Abstract
Purinergic signaling plays distinct and important roles in the CNS, including the transmission of calcium signals between astrocytes. Gap junction hemichannels are among the mechanisms proposed by which astrocytes might release ATP; however, whether the gap junction protein connexin43 (Cx43) forms these “hemichannels” remains controversial. Recently, a new group of proteins, the pannexins, have been shown to form nonselective, high-conductance plasmalemmal channels permeable to ATP, thereby offering an alternative for the hemichannel protein. Here, we provide strong evidence that, in cultured astrocytes, pannexin1 (Panx1) but not Cx43 forms hemichannels. Electrophysiological and fluorescence microscope recordings performed in wild-type and Cx43-null astrocytes did not reveal any differences in hemichannel activity, which was mostly eliminated by treating Cx43-null astrocytes with Panx1-short interfering RNA [Panx1-knockdown (Panx1-KD)]. Moreover, quantification of the amount of ATP released from wild-type, Cx43-null, and Panx1-KD astrocytes indicates that downregulation of Panx1, but not of Cx43, prevented ATP release from these cells.
Introduction
It has been widely proposed that connexin43 (Cx43), the main gap junction protein expressed in astrocytes, can under specific conditions form functional “hemichannels,” providing a transmembrane pathway for the diffusion of ions and relatively large molecules (for review, see Spray et al., 2006; Harris, 2007). Evidence in support is primarily pharmacological, showing blockade of uptake of fluorescent dyes (Lucifer yellow, ethidium bromide, propidium iodide) and of release of intracellular molecules (ATP, glutamate) by compounds known to block gap junction channels; additionally, electrophysiological recordings have demonstrated the presence of large (>200 pS, as expected for Cx43 hemichannels) conductance channels in astrocytes (Contreras et al., 2003; Retamal et al., 2007).
Recently, a newly discovered group of proteins, the pannexins (Panx1, -2, and -3), were cloned from mammalian tissues. Pannexins were classified as gap junction proteins because of their significant but low (∼20%) homology to the innexins, the gap junction proteins of invertebrates; they bear no sequence homology with connexins, the gap junction proteins of chordates (for review, see Scemes et al., 2007).
It is becoming apparent that none of the pannexins readily forms intercellular channels (but see Bruzzone et al., 2003) and that Panx1 in particular forms functional plasmalemmal channels that display properties similar to those that have been attributed to connexin hemichannels (for review, see Dahl and Locovei, 2006; Spray et al., 2006; Scemes et al., 2009).
In the CNS, Panx1 mRNA and protein were reported to be present in both neuronal and glial cells in vivo and in vitro (Ray et al., 2005, 2006; Huang et al., 2007a). Panx1 forms large conductance (400–500 pS) nonselective channels that are permeable to ATP, carboxyfluorescein, and YoPro (Bao et al., 2004; Locovei et al., 2006b, 2007) and are sensitive to compounds known to block connexin channels, including carbenoxolone (CBX), flufenamic acid, and mefloquine (MFQ) (Bruzzone et al., 2005; Iglesias et al., 2008).
Because the overlapping pharmacology reported for pannexins and connexins may confound identification of the molecular substrate of hemichannel activity in astrocytes, we have compared the electrophysiological properties and membrane permeability to dyes of astrocytes prepared from wild-type (WT) and Cx43-null neonatal mice. We here show for the first time that cultured astrocytes display functional Panx1 channels that are activated by membrane depolarization or after P2X7 receptor (P2X7R) stimulation. These channels are sensitive to CBX and MFQ and allow permeation by YoPro and ATP. Because no differences in the activation properties of hemichannels were observed between wild-type and Cx43-null astrocytes and because Panx1 short interfering RNA (siRNA) reduces the occurrence of these channels, we conclude that Panx1 is more likely the molecular substrate for hemichannel activity in these cells.
Materials and Methods
Astrocyte cultures.
We used primary cultures of cortical astrocytes derived from neonatal WT and Cx43-null mice (offspring of Cx43 heterozygotes in C57BL/6J-Gja1 strain; at least two litters per experiment were used). Animals were maintained at the Albert Einstein College of Medicine; the Albert Einstein College of Medicine Animal Care and Use Committee has approved all experimental procedures used in these studies. Cortices were separated from whole-brain embryos [embryonic day 19 (E19) to E20], and after meninges removal, tissues were trypsinized (0.1% trypsin at 37°C for 10 min). Cells from each animal were collected by centrifugation and pellet suspended in DMEM supplemented with 10% FBS and 1% antibiotics and seeded in 60 mm culture dishes. Genotype of individual cultures was determined by PCR on tail DNA (Dermietzel et al., 2000). Astrocytes were maintained for 2–3 weeks in culture (100% humidity; 95% air, 5% CO2; 37°C) at which time ∼95–98% of the cells were immunopositive for glial fibrillary acidic protein.
Electrophysiology.
Solitary WT and Cx43-null astrocytes were plated on coverslips 24–48 h before recordings. Whole-cell patch-clamp recordings were performed as previously described (Iglesias et al., 2008). Briefly, cells were bathed in external solution containing the following (in mm): 147 NaCl, 10 HEPES, 13 glucose, 2 CaCl2, 1 MgCl2, and 5 KCl, pH 7.4. The pipette solution contained the following (in mm): 130 CsCl, 10 EGTA, 10 HEPES, 0.5 CaCl2. Activation of Panx1 channels by voltage was performed using a 10 s ramp protocol from holding a potential of −60 to +100 mV. To analyze the participation of Panx1 channels in agonist-induced P2X7R activation, astrocyte membrane potential was held at −60 mV and the P2X7R agonist 3-O-(4-benzoyl)benzoyl adenosine trisphosphate (BzATP) (50 μm) was superfused for 5–10 s, a condition that was subthreshold for total activation of Panx1 channels. After the first response to the agonist, the gap junction channel blockers CBX (50 μm) and MFQ (100 nm) were superfused for 5 min before the addition of the P2R agonist. Electrophysiological recordings were accomplished using an Axopatch 200B amplifier, and pClamp9 software was used for data acquisition and analysis.
Dye uptake.
Astrocytes (WT and Cx43-null) plated on glass-bottom dishes (MatTek) were bathed for 5 min in phosphate-buffered solution, pH 7.4, containing the cell-impermeant dye YoPro-1 (5 μm). Cells were then exposed to a solution containing 300 μm BzATP and 5 μm of the dye. (Higher concentrations of agonist than those used in the electrophysiological studies are necessary for long-lasting and full activation of Panx1 channels and for optimal detection of YoPro fluorescence.) YoPro fluorescence intensity was measured during 500 s BzATP stimulation, as previously described (Suadicani et al., 2006). The effects of a gap junction channel blocker (MFQ, 10 nm) and of a P2X7 receptor antagonist [brilliant blue G (BBG), 1 μm] on BzATP-induced dye uptake were also tested. YoPro fluorescence was captured using a CoolSNAP-HQ2 CCD camera (Photometrics) attached to an inverted Nikon microscope (Eclipse TE-2000E) equipped with a 20× dry objective and FITC filter set using Metafluor software.
Panx1 siRNA.
Astrocytes were treated with 50 nm small interference RNA corresponding to the mouse pannexin 1 sequence, as well as with scrambled sequences, using 6 μl/1.5 ml oligofectamine reagent (Invitrogen), as previously described (Locovei et al., 2007). After overnight exposure, transfection reagents were removed and cells incubated for 30 h in DMEM–FBS medium before use in electrophysiological and dye uptake studies.
ATP release.
Confluent cultures of WT and Cx43-null astrocytes plated in 35 mm dishes were washed twice in PBS, and then exposed for 3 min to PBS containing 300 μm BzATP. After complete removal and washout of the agonist, cells were bathed in PBS for 2 min before collection of samples of BzATP-induced ATP release. (Agonist concentration and incubation times are based on dye uptake measurements and were optimal for detection of released ATP.) For measurements of intracellular ATP levels, cells were lysed with Tris-buffered solution containing 1% Triton X-100 and supernatants of whole-cell lysates were used. The amount of ATP in samples were measured as previously described (Striedinger et al., 2007), using the luciferin/luciferase assay (Invitrogen) and a plate reader luminometer (Veritas; Turner Instruments). The amounts of ATP in the samples were calculated from standard curves and normalized for the protein concentration, using the BCA assay (Pierce).
Results
Wild-type and Cx43-null astrocytes in culture express functional Panx1 channels
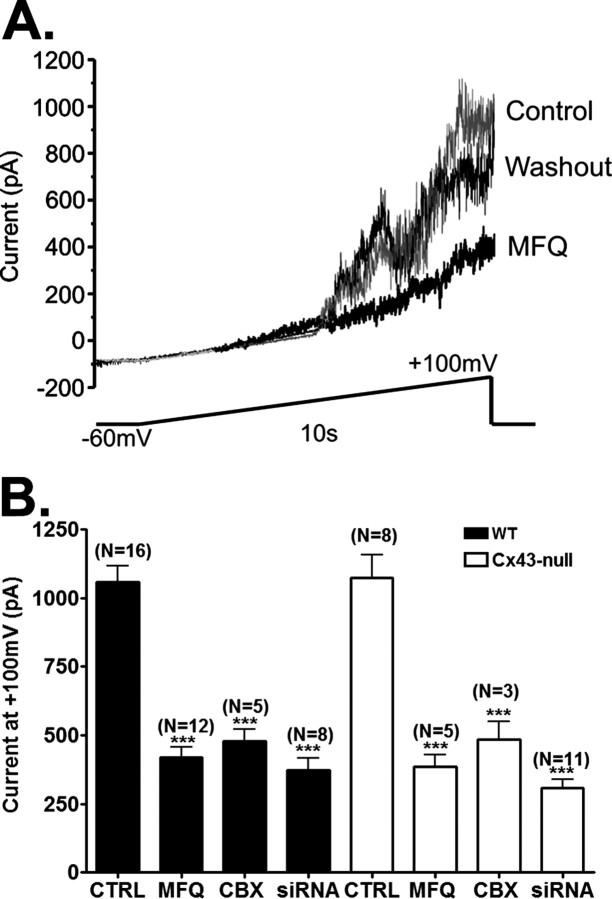
Whole-cell recordings from solitary astrocytes indicated the presence of voltage-activated outward currents in WT cells; the current amplitudes increased at voltages above +20 mV and were significantly attenuated by CBX and MFQ, two compounds previously described to block Panx1 channels more effectively than connexin gap junction channels (Cruikshank et al. 2004; Bruzzone et al., 2005; Iglesias et al., 2008). At membrane potentials of +100 mV, current amplitudes (1062.0 ± 60.6 pA; N = 16 cells) were significantly reduced by 61% (419.0 ± 38.2 pA; N = 12 cells) and by 55% (478.9 ± 41.6 pA; N = 5 cells) after exposure to 100 nm MFQ and 50 μm CBX, respectively (p < 0.01, ANOVA followed by Dunnett's test) (Fig. 1).
Figure 1.
Effects of gap junction channel blockers on voltage-activated Panx1 currents in astrocytes. A, Examples of outward currents recorded from a single astrocyte in the absence and presence of MFQ (100 nm) and after MFQ washout. Currents were obtained in response to voltage ramps (−60 to +100 mV) with −60 mV holding membrane potential. B, Bar histograms showing the mean ± SE values of current amplitudes at the end of the current ramps (+100 mV) recorded from WT (black bars) and Cx43-null (white bars) astrocytes bathed in control (CTRL) solution and solutions containing CBX (50 μm) and MFQ (100 nm). Current amplitudes obtained for astrocytes treated for 48 h with Panx1 siRNA are shown in the last bars. The numbers in parentheses correspond to the number of cells tested. ***p < 0.01.
To verify that channel activity recorded in WT astrocytes was not attributable to activation of currents attributed to Cx43 hemichannels (Contreras et al., 2003; Retamal et al., 2007; Kang et al., 2008), we performed electrophysiological recordings as described above using cortical astrocytes derived from Cx43-null mice. Similarly to what we found for WT astrocytes, Cx43-null cells displayed voltage-activated outward currents (Fig. 1B). At membrane potentials of +100 mV, current amplitudes (1074.0 ± 84.1 pA; N = 8 cells) recorded from Cx43-null cells were significantly reduced by 64% (386.7 ± 41.1 pA; N = 5 cells) and 55% (485.5 ± 62.5 pA; N = 3 cells) after exposure to MFQ (100 nm) and CBX (50 μm), respectively (p < 0.01, ANOVA followed by Dunnett's test). The amplitudes of voltage-activated currents measured in Cx43-null astrocytes (1074.0 ± 84.1 pA; N = 8 cells) were virtually identical with those recorded from WT cells (1062.0 ± 60.6 pA; N = 16 cells; p = 0.89, t test). These data, summarized in Figure 1B, indicate that the channels contributing this outward current at positive potentials are not Cx43 hemichannels.
To obtain evidence for the molecular identity of CBX- and MFQ-sensitive outward currents, we treated astrocytes with Panx1 siRNA. Under the condition of Panx1-knockdown (Panx1-KD), voltage-activated currents recorded from both WT and Cx43-null astrocytes were greatly attenuated. After 48–72 h Panx1-KD, current amplitudes measured at +100 mV were significantly reduced to 308.0 ± 31.1 pA (N = 11 Cx43-null cells) and to 373.8 ± 42.5 pA (N = 8 WT cells) compared with those of untreated (1062.0 ± 60.6 pA; N = 16 cells; p < 0.01, t test) (Fig. 1B) or scrambled Panx1 siRNA-treated (1028.0 ± 89.4 pA; N = 9 cells) (supplemental Fig. S1, available at www.jneurosci.org as supplemental material) WT astrocytes. Exposure of Panx1-KD Cx43-null astrocytes to MFQ (100 nm) did not further attenuate voltage-activated outward currents (230.2 ± 24.2 pA; N = 5 cells; p > 0.05, t test). These data strongly suggest that Panx1 forms the channels responsible for current activation at positive potentials in astrocytes. [Evidence that Panx1 but not Cx43 forms nonjunctional channels in Xenopus oocytes is shown in supplemental Fig. S2 (available at www.jneurosci.org as supplemental material).]
Panx1 channels in wild-type and Cx43-null astrocytes are activated by stimulation of P2X7 receptors
Activation of P2X7 receptors by high ATP concentration and by the synthetic agonist BzATP leads to opening of Panx1 channels in a macrophage cell line and in Xenopus oocytes coexpressing these two proteins (Pelegrin and Surprenant, 2006; Locovei et al., 2007; Iglesias et al., 2008).
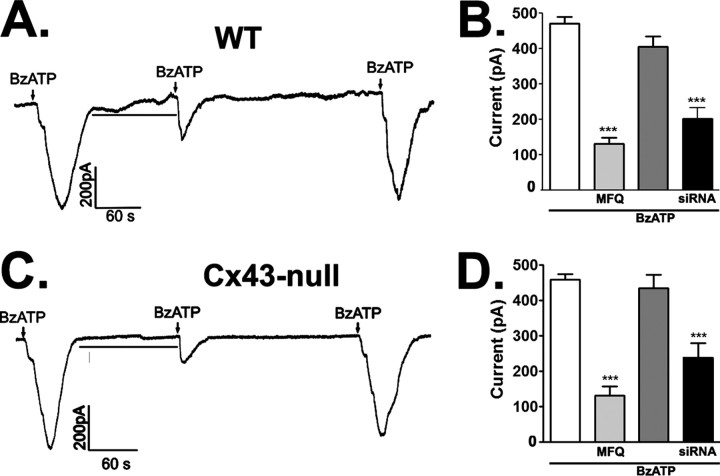
As illustrated in Figure 2A, 5 s exposure to BzATP (50 μm) elicited inward currents in astrocytes held at −60 mV. The inward current elicited by BzATP was biphasic, with an initial small inward current followed by a larger one; the second but not the first component was blocked by 100 nm MFQ (BzATP, 470.6 ± 18.9 pA; N = 10 cells; MFQ, 130.8 ± 17.3 pA, N = 8 cells; p < 0.001, t test) (Fig. 2A,B). The remaining MFQ-insensitive currents are likely mediated by flux of ions through the cation channel of the P2X7R receptor itself, as we have recently shown for the J774 macrophage cell line (Iglesias et al., 2008).
Figure 2.
P2X7R-induced Panx1 currents in astrocytes. A, C, Representative current traces induced by 5 s application of BzATP (50 μm) in WT (A) and Cx43-null (C) astrocytes are shown to be greatly reduced by 5 min preincubation (lines) with 100 nm MFQ; after washout of MFQ, agonist induced currents of similar amplitudes as those recorded after the first application. B, D, Bar histograms show the mean ± SE values (N = 4–10 cells) corresponding to data displayed in A and C, respectively. The traces were obtained by holding the membrane at −60 mV. ***p < 0.01.
Similarly to WT astrocytes, the second phase of BzTAP-induced currents (458.2 ± 15.7 pA; N = 5 cells) in Cx43-null astrocytes was also significantly decreased (131.6 ± 25.4 pA; N = 4 cells; p < 0.01, t test) by 100 nm MFQ (Fig. 2C,D). Knockdown of Panx1 greatly attenuated BzATP-induced currents in Cx43-null (238.9 ± 40.0 pA; N = 5 cells) and in WT astrocytes (202.5 ± 30.10 pA; N = 6 cells), compared with untreated (470.6 ± 18.9 pA; N = 10 cells; p < 0.001, t test) (Fig. 2B,D) and scrambled Panx1 siRNA-treated (431.6 ± 30.1 pA; N = 7 cells) (supplemental Fig. S1, available at www.jneurosci.org as supplemental material) WT cells.
Wild-type and Cx43-null astrocytes are equally permeable to YoPro after BzATP stimulation
To evaluate the contribution of Panx1 to astrocyte membrane permeabilization, we exposed astrocytes to BzATP and measured the influx of YoPro-1. As we previously showed for WT spinal cord astrocytes (Suadicani et al., 2006), BzATP induced YoPro uptake in WT and Cx43-null cortical astrocytes that was prevented by BBG, MFQ, and by Panx1 siRNA (Fig. 3A,B). After 500 s exposure to 300 μm BzATP, YoPro fluorescence intensity increased 1.14 ± 0.005-fold (N = 72 cells) in WT astrocytes and 1.14 ± 0.004-fold (N = 90 cells) in Cx43-null cells. Five minute preincubation with BBG (1 μm) reduced YoPro uptake in WT (1.05 ± 0.002; N = 90 cells) as well as in Cx43-null (1.02 ± 0.003; N = 90 cells). MFQ (10 nm) also prevented YoPro uptake in both WT (1.03 ± 0.003; N = 90 cells) and Cx43-null (1.03 ± 0.003; N = 90 cells) astrocytes. Similarly, knockdown of Panx1 also prevented BzATP-induced YoPro uptake in WT (1.06 ± 0.001; N = 84 cells) and in Cx43-null (1.03 ± 0.01; N = 100 cells) astrocytes (Fig. 3B). Scrambled Panx1 siRNA had no effect on BzATP-induced dye uptake from WT astrocytes (1.13 ± 0.004; N = 300 cells), and there was no YoPro uptake in the absence of BzATP stimulation (1.03 ± 0.002; N = 100 cells).
Figure 3.
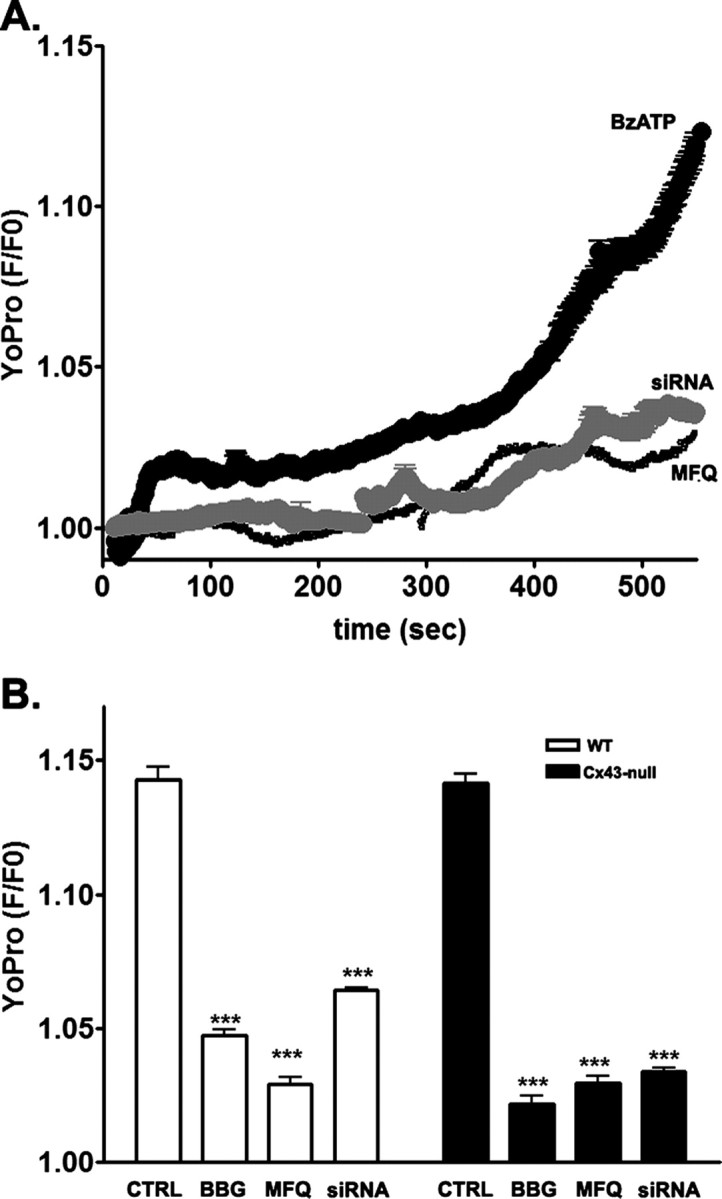
The P2X7R–pannexin 1 complex mediates astrocyte membrane permeabilization. A, Representative time course of YoPro uptake recorded from WT astrocytes exposed to BzATP (300 μm) in the absence and presence of MFQ (100 nm) and after Panx1 knockdown with siRNA. B, Bar histograms showing the mean ± SE values of the relative YoPro fluorescence intensity obtained for wild-type (white bars) and Cx43-null (black bars) astrocytes treated for 500 s with BzATP (300 μm) in the absence and presence of 1 μm BBG and of 100 nm MFQ. ***p < 0.01.
Wild-type and Cx43-null astrocytes express similar levels of Panx1 and P2X7 receptors but release different amounts of ATP
To evaluate whether deletion of the Gja1 gene affected the expression levels of P2X7R and Panx1, Western blot analyses were performed on whole-cell lysates of astrocyte cultures derived from at least three different litters of heterozygous matings. No significant differences in protein expression levels of P2X7 and Panx1 were detected when comparing astrocyte cultures derived from WT and Cx43-null sibling mice (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). Thus, these results strongly suggest that the persistence of hemichannel currents and membrane permeability to YoPro seen in Cx43-null astrocytes (Figs. 1, 3) are not related to increased expression levels of P2X7 receptors and Panx1 that might in principle compensate for the loss of putative Cx43 hemichannels.
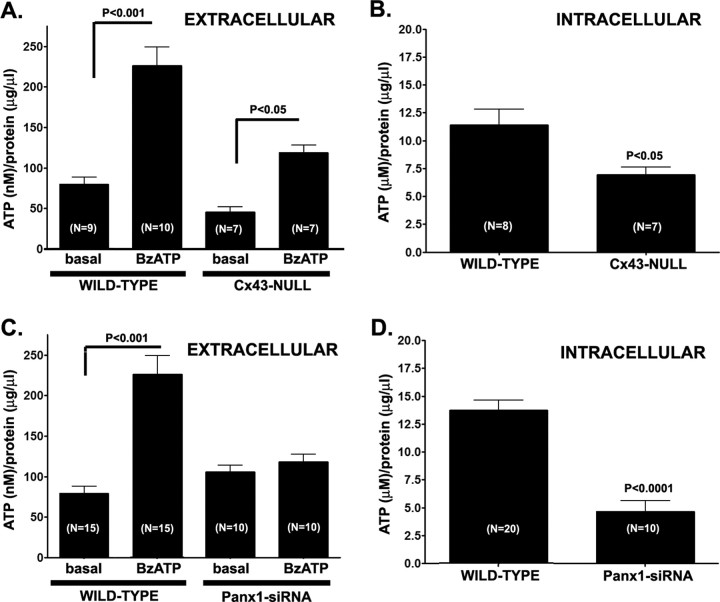
Using the luciferin–luciferase assay, we recorded significantly lower BzATP-induced ATP release from Cx43-null (119.0 ± 18.2 nm; N = 7 experiments) compared with WT astrocytes (205.8 ± 20.5 nm; N = 10 experiments; p < 0.05, t test) (Fig. 4A). However, cytosolic ATP levels in Cx43-null (6.96 ± 0.67 μm; N = 7 experiments) were lower compared with those of WT astrocytes (11.4 ± 1.4 μm; N = 8 experiments; p < 0.05, t test) (Fig. 4B). This result suggests that the lower extracellular ATP levels recorded from Cx43-null astrocytes may reflect their lower cytosolic levels rather than lack of Cx43 hemichannels.
Figure 4.
BzATP-induced ATP release from astrocytes. Bar histograms showing the mean ± SE values of ATP (A, C) present in the extracellular solution in response to BzATP prestimulation of WT and Cx43-null (A) and WT and Panx1-siRNA-treated (C) astrocytes. B and D show the mean ± SE values of intracellular ATP present in the cytosol of WT and Cx43-null astrocytes (B) and in cells untreated and treated with Panx1-siRNA (C). Note that, in A and C, the values of ATP are expressed as nanomolar, and in B and D, in micromolar.
Nevertheless, in terms of fold of ATP release after receptor stimulation in relation to basal nonstimulated condition, no difference was found between WT and Cx43-null astrocytes. After BzATP stimulation, a 2.9-fold increase from basal levels was measured in extracellular ATP from cultured WT astrocytes (from 72.23 ± 7.98 nm ATP to 205.8 ± 20.5 nm ATP; N = 9–10 experiments; p < 0.001, t test) (Fig. 4A). Similarly, a 2.4-fold increase in ATP release was also recorded from Cx43-null astrocytes after BzATP stimulation (from 49.5 ± 6.7 to 119.0 ± 18.2 nm ATP; N = 5 independent experiments) (Fig. 4A) (p < 0.05, t test). This result showing similar fold changes in ATP release in both WT and Cx43-null cells provides additional support that the pathway mediating BzATP-induced ATP release is unlikely to be Cx43 hemichannels.
To evaluate whether Panx1 could provide the pathway for ATP release from astrocytes, experiments were performed on WT astrocytes untreated and treated with Panx1 siRNA. As shown in Figure 4C, knockdown of Panx1 almost completely prevented BzATP-induced ATP release (from 105.6 ± 8.24 to 117.9 ± 9.9 nm; N = 10 experiments; p > 0.05, t test). Similarly to Cx43-null astrocytes (Fig. 4B), cytosolic ATP levels in Panx1 siRNA-treated WT cells (4.7 ± 0.9 μm; N = 10 experiments) were also significantly lower (p < 0.001, t test) than in untreated astrocytes (13.7 ± 0.9 μm; N = 20 experiments) (Fig. 4D). Scrambled Panx1 siRNA reduced cytosolic ATP levels (from 20.7 ± 0.8 to 13.3 ± 0.5 μm; N = 3 experiments; p < 0.0001, t test) but did not prevent BzATP-induced ATP release from WT astrocytes (from 53.8 ± 9.0 to 101.5 ± 1.4 nm; N = 3 experiments; p = 0.0004, t test).
Together, these results support the hypothesis that Panx1 and not Cx43 hemichannels provides a pathway for ATP release from astrocytes.
Discussion
Communication among astrocytes themselves and with other neural cells relies not only on direct gap junctional contacts but is also mediated through the release of paracrine signals. Among the distinct mechanisms by which astrocytes release “gliotransmitters,” hemichannels formed of gap junction (connexin) or gap junction-like (pannexin) proteins have been suggested to be prominent under certain conditions (for review, see Parpura et al., 2004; Dahl and Locovei, 2006; Spray et al., 2006; Scemes et al., 2007).
Connexin43 is the most abundant gap junction protein in astrocytes (Dermietzel et al., 2000; Scemes et al., 2000) and openings of Cx43 hemichannels have been proposed to account for glial release of ATP (Stout et al., 2002; Kang et al., 2008) and glutamate (Ye et al., 2003) as well as for the uptake of fluorescent molecules under ischemic and inflammatory conditions (Contreras et al., 2002; Retamal et al., 2007). Evidence presented favoring the involvement of Cx43 in these processes includes blockade by compounds that inhibit Cx43 gap junction channels, presence of channels with appropriately large unitary conductances (∼200 pS) in membranes of astrocytes and transfected cells (Retamal et al., 2007) (for review, see Spray et al., 2006). The strongest evidence favoring Cx43 hemichannels came from exogenous expression of GFP (green fluorescent protein)-tagged Cx43, in which channel properties reflected the presence of the tag (Bukauskas et al., 2002; Contreras et al., 2003). Interestingly, metabolic inhibition-induced membrane permeabilization was prevented in Cx43de/del but not in Cx43f/f:GFAP-Cre astrocytes (Contreras et al., 2002), suggesting that 90% loss of Cx43 does not abolish hemichannel activity.
Pannexin 1 has been reported to form gap junction channels and also to function as hemi-gap junction channels that are sensitive to gap junction channel blockers, including carbenoxolone and flufenamic acid (Bruzzone et al., 2003, 2005). The nonjunctional Panx1 channels (pannexons) are voltage sensitive, 400–500 pS channels that have been reported to be modulated by intracellular calcium and by mechanical stretch (Bao et al., 2004; Locovei et al., 2006a); these large conductance channels have been proposed to mediate ATP release from erythrocytes, mouse taste buds, and astrocytes (Locovei et al., 2006b; Huang et al., 2007b; Scemes et al., 2007). Panx1 can be activated by ATP through the metabotropic P2Y1 and P2Y2 receptors (Locovei et al., 2006a), as well as through the ionotropic P2X7 receptors (Pelegrin and Surprenant, 2006; Locovei et al., 2007; Iglesias et al., 2008).
Panx1 transcripts are found in astrocytes in vitro and in vivo (Ray et al., 2005, 2006; Huang et al., 2007a); however, the extent to which the Panx1 protein forms functional channels in astroglial cells and their properties has not yet been fully investigated. We here show that Panx1 channels likely perform the hemichannel function in astrocytes. This is based on our quantitative measurements showing no difference between outward currents activated by strong depolarization or by P2X7 receptor stimulation in WT compared with Cx43-null astrocytes. Moreover, knockdown of Panx1 by siRNA is shown to greatly reduce the occurrence of these currents. In addition, MFQ, which has been previously shown not to block Cx43 gap junction channels at concentrations <20 μm (Cruikshank et al., 2004) and to block Panx1 channels at much lower concentration (Iglesias et al., 2008), is here shown to prevent both voltage-activated and BzATP-induced currents in both WT and Cx43-null astrocytes.
We previously showed that the P2X7R–Panx1 complex provides sites of ATP release, amplifying the extent to which intercellular Ca2+ waves (ICWs) spread among WT and Cx43-null astrocytes when exposed to low divalent cation solution (Suadicani et al., 2006; Scemes et al., 2007). In contrast to this view are the reports showing that the amount of ATP released from cells correlates with the levels of Cx43 expression and that gap junction channel blockers attenuate ATP release from astrocytes and transfected C6 glioma cells (Cotrina et al., 1998; Stout et al., 2002; Kang et al., 2008). However, to our knowledge, no previous study has directly measured ATP levels in WT and Cx43-null astrocytes. In agreement with these previous reports, we also found a correlation between Cx43 expression levels and amount of ATP release. However, we attributed this difference to lower cytoplasmic ATP concentration in Cx43-null astrocytes rather than to inhibited release in the absence of Cx43 hemichannels. Moreover, because knockdown of Panx1 prevented ATP release, it is more likely that Panx1 and not Cx43 hemichannels can provide sites of ATP release that amplifies the distance to which calcium signals spread among astrocytes. This possibility is in agreement with our previous studies showing that knockdown of Panx1, but not of Cx43, prevented the amplification of intercellular calcium waves in astrocytes (Suadicani et al., 2006; Scemes et al., 2007).
In summary, our results strongly support the notion that Panx1 and not Cx43 is the main molecular substrate of hemichannel. This is evidenced by the similar hemichannel activity measured in WT and Cx43-null astrocytes and by the prevention of such activity after Panx1 knockdown.
Footnotes
This work was supported by National Institutes of Health Grants NS052245 (E.S.), GM-8610 (G.D.), and NS041282 (D.C.S.). We gratefully acknowledge the technical assistance of Aisha Cordero with cell cultures and mouse genotyping.
References
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Bukauskiene A, Verselis VK. Conductance and permeability of the residual state of connexin43 gap junction channels. J Gen Physiol. 2002;119:171–185. doi: 10.1085/jgp.119.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Sánchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Sáez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci U S A. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Sáez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci U S A. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci U S A. 2004;101:12364–12369. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G, Locovei S. Pannexin: to gap or not to gap, is that a question? IUBMB Life. 2006;58:409–419. doi: 10.1080/15216540600794526. [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, Bennett MV, Spray DC. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Brain Res Rev. 2000;32:45–56. doi: 10.1016/s0165-0173(99)00067-3. [DOI] [PubMed] [Google Scholar]
- Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94:120–143. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Grinspan JB, Abrams CK, Scherer SS. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia. 2007a;55:46–56. doi: 10.1002/glia.20435. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007b;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295:C752–C760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006a;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006b;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Scemes E, Spray DC. Mechanisms of glutamate release from astrocytes: gap junction “hemichannels,” purinergic receptors and exocytotic release. Neurochem Int. 2004;45:259–264. doi: 10.1016/j.neuint.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Zoidl G, Weickert S, Wahle P, Dermietzel R. Site specific and developmental expression of pannexin1 in the mouse nervous system. Eur J Neurosci. 2005;21:3277–3290. doi: 10.1111/j.1460-9568.2005.04139.x. [DOI] [PubMed] [Google Scholar]
- Ray A, Zoidl G, Wahle P, Dermietzel R. Pannexin expression in the cerebellum. Cerebellum. 2006;5:189–192. doi: 10.1080/14734220500530082. [DOI] [PubMed] [Google Scholar]
- Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E, Suadicani SO, Spray DC. Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J Neurosci. 2000;20:1435–1445. doi: 10.1523/JNEUROSCI.20-04-01435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol. 2007;3:199–208. doi: 10.1017/S1740925X08000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E, Spray DC, Meda P. Connexins, pannexins, innexins: novel roles of “hemi-channels”. Pflugers Arch. 2009;457:1207–1226. doi: 10.1007/s00424-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- Striedinger K, Meda P, Scemes E. Exocytosis of ATP from astrocyte progenitors modulates spontaneous Ca2+ oscillations and cell migration. Glia. 2007;55:652–662. doi: 10.1002/glia.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]