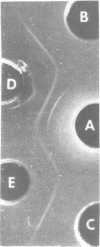
Abstract
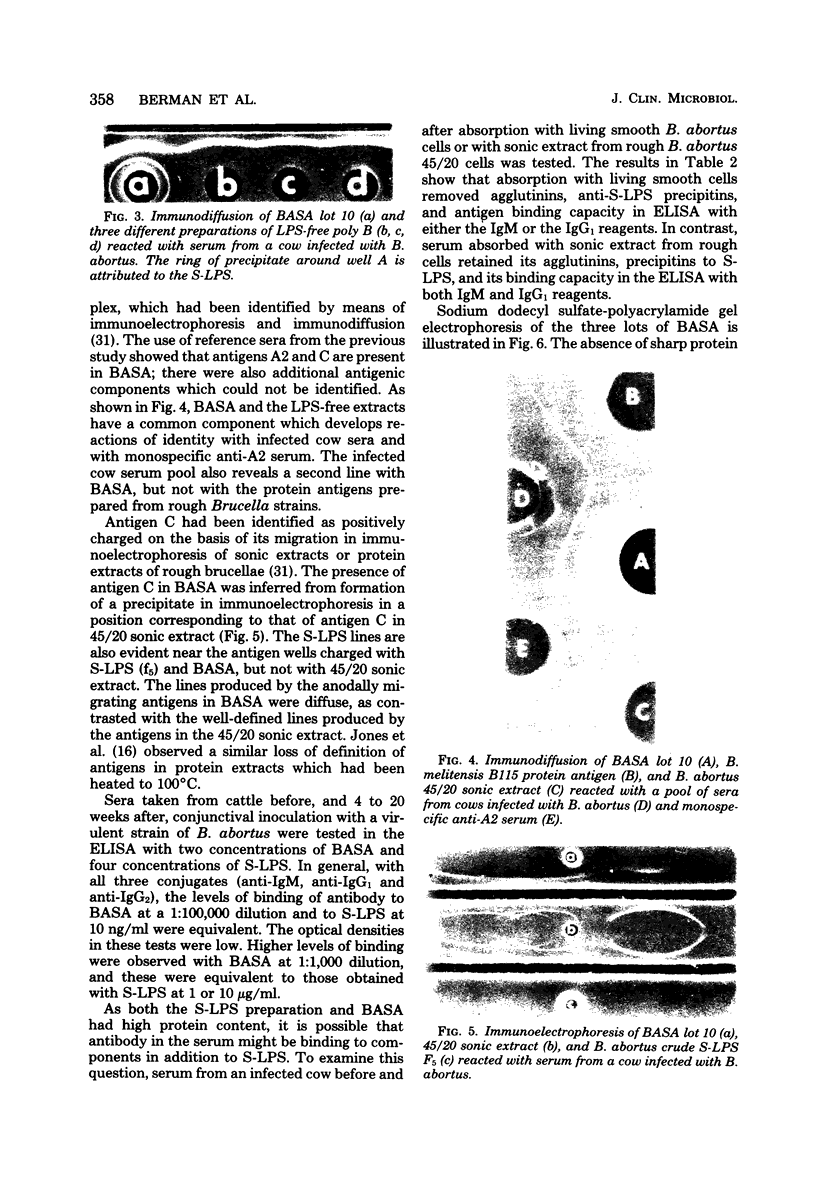
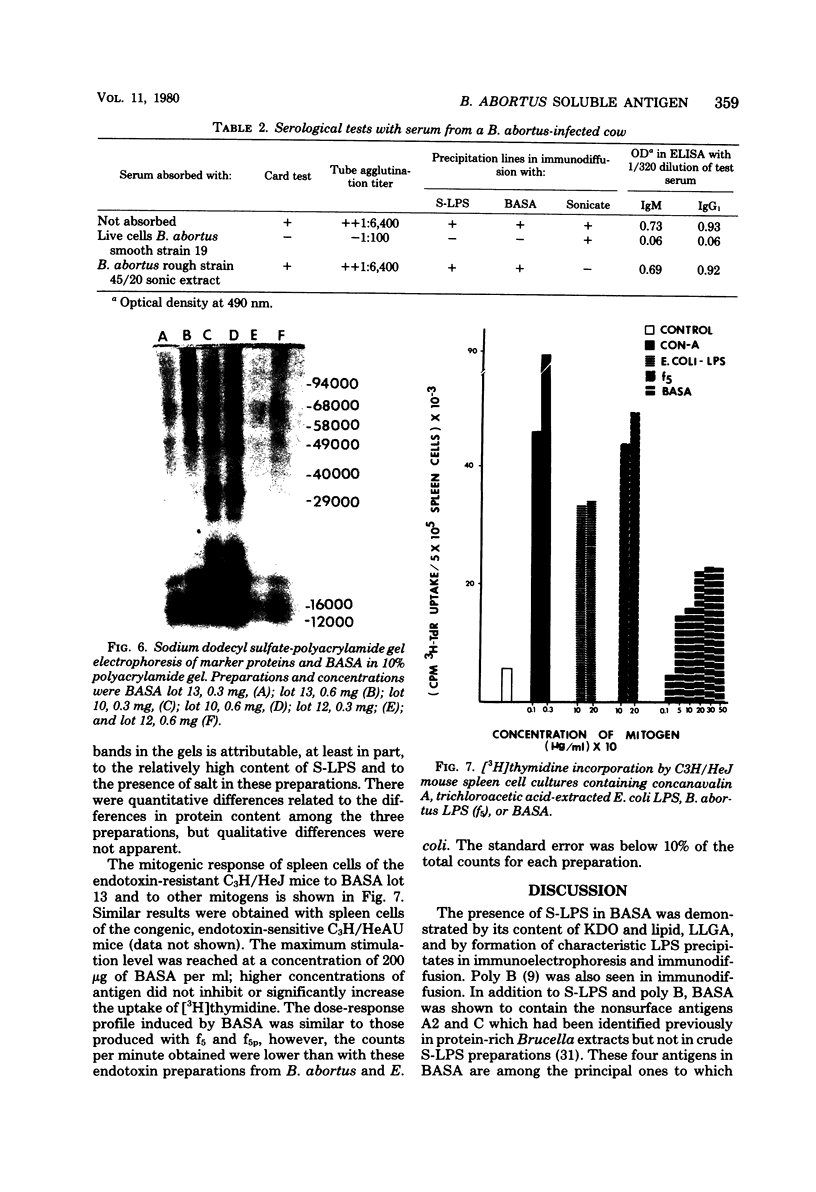
A soluble antigen extract of Brucella abortus (BASA) has been prepared by the National Veterinary Services Laboratories and furnished to a number of workers who are examining antibody-mediated and cell-mediated immune responses of cattle infected with B. abortus. Three lots of BASA were examined. There were quantitative but not qualitative differences among lots by content of protein, total carbohydrate, hexose, fatty acid, and 2-keto-3-deoxyoctonic acid. The presence of smooth lipopolysaccharide was demonstrated by the presence of 2-keto-3-deoxyoctonic acid and lipid, by Limulus lysate gelation activity, and by formation of characteristic lipopolysaccharide precipitates in immunoelectrophoresis. A polysaccharide antigen as well as two nonsurface antigens, A2 and C, were also identified. BASA is a satisfactory antigen for use in the enzyme-linked immunosorbent assay since the smooth lipopolysaccharide component bound to polystyrene and functioned in the test. Normal murine spleen cells showed a mitogenic response to BASA similar to that produced by purified smooth lipopolysaccharide. BASA has been used in other laboratories to stimulate peripheral blood leukocytes from cattle infected with B. abortus. Because BASA is a mixture of antigenic components shown to have mitogenic effects in the mouse system, questions on the nature of its stimulatory effect on bovine cells are raised.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERNATHY R. S., BRADLEY G. M., SPINK W. W. Increased susceptibility of mice with brucellosis to bacterial endotoxins. J Immunol. 1958 Oct;81(4):271–275. [PubMed] [Google Scholar]
- Andersen V., Hansen N. E., Karle H., Lind I., Hoiby N., Weeke B. Sequential studies of lymphocyte responsiveness and antibody formation in acute bacterial meningitis. Clin Exp Immunol. 1976 Dec;26(3):469–477. [PMC free article] [PubMed] [Google Scholar]
- Byrd J. W., Heck F. C., Hidalgo R. J. Evaluation of the enzyme-linked immunosorbent assay for detecting Brucella abortus antibodies. Am J Vet Res. 1979 Jun;40(6):896–898. [PubMed] [Google Scholar]
- Carlsson H. E., Hurvell B., Lindberg A. A. Enzyme-linked immunosorbent assay (ELISA) for titration of antibodies against Brucella abortus and Yersinia enterocolitica. Acta Pathol Microbiol Scand C. 1976 Jun;84(3):168–176. doi: 10.1111/j.1699-0463.1976.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Diaz R., Garatea P., Jones L. M., Moriyon I. Radial immunodiffusion test with a Brucella polysaccharide antigen for differentiating infected from vaccinated cattle. J Clin Microbiol. 1979 Jul;10(1):37–41. doi: 10.1128/jcm.10.1.37-41.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Oyeledun M. A. Studies of some biological activities of "brucella" endotoxin in normal and infected animals and the role of the hypersensitivity factor. Ann Sclavo. 1977 Jan-Feb;19(1):117–130. [PubMed] [Google Scholar]
- Graybill J. R., Alford R. H. Variability of sequential studies of lymphocyte blastogenesis in normal adults. Clin Exp Immunol. 1976 Jul;25(1):28–35. [PMC free article] [PubMed] [Google Scholar]
- Jones L. M., Diaz R., Taylor A. G. Characterization of allergens prepared from smooth and rough strains of Brucella melitensis. Br J Exp Pathol. 1973 Oct;54(5):492–508. [PMC free article] [PubMed] [Google Scholar]
- Kaneene J. M., Anderson R. K., Johnson D. W., Muscoplat C. C. Brucella antigen preparations for in vitro lymphocyte immunostimulation assays in bovine brucellosis. Infect Immun. 1978 Nov;22(2):486–491. doi: 10.1128/iai.22.2.486-491.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneene J. M., Johnson D. W., Anderson R. K., Angus R. D., Pietz D. E., Muscoplat C. C. Kinetics of in vitro bovine lymphocyte immunostimulation with a Brucella abortus antigen. Am J Vet Res. 1978 Feb;39(2):235–239. [PubMed] [Google Scholar]
- Kaneene J. M., Johnson D. W., Anderson R. K., Muscoplat C. C. Utilization of a specific in vitro lymphocyte immunostimulation assay as an aid in detection of brucella-infected cattle not detected by serological tests. J Clin Microbiol. 1978 Nov;8(5):512–515. doi: 10.1128/jcm.8.5.512-515.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Klesius P. H., Kramer T. T., Swann A. I., Christenberry C. C. Cell-mediated immune response after Brucella abortus S19 vaccination. Am J Vet Res. 1978 May;39(5):883–886. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb V. L., Jones L. M., Schurig G. G., Berman D. T. Enzyme-linked immunosorbent assay for bovine immunoglobulin subclass-specific response to Brucella abortus lipopolysaccharides. Infect Immun. 1979 Oct;26(1):240–247. doi: 10.1128/iai.26.1.240-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Berman D. T. Brucella abortus lipopolysaccharide is mitogenic for spleen cells of endotoxin-resistant C3H/HeJ mice. J Immunol. 1979 Dec;123(6):2915–2919. [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C. R., Kiesel G. K., Kramer T. T., Hudson R. S. Cell-mediated and humoral immune responses of cattle to Brucella abortus, Mycobacterium bovis, and tetanus toxoid: evaluation of immunization and assay techniques. Am J Vet Res. 1978 Nov;39(11):1738–1741. [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Host responses to infectious bovine rhinotracheitis virus. III. Isolation and immunologic activities of bovine T lymphocytes. J Immunol. 1974 Nov;113(5):1391–1398. [PubMed] [Google Scholar]
- Schurig G. G., Duncan J. R., Case J., Winter A. J. Blastogenic transformation by lipopolysaccharide of blood leukocytes from immunized but not normal cattle. Infect Immun. 1977 Aug;17(2):463–465. doi: 10.1128/iai.17.2.463-465.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurig G. G., Jones L. M., Speth S. L., Berman D. T. Antibody response to antigens distinct from smooth lipopolysaccharide complex in Brucella infection. Infect Immun. 1978 Sep;21(3):994–1002. doi: 10.1128/iai.21.3.994-1002.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman J. M., Reed N. D. Immune and mitogenic responses by BALB/c, C3H/HeJ, and nude mice to Brucella abortus bacterin and lipopolysaccharide. Infect Immun. 1979 May;24(2):371–378. doi: 10.1128/iai.24.2.371-378.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemshorn B., Nielsen K. The bovine immune response to Brucella abortus I. A water soluble antigen precipitated by sera of some naturally infected cattle. Can J Comp Med. 1977 Apr;41(2):152–159. [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. D., Jr, Watson S. W. Factors affecting the sensitivity of Limulus lysate. Appl Microbiol. 1974 Dec;28(6):1023–1026. doi: 10.1128/am.28.6.1023-1026.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen C. O., Pietz D. E., Armbrust A. L., Harrington R., Jr Enzyme immunoassay for detecting Brucella antibodies in cow's milk. J Clin Microbiol. 1979 Aug;10(2):222–225. doi: 10.1128/jcm.10.2.222-225.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. II. A gene that influences a membrane component involved in the activation of bone marrow-derived lymphocytes by lipipolysaccharides. J Immunol. 1975 May;114(5):1462–1468. [PubMed] [Google Scholar]