SUMMARY
Since enteric microbial composition is a distinctive and stable individual trait, microbial heterogeneity may confer lifelong, non-genetic differences between individuals. Here we report that C57BL/6 mice bearing RF microbiota, a distinct but diverse resident enteric microbial community, are numerically and functionally deficient in marginal zone (MZ) B cells. Surprisingly, MZ B cell levels are minimally affected by germ-free conditions or null mutations of various TLR signaling molecules. In contrast, MZ B cell depletion is exquisitely dependent on cytolytic CD8+ T cells, and includes targeting of a cross-reactive microbial/endogenous MHC class 1B antigen. Thus, members of certain enteric microbial communities link with CD8+ T cells as a previously unappreciated mechanism that shapes innate immunity dependent on innate-like B cells.
INTRODUCTION
The enteric microbial community is astonishingly abundant and diverse in composition [1, 2]. Acquired during infancy, typically of maternal origin, its composition becomes a unique but stable trait of each individual throughout life [3]. Due to its abundance, diversity, and unculturability, there is much to learn about the functionalities and impact of the enteric microbial community on host biology. Since its composition is a distinguishing trait of each individual, it represents a potentially important epigenetic factor that may shape each individual’s physiology and disease susceptibility [4]. The immune system is predicted to be a key target of these epigenetic forces, since it is highly specialized both for microbial sensing and for alterations in these signals.
The innate-like and adaptive arms of the immune system have emerged as a framework for the cellular processes of microbial defense and immunoregulation [5], recently illustrated by the reciprocal roles of dendritic cells in the composition of the enteric microbiota and its contribution to colitis susceptibility [6]. Innate-like functions are associated with the MZ and B-1 B cell subsets [7, 8], which emerge from post-medullary stages of B cell differentiation. Whereas conventional follicular mature (FM) B cells function in adaptive B lymphocyte responses, marginal zone (MZ) and B-1a B cells function in early T-independent (TI) IgM antibody responses, display elevated responsiveness through toll-like receptors (TLRs), and have a limited capacity for immunologic memory [9].
The present study addresses the role of TLR responsiveness, and other modes of enteric microbial sensing, on the levels of MZ B cells in the periphery. We report three observations on the role of endogenous microbial flora in the fate of this B cell population. First, we demonstrate that mice colonized with distinct communities of resident bacteria exhibit striking defects in innate-like B cell populations in the spleen and body cavities (MZ and B-1a B cells, respectively). Second, germ-free mice and genetic studies surprisingly indicate that this phenotype is not due to the absence of enteric microbiota or their products. Third, we show that the expanded memory CD8+ T cells in RF mice dramatically deplete MZ B cells through a cytolytic mechanism. Thus, MZ B cells are preserved in CD8−/− or perforin (Prf1)−/− RF mice, and rapidly depleted in SPF mice transferred with CD8+ T cells from RF mice. Further evidence associates this B cell depletion with CD8+ T cell targeting of cells bearing Qa-1 and a conserved microbial heat shock peptide. Taken together, these observations demonstrate a novel, CD8+ T cell-dependent mechanism linking resident microbiota with the level and function of innate-like effector B cells.
RESULTS
Altered intestinal microbiota inhibit the generation and function of mature B cells
To assess the role of complex resident microbial communities on B cell populations in vivo, we compared two mouse colonies bearing stable but distinct microbial communities. The two enteric microbial communities-termed for simplicity as RF and SPF- have been extensively characterized by conventional microbiologic and molecular phylotyping, each consisting of hundreds of phylotypes spanning the major bacterial and fungal taxa [10, 11]. RF and SPF microbial communities display broad-based compositional differences from species to family levels. For example, Firmicutes and Bacteroidetes phylotypes are preferentially abundant in RF and SPF, respectively, a trait that also distinguishes enteric microbial communities among humans [4]. The microbial composition is stable in individuals and across generations in each colony, and may be conferred to new mouse strains by Caesarian delivery of pups and transfer to adoptive mothers. Notably, functional studies of RF mice reveal their resistance to immune colitis induced by Gαi2−/− T cells (Wei, et al., unpublished data) or CD4+CD45RBhi mice [12].
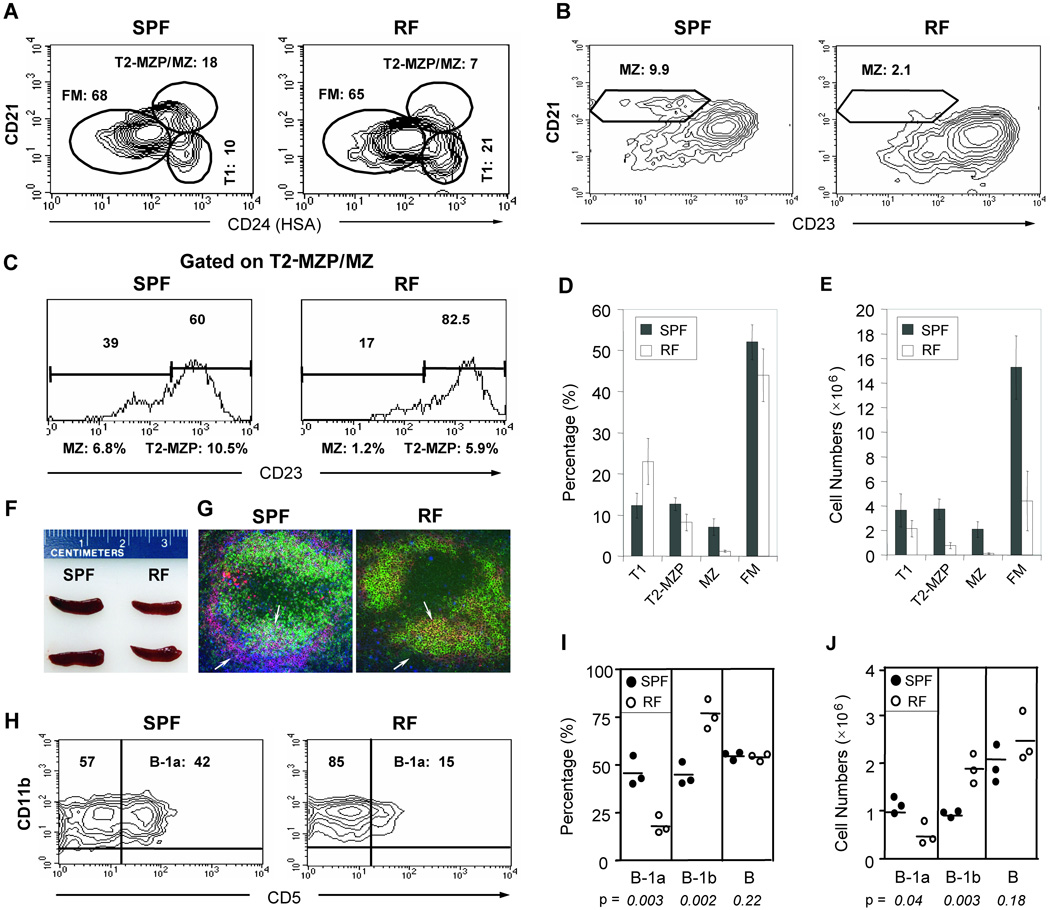
When RF mice were compared to SPF mice for splenic B cell subpopulations, a selective 5-fold reduction in the percentage of MZ B cells was observed (Fig. 1A–D). Using absolute numbers, there was a 10-fold decrease in MZ B cells (Fig. 1E), and a 4–5 fold decrease in FM and the MZ precursor subset, referred to as transitional type 2 marginal zone precursor (T2-MZP) B cells [8, 13]. The more profound diminution by absolute numbers reflects the parallel reduction of naïve CD4+ and CD8+ T cells in RF mice [14]. In contrast, there was no significant change in the percentage or absolute numbers of precursor transitional type 1 (T1) population (Fig. 1A, D, E). Consistent with these reductions of splenic B and T cell populations in RF mice, the spleen size in RF mice was smaller than that in SPF mice (Fig. 1F). Additionally, immunofluorescence staining of splenic MZ B cells confirmed the diminished MZ B cell population in RF mice (Fig. 1G). Thus, in SPF mice (left panel) an abundant layer of IgMhi cells (red) was observed in the marginal zone (immediately outside the layer of marginal zone macrophage, denoted by blue staining with anti-MOMA1). In contrast, RF mice (right panel) were almost entirely depleted for IgMhi cells in the marginal zone. As expected, a more modest reduction was seen in the size of B cell follicles (bearing green IgDhi and or yellow IgM+IgD+ B cells) in RF mice.
Figure 1. Mice bearing distinct resident microbiota (RF mice) exhibit a severe reduction in MZ and B-1a B cells.
(A) CD21/HSA(CD24) and (B) CD21/CD23 profiles of splenic CD19+ B cells from C57BL6 mice maintained in SPF versus RF conditions. The relative percentages of transitional 1 (T1), transitional 2 marginal zone precursor (T2-MZP) + marginal zone (MZ) (T2-MZP/MZ), follicular mature (FM), and marginal zone (MZ) are indicated. (C) CD21/CD23 profiles, gated on the T2-MZP/MZ (CD21hiHSAhi) population in (A) to distinguish (CD23hi) MZP from (CD23lo) MZ cells. (D) Percentage and (E) absolute numbers of T1, T2-MZP, MZ, and FM splenic B cell populations, with averages from five age-matched pairs. The relative percentage of MZP and MZ B cells were derived by multiplying the CD23hi or CD23lo fractions in (C) with the T2-MZP/MZ fractions in (A). (F) Comparison of spleens from age and gender-matched SPF and RF mice. (G) Immunofluorescence staining of splenic tissue sections with IgD (green), IgM (red) and MOMA-1 (blue) against marginal metallophilic macrophages. The arrows indicate marginal zone B cells, based on IgMhigh staining (red) and localization outside the MOMA+ layer (blue). Arrowheads highlight B cells in follicular areas, with IgD and IgM double expression confirmed by yellow staining. (H) Peritoneal cells (PECs) were gated on B220, and the staining contours for CD11b (Mac1) and CD5 are shown for SPF and RF mice. Minimal B-2 B cells (B220-gated, CD11b−CD5−) were detectable in this compartment. (I) Percentage and (J) absolute numbers of B-1 subsets in SPF (solid circles) and RF (open circles) animals. Representative results of 3 or more similar experiments.
In both mesenteric and peripheral (popliteal) lymph nodes, flow cytometric analysis revealed a ~2-fold reduction in absolute numbers of CD19+ B cells (data not shown), indicating that the moderate reduction of splenic B2 B cells is also observed systemically. Analysis of the peritoneal cavity cells (PEC) revealed that they were almost entirely of B-1 phenotype (CD11b+), and showed a dramatic loss of (CD11b+CD5+) B-1a cells in RF mice (Fig. 1H–J). In summary, formation of both innate-like (MZ and B-1a) and adaptive (FM) B cells in vivo were diminished by the distinct resident microbiota in RF mice.
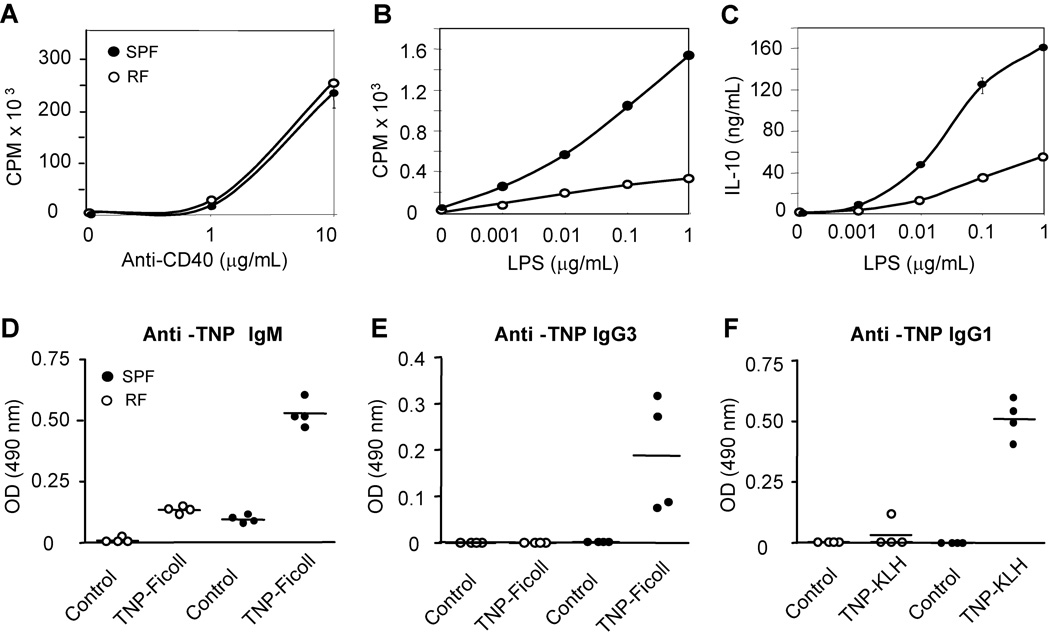
The consequence of these B cell deficits in RF mice was assessed functionally. While CD40-mediated proliferation was similar between SPF and RF mice, LPS-induced proliferation was severely diminished in RF animals (Fig. 2A, B). MZ cells constitute the main splenic B cell population responding to LPS and other TLR ligands (Suppl. Fig. 1). Quantitatively, the decrement in the RF LPS response could be fully attributed to the reduction in the RF MZ population. Similarly, the LPS-induced IL-10 secretion was also severely diminished in RF animals (Fig. 2C), concordant with the diminution of MZ B cells.
Figure 2. RF mice exhibit defective B lineage dependent immune responses.
Proliferation of CD43-depleted splenic B cells in response to (A) anti-CD40 Abs, and (B) LPS. (C) IL-10 production of splenic B cells in response to LPS. (D) Anti-TNP IgM and (E) anti-TNP IgG3 production in serum of mice challenged with the T-independent antigen, TNP-Ficoll. (F) Anti-TNP IgG1 production upon challenge with the T-dependent antigen, TNP-KLH. All the serum samples were diluted at 1:10 and then applied to ELISA assay for detection of antibody responses. The results are representative data from three similar experiments.
Antigen-specific B cell immune responses were determined by serum ELISA, using binding absorbance determined for 1:10 serum dilutions (a concentration yielding middle-range absorbance for samples with any detectable binding activity). Therefore, these data should be considered as relative binding, not linear measures of antibody binding concentration. The T-independent (TNP-Ficoll) induction of IgM and IgG3 (Fig. 2D, E) was deficient in RF mice, consistent with the role of MZ and B-1 B cells in this response and its protective anti-bacterial correlates [15, 16]. Endogenous resident microbiota also affected T-dependent adaptive B cell function, since RF mice were impaired in their T-dependent (TD) antibody responses (Fig. 2F). Evaluation of purified splenic B cell sub-populations from RF mice revealed that they preserved a normal per-cell BCR responses in vitro (data not shown). The impaired TD response in vivo thus may be due to either the numerical B cell deficiency in FM B cells, and/or functional abnormalities of CD4+ and CD8+ T cell populations in RF mice that have been previously reported [14]. These in vitro and in vivo findings provide functional confirmation for the selective deficit in MZ B cells in RF mice.
Microbiota in RF mice affects the formation of MZ B cells
The marked deficiency of innate-like B cells subsets in RF mice suggested either that SPF microbiota are required for MZ B cell development, or that the formation of MZ B cells is affected by microbiota in RF mice. To confirm the role of RF microbiota in MZ B cell development, we tested the effects of both timing and route of bacteria delivery in experiments designed to introduce SPF lumenal bacteria within RF mice. Mice were analyzed at subsequent intervals to see if an alteration of intestinal microbiota could restore the innate-like B cell compartment.
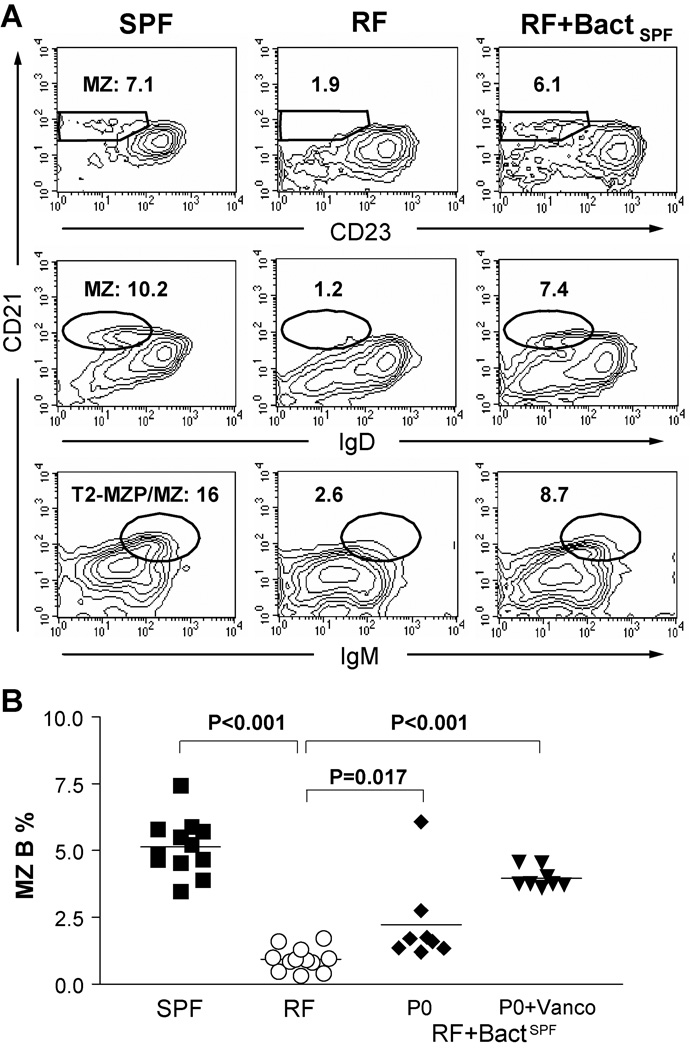
First, lumenal SPF bacteria were introduced into post-weaning (three weeks old) RF mice, and recipient animals were analyzed at monthly intervals for up to six months. Post-weaning exposure to SPF microbiota resulted in no restoration of MZ B cells and B-1a B cells. In contrast, housing of pregnant RF mothers within an SPF animal facility (data not shown), or transfer of SPF lumenal bacteria into RF pups beginning immediately after their birth, led to significant expansion of the innate-like B cell compartment (Fig.3A). MZ B cells were successfully restored in RF offspring, and these populations persisted into adulthood. These results suggest that an early window of exposure (neonatal, but not post-weaning) to specific resident bacteria is crucial for the formation of MZ B cell formation.
Figure 3. Microbiota in RF mice plays a role in the formation of MZ B cells.
RF neonates were treated beginning at post-natal day 0 (P0) with lumenal bacteria from SPF mice (see Methods). The mice were examined for MZ B cell development at age of about 8 weeks old. (A) Phenotypes of splenic CD19+ B-cells; % MZ B cells in CD19+ gate are indicated. (B) Tabulation of % MZ B cells; each symbol represents data from an individual mouse. SPF: specific pathogen free mice; RF: restricted microbiota mice; RF+BactSPF P0: RF pups exposed to SPF cecal bacteria at post-natal day 0 (P0). RF+BactSPF P0+Vanco: Offspring derived from RF parental mice that were treated with vancomycin under SPF condition. These pups were exposed to SPF environment during and immediately after birth. P values by student t test for comparisons of SPF mice to RF and bacteria-treated RF mice. Data was obtained from 3 or more independent experiments.
It should be noted that not all offspring achieved full restoration of their MZ B cells (Fig. 3B) and B-1a B cells (data not shown). In view of this individual variability, we predicted that antibiotic treatment might facilitate more efficient depletion of resident RF microbiota; and thereby promote colonization by SPF derived flora. To test this idea, RF females and males were treated with vancomycin for two weeks, and then bred with SPF male and female under SPF husbandry conditions. Indeed, full restoration of the MZ B cell compartment was achieved in all offspring using this approach (Fig. 3B). Using both conventional bacterial culture and molecular phylotyping criteria [11], innate-like B cell restoration correlated with a shift from RF to SPF enteric microbiota (data not shown). These results suggest that RF microbiota affects the development of MZ B cells.
Limited effect of germ-free conditions and TLRs on MZ B cell development
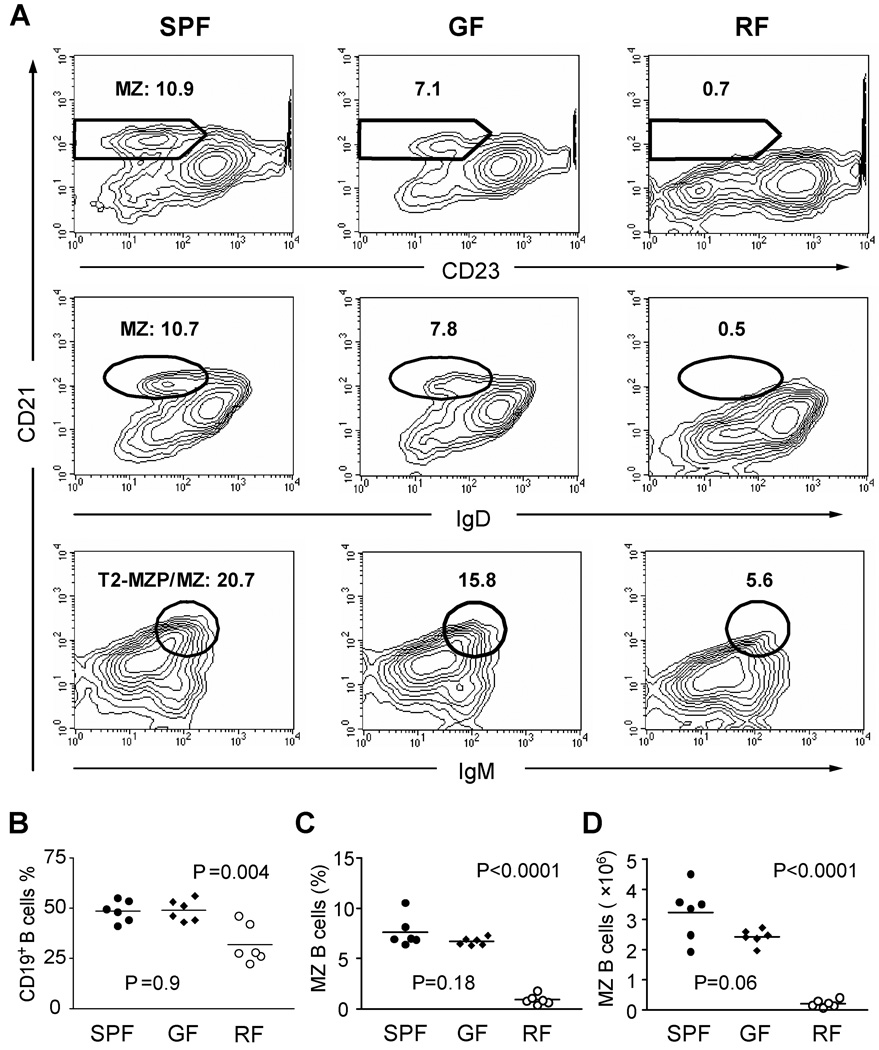
The reduced innate-like B cells in RF mice might in part reflect the deficiency of certain microbial products required for this mode of B cell formation. To test this idea, germ-free (GF) mice were examined for levels of MZ B cells as compared with that in SPF and RF mice (Fig. 4). While the percentage of CD19+ B cells was significantly deceased in RF mice, GF mice showed the equivalent level of B cells as that in SPF mice. MZ B cells were only slightly decreased in GF mice (Fig. 4A), a difference that was not statistically significant when 6 or more GF mice were analyzed (Fig. 4C, D). This agreed with the preservation of MZ B cells previously reported in GF rodents [17, 18]. In contrast, MZ B cells were markedly decreased in RF mice in both percentage and absolute number, suggesting that the innate-like B cell deficiency in RF mice was not due to the lack of products from their resident microbiota.
Figure 4. Profound deficiency of MZ B cells in RF mice but not in germ-free mice.
(A) Splenic lymphocytes were isolated from age and gender-matched SPF, germ-free (GF) and RF mice, and stained to delineate MZ B cells (CD19, CD21, IgM and IgD). The percentages of cell populations with MZ B cell phenotypes are indicated. Data represents at least three independent experiments, and two different strains (C57/BL6 and Swiss Webster) of germ-free mice. (B–D) Tabulation of data from individual age-matched mice (8–10 weeks) reared in SPF, GF, or RF conditions: (B) Percentage of CD19+ B cells; (C) % MZ B cells; (D) total MZ B cells per spleen. P values were calculated using student’s t test comparison of GF or RF to the control SPF group.
Even in germ-free mouse conditions, microbial products in mouse chow might be sufficient to provide positive signals for innate-like B cell formation, particularly in view of their enhanced responses to ligands for various TLR isoforms (Suppl. Fig. 1). To directly assess the role for TLR signaling in innate-like B cell development in vivo, we evaluated B cell populations in mice engineered with null mutations for receptor or signaling components shared in the TLR responses. Mice bearing non-functional TLR2 or TLR4 mutations [19] exhibited normal levels of MZ and B-1a B cells (Suppl. Fig. 2). Since several alternative TLR ligands independently promote T2-MZP or MZ B cell proliferation in vitro, these observations suggested that innate-like B cell generation might be achieved via the redundant function of several TLRs or, possibly, through other pathogen recognition receptors.
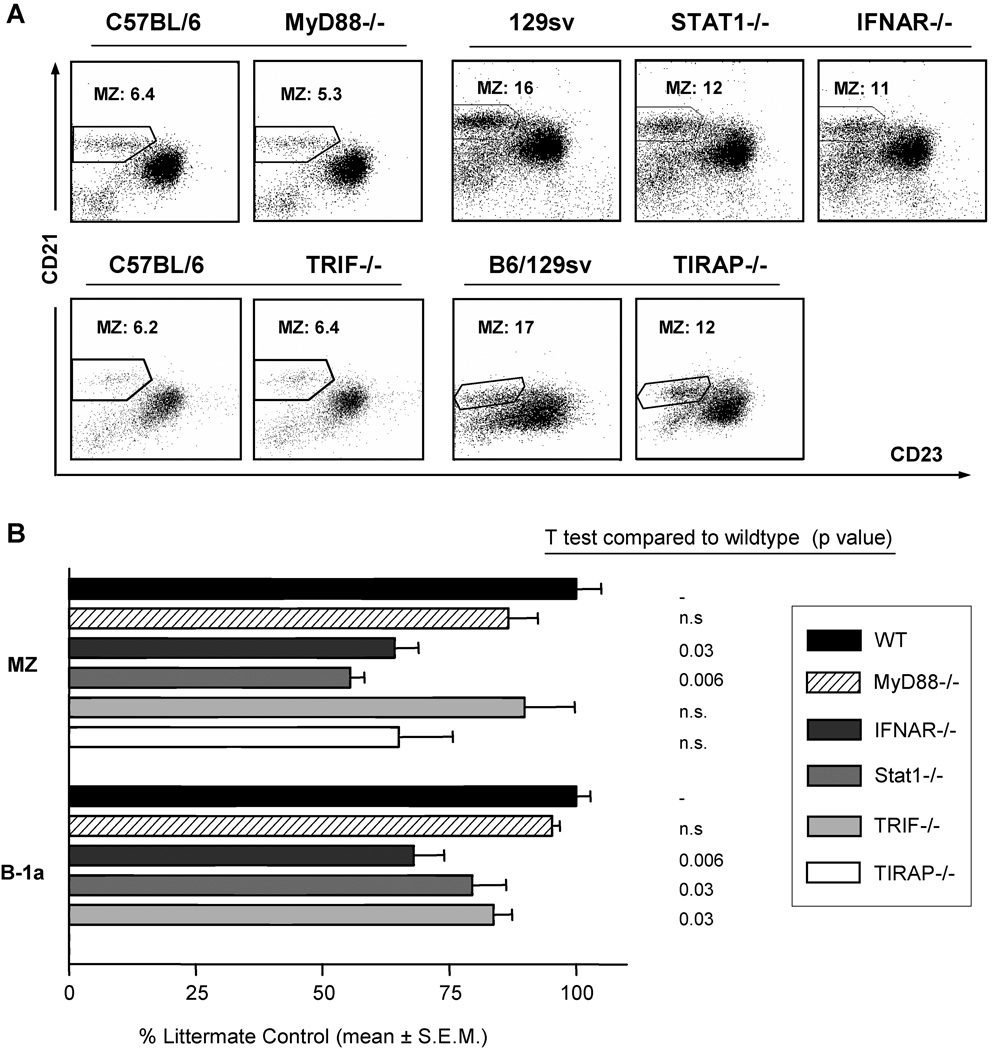
To better understand the role of TLRs in innate-like B cell formation, we evaluated mice bearing TLR adaptor protein null mutations. When we compared age-matched MyD88−/− mice [20] with wildtype littermate controls, there were minimal differences in MZ and B-1a B cells levels, and this change was not statistically significant (Fig. 5A, B). The absence of a significant effect of the MyD88−/− genotype was confirmed in more than 30 mice from five different MyD88−/− SPF colonies. Similarly, mice deficient in the TIRAP/MAL adapter protein (for MyD88-collaborating and -independent TLR signaling [21]), exhibited a slight, non-significant reduction in MZ B cells (Fig. 5B). MZ B cell depletion was not further augmented in MyD88/TIRAP double knockout mice (data not shown). These data do not support MyD88- or TIRAP-dependent signaling in formation of innate-like B cells.
Figure 5. Mice with null mutations in TLR signaling pathways have partial deficiencies in innate-like B cell development.
Splenic cells were prepared from MyD88−/− (and C57BL/6 wildtype littermate control) mice, Stat-1−/− and IFNAR−/− (and 129Sv wildtype littermate control) mice, TRIF−/− and TIRAP−/− mice (and 129Sv × C57BL/6 wildtype littermate control). CD19+ cells were analyzed for MZ B cells as in Fig. 5A. (A) Splenic CD21/23 population (MZ B cells). (B) Relative frequency of innate-like B cells in TLR signaling mutant mouse strains. MZ B cells were measured by flow cytometry in sets of background- and age-matched wildtype controls for MyD88−/− (n= 5), TIRAP−/− (n=3), TRIF−/− (n=5), IFNAR−/− (n=4), and STAT-1−/− (n=4) mice. The ratios of mutant to age-matched controls were calculated for each mouse, and tabulated as % control ± SEM. P values for comparisons of mutant to wildtype controls were determined by student t test.
Another class of MyD88-independent signaling (e.g., TRIF/TICAM-1) involves IRF-3 induction via TLR3 and TLR4 [22]. Accordingly, this adapter is unique in eliciting responses mediated through IFN β expression and IFN β-receptor signaling, and permits full TLR signaling in certain MyD88−/− cell types. Compared to wildtype littermate controls, TRIF-deficient mice retained normal splenic B cell subsets, but exhibited a statistically significant albeit modest reduction in peritoneal B-1a cells. IFNAR−/− and STAT1−/− were significantly deficient in both splenic MZ B cells (Fig. 5A, B) and peritoneal B-1a B cells (Fig. 5B). Taken together, these findings suggest that type-1 interferon signaling plays a limited role in innate-like B cell formation. The partial effect observed in TRIF−/− mice also suggests a possible link between TLRs and this interferon requirement.
RF CD8+ T cells reduce MZ B cells through a perforin-dependent cytolytic mechanism
The foregoing experiments did not implicate reduced RF microbial stimulation in the deficiency of innate-like B cells. We therefore considered the possibility that an active, inhibitory process was involved. In RF mice, splenic memory CD8+ T cells are expanded [14], and this is a systemic feature of the immune system, detectable in liver, mesenteric lymph nodes, and thymus (Suppl. Fig. 3). These findings do not reflect an absence of specific resident microbiota, since no significant differences in the percentage of CD8+ T cells are observed in GF mice (Suppl. Fig. 3A).
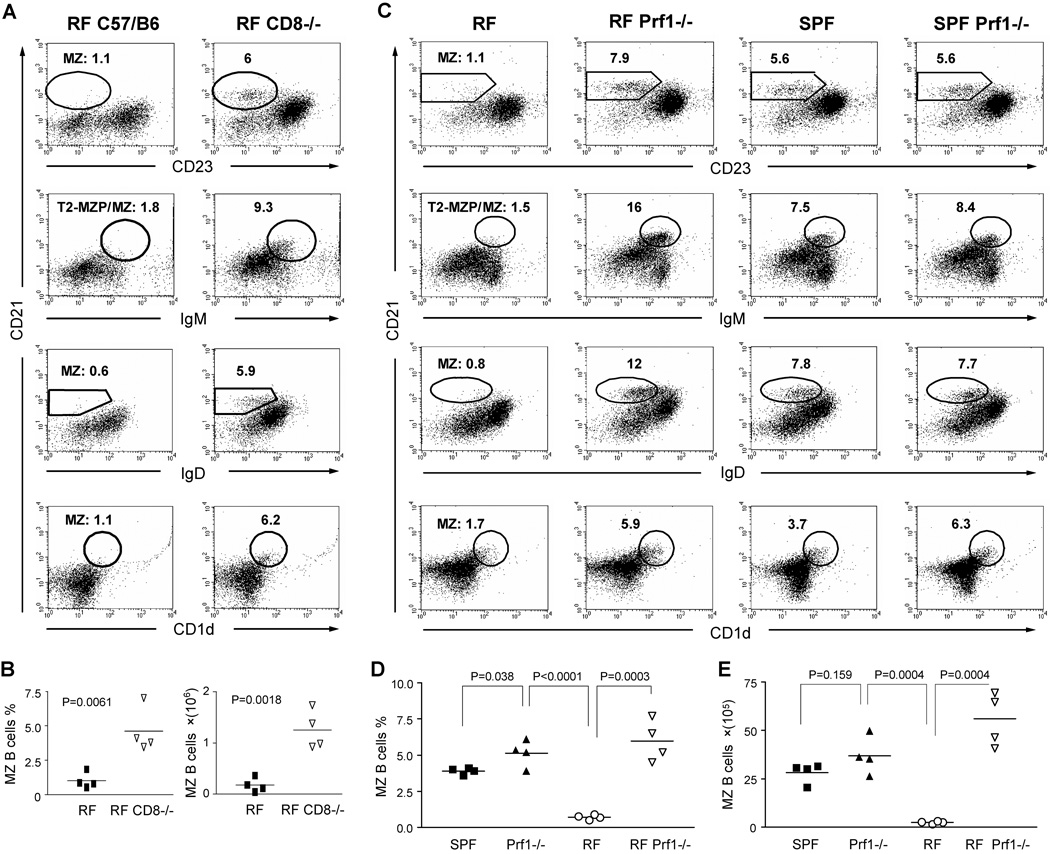
The concordance of CD8+ T cell expansion and MZ B cell depletion specifically within RF mice prompted us to assess whether they might be causally related. In contrast to wildtype RF mice, CD8−/− RF mice exhibited normal numbers of MZ B cells (Fig. 6A). The percentage and absolute number of MZ B cells was significantly increased in CD8−/− RF mice compared with wildtype RF mice (Fig. 6B). This finding suggested that CD8+ T cells within RF animals might function, directly or indirectly, to suppress MZ B cell levels.
Figure 6. CD8+ T cells affect MZ B cells formation through a perforin-dependent cytolytic mechanism.
Splenic lymphocytes were isolated from age- and gender-matched adult C57BL/6 RF mice and stained for CD21, CD23, CD1d, IgM, and IgD in different staining combinations. The MZ B cells were analyzed in CD19+ B cell gate and the percentage of MZ B cells in different staining patterns are indicated. Conventional bacterial culture and molecular phylotyping confirmed that RF mice and RF CD8−/− mice were colonized with the same RF microbiota. (A, B) Comparison of RF mice bearing CD8−/− or wildtype genotypes. (A) Representative flow cytometry of CD19+ gated splenocytes. % MZ B cells are listed. (B) Tabulated percentage and absolute number of MZ B cells in CD19+CD21hiCD23lo gate. The data represents at least three individual experiments. (C–E). Prf1−/− mice bearing SPF or RF microflora were compared with age- and gender-matched wildtype SPF and RF C57/BL6 mice. (C) Representative flow cytometry of CD19+ gated splenocytes. % MZ B cells are listed. Tabulated percentage (D) and absolute number (E) of MZ B cells (CD19+CD21hiCD23lo). P values for the significance of comparisons between different groups were indicated. These data are representative of the results from three independent experiments with nine Pfr1−/− mice and equal numbers of control mice.
We next evaluated mice deficient for perforin (Prf1), a membrane-disruptive protein required for cytolysis driven by CD8+ T cells and NK cells. To determine whether suppression of MZ B cells by CD8+ T cells in RF mice was mediated by CTL cytotoxicity, we established a colony of RF Prf1−/− mice by RF re-derivation (Caesarean section and RF foster mother rearing), and RF breeding and housing. Compared with age- and gender-matched SPF Prf1−/− mice and RF Prf1 +/+ mice, RF Prf1−/− mice displayed normal or even increased MZ B cell levels (Fig. 6C–E). This demonstrated that CD8+ T cells in RF mice reduced levels of MZ B cells by a mechanism involving perforin-mediated cytotoxicity.
In vivo depletion of MZ B cells by CD8+ T cells and microbial antigen from RF mice; relationship to Qa-1
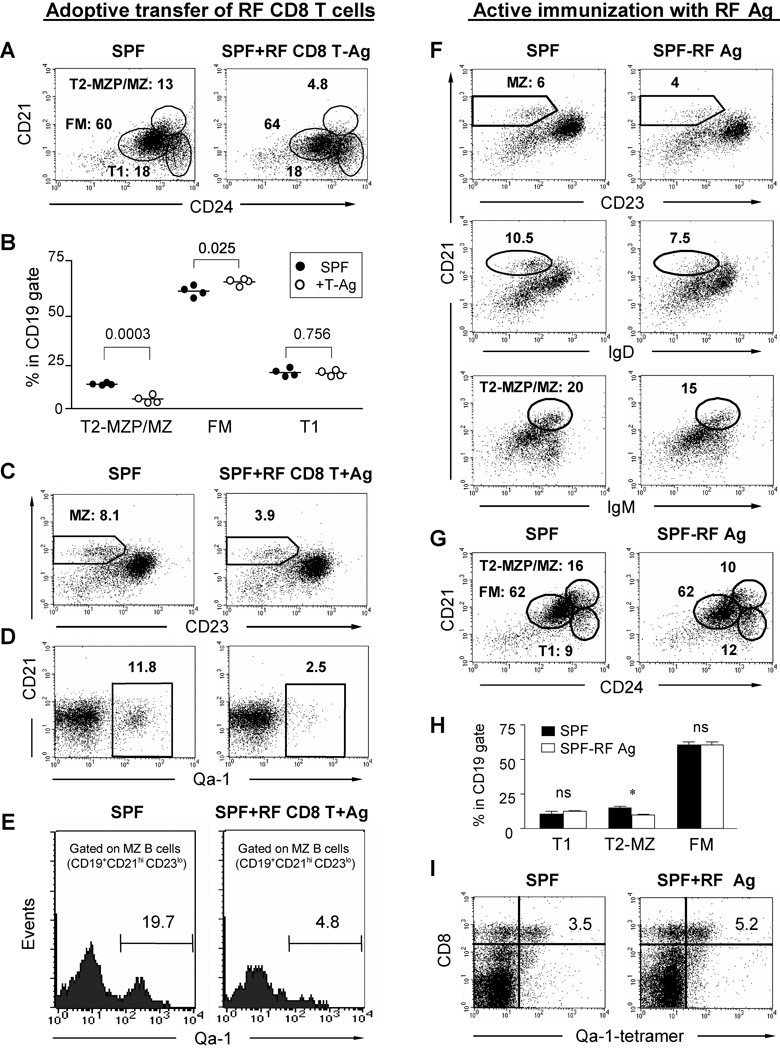
The most direct explanation for the preceding experiments was that RF CD8+ T cells directly target MZ B cells for cytolytic depletion. To test this prediction, we performed an in vivo cytotoxicity experiment. CD8+ T cells were isolated from RF C57BL/6 mice, and transferred into young (4 week old) SPF mice, a stage at which physiologic MZ B cell expansion is underway [23]. SPF recipients of adoptively transferred RF CD8+ T cells exhibited a marked reduction in MZ B cells (Fig. 7A–C, and Suppl. Fig. 4). Both the percentage and absolute number of MZ B cells in RF CD8+ T cell recipients were statistically deceased as compared with SPF control mice (Suppl. Fig. 4). Depletion was augmented by inoculation with RF bacterial antigens, suggesting that bacterial antigen increased the frequency of activated CD8+ T cells. Notably, the reduction occurred rapidly (within 3 days), suggesting that the CD8+ T cells might act through a cytotoxic process.
Figure 7. In vivo depletion of MZ B cells by adoptive transfer of RF CD8+ T cells and active immunization with RF microbial antigens in SPF mice.
Four weeks old SPF mice were transferred i.v. with 4 ×107 of splenic CD8+ T cells and i.p. with 150 µg of enteric bacterial antigens derived from RF mice. On day 3 after cell transfer, splenocytes were collected from SPF mice and SPF mice that received RF CD8+ T cells plus antigens, and stained for analysis of B cell subsets. The data represent three different independent experiments. (A) Representative flow cytometry of CD19+-gated cells stained for B cell subsets. (B) Tabulated percentages of B cell subsets as compared between SPF mice and SPF mice received RF CD8+ T cells and bacterial antigens. (C, D) Flow cytometry of CD19+-gated splenocytes from representative SPF mice with or without RF CD8+ T cell transfer: (C) CD21 and CD23 (MZ B cells); (D) CD21 and Qa-1 (Qa-1+ B cells). (E) Qa-1 expression of MZ-gated splenocytes (CD19+CD21hiCD23lo). Fig. 7 F–I show the reduction of MZ, T2-MZ B cells and increase of Qa-1b tetramer positive CD8+ T cells in liver of SPF mice actively immunized with RF lumenal antigens through i.p injection. (F) CD21 vs. CD23 (MZ B cells), IgM and IgD, respectively; (G) CD21 vs. CD24 (T2-MZP/MZ B cells); (H) Tabulated percentages of T1, T2-MZ B and FM B cell subsets in T2-MZP/MZ gate shown in Fig 7G. “*” indicates the statistic significance (p=0.02); (I) Lymphocytes from liver of immunized SPF mice stained with CD8+ vs. Qa-1b tetramer loaded with a HSP-60 peptide (GMKFDRGYI). All data represents three individual experiments.
We further analyzed mice with regard to the relative sensitivity of various B cell subsets to this rapid CD8+ T cell-mediated depletion. Three days after transfer of RF CD8+ T cells, B cell subsets were analyzed by flow cytometry. Depletion was observed only in MZ B cells and their immediate precursors, T2-MZP cells, whereas T1 and FM B cell subsets were preserved (Fig. 7A–C). The slight increase in FM B cells may reflect the homeostatic expansion of FM cells in response to MZ B cell depletion (Fig. 7B). These findings suggest that T2-MZP and MZ B cells were direct targets of RF CD8+ T cell depletion. In contrast, they suggest that the reduction of FM B cells observed in RF mice represented an indirect, secondary process leading to their steady-state reduction.
Qa-1, a class Ib MHC molecule expressed by B cells [24], can present a Salmonella typhimurium GroEL peptide (GMQFDRGYL), eliciting a cognate CD8+ T cell response during in vivo bacterial infection [25]. A homologous and TCR-cross-reactive peptide is also encoded by mammalian heat shock protein 60 (GMKFDRGYI). Accordingly, we wondered whether this mechanism might be involved in CD8+ T cell cytolysis of innate-like B cells.
First, we measured the levels of Qa-1+ B cells in SPF mice with or without RF CD8+ T cell transfer (Fig. 7D, E). Qa-1 expression was observed in about 12% of total splenic B cells, and 20% of MZ B cells. After RF CD8+ T cell transfer, the levels of Qa-1+ B cells were strikingly depleted, to 2.5 and 4.8 %, respectively. Thus, B cells in each of these compartments were depleted at a four-fold greater rate when associated with Qa-1 expression.
We wished to further confirm the role of RF microbiota in stimulating CD8+ T cells and consequently mediating cytolytic effects on MZ B cells or its progenitors. To do so, 4 weeks old SPF mice were immunized with the microbial antigens isolated from RF mice, and the change of CD8+ T cells and MZ B cells was observed 2–3 weeks after the last immunization. No robust CD8+ T cell expansion were observed in the spleen of RF antigen-immunized SPF mice (data not shown). However, MZ B cells were reduced compared to SPF controls (Fig. 7F–G), and this selectively involved T2-MZP B cells (Fig. 7H). Notably, the reduction of MZ B cells correlated with the moderate increase of liver CD8+ T cells binding to Qa-1 tetramer loaded with a HSP60-derived peptide (Fig. 7I). These results suggest that microbial components in RF enteric microflora could trigger a Qa-1 restricted CD8+ T cells response and play a suppressive role in the formation of MZ B cells. It would be reasonable to speculate that the sustained antigenic stimulation naturally derived from colonized enteric microbiota would better favor the activation and expansion of CD8+ T cells in RF mice.
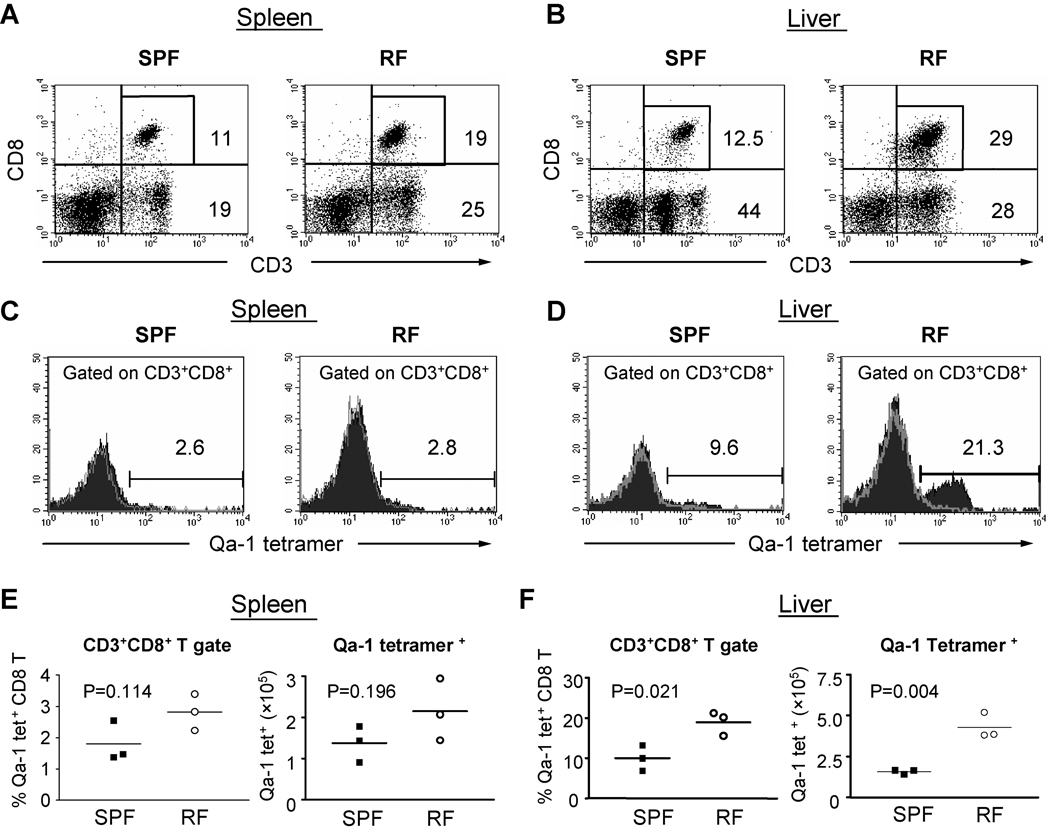
To further clarify whether MZ B cells depletion in RF mice was indeed resulted from the expansion of Qa-1b-restricted CD8+ T cells, we measured the levels of CD8+ T cells binding the Qa-1/microbial peptide tetramer in SPF vs. RF mice. The abundance of CD8+ T cells was increased in RF vs. SPF mice (Fig. 8A–B and Suppl. Fig. 3C), consistent with the previously reported expansion of memory CD8+ T cells in RF mice [14]. This expansion was associated with a preserved (spleen) or increased (liver) frequency of Qa-1 tetramer+ CD8+ T cells (Fig. 8C–D), and a striking increase in the percentage and absolute numbers in liver (Fig. 8D and F). Accordingly, the preferential depletion of Qa-1+ B cells was associated with a selective increase in the abundance of RF CD8+ T cells specific for Qa-1 bearing a conserved peptide encoded by exogenous bacterial or endogenous mammalian heat shock protein family members.
Figure 8. CD8+ T cell binding of Qa-1 tetramer/heat shock peptide.
Single cells were isolated from adult C57BL/6 spleen (A, C, E) and liver (B, D, F) of age- and gender-matched adult RF and SPF mice and stained for CD3, CD8+ and Qa-1b tetramer loaded with the murine HSP60 monamer GMKFDRGYI. Flow cytometry from single RF and SPF animals are shown in (A–D), representative of findings from three different experiments. (A, B) CD3 and CD8 expression. (C, D) Level of Qa-1 tetramer binding by CD8+ T cells. Histograms show staining with Qa-1/tetramer (filled) and unstaining negative control (grey trace). (E, F) Tabulated percentage and absolute number of Qa-1 tetramer-binding CD8+ T cells. The data represents the results from three individual experiments.
DISCUSSION
This study addresses the role of resident microbial heterogeneity in shaping the innate-like B cell compartment. Mice bearing RF microbiota were distinguished from SPF mice by marked depletion of T2-MZP and mature B cell subsets, including, most notably MZ and B-1 B cells, innate-like B cell populations present within the spleen and body cavities, respectively. The minimal change of MZ B cell levels in germ-free conditions, or in mice deficient in TLR signaling, indicated that the depletion was not due to an absence of microbial stimuli. Instead, resident microbiota-associated induction of CD8+ T cells was crucial for this process, which was dependent upon perforin-mediated cytotoxicity, and correlated with Qa-1 antigenic targeting. These findings suggest that different microbial communities can divergently shape the innate-like B cell compartment, through a novel mechanism of resident microbial sensing and resultant immunoregulation by CD8+ T cells.
Limited roles of resident bacteria and TLR signaling on levels of innate-like B cells in vivo
Due to their important role in microbial sensing, the TLR system was our initial focus as the host detection system potentially mediating the deficit of B cell subsets in RF mice. Our in vitro data indeed provided evidence that T2-MZP and MZ B cells were particularly responsive to TLR signaling. However, germ-free conditions supported normal levels of MZ B cells, and genetic studies uncovered negligible effects of several TLR elements (TLR2, TLR4 genes, or the TLR signaling components MyD88 and TIRAP), and only modest diminution of innate-like B cells through deficiency of the type 1 interferon response (TRIF, Stat-1, and IFNAR). With regard to TLR receptors, the limited phenotypes may reflect broad biologic redundancy and in vivo hematagenous delivery of enteric bacteria to the B cell compartment [26, 27]. In dendritic cell maturation, there is a requirement for TLR ligands (double-stranded RNA or viral infection) via type 1 interferon signaling [28], analogous to our findings with innate-like B cells. Taken together, the lack of strong phenotype in mice with mutations in individual TLRs or TLR-associated adapters suggested that the RF mouse phenotype involves a TLR-independent mechanism.
Enteric bacteria of the gastrointestinal tract participate in the development of the murine and human immune system [29, 30]. Thus, in germ-free animals the number of lamina propria lymphocytes and IgA–producing plasma cells, and systemic serum immunoglobulin levels are decreased relative to SPF mice. Secondary lymphatic organs, such as the spleen and lymph node, are also underdeveloped in germ-free animals. Enteric bacteria also influence the abundance of B-cells in both intestinal lymphoid follicles and Peyer’s patches, reflected by their expansion in AID−/− mice (due to impaired microbial control), and their numerical reduction in germ-free and antibiotic-treated mice [31–34]. Peyer’s patch B cells and germinal center formation is also dependent on tonic BCR signaling and the presence of T lymphocytes. Such studies frame the important stimulatory role of resident intestinal bacteria, via BCR signaling and T helper function for germinal center responses, and via less-defined mechanisms for basal cellularity of intestinal and systemic mature B cell populations.
This literature suggested the differentially stimulatory products of resident microbiota of SPF vs. RF microbiota might account for the differential formation of B cell subsets in SPF and RF mice. However, in the present work and previous studies, the formation of innate-like B cells was preserved in germ-free mice [17, 35, 36]. This finding, in association with the limited phenotype on TLR signaling-deficient mice, suggested that the effect of RF microbiota on B cell subset formation was not due to the absence of suitable stimulatory products, but rather through induction of an inhibitory process.
Role of CD8+ T cells in mediating the effect of RF microbiota on the MZ B cell population
Memory and activated CD8+ T cells are expanded in RF mice, including both the spleen and a variety of systemic and mucosal compartments ([14], and present study). Therefore, we evaluated whether CD8+ T cells might be involved in the deficiency of MZ B cells in these mice. Indeed, MZ B cell numbers were preserved in CD8−/− RF mice. Evidence that this phenotype was mediated by CD8+ T cells included the preservation of MZ B cells in Prf1−/− RF mice; and, the rapid depletion of MZ B cells in SPF mice transferred with RF CD8+ T cells. In addition, active immunization of 4 weeks old SPF mice with lumenal microbial antigens also decreased the T2/MZ B cell level and increased a subpopulation of CD8+ T cells binding HSP60 peptide-Qa-1b tetramer. These findings suggest that RF microbiota induce a cytolytic CD8+ T cell population that is necessary for the depletion of MZ B cells in RF mice.
It remains uncertain whether these cytolytic CD8+ T cells directly target B cells, or instead act through intermediate cytolytic targets affecting B cell development or homing. However, our findings are most simply explained by a direct cytotoxic effect: (a) MZ B cells are rapidly and selectively killed by RF CD8+ T cells in an in vivo depletion assay; (b) Depletion preferentially targets Qa-1+ B cells; and, (c) RF mice bear an expanded population of CD8+ T cells binding Qa-1 tetramer loaded with a conserved microbial/host heat shock peptide. Together, these observations suggest that B cells are directly targeted, and implicate this antigenic system in the process.
Adoptive transfer of RF CD8+ T cells with RF antigen administration and active immunization of RF microbial antigens in SPF mice suppressed MZ B cells, indicating that RF microbiota played a crucial role in shaping the formation of MZ B cells through the induction of CD8+ T cells. The correlation (decrease of Qa-1b expressing B cells and increase of Qa-1 tetramer binding CD8+ T cells) suggested that the cytolytic effects of CD8+ T cells on MZ B cells were mediated by Qa-1b in RF mice. It is notable that in natural infection with certain Gram-negative bacteria, Qa-1 is loaded with a highly conserved peptide derived from microbial heat shock protein of the GroEL family, activating a cognate CD8+ T cell response contributing to bacterial clearance [37, 38]. Moreover, this conserved bacterial peptide is also encoded by mammalian HSP60, permitting CD8+ T cells targeting of Qa-1+ host cells expressing cross-reactive peptide endogenously [25]. Accordingly, we speculate that RF resident microbiota are distinguished by an immunogenic bacterial population bearing this conserved peptide, eliciting a cognate anti-bacterial CD8+ T cell response to the Qa-1/peptide. One consequence of this CD8+ T cell population is the depletion of Qa-1/peptide-bearing MZ B cells, targeted either through direct microbial uptake or expression of cross-reactive endogenous Hsp60 peptide. However, we emphasize that the present data demonstrate an association, but not causality, for such antigenic recognition by CD8+ T cells in MZ B cell depletion.
This would be mechanistically equivalent to the rapid cytolytic depletion in vivo of antigen-presenting cells by cytotoxic T lymphocytes (CTL) in natural microbial or tumor immune response [39–41]. Indeed, this process may also account for the depletion of certain dendritic cell subsets by RF CD8+ T cells [11]. Other MHC 1b molecules are also known to shape the host immune system. For example, CD-1 [42, 43] and MR-1 [44–46], both expressed on innate-like or mucosal B cells, elicit effector T cell responses targeting cross-reactive microbial and endogenous ligands. Since antigen-presenting cells vary widely in expression of MHC 1B molecules according to B cell subset and anatomic location [15, 46], it is conceivable that such antigenic targeting may be an unappreciated feature of the B cell subsets elevated for these MHC 1B molecules.
An important issue raised by these findings is the potential role of regulatory CD4+ (or CD8+) T cells in constraining the distinctive cytolytic CD8+ T cell population observed in RF mice. Systemic or enteric antigen can be delivered to the thymus by migratory dendritic cells [47], and Tregs form after birth, perhaps through the stimulus of enteric microbiota ([48], but also see [49]). Mazmanian, Kasper, and colleagues have provided elegant evidence for a discreet enteric microbial product (B. fragilis polysaccharide PSA) that antigenically induces formation of functional CD4+ T cell subsets [50]. Thus, it is conceivable that Tregs, efficiently induced by products of SPF but not RF microbiota, may normally constrain the expansion of cytolytic CD8+ T cells involved in the depletion of MZ B cells. The possibility of a Treg deficiency in RF mice is a potentially important issue to assess in the immunobiology of RF mice.
What are the functional consequences of the immunologic state of RF mice? The present study demonstrates deficient T-independent B cell immunity, and accordingly, reduced host control of capsular bacterial infection. B cells can serve as a key antigen-presenting cell type for CD8+ T cells in microbial infection [51, 52]. The ongoing RF CD8+ T cell-mediated depletion of antigen-presenting cells would probably also result in a numerically reduced, younger, and antigen-sparse antigen-presenting population. One consequence for immunity might be diminished effectiveness in microbial surveillance and anti-microbial CD4+ T cell recruitment. Indeed, RF mice are distinguished from SPF mice for their delayed immune response to and bacterial clearance of enteric Campylobacter jejuni infection [53]. However, this same trait could be beneficial by impairing autoimmune CD4+ T cell recruitment. CD8+ T cells may directly elicit immunoregulation through depletion of antigen-presenting cells [39–41, 54], and a deficiency of intestinal CD8+ regulatory T cells has been reported in a human inflammatory bowel disease cohort [55]. Since RF mice reveal are resistant to immune colitis [12](Wei et al., unpublished data), the B cell and CD8+ T cell interaction uncovered in this study thus may reflect an analogous process.
In summary, this study reveals that distinct resident microbial communities, representative of the human divergence of enteric microbiota, can shape the innate-like B cell population through a novel mechanism involving cytolytic CD8+ T cells. Since such mice are also divergent in their susceptibility to immune or infectious colitis, this observation suggests an unexpected acquired trait that can shape host immunoregulation and immune disease susceptibility.
MATERIALS AND METHODS
Mice
C57BL/6, 129Sv, MyD88−/−[20], TIRAP−/− [21], TRIF−/− [22], IFNAR−/− [56], STAT1−/− [57], CD8α−/− [58] and Prf1−/− mice [59] were bred and maintained in specific pathogen free (SPF) conditions. MyD88−/− mice from additional colonies were generously provided by M. Arditi (Cedars-Sinai Medical Center and UCLA), C. Wilson (University of Washington), and G. Cheng (UCLA). C3H/HeJ mice were purchased from the Jackson Laboratories. TRIF−/− mice were provided by B. Beutler (Scripps Research Institute). Germ-free C57BL/6 and Swiss Webster mice were acquired from the NIH Gnotobiotic Resource at the College of Veterinary Medicine at North Carolina State University, or purchased from Taconic Farms (Hudson, NY), respectively. Sterility of germ-free mice was documented on a monthly basis by fecal Gram stain, and aerobic and anaerobic cultures of the feces and bedding. For selected mice, sterility of cecal contents was documented Gram stain and cultures at the time of necropsy.
C57BL/6, Prf1−/−, and CD8−/− mice were also carried in restricted microbiota (RF) conditions in the RF colony of UCLA Radiation Oncology. RF mice were periodically validated for RF status by aerobic and anaerobic culture of cecal contents, and index molecular phylotypes [11]. With respect to pathogen-free status, both SPF and RF mice were monitored for the absence, by serology or culture, of a panel of viral, fungal, and bacterial pathogenic taxa, including Helicobacter spp. All animal procedures were performed according to the guidelines of the UCLA, University of Washington, Yale University, Scripps Research Institute, and University of Alabama Animal Research Committees.
Flow cytometry
Cell surface staining was performed as previously described [60]. Data were collected on a FACSCalibur flow cytometer (BD Biosciences) and analyzed using CellQuest software. T1 (CD21loHSAhi), T2-MZP (CD23hiCD21hiHSAhi), MZ (CD23loCD21hiHSAhi), and FM (CD21loHSAlo) B cell populations were distinguished by CD21, HSA, and CD23 expression. Anti-CD21, anti-CD24 (HSA), anti-CD23, anti-CD19, anti-B220, anti-CD11b (Mac1), anti-CD9 (KMC8) and anti-CD5 antibodies and streptavidin-APC were purchased from BD PharMingen. All staining profiles were based on live gates, as determined by forward and side scatter. For cell sorting, 5×107 cells per sample were stained in 500 µL of staining medium with anti-CD21, anti-CD24, and anti-CD23 Abs. Cells were sorted on a FACSVantage cell sorter (BD Biosciences). A nine-amino acid murine hsp60 peptide (GMKFDRGYI) [25, 61] with the purity of more than 91% was obtained from Invitrogen (Carlsbad, CA). APC-conjugated Qa-1b tetramer loaded with the peptide was kindly prepared and provided by NIH Tetramer Core Facility at Emory University.
Reagents and cellular assays
Single cell suspensions were prepared from murine splenocytes depleted of erythrocytes by lysis with ammonium chloride solution prior to cell sorting or CD43 depletion. CD43 depletion was performed using magnetic bead-conjugated anti-CD43 and a VarioMacs column according to the manufacturer’s instructions (Miltenyi Biotech). Depleted populations reproducibly contained >95% B220+ B cells. Purified splenocytes and sorted cell populations were cultured in RPMI 1640 with 5% FCS as previously described [62]. Stimulations were performed with 10 µg/mL polyclonal goat F(ab)’2 anti-mouse IgM Ab (Jackson Laboratory), 1 µg/mL of LPS from S. typhosa (Difco) or from S. minnesota (Sigma), or 100ug/ml poly(I:C) (Amersham). Phenol extracted LPS (lipid A) from S. minnesota, the 19 kD lipoprotein from M. tuberculosis, and PSM from S. epidermidis were generous gifts from Dr. Robert Modlin and Dr. Christopher Wilson. Equal numbers of purified B cell populations were cultured at 2.5 ×104 cells/well with various stimuli. Cells were pulsed with 1 µCi 3H-thymidine for 12 h prior to harvesting. Cells were harvested at 48 h and analyzed as previously described. Averages of triplicate samples are presented along with respective standard deviations. For analysis of IL-10 production, splenic B cells were stimulated with LPS and IL-10 levels in culture supernatants were determined by ELISA as previously described [63].
Six to 8 week old SPF and RF mice were immunized intraperitoneally with 10 µg TNP-Ficoll (Biosearch Technologies, Novato, CA) in PBS or 20 µg TNP-KLH (Biosearch Technologies, Novato, CA) precipitated with alum in PBS. Anti-TNP-specific titers were collected at 0, 1, and 2 weeks after immunization and analyzed by ELISA on TNP-BSA with isotype-specific antibodies (anti-mouse IgM, IgG3, and IgG1) (BD PharMingen, San Diego, CA).
Manipulation of enteric microbiota in RF mice
C57 BL/6J RF mice were transferred into specific pathogen free (SPF) condition at various time points: after weaning at the age of 2~3 weeks old; at birth; or, before birth with pregnant mothers. They were fed with the intestinal bacteria derived from SPF mice. Briefly, C57 BL/6J mice housed under SPF condition were sacrificed and intestinal tubes including small and large intestine were flushed with 3 ml/mouse of reduced saline buffer. The homogenized lumen contents were filtered through a 100 µm cell strainer and immediately transferred into RF mice through oral gavage. For each adult mouse, 0.2 ml of suspension was gavaged for three times on days 0, 3, and 5. For neonatal RF mice, the pups were fed with SPF bacterial suspension recurrently every three days. To introduce bacteria into RF offspring, an oral gavage needle with a soft blunt tip was gently put into their mouth to lure sucking for about 5–10 minutes each time. The rest of the bacteria suspension was spread in the bedding materials to generate a SPF condition. To achieve a thorough alteration of RF microflora in the offspring of RF mice, RF females were treated with vancomycin (65 µg/g body weight/mouse/per day) by oral gavage for two weeks, and bred with SPF males under SPF condition to allow the immediate exposure of pups to SPF microflora during and after birth. Enteric microbiota in these mice were monitored by conventional bacterial culture and molecular phylotyping [11].
Adoptive transfer of RF CD8+ T cells
CD8+ T cells from RF mice were transferred into SPF mice to evaluate the effect of RF CD8+ T cell on MZ B cells. Briefly, CD8α+ T cells were isolated from spleen of eight weeks old RF mice by using CD8+ T cell isolation kit (Miltenyi Biotec Inc.). Isolated RF CD8+ T cells with more than 95 % purity were intravenously injected to SPF mice at 4 ×107 cells per mouse. Bacterial lysates prepared from intestinal lumen contents of RF mice were intraperitoneally injected at 100 µg per mouse. Control SPF mice were injected with the same volume of saline. Mice were sacrificed three days after CD8+ T cell transfer, and splenic MZ B cells and other immune phenotypes were examined by FACS.
Enteric bacterial antigens from the lumen contents of RF mice were prepared as previously reported with minor modification [64]. Briefly, the intestinal lumen contents of RF mice were collected and resuspended in 1 mL PBS. 0.25 ml of buffer (PBS containing 100 mM MgCl2 and 1 mg/mL DNase) and 1 mL of glass beads (0.1 mm diameter) were added to the lumen content suspension and then vigorously vortexed for 10 minutes to disrupt bacteria. The glass beads and unlysed cells were removed by centrifugation at 5000× g for 10 minutes. Lumenal lysates were sterilized by using 0.2 µm syringe filtration (Nalgene, Rochester, NY). Protein concentrations were determined using Quick Start Bradford protein assay kit (Bio-Rad, Richmond, CA).
Active immunization of RF microbial antigens
Four weeks old SPF mice were immunized with RF lumenal microbes through i.p. injection. Instead of using RF lumenal lysates, bacterial suspensions were prepared from large intestine of RF mice for intrperitoneal immunization. Briefly, lumen contents of large intestinal were collected from RF mice and re-suspended in 1 mL of saline buffer/per mouse, and then filtered using a 60 µm cell strainer. SPF mice were first intraperitoneally injected with 150 µl thioglycollate broth three days before immunization to recruit and activate peritoneal antigen-presenting cells. Test mice then were immunized with 150 µl of RF microbial suspension i.p. injection, and immunized a second time two weeks later. Control mice received saline rather than bacterial suspension. Two to three weeks after the second immunization, test and control mice were examined for levels of MZ B cells and CD8+ T cells.
Statistical analysis
Statistical analysis was performed by using the programs provided by Prism3 software. In most cases of this study, the statistical analysis were performed by using an unpaired two-tailed Student t test for two data sets or one-way ANOVA for three or more data sets with a 95% confidence interval. Statistical significance was calculated, and p values less than 0.05 were considered to be significantly different.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Jonsson Comprehensive Cancer Center Flow Cytometry Center and Larry Gratland (U.A.B.) for flow cytometry, and Drs. M. Arditi, B. Beutler, G. Cheng, W. McBride, C. McLain, R. Medzhitov, R. Modlin, and C. Wilson for mice and reagents. We thank Dr. Chandrashekhar Pasare for experimental work and advice. The Center for Gastrointestinal Biology and Disease at North Carolina State University provided germ-free C57BL/6 mice, supported by NIH (P30 DK349870). Germ-free mice were kindly provided by Gnotobiotic Rodent Facilities funded by NIH grants P30 DK349870 for the Center for Gastrointestinal Biology and Disease at North Carolina State University and P40 RR018603 for the National Gnotobiotic Rodent Resource Center at UNC. We appreciate the strong support from NIH Tetramer Facility by providing Qa-1b tetramer loaded with peptides. The work was supported by NIH grants DK46763 (J.B.), DK69434 (J.B., B.W.), AI52031 (S.B.), HD37091 (D.J.R.), and CA016042 (Jonsson Comprehensive Cancer Center Flow Cytometry). It was also supported by the Crohn’s and Colitis Foundation of America (B.W.), the Broad Medical Research Foundation (J.B.), and the Elizabeth Campbell Endowment (D.J.R.). D.J.R. was the recipient of a McDonnell Scholar Award, a Leukemia and Lymphoma Society Scholar Award, and the Joan J. Drake Grant for Excellence in Cancer Research. The project was conceived and designed by B.W., D.J.R., and J.B. Specific experiments were designed and performed by B.W., T.T.S, H.W., R.P.S., D.F., T.T.H., and S.B. The paper was written by B.W., J.B., D.J.R., and J.B.
ABBREVIATIONS
- FM
follicular mature
- MZ
marginal zone
- Prf1
perforin 1
- RF
restricted flora
- T1
transitional type 1
- T2-MZP
translational type 2 marginal zone precursor
REFERENCES
- 1.Savage DC. Microbial ecology of the gastrointestinal tract. Annu.Rev.Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 2.Conway PL. Microbial ecology of the human large intestine. In: Gibson GR, editor. Human colonic bacteria: role in nutrition, physiology, and pathology. Boca Raton: CRC Press; 1995. pp. 1–24. [Google Scholar]
- 3.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 4.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Janeway CA., Jr How the immune system works to protect the host from infection: a personal view. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7461–7468. doi: 10.1073/pnas.131202998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nature Rev Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 8.Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K, Pillai S. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14:603–615. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 9.Martin F, Kearney JF. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 2000;12:39–49. doi: 10.1016/s1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
- 10.Scupham AJ, Presley LL, Wei B, Bent E, Griffith N, McPherson M, Zhu F, Oluwadara O, Rao N, Braun J, Borneman J. Abundant and diverse fungal microbiota in the murine intestine. Appl.Environ.Microbiol. 2006;72:793–801. doi: 10.1128/AEM.72.1.793-801.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujiwara D, Wei B, Presley LL, Brewer S, McPherson M, Borneman J, Braun J. Systemic control of plasmacytoid dendritic cells by CD8+ T cells and resident microbiota. J Immunol. 2008;180:5843–5852. doi: 10.4049/jimmunol.180.9.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aranda R, Sydora BC, McAllister PL, Binder SW, Yang HY, Targan SR, Kronenberg M. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol. 1997;158:3464–3473. [PubMed] [Google Scholar]
- 13.Srivastava B, Quinn WJ, III, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J Exp.Med. 2005;202:1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang T, Wei B, Velazquez P, Borneman J, Braun J. Commensal microbiota alter the abundance and TCR responsiveness of splenic naive CD4(+) T lymphocytes. Clin Immunol. 2005;117:221–230. doi: 10.1016/j.clim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 16.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 17.Kumararatne DS, MacLennan ICM. The origin of marginal-zone cells. Adv Exp Med Biol. 1982;149:83–90. doi: 10.1007/978-1-4684-9066-4_12. [DOI] [PubMed] [Google Scholar]
- 18.Makowska A, Faizunnessa NN, Anderson P, Midtvedt T, Cardell S. CD1high B cells: a population of mixed origin. Eur J Immunol. 1999;29:3285–3294. doi: 10.1002/(SICI)1521-4141(199910)29:10<3285::AID-IMMU3285>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 21.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 22.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 23.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 24.Nell LJ, Kastner DL, Rich RR. Qa-1-associated antigens. III. Distribution of Qa-1 region antigens on lymphoid subpopulations. J Immunol. 1980;125:2597–2603. [PubMed] [Google Scholar]
- 25.Lo WF, Woods AS, DeCloux A, Cotter RJ, Metcalf ES, Soloski MJ. Molecular mimicry mediated by MHC class Ib molecules after infection with gram-negative pathogens. Nat Med. 2000;6:215–218. doi: 10.1038/72329. [DOI] [PubMed] [Google Scholar]
- 26.Humphrey JH, Grennan D. Different macrophage populations distinguished by means of fluorescent polysaccharides. Recognition and properties of marginal-zone macrophages. Eur.J Immunol. 1981;11:221–228. doi: 10.1002/eji.1830110311. [DOI] [PubMed] [Google Scholar]
- 27.Amlot PL, Grennan D, Humphrey JH. Splenic dependence of the antibody response to thymus-independent (TI-2) antigens. Eur J Immunol. 1985;15:508–512. doi: 10.1002/eji.1830150516. [DOI] [PubMed] [Google Scholar]
- 28.Honda K, Sakaguchi S, Nakajima C, Watanabe A, Yanai H, Matsumoto M, Ohteki T, Kaisho T, Takaoka A, Akira S, Seya T, Taniguchi T. Selective contribution of IFN-alpha,beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc.Natl.Acad.Sci.U.S.A. 2003;100:10872–10877. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bealmear PM, Mirand EA, Holtermann OA. Miscellaneous immune defects in gnotobiotic and SPF mice. Prog.Clin.Biol.Res. 1983;132C:423–432. [PubMed] [Google Scholar]
- 30.Ouwehand WH, Isolauri E, Salminen S. The role of the intestinal microflora for the development of the immune system in early childhood. Eur.J.Nutr. 2002;41:I32–I37. doi: 10.1007/s00394-002-1105-4. [DOI] [PubMed] [Google Scholar]
- 31.Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat.Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 32.Shikina T, Hiroi T, Iwatani K, Jang MH, Fukuyama S, Tamura M, Kubo T, Ishikawa H, Kiyono H. IgA class switch occurs in the organized nasopharynx-and gut-associated lymphoid tissue, but not in the diffuse lamina propria of airways and gut. J Immunol. 2004;172:6259–6264. doi: 10.4049/jimmunol.172.10.6259. [DOI] [PubMed] [Google Scholar]
- 33.Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J.Immunol. 2003;170:5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki K, Meek B, Doi Y, Honjo T, Fagarasan S. Two distinctive pathways for recruitment of naive and primed IgM+ B cells to the gut lamina propria. Proc Natl Acad Sci U S A. 2005;102:2482–2486. doi: 10.1073/pnas.0409539102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bos NA, Cebra JJ, Kroese FG. B-1 cells and the intestinal microflora. Curr Top.Microbiol.Immunol. 2000;252:211–220. doi: 10.1007/978-3-642-57284-5_22. [DOI] [PubMed] [Google Scholar]
- 36.Sarma-Rupavtarm RB, Ge Z, Schauer DB, Fox JG, Polz MF. Spatial distribution and stability of the eight microbial species of the altered schaedler flora in the mouse gastrointestinal tract. Appl.Environ.Microbiol. 2004;70:2791–2800. doi: 10.1128/AEM.70.5.2791-2800.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lo WF, Ong H, Metcalf ES, Soloski MJ. T cell responses to Gram-negative intracellular bacterial pathogens: a role for CD8+ T cells in immunity to Salmonella infection and the involvement of MHC class Ib molecules. J Immunol. 1999;162:5398–5406. [PubMed] [Google Scholar]
- 38.Lo WF, Dunn CD, Ong H, Metcalf ES, Soloski MJ. Bacterial and host factors involved in the major histocompatibility complex class Ib-restricted presentation of Salmonella Hsp 60: novel pathway. Infect Immun. 2004;72:2843–2849. doi: 10.1128/IAI.72.5.2843-2849.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ludewig B, Bonilla WV, Dumrese T, Odermatt B, Zinkernagel RM, Hengartner H. Perforin-independent regulation of dendritic cell homeostasis by CD8(+) T cells in vivo: implications for adaptive immunotherapy. Eur J Immunol. 2001;31:1772–1779. doi: 10.1002/1521-4141(200106)31:6<1772::aid-immu1772>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Huck SP, McHugh RS, Hermans IF, Ronchese F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proc Natl Acad Sci U S A. 2006;103:147–152. doi: 10.1073/pnas.0509054103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guarda G, Hons M, Soriano SF, Huang AY, Polley R, Martin-Fontecha A, Stein JV, Germain RN, Lanzavecchia A, Sallusto F. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol. 2007;8:743–752. doi: 10.1038/ni1469. [DOI] [PubMed] [Google Scholar]
- 42.Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, Sullivan B, Bodmer-Narkevitch V, Plettenburg O, Kronenberg M, Tsuji M, Ho DD, Wong CH. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc.Natl.Acad.Sci U.S.A. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, III, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 44.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 45.Kawachi I, Maldonado J, Strader C, Gilfillan S. MR1-Restricted V{alpha}19i Mucosal-Associated Invariant T Cells Are Innate T Cells in the Gut Lamina Propria That Provide a Rapid and Diverse Cytokine Response. J.Immunol. 2006;176:1618–1627. doi: 10.4049/jimmunol.176.3.1618. [DOI] [PubMed] [Google Scholar]
- 46.Hansen TH, Huang S, Arnold PL, Fremont DH. Patterns of nonclassical MHC antigen presentation. Nat Immunol. 2007;8:563–568. doi: 10.1038/ni1475. [DOI] [PubMed] [Google Scholar]
- 47.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 48.Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006;36:2336–2346. doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- 49.Min B, Thornton A, Caucheteux SM, Younes SA, Oh K, Hu-Li J, Paul WE. Gut flora antigens are not important in the maintenance of regulatory T cell heterogeneity and homeostasis. Eur J Immunol. 2007;37:1916–1923. doi: 10.1002/eji.200737236. [DOI] [PubMed] [Google Scholar]
- 50.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 51.McClellan KB, Gangappa S, Speck SH, Virgin HWt. Antibody-independent control of gamma-herpesvirus latency via B cell induction of anti-viral T cell responses. PLoS Pathog. 2006;2:e58. doi: 10.1371/journal.ppat.0020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Homann D, Tishon A, Berger DP, Weigle WO, von Herrath MG, Oldstone MB. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice. J Virol. 1998;72:9208–9216. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang C, Miller JF. Campylobacter jejuni colonization of mice with limited enteric flora. Infect Immun. 2006;74:5261–5271. doi: 10.1128/IAI.01094-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei B, McPherson M, Turovskaya O, Velazquez P, Fujiwara D, Brewer S, Braun J. Integration of B cells and CD8+ T in the protective regulation of systemic inflammation. Clin Immunol. 2008;127:303–312. doi: 10.1016/j.clim.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ Regulatory T Cells in the Lamina Propria of Patients with Inflammatory Bowel Disease. J Immunol. 2005;174:5814–5822. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 56.Lin Q, Dong C, Cooper MD. Impairment of T and B cell development by treatment with a type I interferon. J Exp Med. 1998;187:79–87. doi: 10.1084/jem.187.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D, Carver-Moore K, DuBois RN, Clark R, Aguet M, Schreiber RD. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 58.Fung-Leung WP, Schilham MW, Rahemtulla A, Kundig TM, Vollenweider M, Potter J, van Ewijk W, Mak TW. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 59.van den Broek ME, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJ, Zinkernagel RM, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dalwadi H, Wei B, Schrage M, Spicher K, Birnbaumer L, Su TT, Rawlings DJ, Braun J. B cell developmental requirement for the Gài2 gene. J Immunol. 2003;170:1707–1715. doi: 10.4049/jimmunol.170.4.1707. [DOI] [PubMed] [Google Scholar]
- 61.Davies A, Kalb S, Liang B, Aldrich CJ, Lemonnier FA, Jiang H, Cotter R, Soloski MJ. A peptide from heat shock protein 60 is the dominant peptide bound to Qa-1 in the absence of the MHC class Ia leader sequence peptide Qdm. J Immunol. 2003;170:5027–5033. doi: 10.4049/jimmunol.170.10.5027. [DOI] [PubMed] [Google Scholar]
- 62.Su TT, Rawlings DJ. Transitional B lymphocyte subsets operate as distinct checkpoints in murine splenic B cell development. J Immunol. 2002;168:2101–2110. doi: 10.4049/jimmunol.168.5.2101. [DOI] [PubMed] [Google Scholar]
- 63.Dalwadi H, Wei B, Kronenberg M, Sutton CL, Braun J. The Crohn's disease-associated bacterial protein I2 is a novel enteric T cell superantigen. Immunity. 2001;15:149–158. doi: 10.1016/s1074-7613(01)00164-9. [DOI] [PubMed] [Google Scholar]
- 64.Cong Y, Brandwein SL, McCabe RP, Lazenby A, Birkenmeier EH, Sundberg JP, Elson CO. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–864. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.