Abstract
Background and Purpose
Stroke is a major cause of death and disability, and it is imperative to develop therapeutics to mitigate stroke-related injury. Despite many promising prospects, attempts at translating neuroprotective agents that show success in animal models of stroke have resulted in very limited clinical success.
Summary of Review
This review discusses reasons for the lack of translational success based on the therapeutic targets tested and the pathophysiology of stroke. New recanalization therapies and alternative therapeutic strategies are discussed concerning mitochondria-mediated cell death. Mitochondrial death-regulation pathways are divided into 3 categories: Upstream signaling pathways, agents that target mitochondria directly, and downstream death-execution effectors. The apoptosis signal-related kinase/c-Jun–terminal kinase pathway is used as an example to provide rationale as to why inhibiting signaling pathway upstream of mitochondrial dysfunction is a promising therapeutic approach. Finally, the mechanisms of autophagy and mitochondrial biogenesis are discussed in relation to stroke.
Conclusions
Increasing evidence suggests that reperfusion is necessary for improved neurological outcomes after stroke. Development of improved recanalization methods with increased therapeutic windows will aid in improving clinical outcome. Adjunct neuroprotective interventions must also be developed to ensure maximal brain tissue salvage. Targeting prodeath signaling pathways upstream of mitochondrial damage is promising for potential clinically effective treatment. Further understanding of the roles of equilibrium of autophagy and mitochondrial biogenesis in the pathogenesis of stroke could also lead to novel therapeutics.
Keywords: cerebral ischemia, therapeutics, neuroprotection, mitochondria
Each year approximately 795 000 people are afflicted with stroke, which ranks as the third leading cause of death in the United States when considered independently from other cardiovascular diseases.1 Moreover, stroke is a leading cause of severe long-term disability in this country,2 with approximately 30.7% of stroke survivors receiving outpatient rehabilitation for stroke-related disability.1 Accordingly, the estimated cost of stroke and stroke-related disability for 2009 is $68.9 billion.3 Stroke is therefore a substantial health problem in the United States and warrants extensive attention to mitigate stroke-related death and disability.
Tremendous progress has been made in our understanding of the pathophysiology of stroke. However, to date, drugs that have been effective in preclinical trials have not been effective in stroke patients. There are many reasons for these failures at the clinical level, including inappropriate subject selection, outcome measures, and time and dosage of medication administration.4 Another major problem lies in the therapeutic targets selected for clinical trials. This review examines the source of the failure of clinical trials based on the therapeutic strategies used and provides rationale and alternative molecular targets for alternate potential clinical therapies.
Past Clinical Strategies
Analysis of the Stroke Trials Registry (http://www.strokecenter.org/trials/) in October 2008 revealed 235 completed trials involving acute ischemic stroke. Of these trials, 81 (34%) used thrombolytic or anticoagulant therapies, 12 (5%) examined the effect of mechanical intervention (ie, surgery), and 5 (2%) examined the effect of the combined modalities. Thus, approximately 42% of clinical trials conducted have focused on mechanisms to recanalize occluded vessels. The other major category of clinically examined therapy has targeted excitotoxicity after ischemia and reperfusion. Thirty-five trials (15%) have used glutamate, AMPA, GABA, glycine, and ion channel antagonists to inhibit excitoxicity after ischemia.5
Prompt reestablishment of blood flow to restore oxygen and glucose to deprived brain tissue is important for minimizing infarct area. It is also critical to understand the pathophysiological events induced by ischemia and reperfusion both in the core area and in the penumbral area of the ischemic insult. Brain tissue immediately supplied by the occluded vessel dies rapidly, and this tissue death has been traditionally thought to be attributable to uncontrolled necrosis. In this area of irreversible damage, or core of the infarct, the extent of the damage is a function of the breadth and depth of the infarct. In areas adjacent to the core, collateral blood vessels maintain sufficient flow and brain tissue experiences a less severe insult. Neurons in this penumbral area have impaired function but remain viable for a period of time before succumbing to the insult. Because these neurons die in a delayed manner, targeting this damaged but still salvageable brain region will potentially reduce infarct volume and improve neurological outcome.6
Reperfusion Therapy
As stated above, 42% of completed clinical trial focus has been on reperfusion therapy. Despite this attention, tissue plasminogen activator (tPA) is the only approved therapy for thrombolysis.7 Unfortunately, because of the limited therapeutic window (≤3 hours, wherein the greatest therapeutic benefit is seen within 90 minutes), tPA is given to only about 3% to 8.5% of ischemic stroke patients.8 This is further frustrated by the marginal therapeutic benefit seen by tPA. The recent ECASS III study attempted to examine the effect of tPA on ischemic stroke outcome using a time window of 3 to 4.5 hours for administration. Although the study showed that tPA retained a modest favorable outcome when administered between 3 to 4.5 hours (52.4% tPA-treated versus 45.2% placebo), there was also a significant increase in intracranial hemorrhage.9 Therefore, despite an overall benefit in delayed thrombolytic therapy, it is diminished with greater ischemic periods.
Alternative recanalization therapies tested include intraarterial therapy with thrombolytics and mechanical embolectomy. The PROACT II study realized a 15% increase in improved neurological outcome over control patients when intraarterial administration of the thrombolytic prourokinase was received within 6 hours of ischemic stroke onset.10 In the MERCI trial, use of a mechanical embolectomy device within 8 hours of stroke onset resulted in significant improvement in recanalization percentage and good neurological outcome.11
Accumulating evidence suggests that reperfusion consistently results in better outcomes. Without reperfusion it is unlikely any neuroprotective strategy will have much measurable benefit. This is because it may be difficult for any therapy to reach the affected area, and, in addition, the clinical deficits from the occlusion may overwhelm any protective benefit. Thus, neuroprotective therapy will likely be most effective as an adjunct to reperfusion therapy. However, although restoring blood flow is important for infarct reduction, brain tissue in the penumbra has already been damaged, and restoration of blood flow will not protect all of the brain tissue destined to die. To maximize brain tissue salvation, concomitant therapies must be developed. Development of such therapies will be aided by an understanding of the pathophysiological events underlying programmed cell death (PCD) in the penumbra.
Antiexcitotoxicity Therapy
Initial neuroprotective clinical stroke trials primarily used antiexcitotoxic drugs to limit acute calcium-mediated injury attributable to cerebral ischemia. The premise for this strategy is that after ischemia there is an immediate loss of energy leading to uncontrolled depolarization of neurons. Massive release of glutamate to the surrounding tissue ensues, causing glutamate receptor activation with consequential intracellular calcium overload and impaired ion homeostasis. Increased calcium produces acute energy failure via severe disruption of mitochondria and activation of proteases, resulting in acute necrosis. Less severe excitotoxicity may initiate the cascade of events leading to programmed cell death (PCD). It is accepted that excitotoxicity is the basis for necrotic damage in the ischemic core and the initiator of PCD in the penumbra. However, the excitotoxic phase mediated by release of excitatory acids into brain peaks within to 1 to 2 hours after ischemia, and treatments with antiexcitotoxic drugs are only effective when given within 1 to 2 hours after focal ischemia in animal models.12 Considering that the median hospital arrival time for stroke patients is 3 to 6 hours,13 and all of the trials of antiexcitotoxic drugs have treated patients up to at least 6 hours after onset of symptoms, the negative outcomes of the 35 completed trials of antiexcitotoxic agents are not surprising.
As noted above, reperfusion therapy and antiexcitotoxic agents have limited therapeutic benefit mainly because of feasibility of administration within an acceptable therapeutic window. In the development of new therapies, focus must be placed on targets producing long-lasting protection and efficacy even when treatments are administered during or after 4 to 6 hours after symptom onset, which is when patients actually arrive in the hospital. A promising strategy for the development of such therapeutics is to inhibit the mechanisms that destabilize mitochondria. The remainder of this review focuses on the rationale for targeting mitochondria to develop stroke therapeutics and discusses specific targets that have potential to exhibit long-lasting protection in the clinic.
Mitochondrial Stroke Targets
Mitochondria are essential organelles involved with oxidative phosphorylation, calcium homeostasis, reactive oxygen species (ROS) management, and PCD. Convergence of a number of cell death pathways emanating from membrane receptors, the cytosol, nucleus, lysosome, and endoplasmic reticulum on the mitochondria results in mitochondrial destabilization.14 A common consequence of these death pathways is damage to the mitochondria, resulting in mitochondrial membrane permeabilization (MMP). Indeed, a number of assays have been developed to measure MMP as an indicator of cytotoxicity.15 Mitochondrial membrane destabilization results in the release of mitochondrial components (ie, cytochrome c and apoptosis-inducing factor), which in turn initiate the caspase-dependent and -independent intrinsic PCD pathways.
The effectors of mitochondria-related PCD can be divided into 3 categories: (1) downstream mitochondrial death effectors; (2) agents that directly target and destabilize mitochondrial membranes; and (3) upstream signaling mechanisms. Identifying the category of mitochondria-related PCD is critical for focusing the search on targets that will provide optimal protection against ischemia. The following section will discuss the 3 categories and their therapeutic potential.
Downstream Mitochondrial Death Effectors
Perturbation of the mitochondrial membrane results in release into the cytosol of cytotoxic molecules such as cytochrome c and apoptosis inducing factor (AIF) initiating caspase-dependent and -independent forms of cell death, respectively. In caspase-dependent cell death, released cytochrome c activates the caspase cascade, causing cleavage of many proteins, DNA damage, and ultimately cell death (for review see reference16). Other mitochondrial proteins are also released to negatively regulate the endogenous inhibitors of caspases such as X-lined inhibitor of apoptosis (XIAP). Caspase-independent cell death occurs via calpain- or poly (ADP-ribose) polymerase-1 (PARP1)-mediated AIF release from the mitochondria, with subsequent translocation to the nucleus.17
Attempts at inhibiting these downstream mitochondrial death effectors have been successful at producing robust neuroprotection. A caveat is that neuroprotection as measured by both decreased infarct volume and neurobehavioral recovery has been assessed only after brief periods of reperfusion. Examining a recent review analyzing the neuroprotection afforded by downstream mitochondrial death effectors reveals that neuroprotection was assessed only between 24 hours to 1 week after insults of neonatal hypoxia/ischemia and focal and global ischemia in adult animals (see Table 1 in Galluzzi et al17). Assessment included caspase inhibition, XIAP overexpression, and protein transduction domain (PTD)-fused XIAP, HSP70 overexpression (which binds and sequesters AIF released from the mitochondria), AIF deletion, and peptide inhibition.
Two reasonable conclusions can be drawn from these data: (1) neuroprotection was not assessed longer than 1 week after the insult; or (2) the interventions were no longer neuroprotective at later times. Using the transient global ischemia model in rats, we designed an experiment to answer this question. Comparison of PTD-fused Apaf-1 interacting protein (AIP), an inhibitor of Apaf-1 and caspase-dependent PCD, and PTD-fused Bcl-xL was performed to determine whether inhibition of PCD at the mitochondria (Bcl-xL) or inhibition of a downstream death effector (AIP) could confer long-term neuroprotection. We found that although PTD-AIP administration decreased death of hippocampal CA1 neurons 4 days after ischemia, there was no neuroprotection or improved spatial learning or memory 60 days after ischemia. Alternatively, administration of Bcl-xL increased CA1 survival up to 60 days after ischemia and was associated with improved spatial learning and memory.18 Thus, inhibition of downstream effectors affords merely short-term protection, likely attributable to the presence of compensatory mechanisms of the mitochondria (ie, AIF release) or to mitochondria-independent mechanisms including genomic alterations and increased oxidative stress.
Direct Targets of Mitochondria
Direct effectors of mitochondrial membrane disruption act further upstream in the mitochondrial death decision pathway. The foremost proteins involved with mitochondrial impairment are the prodeath Bcl-2 family proteins including Bax, Bak, Bid, Bad, Bim, and PUMA, among others. The Bcl-2 multidomain proteins Bax and Bak directly cause mitochondrial membrane disruption via channel formation in the outer mitochondrial membrane. The BH3-only proteins Bid and PUMA act to facilitate Bax and Bak channel formation, whereas Bad and Bim inhibit prosurvival Bcl-2 and Bcl-xL.19
Analysis of a number of studies shows neuroprotection is afforded when either inhibition of prodeath Bcl-2 family proteins or increased prosurvival Bcl-2 family expression is performed (see Table 2 in Galluzzi et al17). However, none of these studies examined neuroprotection past 7 days. Examination of infarct volume 48 hours after focal ischemia shows that single gene deletion of prodeath Bcl-2 family proteins Bax, Bid, Bim, and PUMA produces limited neuroprotection. Even double knockout Bax/Bid mice provide just 48 hours of protection, as infarct volume is similar to controls at 14 days. However, triple knockout mice with deletions of Bax, Bid, and PUMA show prolonged neuroprotection up to 14 days (authors’ unpublished results, 2008). Single gene targeting of prodeath Bcl-2 family proteins is not sufficient to attenuate prodeath signaling, indicating that targeting 3 or more of this family of proteins is necessary to confer neuroprotection. Therefore, there is a functional redundancy in prodeath Bcl-2 family proteins, which is not surprising because different cell death stimuli can activate different prodeath Bcl-2 family proteins.20
Because there is a functional redundancy in the prodeath Bcl-2 family proteins, it is very probable that the broad insult of ischemia initiates numerous mechanisms activating multiple prodeath Bcl-2 family proteins. Therapeutically, one can look upstream to the natural inhibitors of the prodeath proteins. Prosurvival Bcl-2 and Bcl-xL sequester Bax and Bak, inhibiting oligomerization and mitochondrial membrane permeabilization. Recent studies have revealed that administration of PTD-fused Bcl-xL can provide robust neuroprotection 60 days after global ischemia18 and up to 8 weeks after neonatal hypoxia-ischemia.21
Upstream Signaling Mechanisms
c-Jun N-Terminal Kinase
Using the reasoning that selecting targets further upstream provides neuroprotection superior to that of downstream targets, we consider signaling pathways upstream of mitochondria-induced neuronal death. An important kinase consistently activated after cerebral-ischemia is the MAP kinase c-Jun N-terminal kinase (JNK; for review see reference22). Inhibition of JNK signaling has high therapeutic potential because of its diverse mechanisms of inducing PCD.
Activation of JNK signaling directly and indirectly regulates prodeath Bcl-2 family proteins to cause mitochondrial membrane disruption. JNK phosphorylates the scaffolding protein 14−3−3, causing release and subsequent translocation of Bax to mitochondria.23 Bax activation is further induced by JNK-mediated phosphorylation of Bad and Bim, resulting in inhibition of prosurvival Bcl-xL and Bcl-2. Another consequence of JNK signaling is alterations in transcription of the transcription factor c-Jun. Activation of c-Jun promotes upregulation of Fas, Bim, and TNFα while decreasing expression of prosurvival Bcl-xL.24 Thus, JNK can induce neuronal death directly through mitochondrial death pathways, and alterations in the genomic response can shift gene expression toward production of prodeath mediators.
Importantly, the time frame for JNK activation coincides with the optimal time for maximal therapeutic potential. Elevated JNK activity begins at 6 hours and continues to 3 days in hippocampal CA1 neurons after global ischemia25 and between 0.5 and 24 hour following transient focal ischemia23,26 in rats. Indeed, compelling evidence has shown that inhibition of JNK using intracerebroventricular injection of the pharmacological inhibitor SP60012523 or the small peptide inhibitor D-JNKI-126 provides robust neuroprotection after 60 or 30 minutes of focal ischemia, respectively. Long-lasting neuroprotection and neurobehavioral recovery are realized up to 14 days of reperfusion when D-JNKI-1 is administered 6 hours after ischemia.26 Remarkably, recent evidence also shows D-JNKI-1 administered i.v. 3 hours after 90-minute focal ischemia resulted in improved sensorimotor and cognitive outcome up to 10 days.27 In sum, this neuroprotective agent demonstrates robust and long-lasting neuroprotection and neurobehavioral recovery, and it can concurrently be administered systemically at delayed times. This is an exciting prospect for realizing clinical neuroprotection.
Apoptosis Signal-Regulating Kinase
Keeping with the rationale that targeting upstream effectors of neuronal death has greater potential to afford long-lasting neuroprotection, we consider targeting the MAP kinase kinase kinase upstream of JNK activation, apoptosis signal-regulating kinase 1 (ASK1). Oxidative stress, endoplasmic reticulum (ER) stress, calcium overload, and TNFα receptor (TNFR) stimulation—cytotoxic stressors that are implicated in ischemic damage—can all activate ASK1.28
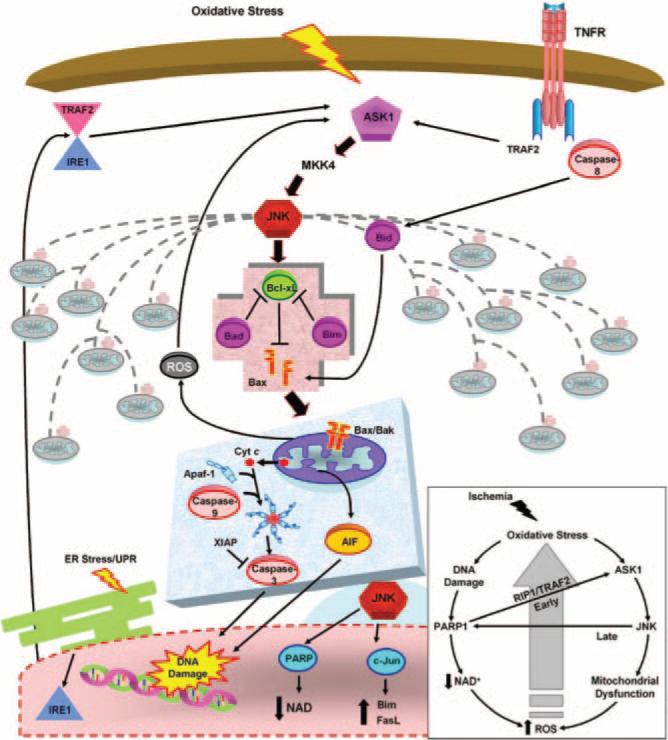
Another important factor involved with ischemia, PARP1, can enhance and be activated by ASK1/JNK signaling. Initially after ischemia, oxidative stress causes DNA damage activating PARP1 (for review, see29). Activation of PARP1 can stimulate JNK via receptor-interacting protein 1 (RIP1) and TNF receptor–associated factor 2 (TRAF2) activation of ASK1. Activated JNK, in turn, can enable PARP1 activation,30 creating a feed-forward cycle. Thus, oxidative damage immediately causes ASK1 activation in the cytosol leading to JNK activation, mitochondrial damage with release of downstream death effectors, and increased ROS production. At the same time, oxidative stress causes DNA damage, PARP1 activation, and depletion of NAD. Depletion of NAD, an ATP precursor, results in increased ATP demand and further ROS production. PARP activation can also induce ASK1/JNK signaling, which can further perpetuate its own activity. Stresses occurring at later times after injury such as ER stress and inflammation-activated TNFR also stimulate ASK1/JNK, propagating PCD after a lethal ischemic insult (Figure 1). Thus, ASK1 is an attractive therapeutic target because of its extensive role in enacting and perpetuating cell death. The kinase domain of ASK1 is also evolutionarily conserved,28 facilitating development of small peptide inhibitors that have clinical potential.
Figure 1.
Targeting the ASK1/MKK4/JNK pathway upstream of mitochondrial prodeath signaling. Oxidative stress caused by cerebral ischemia results in activation of mitochondrial death signaling. Initially activated by oxidative stress, ASK1 signals JNK activation via MKK4. Activated JNK triggers prodeath Bcl-2 proteins to disrupt the mitochondrial membrane. Subsequent release of downstream mitochondrial death effectors ensues, causing DNA damage and ultimate neuronal death. Alternative stresses that act at later times after ischemia, such as ER stress and inflammation, can also activate ASK1/MKK4/JNK signaling. In addition to the direct signaling consequences, JNK activation results in a genomic response, altering the expression of key regulators of neuronal death. Inset: Oxidative stress simultaneously causes DNA damage and ASK1 activation. DNA damage activates PARP1, leading to NAD depletion and increased ROS. ASK1 results in mitochondrial damage and increased ROS. The increase in ROS causes further oxidative stress and propagation of the cycle. Cross-talk also occurs as PARP1 activation can activate ASK1, and JNK activation can also activate PARP at later times after ischemia. Progressive ROS accumulation causes increased JNK activation and damage to multiple mitochondria, ensuring neuronal demise. AIF indicates apoptosis-inducing factor; Apaf-1, apoptosis-activating factor-1; ASK1, apoptosis signal-regulating kinase 1; cyt c, cytochrome c; ER, endoplasmic reticulum; FasL, Fas ligand; IRE1, inositol requirement 1; JNK, c-Jun–terminal kinase; NAD, nicotinamide adenine dinucleotide; PARP, poly (ADP-ribose) polymerase; RIP1, receptor interacting protein 1; ROS, reactive oxygen species; TRAF2, TNF receptor-associated factor 2; TNFR, tumor necrosis factor receptor; XIAP, X-linked inhibitor of apoptosis.
Future Targets for Stroke-Therapy Development
The previous sections provide an argument for targeting upstream effectors of mitochondrial death pathways based on extensive mechanistic information. Burgeoning evidence also implicates the regulatory processes of mitochondrial biogenesis and autophagy in the pathogenesis of stroke. The next section discusses these topics in relation to the current information on their role in stroke and how targeting these processes may lead to alternative therapeutics.
Autophagy
Ischemic injury causes extensive and progressive damage to mitochondria, and the prevailing mechanism for eliminating damaged mitochondria is autophagy. Autophagy is a highly regulated process that breaks down organelles and macromolecules through lysosomal degradation and is essential for maintenance of intracellular homeostasis. It serves as a survival mechanism during times of limited nutrients, as macromolecules are recycled for ATP generation and nascent macromolecule synthesis. Autophagy can also cause autophagic or type II PCD, which is in contrast to type I PCD, or more classical caspase- and AIF-dependent PCD (for review see reference31).
The role of autophagy after cerebral ischemia is beginning to be elucidated. An ischemic insult causes oxidative stress that damages multiple intracellular targets. Thus, efficient clearance of damaged organelles and macromolecules would be protective. In contrast, uncontrolled autophagy would lead to progressive digestion of affected neurons and neuronal death.
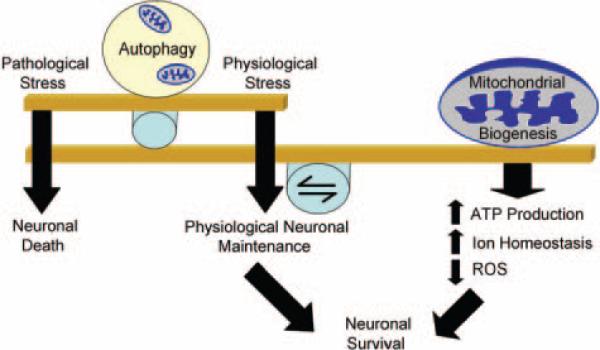
Genetic deletion of essential autophagy genes Atg 5 and 7 in mice results in neurodegeneration, suggesting that autophagy is important for normal neuronal function.32 Evidence of autophagy-induced PCD is also found in ischemia-effected neurons. Abrogation of Atg7 expression resulted in hippocampal CA1 neuron protection after neonatal hypoxic-ischemia,33 and inhibition of autophagy using pharmacological inhibitors reduced infarct volume after permanent focal ischemia.34 Beclin-1, a protein involved in autophagosome formation, is also upregulated after ischemia.32 Beclin-1 may be activated in ischemia via JNK inhibition of Beclin-1 binding protein, Bcl-2, allowing for autophagy activation.35 Thus, autophagy can be viewed as a double-edged sword; it is protective when activated by mild physiological stressors, but it can be detrimental to neuronal survival because of overactivation caused by a severe pathological stress such as ischemia (Figure 2).
Figure 2.
Mitochondrial biogenesis-autophagy equilibrium. A delicate balance exists between the formation of new and disposal of damaged mitochondria. After a physiological stress such as starvation, autophagy is induced to clear damaged organelles (including mitochondria) and macromolecules. Concomitant activation of mitochondrial biogenesis ensures adequate ATP production and ion homeostasis. A pathological insult such as ischemia does initiate mitochondrial biogenesis; however, it is insufficient to counteract the damage, and the balance is tipped because of uncontrolled autophagy. Regulating autophagy and enhancing mitochondrial biogenesis are novel neuroprotective strategies that should receive focus in the near future.
Mitochondrial Biogenesis
Mitochondria are important for cellular homeostasis. However, mitochondria are not static organelles. Fluctuating homeostatic demands and inherent production of ROS by mitochondria cause progressive damage and require dynamic regulation of turnover, content, function, and number.36 This is especially essential for proper function of postmitotic neurons.
Information is limited regarding the role of mitochondrial biogenesis in neurons. Initially, focus has been directed at transcriptional regulation of mitochondrial biogenesis. Neurodegeneration caused by oxidative stress is increased in a ROS-mediated manner in mitochondrial transcription factor peroxisome proliferator–activated receptor coactivator (PGC) 1α null mice.37 Hypoxic preconditioning stimulates neuronal nitric oxide synthase (nNOS)-dependent upregulation of PGC-1α expression.38 Oxidative stress also causes extensive mitochondrial fission, an event that precedes neuronal death. Interestingly, neuronal death can be abrogated by overexpression of the mitochondrial fusion protein mitofusin 2.39 This suggests that tight regulation of fission and fusion is important for neuronal viability. Finally, a recent study demonstrates that hypoxia-ischemia induces mitochondrial biogenesis. After hypoxia, increases are seen in mitochondrial DNA, total mitochondrial number, expression of the mitochondrial transcription factors downstream of PGC-1α (nuclear respiratory factor 1 and mitochondrial transcription factor A), and the mitochondrial protein HSP60.40 This is an exciting finding that suggests mitochondrial biogenesis is a novel endogenous neuroprotective response.
In summary, after a lethal ischemic insult, mitochondria are damaged, and autophagy is induced to remove damaged organelles. However, maintenance of energy levels and zinc and calcium homeostasis is necessary for neuronal function. Under stress, mitochondrial biogenesis becomes an essential endogenous neuroprotective response that creates new functional mitochondria. Therefore, stimulation or enhancement of mitochondrial biogenesis is a novel neuroprotective strategy (Figure 2).
Clearly, more research is necessary to determine the mechanistic underpinnings of mitochondrial biogenesis in relation to stroke. Future studies could investigate the role of alternate transcriptional mechanisms, mitochondrial fission and fusion mechanisms, and mitochondrial turnover after ischemia.36
Conclusion
Discovery of viable neuroprotective agents is imperative to mitigate the death and disability caused by stroke. Efforts to optimize recanalization therapy are important, as patients with restored blood flow show improved neurological function. Moreover, it is unlikely that neuroprotective strategies would be beneficial without adequate blood flow to damaged tissue. Neuroprotective strategies should therefore be used as an adjunct therapy to provide maximal brain tissue salvation. Because the majority of patients arrive at the hospital later than 3 hours after stroke, basic research should focus on identifying and inhibiting mediators of PCD to provide neuroprotection and neurobehavioral recovery when administered at delayed intervals after ischemia.
Mitochondrial death signaling is essential for programmed cell death, and targeting upstream signaling mechanisms has the greatest potential for long-term neuroprotection, even with delayed administration. Research should also focus on the roles of mitochondrial biogenesis and autophagy in the pathogenesis of cerebral ischemia. Damaged mitochondria must be discarded, but the ischemic insult disrupts the delicate balance of biogenesis and autophagy. Therefore, a multi-pronged strategy is needed, aimed at enhancing biogenesis, regulating autophagy, and inhibiting the deleterious actions of signaling pathways detrimental to mitochondrial function. Delineation of the key mediators of autophagy and mitochondrial biogenesis will provide novel targets for therapeutic development to enhance the beneficial effects of mitochondrial preservation.
Sources of Funding
This work was supported by NIH/NINDS grants (NS43802, NS45048, NS44178, NS56118, NS36736, and NS37459), VA Merit Reviews to J.C. and S.H.G., and a NINDS NRSA predoctoral fellowship grant to P.S.V. (F30NS057886).
Footnotes
Disclosures
None.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics–2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Prevalence of disabilities and associated health conditions among adults–United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:120–125. [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics–2007 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 4.Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure: Lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 5.2008 Stroke trials registry. http://www.strokecenter.org/trials/
- 6.Lo EH. A new penumbra: Transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 7.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 8.Reeves MJ, Arora S, Broderick JP, Frankel M, Heinrich JP, Hickenbottom S, Karp H, LaBresh KA, Malarcher A, Mensah G, Moomaw CJ, Schwamm L, Weiss P. Acute stroke care in the US: Results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke. 2005;36:1232–1240. doi: 10.1161/01.STR.0000165902.18021.5b. [DOI] [PubMed] [Google Scholar]
- 9.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 10.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM, Silver F, Rivera F. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: A randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 11.Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, Lutsep HL, Rymer MM, Higashida RT, Starkman S, Gobin YP, Frei D, Grobelny T, Hellinger F, Huddle D, Kidwell C, Koroshetz W, Marks M, Nesbit G, Silverman IE. Mechanical thrombectomy for acute ischemic stroke: Final results of the Multi MERCI Trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 12.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 13.Evenson KR, Rosamond WD, Morris DL. Prehospital and in-hospital delays in acute stroke care. Neuroepidemiology. 2001;20:65–76. doi: 10.1159/000054763. [DOI] [PubMed] [Google Scholar]
- 14.Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: A matter of life or death. Free Radic Biol Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 15.Galluzzi L, Zamzami N, de La Motte Rouge T, Lemaire C, Brenner C, Kroemer G. Methods for the assessment of mitochondrial membrane permeabilization in apoptosis. Apoptosis. 2007;12:803–813. doi: 10.1007/s10495-007-0720-1. [DOI] [PubMed] [Google Scholar]
- 16.Taylor RC, Cullen SP, Martin SJ. Apoptosis: Controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 17.Galluzzi L, Morselli E, Kepp O, Kroemer G. Targeting post-mitochondrial effectors of apoptosis for neuroprotection. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbabio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y, Cao G, Iwai M, Signore A, Chen J. Antiapoptotic intervention targeting the mitochondrial signaling pathway attenuates Ca1 neuronal loss and cognitive dysfunction after transient global ischemia. Stroke. 2007;38:560. Abstract. [Google Scholar]
- 19.Youle RJ, Strasser A. The bcl-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 20.Steckley D, Karajgikar M, Dale LB, Fuerth B, Swan P, Drummond-Main C, Poulter MO, Ferguson SS, Strasser A, Cregan SP. PUMA is a dominant regulator of oxidative stress induced Bax activation and neuronal apoptosis. J Neurosci. 2007;27:12989–12999. doi: 10.1523/JNEUROSCI.3400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin W, Cao G, Johnnides MJ, Signore AP, Luo Y, Hickey RW, Chen J. Tat-mediated delivery of Bcl-XL protein is neuroprotective against neonatal hypoxic-ischemic brain injury via inhibition of caspases and AIF. Neurobiol Dis. 2006;21:358–371. doi: 10.1016/j.nbd.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Johnson GL, Nakamura K. The c-Jun kinase/stress-activated pathway: Regulation, function and role in human disease. Biochim Biophys Acta. 2007;1773:1341–1348. doi: 10.1016/j.bbamcr.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Y, Signore AP, Yin W, Cao G, Yin XM, Sun F, Luo Y, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J Cereb Blood Flow Metab. 2005;25:694–712. doi: 10.1038/sj.jcbfm.9600062. [DOI] [PubMed] [Google Scholar]
- 24.Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Wang XT, Pei DS, Xu J, Guan QH, Sun YF, Liu XM, Zhang GY. Opposing effects of bad phosphorylation at two distinct sites by Akt1 and JNK1/2 on ischemic brain injury. Cell Signal. 2007;19:1844–1856. doi: 10.1016/j.cellsig.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- 27.Esneault E, Castagne V, Moser P, Bonny C, Bernaudin M. D-JNKI, a peptide inhibitor of c-Jun N-terminal kinase, promotes functional recovery after transient focal cerebral ischemia in rats. Neuroscience. 2008;152:308–320. doi: 10.1016/j.neuroscience.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 28.Takeda K, Noguchi T, Naguro I, Ichijo H. Apoptosis signal-regulating kinase 1 in stress and immune response. Annu Rev Pharmacol Toxicol. 2008;48:199–225. doi: 10.1146/annurev.pharmtox.48.113006.094606. [DOI] [PubMed] [Google Scholar]
- 29.Chiarugi A. Poly(ADP-ribosyl)ation and stroke. Pharmacol Res. 2005;52:15–24. doi: 10.1016/j.phrs.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, Lin Y, Kim YS, Hande MP, Liu ZG, Shen HM. C-Jun N-terminal kinase mediates hydrogen peroxide-induced cell death via sustained poly(ADP-ribose) polymerase-1 activation. Cell Death Differ. 2007;14:1001–1010. doi: 10.1038/sj.cdd.4402088. [DOI] [PubMed] [Google Scholar]
- 31.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 32.Rami A, Kogel D. Apoptosis meets autophagy-like cell death in the ischemic penumbra: Two sides of the same coin? Autophagy. 2008;4:422–426. doi: 10.4161/auto.5778. [DOI] [PubMed] [Google Scholar]
- 33.Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E, Tanaka K, Uchiyama Y. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol. 2008;172:454–469. doi: 10.2353/ajpath.2008.070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, Han F, Fukunaga K, Qin ZH. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 2008;4:762–769. doi: 10.4161/auto.6412. [DOI] [PubMed] [Google Scholar]
- 35.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diaz F, Moraes CT. Mitochondrial biogenesis and turnover. Cell Calcium. 2008;44:24–35. doi: 10.1016/j.ceca.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 38.Gutsaeva DR, Carraway MS, Suliman HB, Demchenko IT, Shitara H, Yonekawa H, Piantadosi CA. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci. 2008;28:2015–2024. doi: 10.1523/JNEUROSCI.5654-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jahani-Asl A, Cheung EC, Neuspiel M, MacLaurin JG, Fortin A, Park DS, McBride HM, Slack RS. Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death. J Biol Chem. 2007;282:23788–23798. doi: 10.1074/jbc.M703812200. [DOI] [PubMed] [Google Scholar]
- 40.Yin W, Signore AP, Iwai M, Cao G, Gao Y, Chen J. Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury. Stroke. 2008;39:3057–3063. doi: 10.1161/STROKEAHA.108.520114. [DOI] [PMC free article] [PubMed] [Google Scholar]