentration were substantially decreased in the ipsilateral hippocampus, which confirmed previous study findings. In addition, N-acetylaspartate measures were decreased in the contralateral hippocampus in patients with temporal lobe epilepsy as compared with the measures in control subjects.
To determine the best lateralization criteria, we looked for (a) which measure was in best agreement with EEG results by comparing the sides with the lower MR spectroscopic imaging measures with the EEG lateralization findings and (b) which measure had the largest difference between the mean values in the patients compared with those in the control subjects. N-acetylaspartate/(creatine + choline) provided concordance with EEG lateralization findings in a left-versus-right hemisphere comparison in all patients. The largest difference in means was in the N-acetylaspartate concentration. The N-acetylaspartate concentration did not allow correct lateralization in three patients without atrophy in a left-versus-right comparison but indicated greater bilateral abnormalities (ie, abnormalities in both the ipsilateral and contralateral hippocampi) in a comparison with control subject data. In 15 of 16 patients, ipsilateral N-acetylaspartate concentration and N-acetylaspartate/(creatine + choline) were below the mean control values.
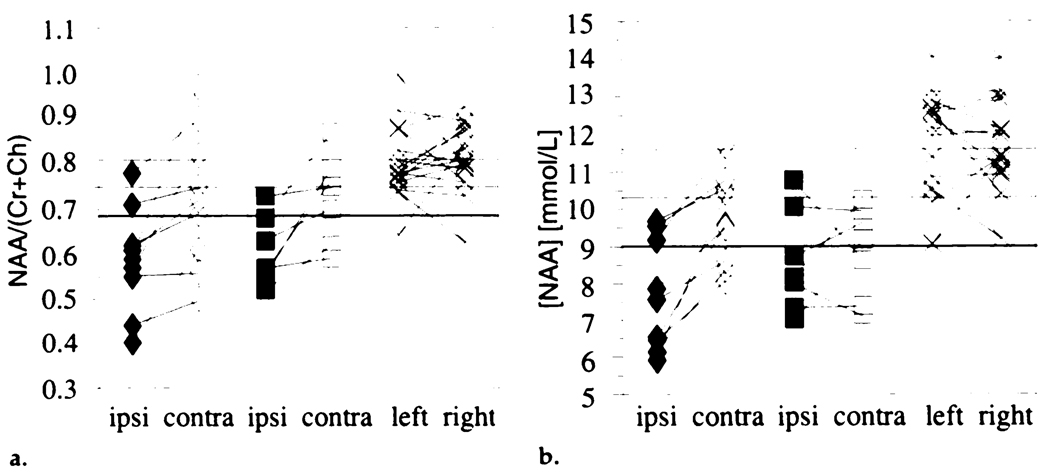
N-acetylaspartate/(creatine + choline) and N-acetylaspartate concentration in an ipsilateral versus contralateral comparison in patients and in a left-versus-right comparison in control subjects are plotted in Figure 2. Table 1 lists the mean values for all MR spectroscopic imaging measures in the control group and for the same and opposite sides in patients with and in patients without hippocampal atrophy.
Figure 2.
(a) Individual N-acetylaspartate/(creatine + choline) ratios and (b) N-acetylasparate concentrations in the same side as the seizure focus in patients with (◆) and in patients without (■) hippocampal atrophy and in the opposite side in patients with (◇) and in patients without (□) hippocampal atrophy and in healthy control subjects (×). The control mean (top line) and mean – 1 (middle line) or 2 (lower bold line) standard deviations are indicated. Ch = choline, contra = opposite side, Cr = creatine, ipsi = same side, NAA = N-acetylaspartate.
Table 1.
Metabolite Concentrations and Metabolite Peak Ratios in Patients and Control Subjects
| Metabolite Concentration |
Metabolite Peak Ratio |
|||||
|---|---|---|---|---|---|---|
| Subjects | NAA | Choline | Creatine | NAA/(Creatine + Choline) | NAA/Choline | NAA/Creatine |
| Control subjects (n = 16) | 11.6 ± 13 | 2.6 ± 0.3 | 9.7 ± 1.3 | 0.81 ± 0.06 | 1.63 ± 0.18 | 1.66 ± 0.16 |
| All patients (n = 16) | ||||||
| Ipsilateral hemisphere | 8.1 ± 1.5 (−30.2)* | 2.6 ± 0.4 (0) | 8.7 ± 1.8 (−10.3) | 0.59 ± 0.10 (−27.2)* | 1.14 ± 0.27 (−30.1) | 1.29 ± 0.20 (−22.3) |
| Contralateral hemisphere | 9.4 ± 1.3 (−19.0)* | 2.4 ± 0.5 (−11.1) | 9.0 ± 1.6 (−7.2) | 0.73 ± 0.13 (−9.9) | 1.46 ± 0.36 (−10.4) | 1.51 ± 0.29 (−9.0) |
| Patients with hippocampal atrophy (n = 9) | ||||||
| Ipsilateral hemisphere | 7.6 ± 1,5 (−34.5)* | 2.5 ± 0.4 (−3.8) | 8.7 ± 2.0 (−10.3) | 0.59 ± 0.12 (−27.2)* | 1.13 ± 0.30 (−30.7) | 1.28 ± 0.25 (−22.9) |
| Contralateral hemisphere | 9.7 ± 1.2 (−16.3)* | 2.5 ± 0.5 (−3.8) | 9.3 ± 1.2 (−4.1) | 0.72 ± 0.16 (−11.1) | 1.45 ± 0.37 (−11.0) | 1.49 ± 0.30 (−10.2) |
| Patients without hippocampal atrophy (n = 7) | ||||||
| Ipsilateral hemisphere | 8.6 ± 1.4 (−25.9)* | 2.7 ± 0.4 (+3.8) | 8.8 ± 1.5 (−9.3) | 0.61 ± 0.08 (−24.7)* | 1.16 ± 0.25 (−28.8) | 1.31 ± 0.13 (−21.1) |
| Contralateral hemisphere | 8.9 ± 1.2 (−23.35)* | 2.4 ± 0,6 (−11.1) | 8.6 ± 2.0 (−11.3) | 0.73 ± 0.10 (−9.9) | 1.46 ± 0.38 (−10.4) | 1.53 ± 0.30 (−7.8) |
Note.—Data are mean ± standard deviation. Numbers in parentheses are percentage change. NAA = N-acetylaspartate.
Significantly different from the control mean (P < .05) with Duncan multiple range test.
Statistical analysis was performed for N-acetylaspartate concentration and N-acetylaspartate/(creatine + choline). No statistically significant left-versus-right differences in the control group were found. Within the control and patient groups, no age- or sex-related differences were found (all P values > .05).
There was no difference in the concentration of choline between patients and control subjects. A trend for decreased creatine concentration was found; the mean ipsilateral creatine concentration in patients was 10% less than that in control subjects. The coil-loading-corrected metabolite signals (not shown) yielded a slight increase in the ipsilateral choline signal of 6.7% in patients. Furthermore, although a decreased ipsilateral creatine-choline ratio was found in the patients (ipsilateral mean creatine-choline ratio = 0.90, control mean creatine-choline ratio = 1.00), lateralization on the basis of the creatine-choline ratio failed in eight of 16 patients.
Patient characteristics, EEG lateralization, and MR imaging findings are given in Table 2. This table also shows findings of MR spectroscopic imaging lateralization on the basis of N-acetyl-aspartate concentration and N-acetyl-aspartate/(creatine + choline); lateralization criteria were a comparison of left and right MR spectroscopic imaging measures in patients with temporal lobe epilepsy and a comparison of temporal lobe epilepsy MR spectroscopic imaging data with control data.
Table 2.
Patient Characteristics and Lateralization of Seizure Focus
| MR Spectroscopic Imaging |
|||||||
|---|---|---|---|---|---|---|---|
| Left vs Right Hemisphere |
Patient vs Control Subject* |
||||||
| Patient No./Age (y)/Sex | Histopathologic Diagnosis | EEG | NAA/(Creating and Choline) | NAA Concentration | NAA/(Creating and Choline) | NAA Concentration | Engel Classification at Surgery |
| Patients with hippocampal atrophy | |||||||
| 1/38/M | MTS | R | R | R | N | N | I |
| 3/24/F | MTS | L | L | L | L | N | I |
| 6/35/F | N/A | L | L | L | B:L | B:L | N/A |
| 7/33 F | MTS | L | L | L | B:L | L | III |
| 8/49/F | MTS | L | L | L | L | L | I |
| 10/28/M | MTS | R | R | R | B:R | B:R | I |
| 14,21/M | N/A | R | R | R | R | R | N/A |
| 15/26/M | N | R | R | R | B:R | B:R | I |
| 16/43/F | N/A | R | R | R | N | N | N/A |
| Patients without hippocampal atrophy | |||||||
| 2/49/F | MTS | L | L | R | N | R | III |
| 4/43/M | MTS | L | L | R | L | N | II |
| 5/28/M | N | L | L | L | L | B:L | IV |
| 9/36/M | N/A | L | L | L | B:L | L | N/A |
| 11/41/F | N/A | L | L | R | B:L | B:R | N/A |
| 12/28/M | MTS | R | R | R | R | R | I |
| 13/44/M | MTS | R | R | R | R | R | I |
Note.—B = bilateral. B:L = bilateral with dominant left hemispheric lesions, B:R = bilateral with dominant right hemispheric lesions, L. = left, MTS = mesial temporal sclerosis, N = normal, N/A = not applicable, NAA = N-acetylasprtate, R = right.
Determination of hemisphere on the basis of patient data at least 2 standard deviations below the control subject data.
Relationship of Decreased N-Acetylaspartate Concentration to Hippocampal Atrophy
The second specific aim of this study was to determine whether decreased hippocampal N-acetylaspartate concentration in temporal lobe epilepsy is simply due to hippocampal atrophy. Figure 2b shows that five of seven patients without hippocampal atrophy, as assessed at MR imaging, had decreased ipsilateral hippocampal N-acetylaspartate concentrations of more than 2 standard deviations below the control mean.
Because the water signal was used for absolute quantitation, the magnitude of the hippocampal water signal was analyzed. The patient groups showed slightly increased mean ipsilateral hippocampal water signals (the mean increase in patients with hippocampal atrophy was 7.9%; that in patients without hippocampal atrophy, 4.4%). This might be due to either an increase in the amount of cerebrospinal fluid (CSF) in the spectroscopic volume of interest or an increase in the T2 of water in the hippocampus (31,32). However, the mean ipsilateral water signal increase of 7.9% in the patients with atrophy accounts for only 6.5% of the 34.2% N-acetylaspartate concentration decrease. Furthermore, no negative correlation between the water and the N-acetylaspartate signal was found (correlation coefficient, .26).
Bilateral Abnormalities
To determine the presence of bilateral hippocampal disease in temporal lobe epilepsy, MR spectroscopic imaging data from the contralateral hippocampus were compared with control data. Mean contralateral N-acetylaspartate concentration was significantly reduced below that in control subjects (P < .001). Compared with the control group data, contralateral N-acetylaspartate concentration and N-acetylaspartate/(creatine + choline) were below the control mean in 15 of 16 and 12 of 16 patients, respectively. Contralateral values of N-acetylaspartate concentration or N-acetylaspartate/(creatine + choline) more than 2 standard deviations below the control mean were detected in six of 16 (38%) patients for each measure; in total, N-acetylaspartate concentration or N-acetylaspartate/(creatine + choline) was more than 2 standard deviations below the control mean in eight patients (50%) (Fig 2).
Relationship of MR Spectroscopic Imaging Data to Surgical Outcome
The final aim of the study was to investigate the correlation between MR spectroscopic imaging findings and seizure surgery outcome. Six patients with hippocampal atrophy underwent surgery; five became seizure free (five patients, class I; one patient, class III). Both N-acetylaspartate concentration and N-acetylaspartate/(creatine + choline) in these six patients provided lateralization findings concordant with EEG findings.
Five patients without hippocampal atrophy underwent surgery. In two of them, N-acetylaspartate concentration and N-acetylaspartate/(creatine + choline) findings were concordant with EEG findings and the patients became seizure free (class I). In the three remaining patients, the N-acetylaspartate/(creatine + choline) ratio resulted in lateralization findings concordant with those of the EEG left-versus-right comparison; however, the N-acetylaspartate concentration was equally low on both sides or was even lower on the opposite side, and in two patients it was ipsilateral within the control mean ± 2 standard deviation range. These three patients continued to have seizures after surgery, although the seizure frequency decreased.
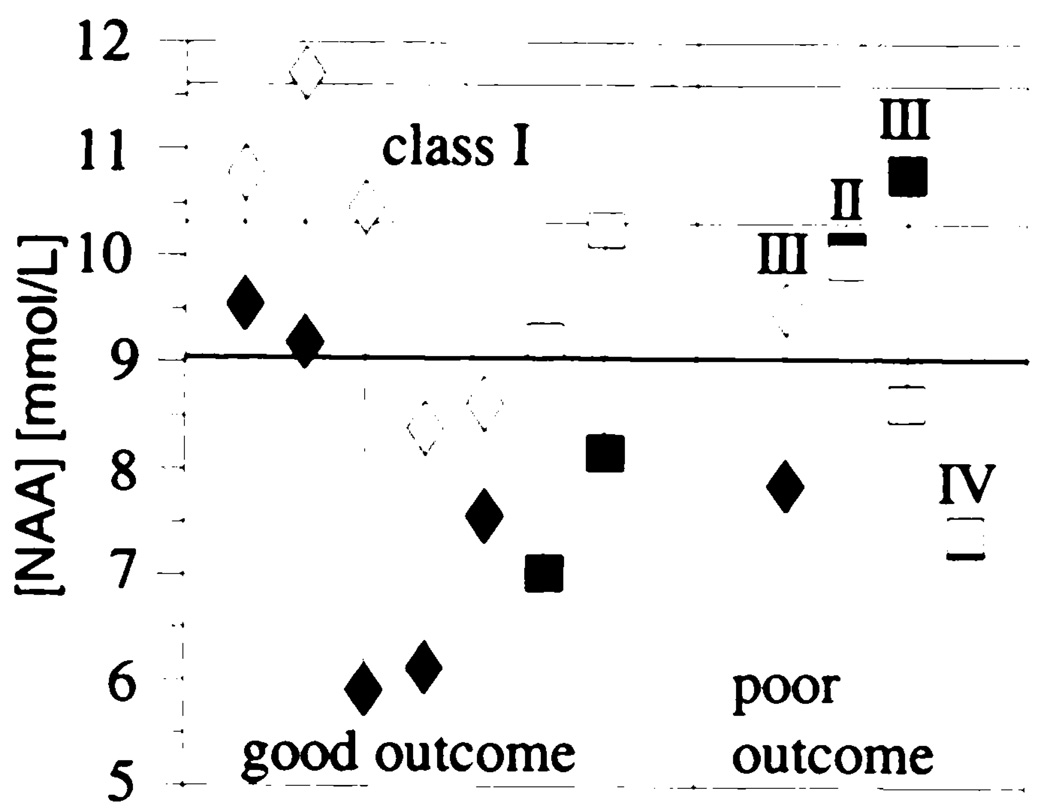
Two of the seven seizure-free patients also showed contralateral abnormally low N-acetylaspartate concentrations, but these decreases were less than the ipsilateral decrease. The individual asymmetries between ipsi- and contralateral N-acetylaspartate concentrations in the patients who were followed up after seizure surgery are shown in Figure 3.
Figure 3.
Individual N-acetylaspartate concentrations in the same side as the seizure focus in patients with (◆) and in patients without (■) hippocampal atrophy and in the opposite side in patients with (◇) and in patients without (□) hippocampal atrophy who were followed up after surgery for a minimum of 8 months. Good outcome corresponds to Engel classification I. The classification for poor outcome (Engel classification II–IV) is indicated individually. The control mean (top line) and mean – 1 (middle line) or 2 (lower bold line) standard deviations are indicated.
In summary, in seven patients with unilateral temporal lobe epilepsy who underwent surgery, a concordant decrease in N-acetylaspartate concentration, in N-acetylaspartate/(creatine + choline), or in both in the ipsilateral hippocampus was predictive of surgical success. Five of these seven patients had ipsilateral atrophy. In three patients without ipsilateral atrophy, a bilateral equally decreased or contralateral lower N-acetylaspartate concentration was associated with poor surgical outcome.
DISCUSSION
The results confirm previous study findings that proton MR spectroscopic imaging measures allow lateralization of the seizure focus in temporal lobe epilepsy. Statistically significant decreases in N-acetylaspartate concentration and N-acetylaspartate/(creatine + choline) as compared with control data were found in the ipsilateral hippocampus in patients with and in patients without hippocampal atrophy. N-acetylaspartate/(creatine + choline) was found to be the most sensitive measure for lateralization in concordance with EEG results. Contralateral low N-acetylaspartate concentration or N-acetylaspartate/(creatine + choline) was detected in 50% of the patients. Furthermore, in this study, MR spectroscopic imaging findings of decreased contralateral N-acetylaspartate concentration were related to surgical outcome.
Metabolite Measures That Allow Optimal Lateralization
N-acetylaspartate/(creatine + choline) was in complete concordance with EEG lateralization in left-versus-right comparisons (Fig 2a). The N-acetylaspartate-choline ratio was not concordant in one case, and the N-acetylaspartate-creatine ratio and N-acetylaspartate concentration (Fig 2b) were discordant in three cases. The differences in concordance between these measures are suggestive of a greater reliability of the sum of creatine and choline than either one alone, although not to a level of statistical significance. A possible reason is that the creatine and choline signals often partially overlap; this results in inaccuracies in the individual line fits for these metabolites, even though the sum of the fits for these two metabolites remains accurate.
All previous proton MR spectroscopic studies of temporal lobe epilepsy found decreased ipsilateral N-acetylaspartate as an absolute decrease in the N-acetylaspartate signal (4,7), as a decrease in the N-acetylaspartate concentration (8), or as a decrease in the N-acetylaspartate-creatine ratio (5,6,9,13), in the N-acetylaspartate-choline ratio, or in both (10–12). Unfortunately, no unique measure was entirely successful in lateralization in these previous studies. For example, Breiter and colleagues (8) found an ipsilateral decreased N-acetylaspartate concentration in all their patients but no statistically significant decrease in the N-acetylaspartate-creatine ratio. Cendes and co-workers (9) preferred to use the N-acetylaspartate-creatine ratio as the criterion for lateralization, whereas Ng and colleagues (12) found the N-acetylaspartate-choline ratio to be the most sensitive measure.
Only a few studies have reported changes in creatine and choline, and these studies have had contradictory results. Layer and colleagues (7) reported ipsilaterally decreased creatine in temporal lobe epilepsy, whereas Connelly and colleagues (10) and Gadian and colleagues (11) found a 15% increase. By using the method of Connelly et al (10) and Gadian et al (11) (that is, by correcting the metabolite signal for coil loading), we did not find a change in the creatine signal in the seizure focus. However, our calculations of the creatine concentration on the basis of the water signal did show a trend for a decreased concentration, which supports the findings of Layer et al (7).
The choline signal was found to be increased in the seizure focus in three previous studies (10–12). Connelly and co-workers (10) reported a 25% increase in the coil-loading-corrected ipsilateral choline signal in 25 patients with temporal lobe epilepsy compared with that in 13 control subjects. We found a trend for an increased coil-loading-corrected choline signal of 6.7% but no changes in the calculated choline concentrations.
Absolute Quantitation
In the past, to our knowledge, most investigators have expressed MR spectroscopic results in terms of peak ratios, which resulted in ambiguity about whether one metabolite is elevated or another decreased. The ability to obtain absolute concentration values rather than ratios eliminates such problems; this is especially important when metabolite changes in disease processes are investigated and when intersubject comparisons are being used. To our knowledge, absolute quantitation of metabolite concentrations in patients with temporal lobe epilepsy has been reported on only by Breiter and colleagues (8), who used water as an internal reference in single-voxel spectroscopy in an 8-cm3 voxel that included temporal lobe gray and white matter and portions of the hippocampus. They found the concentration of N-acetylaspartate was reduced ipsilaterally in patients with temporal lobe epilepsy, which is in agreement with our findings.
The major advantage of quantitating MR spectroscopic imaging data by using the in vivo water signal is that water signals are obtained under exactly the same conditions as the metabolite signals. The signals are obtained from the same anatomic region; therefore, the method is insensitive to variations in B0 and B1. There is no need to reshim or recalibrate flip angles, and long-term time-dependent instrument instabilities are inherently accounted for. Another advantage of this method is that it is possible to determine the extent to which the decrease in the N-acetylaspartate signal is due to an increase in the amount of water in the voxel. The absence of a statistically significant negative correlation between the water signal and the N-acetylaspartate signal rules out statistically significantly varying partial volumes of perihippocampal CSF in the analyzed MR spectroscopic imaging voxels, because CSF contains no MR-detectable N-acetylaspartate concentration (33,34).
Nevertheless, there are several potential error sources in the use of absolute units. The disadvantage that corrections for metabolite relaxation times T1 and T2 are necessary is inherent in MR spectroscopic imaging quantitation methods, because time limitations for in vivo studies do not allow collection of fully relaxed spectra. In addition to the uncertainties in the evaluation of T1 and T2 (no hippocampal metabolite relaxation times have been reported so far), a bi- or multiexponential T2 decay for water is caused by different types of brain material (CSF, gray or white matter, blood vessels), especially in CSF because of the long T2 (35). Furthermore, pathologic conditions can alter metabolite relaxation rates.
In contrast with single-voxel MR spectroscopic studies, the determination of the metabolite T1 and T2 in the individual MR spectroscopic imaging voxel is much too time-consuming to be applicable in a patient examination. Shorter TEs would reduce the error introduced by the estimated T2 values. The reasons for using a TE of 135 msec for both MR spectroscopic imaging measurements were that we wanted to use identical sequence and instrumentation parameters and that the metabolite resonance evaluation of short-TE spectra is more difficult because of interfering resonances from macromolecules and substantially worse water and lipid suppression.
An MR spectroscopic imaging–specific disadvantage of the absolute quantitation method presented here is that Gibb ringing effects may lead to over- or underestimation of the water content in a specific voxel. However, Gibb ringing can be minimized by means of data processing with nonuniform k-space weighting functions (36) (although some spatial resolution is sacrificed with this approach), by means of a further increase in the number of phase-encoding steps, or by means of a combination of both methods. One particular disadvantage of the water reference method is that measurement time is prolonged because of the need to collect the water signal. The higher probability of patient movement with prolonged measurement time does indirectly affect the accuracy of the method.
The water content is usually not uniform over the whole volume of interest. The concentration varies with the composition of brain tissue, CSF, and, possibly, abnormalities. This affects any quantitation method. For the water reference method, this has the disadvantage that the concentration of the water standard is not precisely known. Because of all these limitations, a large error range is intrinsic in the quantitation of metabolites in absolute units in MR spectroscopic and MR spectroscopic imaging studies.
In addition to these general obstacles, the hippocampus is a small structure that mainly consists of gray matter surrounded by temporal lobe white matter and CSF in the temporal horn. It should be emphasized that even though this method reports quantitative data in millimoles per liter, the current method does not quantitate the heterogeneous tissue compartmentation of the voxel from which the signals arise. Nevertheless, our results demonstrate that any intrusion of CSF associated with hippocampal atrophy is too small to be statistically significant in the quantitation method presented here.
A possible improvement could be attained from an MR image segmentation of the tissue in each volume of interest (37). In theory, the tissue composition of gray and white matter and CSF and the amount of tissue water used to quantitate the tissue N-acetylaspartate concentration could be determined. Also, the effective MR spectroscopic imaging voxel size could be reduced by extending the number of phase-encoding steps to 32 × 32 instead of 24 × 24 and by applying a less effective k-space filter.
Relationship of Decreased N-Acetylaspartate Concentration to Hippocampal Atrophy
The finding that N-acetylaspartate was also reduced in ipsilateral nonatrophic hippocampi and without a simultaneous decrease in creatine and choline in all patients further indicates that the reduced N-acetylaspartate concentration is not due to atrophy alone. The decrease in the hippocampal N-acetylaspartate concentration can be explained neither in terms of atrophy alone nor in terms of the small increase in the hippocampal water signal.
These results suggest that MR spectroscopic imaging depicts neuron loss and that growth of glial cells (gliosis) has replaced lost neurons, which prevents atrophy. This is supported by histopathologic evidence of gliosis in temporal lobe epilepsy. The finding that MR spectroscopic imaging measures allowed lateralization in patients without atrophy or other abnormalities at MR imaging suggests that MR spectroscopic imaging is a more sensitive marker of hippocampal abnormality in temporal lobe epilepsy than qualitative MR imaging.
Bilateral Abnormalities
Articles on previous neuroimaging studies (eg, on positron emission tomography, MR imaging) have not reported much bilateral disease (38—41). The reduced N-acetylaspartate concentration, N-acetylaspartate/(creatine + choline), or both in the contralateral hippocampus in 50% of patients with unilateral seizure foci suggests that bilateral hippocampal abnormalities are more common than previously thought. Contralateral abnormalities in proton spectra from the temporal lobe were also found by Connelly and colleagues (10) in 40% and by Ng and colleagues (12) in 18% of their patients with unilateral temporal lobe epilepsy. In a carefully performed autopsy study of temporal lobe epilepsy, Margerison and Corsellis (42) found structural abnormalities of both hippocampi in 30% of their patients.
The importance and interpretation of the contralateral decreases in N-acetylaspartate are speculative at this time. These changes could be nonspecific and unrelated to epilepsy. However, contralateral metabolite changes are defined in comparison with data obtained in healthy control subjects age matched to the patients, and we cannot identify any factor other than epilepsy or its treatment that might have caused these changes. We determined that patients with neocortical epilepsy do not show decreased hippocampal N-acetylaspartate (43). Therefore, it seems that these hippocampal changes are not a nonspecific response to seizure activity or treatment but are associated with mesial temporal lobe epilepsy.
Furthermore, it is possible that the observed bilateral abnormalities are associated with (and possibly responsible for) poor surgical outcome.
Relationship of MR Spectroscopic Imaging Data to Surgical Outcome
MR spectroscopic imaging findings were predictive of poor outcome in three patients who underwent seizure surgery. Contralateral abnormalities were not always associated with poor surgical outcome, but the three patients who had either bilaterally equal N-acetylaspartate concentrations or a more marked reduction in N-acetylaspartate concentration on the opposite as compared with the same side did not become seizure free after surgery. This emphasizes the value of absolute quantitation of MR spectroscopic imaging data. However, these results need to be confirmed in a larger cohort with longer postsurgical follow-up.
In conclusion, the results presented here show that decreased N-acetylaspartate concentration is not due solely to hippocampal atrophy and that contralateral abnormalities are much more common than previously thought. More important, our findings emphasize the value of MR spectroscopic imaging in the presurgical evaluation of epilepsy.
Acknowledgment
We thank Diane Amend, PhD, for her help with the statistical analysis.
G.R.E., K.D.L., G.B.M., and M.W.W. supported by U.S. National Institutes of Health grant ROI-NS31966-01.
Abbreviations
- CSF
cerebrospinal fluid
- EEG
electroencephalography
- FLASH
fast low-angle shot
- PRESS
point-resolved spectroscopy
- TE
echo time
- TR
repetition time
References
- 1.Garcia PA, Laxer KD, Barbaro NM, Dillon WP. The prognostic value of qualitative magnetic resonance imaging hippocampal abnormalities in patients undergoing temporal lobectomy for medically refractory seizures. Epilepsia. 1994;35:520–524. doi: 10.1111/j.1528-1157.1994.tb02471.x. [DOI] [PubMed] [Google Scholar]
- 2.Kuzniecky R, Burgard S, Faught E, Morawetz R, Bartolucci A. Predictive value of magnetic resonance imaging in temporal lobe epilepsy surgery. Arch Neurol. 1993;50:65–69. doi: 10.1001/archneur.1993.00540010059018. [DOI] [PubMed] [Google Scholar]
- 3.Jack CR, Sharbrough FW, Cascino GD, et al. Magnetic resonance image–based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol. 1992;31:138–146. doi: 10.1002/ana.410310204. [DOI] [PubMed] [Google Scholar]
- 4.Hugg JW, Laxer KD, Matson GB, Maudsley AA, Weiner MW. Neuron loss localizes human temporal lobe epilepsy by in vivo proton magnetic resonance spectroscopic imaging. Ann Neurol. 1993;34:788–794. doi: 10.1002/ana.410340606. [DOI] [PubMed] [Google Scholar]
- 5.Garcia PA, Laxer KD, van den Grond J, Hugg JW, Matson GB, Weiner MW. Proton magnetic resonance spectroscopic imaging in patients with frontal lobe epilepsy. Ann Neurol. 1995:279–281. doi: 10.1002/ana.410370222. [DOI] [PubMed] [Google Scholar]
- 6.Matthews PM, Andermann F, Arnold DL. A proton magnetic resonance spectroscopy study of focal epilepsy in humans. Neurology. 1990;40:985–989. doi: 10.1212/wnl.40.6.985. [DOI] [PubMed] [Google Scholar]
- 7.Layer G, Traeber F, Mueller-Lisse U, Bunke J. Spectroscopy: a new magnetic resonance technique for the diagnosis of epilepsy. Radiologe. 1993;33:178–184. [PubMed] [Google Scholar]
- 8.Breiter SN, Arroyo S, Mathews VP, Lesser RP, Bryan RN, Barker PB. Proton MR spectroscopy in patients with seizure disorder. AJNR. 1994;15:373–384. [PMC free article] [PubMed] [Google Scholar]
- 9.Cendes F, Andermann F, Preul MC, Arnold DL. Lateralization of temporal lobe epilepsy based on regional metabolite abnormalities in proton magnetic resonance spectroscopic images. Ann Neurol. 1994;35:211–216. doi: 10.1002/ana.410350213. [DOI] [PubMed] [Google Scholar]
- 10.Connelly A, Jackson GD, Duncan JS, King MD, Gadian DG. Magnetic resonance spectroscopy in temporal lobe epilepsy. Neurology. 1994;44:1411–1417. doi: 10.1212/wnl.44.8.1411. [DOI] [PubMed] [Google Scholar]
- 11.Gadian DG, Connelly A, Duncan JS, et al. 1H magnetic resonance spectroscopy in the investigation of intractable epilepsy. Acta Neurol Scand. 1994;152:116–121. doi: 10.1111/j.1600-0404.1994.tb05202.x. [DOI] [PubMed] [Google Scholar]
- 12.Ng TC, Comair YG, Xue M, et al. Temporal lobe epilepsy: presurgical localization with proton chemical shift imaging. Radiology. 1994;193:465–472. doi: 10.1148/radiology.193.2.7972764. [DOI] [PubMed] [Google Scholar]
- 13.Hetherington H, Kuzniecky R, Pan J, et al. Proton nuclear magnetic resonance spectroscopic imaging of human temporal lobe epilepsy at 4.1 T. Ann Neurol. 1995;38:396–404. doi: 10.1002/ana.410380309. [DOI] [PubMed] [Google Scholar]
- 14.Engel JJ, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J, editor. Surgical treatment of the epilepsies. New York, NY: Raven; 1993. pp. 609–621. [Google Scholar]
- 15.Maudsley AA, Matson GB, Hugg JW, Weiner MW. Reduced phase encoding in spectroscopic imaging. Magn Reson Med. 1994;31:645–651. doi: 10.1002/mrm.1910310610. [DOI] [PubMed] [Google Scholar]
- 16.Ende G, Laxer KD, Knowlton R, Schuff N, Matson GB, Weiner MW. Water referenced quantitative 2D 1H SI in the hippocampus of healthy controls and TLE patients (abstr). Proceedings of the Society of Magnetic Resonance 1995; Society of Magnetic Resonance; Berkeley, Calif. 1995. p. 1962. [Google Scholar]
- 17.Alger JR, Symko SC, Bizzi A, Posse S, Des-Pres DJ, Armstrong MR. Absolute quantitation of short TE brain 1H-MR spectra and spectroscopic imaging data. J Comput Assist Tomogr. 1993;17:191–199. doi: 10.1097/00004728-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Hennig J, Pfister H, Ernst T, Ott D. Direct absolute quantification of metabolites in the human brain with in vivo localized proton spectroscopy. NMR Biomed. 1992;5:193–199. doi: 10.1002/nbm.1940050406. [DOI] [PubMed] [Google Scholar]
- 19.Barker PB, Soher BJ, Blackband SJ, Chatham JC, Mathews VP, Bryan RN. Quantitation of proton NMR spectra of the human brain using tissue water as an internal concentration reference. NMR Biomed. 1993;6:89–94. doi: 10.1002/nbm.1940060114. [DOI] [PubMed] [Google Scholar]
- 20.Christiansen P, Henriksen O, Stubgaard M, Gideon P, Larsson HB. In vivo quantification of brain metabolites by 1H-MRS using water as an internal standard. Magn Reson Imaging. 1993;11:107–118. doi: 10.1016/0730-725x(93)90418-d. [DOI] [PubMed] [Google Scholar]
- 21.Kreis R, Ernst T, Ross BD. Absolute quantitation of water and metabolites in the human brain. II. Metabolite concentrations. J Magn Reson. 1993;102:9–19. [Google Scholar]
- 22.Danielsen E, Henriksen O. Absolute quantitative proton NMR spectroscopy based on the amplitude of the local water suppression pulse: quantification of brain water and metabolites. NMR Biomed. 1994;7:311–318. doi: 10.1002/nbm.1940070704. [DOI] [PubMed] [Google Scholar]
- 23.Hagberg G, Seelig J. Localized proton spectroscopy of the human brain: comparison of different methods for absolute quantitation (abstr). Proceedings of the Society of Magnetic Resonance in Medicine 1993; Society of Magnetic Resonance in Medicine; Berkeley, Calif. 1993. p. 979. [Google Scholar]
- 24.Hänicke W, Michaelis T, Merboldt KD, Frahm J. Book of abstracts: Society of Magnetic Resonance in Medicine 1993. Berkeley, Calif: Society of Magnetic Resonance in Medicine; 1993. On the use of a fully automated data analysis method for in vivo MRS (abstr) p. 977. [Google Scholar]
- 25.Blüml S, Schad LR, Stepanow B, Lorenz WJ. Spin-lattice relaxation time measurement by means of a TurboFLASH technique. Magn Reson Med. 1993;30:289–295. doi: 10.1002/mrm.1910300304. [DOI] [PubMed] [Google Scholar]
- 26.Norton WT, Cammer W. Isolation and characterization of myelin. In: Morell P, editor. Myelin. London, England: Plenum; 1984. pp. 147–195. [Google Scholar]
- 27.Hoult DI, Richards RE. The signal-to-noise ratio of the nuclear magnetic resonance experiment. J Magn Reson. 1976;24:71–85. doi: 10.1016/j.jmr.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Austin SJ, Connelly A, Gadian DG, Benton JS, Brett EM. Localized 1H NMR spectroscopy in Canavans disease: a report of two cases. Magn Reson Med. 1992:1913. doi: 10.1002/mrm.1910190235. [DOI] [PubMed] [Google Scholar]
- 29.Michaelis T, Merboldt KD, Bruhn H, Hänikcke W, Frahm J. Absolute concentration of metabolites in the adult human brain in vivo: quantification of localized proton MR spectra. Radiology. 1993;187:219–227. doi: 10.1148/radiology.187.1.8451417. [DOI] [PubMed] [Google Scholar]
- 30.SAS Institute. SAS/STAT users guide, release 6.03 edition, software version 6.09. Cary, NC: SAS Institute; 1988. [Google Scholar]
- 31.Jackson GD, Connelly A, Duncan JS, Grunewald RA, Gadian DG. Detection of hippocampal pathology in intractable partial epilepsy: increased sensitivity with quantitative magnetic resonance T2 relaxometry. Neurology. 1993;43:173–179. doi: 10.1212/wnl.43.9.1793. [DOI] [PubMed] [Google Scholar]
- 32.Ende G, Laxer KD, Knowlton R, Tanabe JL, Matson GB, Weiner MW. T2 in the hippocampus of TLE patients: no changes are detected in the absence of hippocampal atrophy (abstr). Proceedings of the Society of Magnetic Resonance 1995; Society of Magnetic Resonance; Berkeley, Calif. 1995. p. 1230. [Google Scholar]
- 33.Lynch J, Peeling J, Auty A, Sutherland GR. Nuclear magnetic resonance study of cerebrospinal fluid from patients with multiple sclerosis. Can J Neurol Sci. 1993;20:194–198. [PubMed] [Google Scholar]
- 34.Tallan HH. Studies on the distribution of N-acetyl-L-aspatic acid in brain. J Biol Chem. 1957;224:41–45. [PubMed] [Google Scholar]
- 35.Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain. I. Compartments and water. J Magn Reson. 1993;102:1–9. [Google Scholar]
- 36.Brooker HR, Mareci TH, Mao JT. Selective Fourier transform localization. Magn Reson Med. 1987;5:417–433. doi: 10.1002/mrm.1910050503. [DOI] [PubMed] [Google Scholar]
- 37.Meyerhoff DJ, MacKay S, Ezekiel JMF, et al. Metabolite changes in Alzheimers disease are not due to atrophy: combined 1H MRSI and MRI segmentation (abstr). Proceedings of the Society of Magnetic Resonance 1995; Society of Magnetic Resonance; Berkeley, Calif. 1995. p. 388. [Google Scholar]
- 38.Spencer SS, McCarthy G, Spencer DD. Diagnosis of medial temporal lobe seizure onset: relative specificity and sensitivity of quantitative MRI. Neurology. 1993;43:2117–2124. doi: 10.1212/wnl.43.10.2117. [DOI] [PubMed] [Google Scholar]
- 39.Jack JR, Sharbrough FW, Twomey CK, et al. Temporal lobe seizures: lateralization with MR volume measurements of the hippocampal formation. Radiology. 1990;175:423–429. doi: 10.1148/radiology.175.2.2183282. [DOI] [PubMed] [Google Scholar]
- 40.Kuzniecky R, Burgard S, Faught E, Morawetz R, Bartolucci A. Predictive value of magnetic resonance imaging in temporal lobe epilepsy surgery. Arch Neurol. 1993;50:65–69. doi: 10.1001/archneur.1993.00540010059018. [DOI] [PubMed] [Google Scholar]
- 41.Spencer SS. The relative contributions of MRI, SPECT, and PET imaging in epilepsy. Epilepsia. 1994;35 suppl 6:72–89. doi: 10.1111/j.1528-1157.1994.tb05990.x. [DOI] [PubMed] [Google Scholar]
- 42.Margerison JH, Corsellis JA. Epilepsy and the temporal lobes: a clinical electroen-cephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- 43.Vermathen P, Ende G, Laxer KD, et al. Patients with neocortical epilepsy show no spectral changes in the hippocampus: a 1H SI study (abstr). Proceedings of the Society of Magnetic Resonance 1996; Society of Magnetic Resonance; Berkeley, Calif. 1996. p. 138. [Google Scholar]