Abstract
Peptide analogues targeting various neuropeptide receptors have been used effectively in cancer therapy. A hallmark of adrenocortical tumor formation is the aberrant expression of peptide receptors relating to uncontrolled cell proliferation and hormone overproduction. Our microarray results have also demonstrated a differential expression of neuropeptide hormone receptors in tumor subtypes of human pheochromocytoma. In light of these findings, we performed a comprehensive analysis of relevant receptors in both human adrenomedullary and adrenocortical tumors and tested the antiproliferative effects of peptide analogues targeting these receptors. Specifically, we examined the receptor expression of somatostatin-type-2 receptor, growth hormone-releasing hormone (GHRH) receptor or GHRH receptor splice variant-1 (SV-1) and luteinizing hormone-releasing hormone (LHRH) receptor at the mRNA and protein levels in normal human adrenal tissues, adrenocortical and adrenomedullary tumors, and cell lines. Cytotoxic derivatives of somatostatin AN-238 and, to a lesser extent, AN-162, reduced cell numbers of uninduced and NGF-induced adrenomedullary pheochromocytoma cells and adrenocortical cancer cells. Both the splice variant of GHRH receptor SV-1 and the LHRH receptor were also expressed in adrenocortical cancer cell lines but not in the pheochromocytoma cell line. The GHRH receptor antagonist MZ-4–71 and LHRH antagonist Cetrorelix both significantly reduced cell growth in the adrenocortical cancer cell line. In conclusion, the expression of receptors for somatostatin, GHRH, and LHRH in the normal human adrenal and in adrenal tumors, combined with the growth-inhibitory effects of the antitumor peptide analogues, may make possible improved treatment approaches to adrenal tumors.
Keywords: targeted therapy, tumor endocrinology, aberrant receptors, cytotoxic peptide derivatives
The over-expression or aberrant expression of G protein-coupled receptors for neuropeptides in human adrenal tissue has been linked to adrenal tumor formation and excessive hormone production (1, 2). This includes ectopic receptors for gastric inhibitory polypeptide, ghrelin, luteinizing hormone, IL-1, and corticotrophin-releasing hormone (3, 4), as well as altered activity of eutopic receptors for vasopressin (V1-AVPR) and serotonin (5-HT4) (5–7). Cell transplantation studies inducing the expression of some of these receptors have resulted in the formation of hyperplastic adrenal tissues (2). The identification of these receptors may make possible pharmacological interventions as an alternative approach to adrenalectomy. Furthermore, peptide hormone receptors have been detected in adrenocortical cancers as well as in malignant pheochromocytomas (PHEO) (3, 8, 9), a potentially important observation in as much as the therapeutic options for both of these malignancies are limited (10).
The elucidation of specific molecular characteristics of tumor cells facilitates the development of potential targeted therapies. Modern targeted anticancer drugs include antibodies against surface structures on malignant cells and conjugates consisting of receptor-specific ligands linked to toxins, radionuclides, and chemotherapeutic agents (11). The much higher intratumoral concentrations of such antineoplastic drugs, compared with the surrounding tissue, are expected to result in a greater antitumor efficacy and reduced systemic toxicity. This approach might also help to overcome the chemoresistance shown by some malignant cell types (12).
Several targeted cytotoxic hormone analogues have been synthesized by Schally et al. to yield new classes of antineoplastic agents (13). These compounds include the cytotoxic analogues of bombesin (AN-215), of somatostatin AN-238 and AN-162, of luteinizing hormone-releasing hormone (LHRH) AN-152, AN-207, and others, synthesized by coupling doxorubicin or 2 pyrrolinodoxorubicin (2-pyrrolino-DOX) (AN-201) to the respective hormone analogues (14, 15). A possible role in the therapy of benign and malignant PHEO (16), as well as in treating tumors in patients with other endocrine malignancies (17, 18), was suggested for somatostatin analogues. Of particular interest is the finding that somatostatin is capable of inhibiting cell proliferation in a large variety of cell types through interactions with G protein-coupled receptors. This regulatory effect makes somatostatin and its analogues potentially important agents in cancer cell growth regulation (19, 20).
The octapeptide somatostatin analogues display high affinity to somatostatin-type (sst) 2 and sst5, moderate affinity to sst3, and poor affinity to sst1 and sst4 (21). Immunoreactive growth hormone-releasing hormone (GHRH) is present in several tumors, including carcinoids, pancreatic cancers, small-cell lung cancers, and endometrial tumors. Adrenal adenomas and PHEO have also been reported to secrete GHRH (22). Additionally LHRH and its receptors have been demonstrated in a number of malignant human tumors, including those of the breast, ovary, endometrium, and prostate. Although analogs of somatostatin and LHRH have been successfully used in the treatment of various types of malignancy, no consistently effective therapy is currently available for inhibiting proliferation and metastasis in adrenal tumors.
Previous studies from our laboratories have shown that peptide analogues, which are conjugated to chemotherapeutic agents, such as doxorubicin, have less toxicity and are more effective in a variety of endocrine, as well as nonendocrine tumors, than free cytotoxic compounds. Furthermore, our analysis of a huge PHEO microarray database with 70 benign and 20 malignant metastatic specimens has shown that up to 89% of genes were under-expressed in malignant primary tumors as compared to benign tumors (23). Interestingly, further detailed analysis of this database also revealed a differential expression of neuropeptide hormone receptors in subtypes of human PHEO. In this study we have analyzed the expression of the respective receptors in human adrenal tumors and tumor cell lines, focusing on the sst2 and the GHRH-receptor, including its splice variant SV-1, which is known to be present in various human malignancies (24). We also examined the expression of the LHRH-receptor. We then tested the targeted cytotoxic somatostatin analogues AN-162 and AN-238, the octapeptide somatostatin analogue RC-160, the GHRH antagonist MZ-4–71, and the LHRH antagonist Cetrorelix for effects on growth in cell culture.
Results
Oligonucleotide Microarray Analyses of PHEO Subtypes.
Tumors were classified and analyzed for differentially regulated genes in the following subgroups: benign vs. malignant, adrenergic vs. noradrenergic, primary tumor vs. metastases, and von Hippel–Lindau (VHL) vs. multiple endocrine neoplasia, type 2A (MEN2A). The expression of receptors for sst1 to -5 and GHRH differed significantly in PHEO specimens of our patients' cohort (23). Microarray data demonstrated relevant expression of sst2, -4, and -5, as well as GHRH-R. Interestingly, the expression of GHRH-R was significantly lower in malignant vs. benign PHEO, as well as in the primary tumors compared to metastases. In addition, GHRH-R expression was also lower in noradrenergic vs. adrenergic PHEO. Sst5 showed variable expression, depending on the genetic background of the PHEO and its biochemical activity, with higher expression in VHL compared to MEN2 and in noradrenergic compared to adrenergic PHEO. We concentrated on the expression of sst2, GHRH SV-1-R, and LHRH-R during our subsequent analysis (Table 1).
Table 1.
Oligonucleotide microarray: Differential expression of neuropeptide hormone receptors in human PHEO subtypes
| Gene symbol | Gene ID | Malignant (all) vs. benign |
Primary malignant vs. benign |
Primary malignant vs. metastases |
Adrenergic vs. noradrenergic |
MEN2 vs VHL |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Direction | P-value | Direction | P-value | Direction | P-value | Direction | P-value | Direction | P-value | ||
| sst2 | 6752 | 0.65972 | 0.85655 | 0.23083 | 0.80935 | 0.51129 | |||||
| sst4 | 6754 | 0.78704 | 0.65491 | 0.61670 | 0.99411 | 0.38928 | |||||
| sst5 | 6755 | 0.20848 | 0.43863 | 0.32459 | ↓ | 0.00000 | ↓ | 0.00042 | |||
| GHRHR | 2692 | 0.06917 | ↓ | 0.01745 | ↓ | 0.00041 | ↑ | 0.02035 | 0.65040 | ||
| GHRHR* | 2692 | ↓ | 0.01466 | ↓ | 0.00336 | ↓ | 0.00009 | 0.29209 | 0.45467 | ||
Direction of difference indicates higher (↑) or lower (↓) expression in the first compared to second tumor subtype
*GHRHR SV 1
mRNA Expression of Neuropeptide Receptors in Adrenal, Medullary, and Cortical Specimens and Cell Lines.
To confirm the microarray data, mRNA of PHEO and related cell lines was investigated by RT-PCR, using adrenal cortex and related tumors and cell lines for comparison. Sst2 was expressed in the normal adrenal cortex and in adrenal tumors of both the cortex and medulla, as well as in SW-13 adrenocortical tumor cells (Table 2) and PC-12 pheochromoytoma cells (Table 2). GHRH-R was detected in benign and malignant PHEO, whereas SV-1-R was found in the highly malignant SW-13 adrenocortical tumor cell line (Table 2). The receptor for LHRH was expressed in normal adrenal gland, in cortical adenoma, and in the SW-13 cortical tumor cell-line (Table 2). While sst2 and GHRH-R were expressed in malignant tumors of both the medulla and the cortex, LHRH-R appeared to be limited to cortical tumors (Table 2).
Table 2.
mRNA expression of neuropeptide hormone receptors in the normal adrenal, adrenal tumors, and adrenal tumor cell lines
| sst2 | GHRHR/SV-1-R | LHRHR | |
|---|---|---|---|
| Normal adrenal medulla | – | – | + |
| Normal adrenal cortex | + | – | + |
| Benign PHEO | + | + | – |
| Malignant PHEO | + | + | – |
| Adrenocortical adenoma | + | – | + |
| Adrenocortical carcinoma | + | – | – |
| Adrenomedullary PC-12 tumor cells | + | – | – |
| Adrenocortical SW-13 tumor cells | + | + | + |
Immunohistochemistry Demonstrates the Expression of sst2-Protein in Normal Adrenal, Adrenal Tumors, and in the SW-13 Adrenocortical Cancer Cell Line.
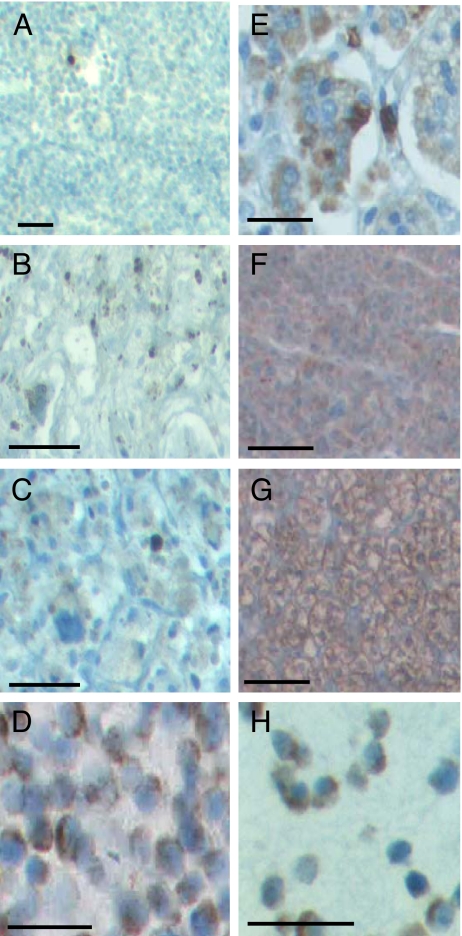
In addition to mRNA expression of sst2 receptor, sst2 protein was detected by immunohistochemistry. Normal adrenal medullary tissue (Fig. 1A) did not immunostain for sst2. The tissue from human PHEO, however, abundantly expressed sst2 in both benign (Fig. 1B) and malignant tumors (Fig. 1C). Furthermore, cells of the PC-12 adrenomedullary tumor cell line also immunostained for sst2 (Fig. 1D). Normal adrenal cortex (Fig. 1E), cortical adenoma (Fig. 1F), cortical carcinoma (Fig. 1G), and the SW-13 malignant tumor cell line (Fig. 1H) were all positive for sst2.
Fig. 1.
Immunohistochemical analysis. (A–D) Adrenal medullary tissue and PC-12 cell line: (A) no sst2 expression was found in normal adrenal medulla; (B) relatively weak sst2 staining was seen in benign PHEO; (C) more pronounced sst2 receptor expression in malignant PHEO and (D) PC-12 cells. (E–H) Adrenal cortical tissue and SW-13 cell line: (E) normal cortex is strongly immunostained for sst2; (F) cortical adenoma, (G) cortical carcinoma, and (H) malignant SW-13 cells show even more pronounced sst2 expression (n = 3 for all tissues and cell lines analyzed). (Scale bars, 20 μm.)
Ultrastructural Analysis of Tumor Cell Lines Before and After Incubation with Somatostatin Analogues.
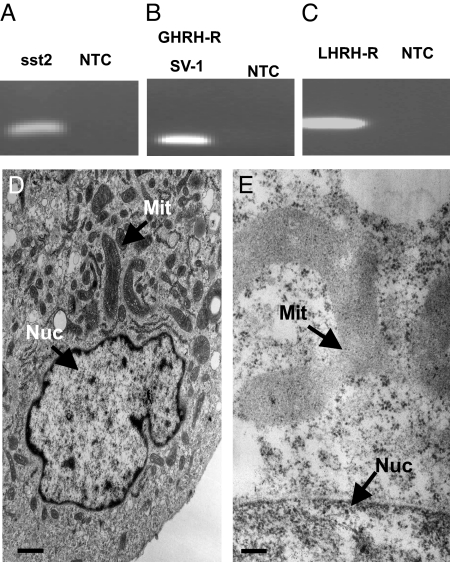
The steroidogenic potential of SW-13 cells is very limited and ultrastructural analysis of SW-13 cells showed primarily cristae-like mitochondria and ample rough endoplasmic reticulum under normal culture conditions. The human SW-13 cell is thus a suitable in vitro model of an undifferentiated malignant adrenocortical tumor (Fig. 2D). These cells express sst2 (Fig. 2A), GHRH-R (Fig. 2B), and LHRH-R (Fig. 2C). PC-12 cells demonstrated the morphology typical of PHEO cells, with a reduced number of large, dense core vesicles as compared to normal adrenal chromaffin cells (Fig. 4B). These cells express sst2 (Fig. 4A). Treatment of the SW-13 adrenocortical cancer cell line with RC-160 (Fig. 2E) did not lead to major apoptotic events, but cells exhibited swollen mitochondria lacking the typical tubulovesicular cristae and showed amorphous inclusions, as well as the disruption of nuclear and mitochondrial membranes, indicating primarily a necrotic mode of cell death. In contrast, the treatment of PC-12 PHEO cell line with AN-238 (Fig. 4C) induced characteristic apoptotic changes, indicated by shrinking of the cytoplasm away from the cell wall, apoptotic bodies, internucleosomal DNA fragmentation, and condensation of the cytoplasm, while retaining mitochondria and endomembrane structure. Interestingly, there was a conspicuous increase in filopodia in the treated cells, suggesting further membrane alterations.
Fig. 2.
Receptors for sst2 (168 bp), SV-1 (121 bp), and LHRH (319 bp) are expressed in SW-13 cells as shown by RT-PCR (A–C). (D and E) Ultrastructural analysis demonstrates the morphology of an undifferentiated cell, with abundant characteristic elongated cristae-like mitochondria, as well as ample endoplasmic reticulum under normal culture conditions (D). (Scale bar, 3 μm.) SW-13 cells (E) are used as a model for malignant adrenocortical tumors. Cells treated with RC-160 exhibited swollen mitochondria, containing amorphous inclusions, and showing disruption of nuclear and mitochondrial membranes, indicating primarily a necrotic mode of cell death. (Scale bar, 0.1 μm.) DCV, dense core vesicles; Mit, mitochondria; Nuc, nucleus.
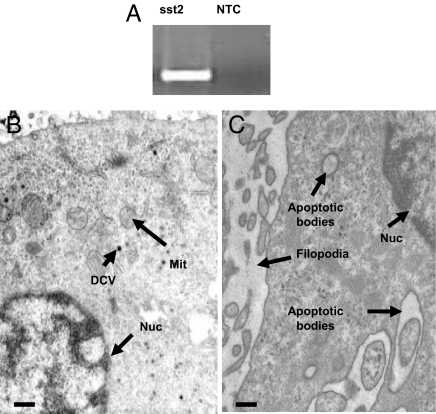
Fig. 4.
The somatostatin receptor subtype 2 (276 bp) is expressed in PC-12 cells as shown by RT-PCR (A). Ultrastructural analysis shows a relatively small number of dense core vesicles, typical of chromaffin cells under normal culture conditions (B). (Scale bar, 0.2 μm.) Incubation of PC-12 cells with AN-238 (C) induces characteristic apoptotic changes, including shrinking of the cytoplasm away from the cell wall, apoptotic bodies, internucleosomal DNA fragmentation, or condensation of the cytoplasm while retaining mitochondria and endomembrane structure. (Scale bar, 0.2 μm). DCV, dense-core vesicles; MIT, mitochondria; NTC, no template control; Nuc, nucleus.
Effects of Somatostatin RC-160 Analog and Antagonists of GHRH and LHRH in SW-13 Cells.
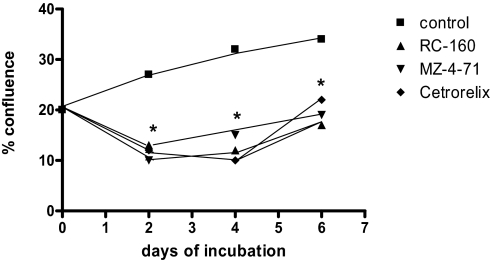
The somatostatin analog RC-160 had a significant effect on growth and survival of SW-13 human adrenocortical cells under proliferative culture conditions (10% FCS). In control cultures, the percent-confluence continually increased from a starting value of 20 ± 2% to 27 ± 3% on day 2, to 32 ± 4% and 34 ± 5% on days 4 and 6, respectively. The presence of RC-160 in the culture medium resulted in a statistically significant fall (P < 0.05) in the percent-confluence of cultures to 13 ± 3% on day 2, 12 ± 5% on day 4, and 17 ± 7% on day 6. The effects of the GHRH antagonist MZ-4–71 on proliferation of SW-13 tumor cells were also studied. In control cultures, the percent-confluence continually increased from a starting value of 27 ± 3% on day 2 to 30 ± 3% on day 4, to 34 ± 5% on day 6. The presence of MZ-4–71 in the culture medium resulted in a statistically significant reduction (P < 0.05) in the percent-confluence of cultures to 10 ± 4 % on day 2, 15 ± 6 % on day 4, and 19 ± 7 % on day 6. The effect of the LHRH antagonist Cetrorelix on growth and survival of SW-13 cells was similarly studied. In control cultures, the percent-confluence continually increased from a starting value of 27 ± 3% on day 2 to 30 ± 3% on day 4, to 34 ± 5% on day 6. The presence of Cetrorelix in the culture medium resulted in a statistically significant fall (P < 0.01) in the percent-confluence of cultures to 12 ± 2% on day 2, to 10 ± 1% on day 4, to 22 ± 3% on day 6. (Data indicate the mean ± SEM of 4 to 6 determinations from 2 separate experiments.) All compounds were added at day 0 at a concentration of 10−6 M (Fig. 3).
Fig. 3.
Effects of somatostatin analogue RC-160, GHRH antagonist MZ-4–71, and LHRH antagonist Cetrorelix on proliferation of human adrenocortical carcinoma cells in culture. This figure illustrates changes in the percent-confluence over 6 days incubation of SW-13 cell cultures under proliferative culture conditions (10% FCS), with and without the addition of the peptides MZ-4–71 and RC-160 (P < 0.05 for both) and for Cetrorelix (P < 0.01), relative to control for each day.
Effects of the Somatostatin Octapeptide Analog RC-160 and Targeted Cytotoxic Somatostatin Analogs AN-162 and AN-238 on Adrenomedullary PC-12 Tumor Cells.
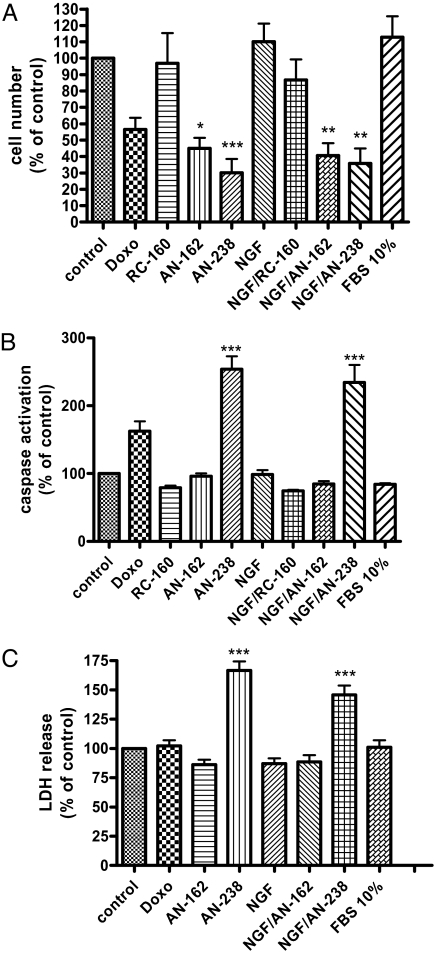
The targeted cytotoxic somatostatin analogs AN-238 and AN-162 significantly reduced the cell number of uninduced and NGF-induced tumor cells by more than 50% after incubation for 24 h; however, the noncytotoxic analog RC-160 did not affect PC-12 cell numbers (Fig. 5A). Furthermore, AN-238 had a strong proapoptotic effect on uninduced and NGF-induced tumor cells, as demonstrated by a highly significant caspase 3/7 activation to more than 250% (Fig. 5B) and a significant lactate dehydrogenase (LDH) release from these cells to more than 150% of untreated control cultures after 24 h (Fig. 5C); however, AN-162 and RC-160 did not affect caspase 3/7 activation nor LDH release under our experimental conditions.
Fig. 5.
Effects of RC-160, AN-162, and AN-238 on uninduced and NGF (20 ng/ml)-induced PC-12 cells. (A) Cell-count measurements. AN-238 and, to a lesser extent, AN-162 significantly reduced cell number of uninduced and NGF-induced PC-12 cells after 24 h (n = 4). RC-160 did not affect PC-12 cell number. (B) Apoptosis assays. AN-238 led to a highly significant activation of caspase 3/7 activation after 24 h (n = 4). There was no effect of RC-160 and AN-162. (C) Cytotoxicity assays. LDH release was significantly increased after incubation with AN-238 for 24 h of induced or NGF-induced cells as compared to control (n = 6) *P < 0.05, **P < 0.01, ***P < 0.001 as compared to control for all assays.
Discussion
In this study, we evaluated specific receptor-targeted chemotherapeutic peptide antagonists and agonists for their potential future use in the antineoplastic therapy of adrenal tumors. As with peptide receptor-targeted radiotherapy (25, 26) or gene-transfer methods for up-regulation of tumor-associated receptor expression, targeted chemotherapy represents a promising approach for delivering therapeutic compounds selectively to tumor cells (27, 28).
Based on our microarray data (23), which demonstrated a significant differential expression of neuropeptide hormone receptors in human PHEO specimens, we performed a comprehensive analysis of the expression of relevant receptors in human adrenal tumors and cell lines. Both adrenal tumor tissue and 2 adrenal tumor cell lines (PC-12 and SW-13) expressed receptors for somatostatin, GHRH, or the SV-1 splice variant, as well as for LHRH. The immunohistochemical staining of adrenal tissue showed strong staining for sst2 in normal adrenal cortex, adrenocortical adenoma and carcinoma, as well as in the SW-13 adrenocortical cell line. Regarding the adrenal medulla, both benign and malignant PHEO specimens were moderately positive for sst2, as were PC-12 PHEO cells; however, no sst2 staining could be detected in the normal adrenal medulla.
Recently, Unger et al. (16) obtained results similar in an immunohistochemical determination of sst1, -2, -3, -4, and -5 in various adrenal tumors. They were able to show that all benign PHEO were positive for sst3, 29% were positive for sst1, -2, and -5, while sst4 was not expressed. Regarding malignant PHEOs, 75% were positive for sst3, 13% and 38% positive for sst4 and -5, respectively, while none of the malignant PHEOs expressed sst1 or sst2. Most adrenocortical adenomas were positive for all 5 subtypes. Furthermore, a high expression of sst4 was found in cortisol-secreting adenomas, while only very few cortical carcinomas exhibited somatostatin immunostaining. Whereas the in vivo detection rate of somatostatin receptors by octreotide scintigraphy is disappointingly low in benign PHEO, a much higher sensitivity of 88% has been demonstrated in metastases of malignant PHEO, exceeding the sensitivity of 123I-metaiodobenzylguanidine scintigraphy (29). A recent study evaluated the effectiveness of the radiolabeled somatostatin analogue [DOTA-Tyr (3)]-octreotide in patients with metastatic paraganglioma and PHEO. Restaging 8 to 12 weeks after the last treatment cycle revealed partial remission or minor response in 25% of patients and disease stabilization in another 46% (30). Intriguingly, the binding of both octreotide and a universal somatostatin analogue SOM230 to sst1 to sst3 and sst5 significantly reduced cell viability in primary PHEO cell culture (31).
As a general rule, adrenocortical and adrenomedullary tumor cells respond to somatostatin analogues. In our study, somatostatin octapeptide RC-160 significantly reduced growth and survival of SW-13 human adrenocortical cells under proliferative culture conditions. Our ultrastructural analysis confirmed a necrotic mode of cell death, rather than a proapoptotic mechanism. At the molecular level, RC-160 might have a strong antiproliferative effect by promoting cell cycle arrest, as shown in a recent study on Chinese hamster ovary cells (31).
In addition to somatostatin analogues, potential antitumor effects of antagonists for GHRH and LHRH receptors were tested in the SW-13 adrenocortical tumor model. Immunoreactive GHRH receptor was also present in several other neoplasms, including carcinoid tumors, pancreatic cell tumors, small-cell lung cancers, and endometrial neoplasms. Furthermore, adrenal adenomas and PHEO have been shown to secrete GHRH and antagonists of GHRH were found to suppress the growth of human cancer lines, including those from breast, ovary, uterine endometrium, and prostate xenografted into nude mice (13, 19, 32). In addition, splice variants of GHRH-R have been detected on numerous tumors and found to be distinct from the pituitary GHRH receptors (24). These splice variants could mediate the antiproliferative effects of GHRH antagonists on various cancers. In our study, we were able to demonstrate a significant reduction of growth of SW-13 cells in culture. (Similar results were obtained in the NCI 295R adrenocortical cancer cell line.) The suppressive effect of GHRH antagonists on adrenal malignancies are assumed to be mediated by a reduced production of tumor growth factors, such as IGF1 and IGF2, as shown in previous studies (20). To further analyze effects thought to be mediated through GHRH signaling, we recently established an orthotopic intra-adrenal transplantation technique for adrenocortical cells that could be of value in future studies (33).
Potent antagonistic LHRH analogues, such as Cetrorelix, were recently found to interfere with mitogenic signal transduction of growth-factor receptors and related oncogene products associated with tyrosine kinase activity in some tumors, particularly those of breast, ovary, and uterine endometrium (34). In our study, Cetrorelix was found to significantly reduce the percent-confluence of SW-13 tumor cells in culture under proliferative conditions. Based on our data and those of other groups, LHRH and GHRH appear to act as important tumoral growth factors. We believe that blocking their receptors with potent antagonists could provide new approaches to therapy of endocrine tumors. Preclinical evaluation of antagonists of GHRH could prove to be very important, as somatostatin analogues do not adequately suppress GH and IGF1 levels in patients with neoplasms potentially dependent on IGF1 (19). The expression of GHRH and LHRH receptors, in addition to somatostatin receptors in the human adrenocortical SW-13 cell line, and the susceptibility of these cells to the specific antagonists Cetrorelix and MZ-4–71, indicate potential treatment approaches for adrenocortical carcinoma. In support of this possibility is a recent report, where both LHRH and GHRH antagonists were found to be very effective in the inhibition of prostate cancer (35).
In the normal adrenal medulla, no sst2 staining was found. Because PHEO specimens and PC-12 cells showed pronounced staining for sst2, it is not surprising that these cells respond to somatostatin analogues. Interestingly, a recent study of Reubi et al. (36) demonstrated that almost 90% of PHEO express sst2. We could demonstrate that AN-238, and to a lesser extent AN-162, reduced the number of uninduced PC-12 cells and of cells induced with anti-apoptotic NGF; however, in these experiments RC-160 was not effective in reducing PC-12 cell number. Because the number of PC-12 cells was significantly reduced by the cytotoxic analogs, we evaluated whether AN-238 and AN-162 might increase apoptosis or necrosis of PC-12 cells. The hybrid cytotoxic conjugate AN-238 led to a significant activation of caspases 3/7 and a strong release of LDH from uninduced and NGF-induced PC-12 cells, indicating an increase in apoptosis and necrosis. Similarly, our ultrastructural analysis supported the induction primarily of apoptotic pathways as the mode of action for cell death induced by AN-238. However, there was little effect of AN-162. Both compounds consist of the octapeptide somatostatin analogues, but AN-162 is conjugated to DOX and AN-238 to 2-pyrrolino-DOX (AN-201). AN-201 is 200 to 500 times more potent than DOX in vitro (37). In other studies, a strong effect of AN-238, possibly mediated via its cytotoxic radical AN-201, has been documented in many human and rodent experimental cancer models, especially in neoplastic cells with high mitotic activity (29, 37, 38). Furthermore, AN-238 has been reported to significantly increase the number of cells undergoing apoptosis (13). In agreement with the previous findings on PC-3 human prostate cancer cells, our study provides evidence that AN-238 leads to an increased number of apoptotic PC-12 cells.
All together, our results raise hope for improved targeted treatment strategies for adrenal diseases. An approach based on antineoplastic therapy of specific molecular pathways or targeted to receptors expressed by malignant cells, while leaving normal cells unaffected, is promising (28, 39). Furthermore, differential expression of somatostatin acceptors, GHRH receptor SV-1, and LHRH receptors in various adrenal tumors may point to new aspects of pathogenesis of these malignancies.
In conclusion, our studies show that normal adrenal cortex, adrenocortical adenomas and carcinomas, and SW-13 adrenocortical tumor cells are strongly sst2 positive. SW-13 cells were found to express receptors for GHRH SV-1 and LHRH. The somatostatin analog RC-160 had a pronounced cytostatic effect on SW-13 cells exposed to this agent during rapid cell growth. We also demonstrated a significant effect of the GHRH antagonist MZ-4–71, as well as the LHRH antagonist Cetrorelix, through a decrease in cell proliferation. In adrenomedullary specimens, we could show the expression of sst2 in human PHEO tissues and PC-12 tumor cells, although this was not found in the normal adrenal medulla. The targeted chemotherapeutic agents AN-238 and to a lesser extent AN-162 were effective in reducing cell number, most likely through the induction of cell necrosis and apoptosis. Future in vivo studies on adrenal tumors in rodents, and eventually in humans, are warranted to further evaluate AN-238 and other tumor-targeted peptides for possible therapeutic use. This generation of peptide analogues might lead to improved therapy of various neoplasms considered untreatable by current therapeutic modalities, including rare but frequently lethal adrenal tumors.
Materials and Methods
The tissue samples analyzed in this study were obtained from individuals in whom an adrenocortical adenoma or benign adrenomedullary tumor was detected at autopsy and specimens from patients with surgically removed adrenal carcinoma or malignant pheochromocytoma, as demonstrated by metastases. The cortical origin of the tumors was confirmed by immunohistochemical staining against 17 alpha-hydroxylase, cytokeratin, vimetin, and D11 protein. The medullary origin of tumors was confirmed by immunohistochemical staining against synaptophysin and chromogranins. Adenomas ranged in size between 1 and 5 cm and carcinomas between 4 and 19 cm. The patients (mean age 56.3 years) had no history of autoimmune diseases (40–42). Tumors from at least 3 different patients were analyzed for neuropeptide mRNA and protein expression studies. All human samples were received from the tumor centers in Essen, Dresden, and Düsseldorf, Germany, with the protocols being approved by the local ethical committees. All other methods and techniques are described in detail in the supporting information (SI) Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Martina Kohl for cell count measurements, Silke Langer for her assistance with immunohistochemistry, and Doreen Streichert for help with electron microscopy. We thank Dr. J. Varga for helpful comments and suggestions and Kathleen Eisenhofer and Martina Haberland for technical help. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 655 From Cells to Tissues) (to M.E.-B. and S.R.B.), and the Dresden Tumor Center of Excellence, Center for Regenerative Therapies Dresden.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907843106/DCSupplemental.
References
- 1.Lacroix A, Ndiaye N, Tremblay J, Hamet P. Ectopic and abnormal hormone receptors in adrenal Cushing's syndrome. Endocr Rev. 2001;22:75–110. doi: 10.1210/edrv.22.1.0420. [DOI] [PubMed] [Google Scholar]
- 2.Mazzuco TL, Chabre O, Feige JJ, Thomas M. Aberrant GPCR expression is a sufficient genetic event to trigger adrenocortical tumorigenesis. Mol Cell Endocrinol. 2007;265–266:23–28. doi: 10.1016/j.mce.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 3.Willenberg HS, et al. Corticotropin-releasing hormone receptor expression on normal and tumorous human adrenocortical cells. Neuroendocrinology. 2005;82:274–281. doi: 10.1159/000093126. [DOI] [PubMed] [Google Scholar]
- 4.Willenberg HS, et al. Aberrant interleukin-1 receptors in a cortisol-secreting adrenal adenoma causing Cushing's syndrome. N Engl J Med. 1998;339:27–31. doi: 10.1056/NEJM199807023390105. [DOI] [PubMed] [Google Scholar]
- 5.Lampron A, et al. Regulation of aldosterone secretion by several aberrant receptors including for glucose-dependent insulinotropic peptide in a patient with an aldosteronoma. J Clin Endocrinol Metab. 2009;94:750–756. doi: 10.1210/jc.2008-1340. [DOI] [PubMed] [Google Scholar]
- 6.Ueberberg B, et al. Differential expression of ghrelin and its receptor (GHS-R1a) in various adrenal tumors and normal adrenal gland. Horm Metab Res. 2008;40:181–188. doi: 10.1055/s-2007-1004574. [DOI] [PubMed] [Google Scholar]
- 7.Cartier D, et al. Expression profile of serotonin4 (5-HT4) receptors in adrenocortical aldosterone-producing adenomas. Eur J Endocrinol. 2005;153:939–947. doi: 10.1530/eje.1.02051. [DOI] [PubMed] [Google Scholar]
- 8.Bornstein SR, Stratakis CA, Chrousos GP. Adrenocortical tumors: recent advances in basic concepts and clinical management. Ann Intern Med. 1999;130:759–771. doi: 10.7326/0003-4819-130-9-199905040-00017. [DOI] [PubMed] [Google Scholar]
- 9.Wolf A, et al. Adrenal pheochromocytoma with contralateral cortisol-producing adrenal adenoma: diagnostic and therapeutic management. Horm Metab Res. 2005;37:391–395. doi: 10.1055/s-2005-870159. [DOI] [PubMed] [Google Scholar]
- 10.Bornstein SR, Wirth MP, Schally AV. Update on endocrine-related tumors. Horm Metab Res. 2008;40:299–301. doi: 10.1055/s-2008-1076696. [DOI] [PubMed] [Google Scholar]
- 11.Abou-Jawde R, Choueiri T, Alemany C, Mekhail T. An overview of targeted treatments in cancer. Clin Ther. 2003;25:2121–2137. doi: 10.1016/s0149-2918(03)80209-6. [DOI] [PubMed] [Google Scholar]
- 12.Buchholz S, et al. Therapy of ovarian cancers with targeted cytotoxic analogs of bombesin, somatostatin, and luteinizing hormone-releasing hormone and their combinations. Proc Natl Acad Sci USA. 2006;103:10403–10407. doi: 10.1073/pnas.0602971103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schally AV, et al. Hypothalamic hormones and cancer. Front Neuroendocrinol. 2001;22:248–291. doi: 10.1006/frne.2001.0217. [DOI] [PubMed] [Google Scholar]
- 14.Plonowski A, et al. In vivo inhibition of PC-3 human androgen-independent prostate cancer by a targeted cytotoxic bombesin analogue, AN-215. Int J Cancer. 2000;88:652–657. doi: 10.1002/1097-0215(20001115)88:4<652::aid-ijc21>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Sun B, Schally AV, Halmos G. The presence of receptors for bombesin/GRP and mRNA for three receptor subtypes in human ovarian epithelial cancers. Regul Pept. 2000;90:77–84. doi: 10.1016/s0167-0115(00)00114-2. [DOI] [PubMed] [Google Scholar]
- 16.Unger N, et al. Immunohistochemical localization of somatostatin receptor subtypes in benign and malignant adrenal tumours. Clin Endocrinol (Oxf) 2008;68:850–857. doi: 10.1111/j.1365-2265.2007.03124.x. [DOI] [PubMed] [Google Scholar]
- 17.Barkan AL. New options for diagnosing and treating acromegaly. Cleve Clin J Med. 1998;65:347–349. doi: 10.3949/ccjm.65.7.343. [DOI] [PubMed] [Google Scholar]
- 18.Drange MR, Melmed S. Long-acting lanreotide induces clinical and biochemical remission of acromegaly caused by disseminated growth hormone-releasing hormone-secreting carcinoid. J Clin Endocrinol Metab. 1998;83:3104–3109. doi: 10.1210/jcem.83.9.5088. [DOI] [PubMed] [Google Scholar]
- 19.Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: an emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab. 2008;4:33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
- 20.Schally AV. New approaches to the therapy of various tumors based on peptide analogues. Horm Metab Res. 2008;40:315–322. doi: 10.1055/s-2008-1073142. [DOI] [PubMed] [Google Scholar]
- 21.Nagy A, et al. Synthesis and biological evaluation of cytotoxic analogs of somatostatin containing doxorubicin or its intensely potent derivative, 2-pyrrolinodoxorubicin. Proc Natl Acad Sci USA. 1998;95:1794–1799. doi: 10.1073/pnas.95.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gola M, Bonadonna S, Mazziotti G, Amato G, Giustina A. Resistance to somatostatin analogs in acromegaly: an evolving concept? J Endocrinol Invest. 2006;29:86–93. doi: 10.1007/BF03349183. [DOI] [PubMed] [Google Scholar]
- 23.Brouwers FM, et al. Gene expression profiling of benign and malignant pheochromocytoma. Ann N Y Acad Sci. 2006;1073:541–556. doi: 10.1196/annals.1353.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rekasi Z, Czompoly T, Schally AV, Halmos G. Isolation and sequencing of cDNAs for splice variants of growth hormone-releasing hormone receptors from human cancers. Proc Natl Acad Sci USA. 2000;97:10561–10566. doi: 10.1073/pnas.180313297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchsbaum DJ, Chaudhuri TR, Zinn KR. Radiotargeted gene therapy. J Nucl Med. 2005;46:179–186. [PubMed] [Google Scholar]
- 26.van Essen M, et al. Peptide-receptor radionuclide therapy for endocrine tumors. Nat Rev Endocrinol. 2009 doi: 10.1038/nrendo.2009.105. Jun 2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Hofland LJ, Lamberts SW. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev. 2003;24:28–47. doi: 10.1210/er.2000-0001. [DOI] [PubMed] [Google Scholar]
- 28.Schally AV, Nagy A. Cancer chemotherapy based on targeting of cytotoxic peptide conjugates to their receptors on tumors. Eur J Endocrinol. 1999;141:1–14. doi: 10.1530/eje.0.1410001. [DOI] [PubMed] [Google Scholar]
- 29.van der Harst E, et al. [(123)I]metaiodobenzylguanidine and [(111)In]octreotide uptake in begnign and malignant pheochromocytomas. J Clin Endocrinol Metab. 2001;86:685–693. doi: 10.1210/jcem.86.2.7238. [DOI] [PubMed] [Google Scholar]
- 30.Forrer F, Riedweg I, Maecke HR, Mueller-Brand J. Radiolabeled DOTATOC in patients with advanced paraganglioma and pheochromocytoma. Q J Nucl Med Mol Imaging. 2008;52:334–340. [PubMed] [Google Scholar]
- 31.Pasquali D, et al. Effects of somatostatin analog SOM230 on cell proliferation, apoptosis, and catecholamine levels in cultured pheochromocytoma cells. J Mol Endocrinol. 2008;40:263–271. doi: 10.1677/JME-08-0012. [DOI] [PubMed] [Google Scholar]
- 32.Pages P, et al. sst2 somatostatin receptor mediates cell cycle arrest and induction of p27(Kip1). Evidence for the role of SHP-1. J Biol Chem. 1999;274:15186–15193. doi: 10.1074/jbc.274.21.15186. [DOI] [PubMed] [Google Scholar]
- 33.Cardoso CC, Bornstein SR, Hornsby PJ. New methods for investigating experimental human adrenal tumorigenesis. Mol Cell Endocrinol. 2009;300:175–179. doi: 10.1016/j.mce.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emons G, Ortmann O, Schulz KD, Schally AV. Growth-inhibitory actions of analogues of luteinizing hormone releasing hormone on tumor cells. Trends Endocrinol Metab. 1997;8:355–362. doi: 10.1016/s1043-2760(97)00155-0. [DOI] [PubMed] [Google Scholar]
- 35.Stangelberger A, et al. The combination of antagonists of LHRH with antagonists of GHRH improves inhibition of androgen sensitive MDA-PCa-2b and LuCaP-35 prostate cancers. Prostate. 2007;67:1339–1353. doi: 10.1002/pros.20605. [DOI] [PubMed] [Google Scholar]
- 36.Reubi JC, Waser B, Liu Q, Laissue JA, Schonbrunn A. Subcellular distribution of somatostatin sst2A receptors in human tumors of the nervous and neuroendocrine systems: membranous versus intracellular location. J Clin Endocrinol Metab. 2000;85:3882–3891. doi: 10.1210/jcem.85.10.6864. [DOI] [PubMed] [Google Scholar]
- 37.Koppan M, et al. Targeted cytotoxic analogue of somatostatin AN-238 inhibits growth of androgen-independent Dunning R-3327-AT-1 prostate cancer in rats at nontoxic doses. Cancer Res. 1998;58:4132–4137. [PubMed] [Google Scholar]
- 38.Schally AV, Nagy A. Chemotherapy targeted to cancers through tumoral hormone receptors. Trends Endocrinol Metab. 2004;15:300–310. doi: 10.1016/j.tem.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30:781–793. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 40.Merke DP, et al. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N Engl J Med. 2000;343:1362–1368. doi: 10.1056/NEJM200011093431903. [DOI] [PubMed] [Google Scholar]
- 41.Marx C, Wolkersdorfer GW, Brown JW, Scherbaum WA, Bornstein SR. MHC class II expression–a new tool to assess dignity in adrenocortical tumours. J Clin Endocrinol Metab. 1996;81:4488–4491. doi: 10.1210/jcem.81.12.8954065. [DOI] [PubMed] [Google Scholar]
- 42.Marx C, et al. Adrenocortical hormones in survivors and nonsurvivors of severe sepsis: diverse time course of dehydroepiandrosterone, dehydroepiandrosterone-sulfate, and cortisol. Crit Care Med. 2003;31:1382–1388. doi: 10.1097/01.CCM.0000063282.83188.3D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.