Abstract
There are a number of neurologically active ion channel blocking peptides derived from cone snail venom, such as conantokin-G and ω-conotoxin MVIIA. Conantokin-G inhibits NMDA receptors containing the NR2B subunit whereas ω-conotoxin MVIIA blocks N-type Ca2+ channels. Separately, these peptides induce antinociceptive effects in pre-clinical pain models following intrathecal injection. In the current study, the efficacies of these peptides were determined separately and in combination by intrathecal injection into rats with a spinal nerve ligation, in rats with a spinal cord compression injury and in the formalin test. Separately, both conantokin-G and ω-conotoxin MVIIA dose-dependently attenuated nociceptive responses in all of these models. However, at high antinociceptive doses for both formalin and nerve injury models, ω-conotoxin MVIIA evoked untoward side-effects. Using isobolographic analysis, the combination of sub-antinociceptive doses of peptides demonstrated additive antinociception in rats with a nerve ligation and in the formalin test, without apparent adverse side-effects. In a model of neuropathic spinal cord injury pain, which is clinically difficult to treat, the combination of conantokin-G and ω-conotoxin MVIIA resulted in robust synergistic antinociception. These data suggest that a combination of these peptides may be analgesic across diverse clinical pains with limited untoward side effects, and particularly potent for reducing spinal cord injury pain.
1. Introduction
A number of voltage-gated and ligand-gated ion channels have been implicated in both normal pain processing and pathological pain (Millan, 1999). In the normal, uninjured state, at the level of the spinal dorsal horn, cation influx through voltage-gated ion channels accompanies depolarization at the primary afferent nociceptor central terminal, followed by neurotransmitter release. The released excitatory neurotransmitters, in turn, depolarize post-synaptic dorsal horn interneurons and neurons that project to the brain, sending the nociceptive “signal” supraspinally. Following tissue injury, the functioning of dorsal horn ligand-gated as well as voltage-gated ion channels is altered such that neurons exhibit persistent spontaneous firing or activation to normally non-noxious stimuli. The abnormal activity of primary afferents and dorsal horn neurons are in part responsible for the pain and cutaneous hypersensitivity that accompanies post-injury recovery and persists long after healing. Thus, one possible method to treat pathological pain is to block cation channels at the level of the dorsal horn.
There are several peptide venoms that have been isolated from Conus marine snails that have significant biological effects. These peptides have been extensively characterized in in vitro assays and have been found to have selectivity to particular ligand- and voltage-gated ion channels (Olivera and Teichert, 2007). For example, intrathecal (i.t.) injection of ω-conotoxin MVIIA (MVIIA; ziconotide) potently blocks neuronal N-type voltage-gated Ca2+ channels (CaV 2.2) and is antinociceptive in a number of preclinical pain models (Chaplan et al., 1994b; Malmberg and Yaksh, 1994). Ziconotide (Prialt®) has been recently approved by the FDA for use in severe pain. However, the therapeutic index of intrathecal MVIIA is narrow and the severity of adverse side-effects appears dose-dependent (Horvath et al., 2002; Scott et al., 2002; Wermeling et al., 2003).
The N-methyl-D-aspartate (NMDA) receptor is a ligand-gated ion channel complex that is sensitive to block by conantokins which are also found in Conus venom (Layer et al., 2004). Activation of spinal dorsal horn NMDA receptors following tissue injury appears to be a key mechanism in tissue injury-associated pain and hypersensitivity (Millan, 1999). There are several binding sites that modulate NMDA receptor function that are potential targets for analgesic drugs, such as the glutamate binding site and the PCP binding site within the channel itself. Antagonists that bind within the channel and block ion influx are analgesic in neuropathic pain (Kristensen et al., 1992; Kvarnstrom et al., 2004; Max et al., 1995). However, psycho-mimetic side-effects do appear even after intrathecal injection. Antagonists that bind to a specific subunit of the NMDA receptor, such as the NR2B subunit, appear to decrease pathological pain but not normal pain perception and do not have the adverse side-effects associated with other subunit non-selective NMDA receptor antagonists (Boyce et al., 1999; Taniguchi et al., 1997). In vitro, conantokin-G (conG), isolated from C. geographus, potently inhibits NMDA receptor function, particularly to receptors containing the NR2B subunit (Layer et al., 2004). In mice, intrathecal injection of conG significantly suppresses the tonic phase (late phase) in the formalin test and neuropathic and inflammation-evoked hind paw hypersensitivity (Malmberg et al., 2003). The antinociceptive doses were well below that which caused motor impairment as measured in the rotarod test.
The current study evaluated the effects of conG and MVIIA in rat models of chronic pain. In particular, pain following spinal cord injury (SCI) is a notoriously difficult therapeutic indication. Since there are numerous mechanisms maintaining the chronic pain state, a combination pharmacological approach may lead to greater pain relief than a drug given alone and result in fewer side effects if lower doses can be used. Thus, the current study also evaluated the efficacy of sub-antinociceptive doses of the peptides combined.
2. Methods
Male Sprague-Dawley rats (Harlan, IN) were used for these experiments. Upon arrival at the vivarium, rats were 100–125 g, were housed two per cage and allowed free access to food and water. Procedures were reviewed and approved by the University of Miami Animal Care and Use Committee and followed recommendations of the “Guide for the Care and Use of Laboratory Animals”. At the end of the studies, rats were euthanized with CO2.
2.1 Surgical procedures
2.11 Spinal nerve ligation
Ligation of the left L5 and L6 spinal nerves was performed as described elsewhere (Kim and Chung, 1992). Rats were anesthetized in isoflurane in O2, and the back of the rat was shaved. Using aseptic technique, the transverse process of the L6 vertebrae was identified and removed. The L5 and L6 spinal nerves were tightly ligated with 4-0 silk. The musculature was sutured shut and the skin incision closed with wound clips. One week following spinal nerve ligation surgery, rats underwent intrathecal catheter surgery.
2.12 Spinal cord injury
Rats were anesthetized with isoflurane in O2 (Hama and Sagen, 2007). Their backs were shaved and, using aseptic surgical technique, a laminectomy was performed to expose spinal segment T6-T7. A micro-vascular clip (Harvard Apparatus, MA) was placed vertically on the exposed thoracic spinal cord and then left in place for 60 sec. Care was taken not to cut the dura or disturb near-by spinal nerve roots. Following spinal compression, the clip was removed, the muscles sutured shut and the skin closed with wound clips. Three weeks following compression surgery, rats underwent intrathecal surgery.
2.13 Intrathecal catheters
Rats were anesthetized with isoflurane in O2, the back of the rat’s head was shaved and the head was secured in a stereotaxic unit. Rats were maintained on isoflurane in O2 via a nose cone. Using aseptic surgical technique, the atlantooccipital membrane was exposed and cut (Yaksh and Rudy, 1976). An intrathecal catheter (8 cm; ReCathCo, PA) was threaded down the intrathecal space and secured to the neck muscles with sutures. The skin incision was closed with cyanoacrylate and the externalized catheter was melted shut. Rats were allowed to recover at least three days following intrathecal surgery prior to use in experiments. At the end of testing, prior to euthanasia, 10 μl of 1.5% lidocaine was intrathecally injected to assess the location of the catheter tip. A bilateral flaccid paralysis of the hind limbs indicated that the catheter tip was in the correct spinal position.
2.2 Testing procedures
2.21 Tactile sensitivity
Hind paw responsiveness to innocuous tactile stimulation were measured with von Frey filaments. Rats were placed on an elevated wire mesh surface and enclosed in Plexiglas. Using the up-down method, filaments were pressed on the plantar hind paw until they slightly bent (Chaplan et al., 1994a). The response series determined the withdrawal threshold (g). In uninjured rats, the highest filament, 15 g, did not evoke a response. Thresholds were measured prior to i.t. injection and 30, 60, 90, 120 post-injection.
2.22 Formalin test
Uninjured rats were given i.t. catheters and allowed at least three days of recovery. Either the peptides or the vehicle was intrathecally injected in a volume of 5 μl, followed by a 5 μl flush with saline. Ten min. after injection, 50 μl of 5% formalin was subcutaneously injected into the plantar left hind paw and rats were immediately placed in a clear Plexiglas chamber (Wheeler-Aceto and Cowan, 1991). The number of hind paw flinches and licking occurring in one min were counted in five min intervals up to 60 min post-formalin injection. Phase 1 was defined as the first min following formalin injection (0–1 min) and phase 2 was defined as 15–61 min post-formalin injection.
2.23 Rotarod test
The effect of the peptides alone and in combination on coordinated motor function was assessed with an accelerating rotarod apparatus (Columbus Instruments, OH; (Hama et al., 2003)). A group of uninjured rats (n = 12) with i.t. catheters were trained once a day for two days on the apparatus prior to testing. The rotarod accelerated 5–25 rpm over 60 sec. Rats were tested prior to and 30, 60, 90, 120 min following i.t. injection. At each time point, the latency to fall (sec) off the rotarod was recorded. After a two day wash-out period, rats were reused. Following the second test session, placement of the i.t. catheter was confirmed and the rats were euthanized.
2.3 Peptides
Peptides were obtained from Bachem, Inc. (CA) and dissolved in saline (vehicle). The peptides, both separately and in combination, were injected in a volume of 5 μl, followed by a 5 μl vehicle flush.
2.4 Statistical analysis
To plot the dose-response curves, the behavioral effects of the peptides were converted to percent effect.
The withdrawal thresholds were converted to percent maximum possible effect:
The formalin-evoked pain-related behaviors of peptide-treated were converted to a percent of vehicle:
The A50 (50% antinociceptive dose) of the peptides alone were calculated from the dose-response curves using a computer program (Tallarida and Murray, 1981). In testing the combination, multiple doses were used, based on the fixed ratio of the A50 values of the peptides. Although the A50 of the peptides alone differed according to the test, the same dose-ratio was used for each test in order to minimize the amount of MVIIA used and to be able to make comparisons across the tests. It is possible that other ratios for each test may lead to super-additive effects (Tallarida and Raffa, 1996).
To asses whether the effect of a peptide combination was greater than that of either peptide alone an isobologram was constructed on a Cartesian plane. The A50 of each peptide was plotted on the horizontal and vertical axis. The line connecting the two A50 was the line of additivity, the theoretical effect locus of the combination “dose pairs”. A dose-response curve of the combination was constructed and the A50 was calculated. The combination was merely additive if the experimentally determined A50 fell on the line of additivity (or within its S.E.M.). An A50 that was below the line of additivity indicated that lesser amounts were needed to obtain the effect whereas an A50 that was above the line of additivity indicated that greater amounts were needed (Tallarida, 2001). Thus, if the A50 of the combination was less than the theoretical additive A50, the combination was superadditive (synergistic) but if the A50 was greater, then the combination was sub-additive. The theoretical and experimentally determined A50 were compared using Student’s t-test to determine statistical significance (P < 0.05). Details of the statistical analysis of drug synergism have been elaborated elsewhere (Tallarida et al., 1989).
3. Results
3.1 General behavioral observations
Following i.t. injection of 300 and 1000 pmol MVIIA, movement-evoked tremors and tail writhing were observed for the duration of the observation period (Malmberg and Yaksh, 1994). Visible adverse side-effects were not apparent at lower doses of MVIIA. Although performance on the rotarod test was diminished (see 3.5 Rotarod test), no visible adverse side-effects were observed with conG at any of the doses tested.
3.2 Formalin test
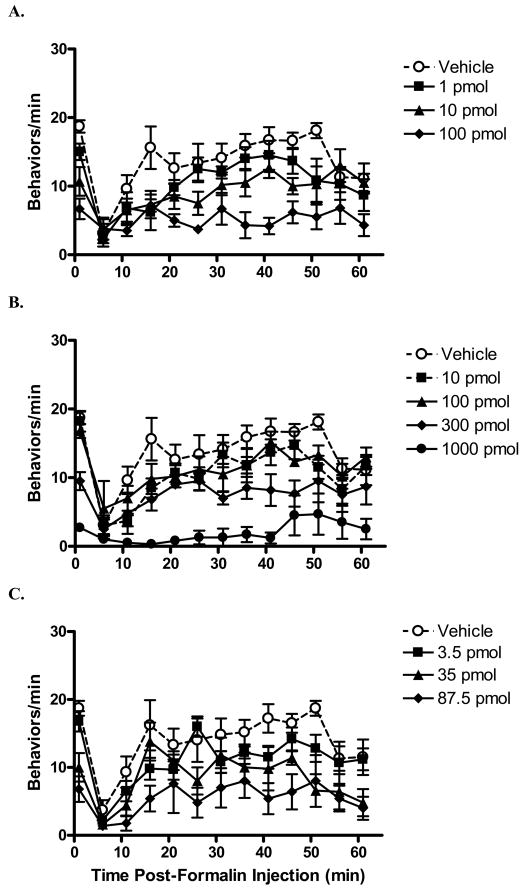
Increasing doses of both MVIIA and conG resulted in significantly decreased formalin-evoked pain related behaviors in both phases (Table 1Fig. 1 A,B ). Although there were no significant differences in potencies of the peptides between phases, ConG was more potent than MVIIA in both phase 1 and phase 2 (P < 0.05). A high dose of MVIIA (1000 pmol) was needed to produce substantial antinociception in the formalin test (both phases), but this dose resulted in tremors and tail writhing.
Table 1.
50% antinociceptive doses of the peptides in rat models of pain.
| ConG | MVIIA | Theoretical additive | Combined | |
|---|---|---|---|---|
| Formalin test | ||||
| Phase 1 (0–1 min) | 21+ (22) | 343 (241) | 116 (90) | 70 (51) |
| Phase 2 (15–61 min) | 36+ (38) | 241 (180) | 138 (91) | 99 (95) |
| Spinal nerve ligation 60 min | 183 (211) | 277 (367) | 203 (192) | 125 (132) |
| Spinal cord injury 90 min | 160 (143) | 72 (36) | 118 (64) | 19* (23) |
50% antinociceptive doses (A50) are expressed in pmol. Values in parenthesis are 95% confidence limits. The A50 were determined from the dose response curves. The “theoretical additive” A50 were calculated based on the dose response curves of the peptides alone. The “combined” A50 were determined from the experimentally determined dose response curves of conG+MVIIA.
Synergistic interaction (P < 0.05 vs. theoretical additive).
P < 0.05 vs. A50 for MVIIA.
Fig. 1.
Time course of the intrathecally injected peptides in the formalin test. Peptide or vehicle was injected 10 min prior to hind paw formalin injection. Following formalin injection, the numbers of flinches and hind paw licks within one minute were counted at five minute intervals. In rats pretreated with vehicle, an initial increase in formalin-evoked behaviors was observed, followed by prolonged period of pain-related behaviors. Pre-treatment with either conantokin-G (conG; A) or conotoxin MVIIA (MVIIA; B) markedly attenuated in a dose-dependent manner the formalin-evoked behaviors. C: Intrathecal injection of the combination of conG and MVIIA dose-dependently decreased formalin-evoked behaviors. The total dose (pmol) of the combination is shown. N = 6–7/group. Data are expressed as mean ± S.E.M.
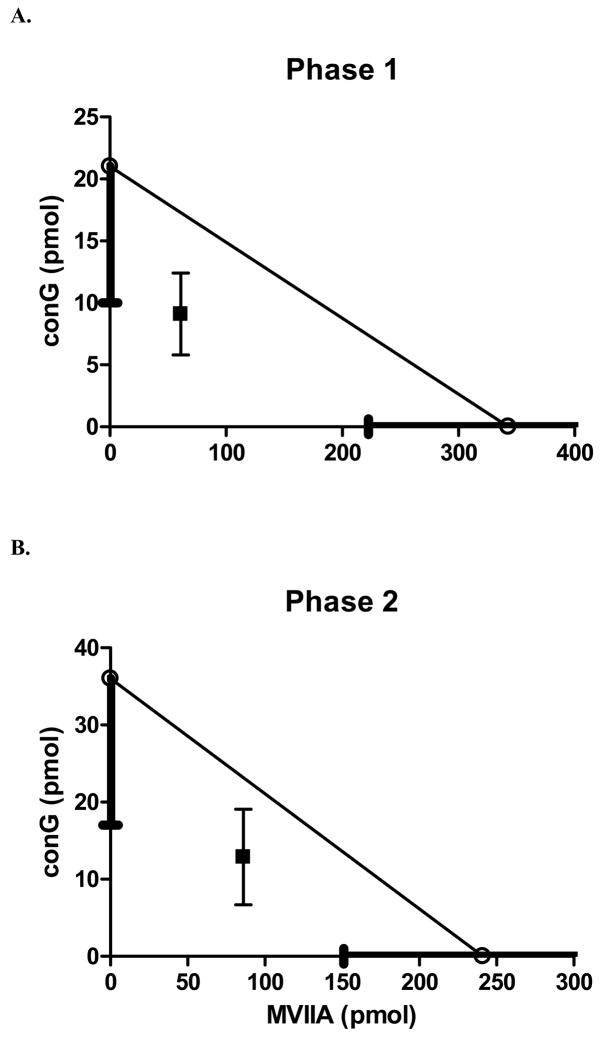
The theoretical additive A50 values for phase 1 and phase 2 were 116 pmol and 138 pmol, respectively. The A50 values of the combination (phase 1: 70 pmol, phase 2: 99 pmol) were not significantly different from the theoretical additive A50. Therefore, the combination was additive (Fig. 1C, Fig. 4). The highest doses of the combination used (62.5 pmol conG and 25 pmol MVIIA), which produced robust antinociception in both phases, did not result in either tremors or tail writhing.
Fig. 4.
Isobolograms of the combination of conantokin-G and conotoxin MVIIA in the formalin test. The A50 of MVIIA (pmol) is demarcated on the horizontal axis and the A50 of conG (pmol) is marked on the vertical axis. The S.E.M. is the thick line overlying the axis. The line connecting the A50 values is the theoretical additive line. A: Isobologram of phase 1. The experimentally determined A50 of the combination falls within the S.E.M. of the line of additivity. Thus, the combination in phase 1 is merely additive. B: Isobologram of phase 2. The combination in phase 2, similar to phase 1, is merely additive.
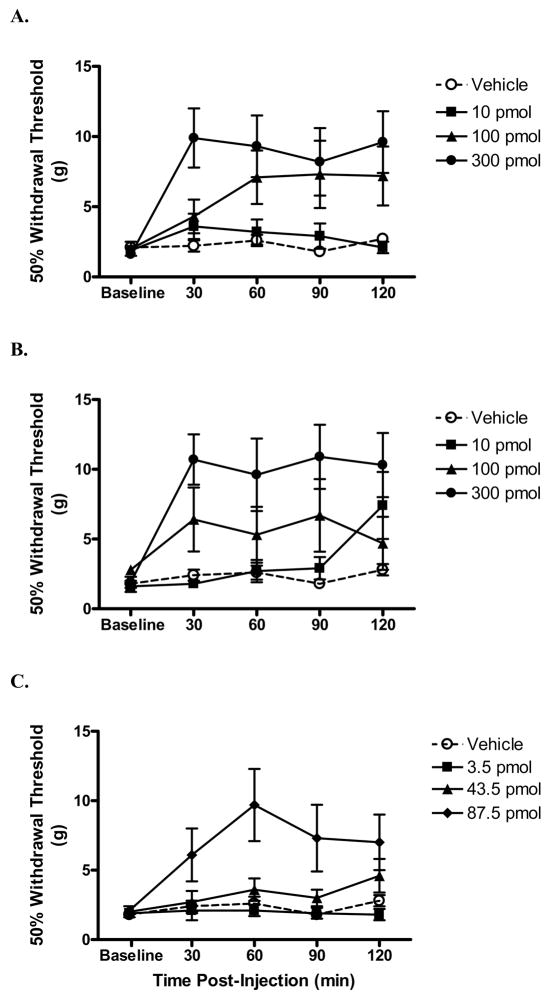
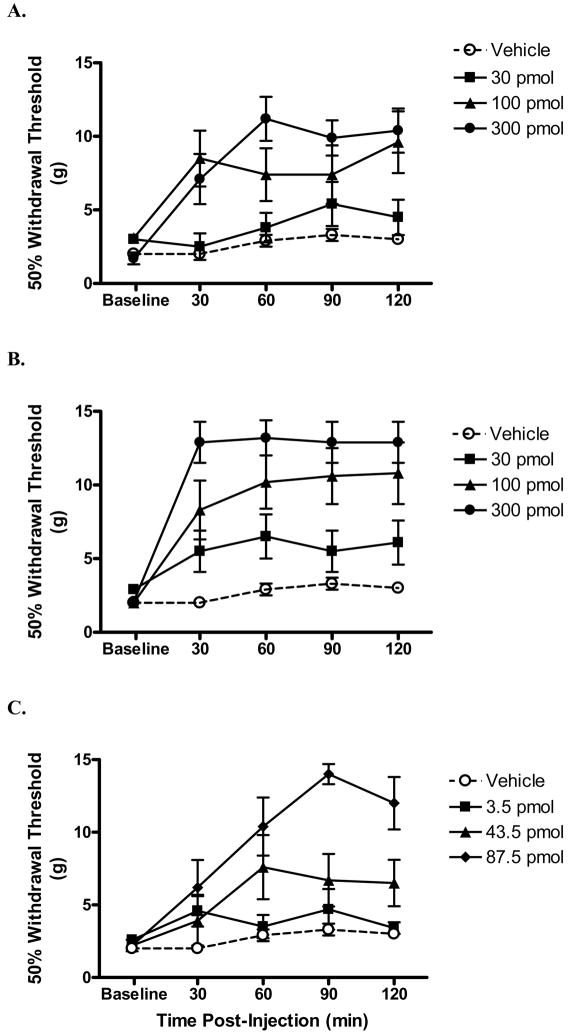
3.3 Spinal nerve ligation
In a peripheral neuropathic pain model, increasing doses of both MVIIA and conG resulted in significantly increased withdrawal thresholds (Table 1, Fig. 2 A, B). Since the peak antinociceptive effects were observed at 60 min post-injection, the A50s were calculated at that time point. The potencies of conG and MVIIA, 183 pmol and 277 pmol, respectively, were not significantly different, but effective doses of MVIIA also results in untoward side effects. ConG appeared to be markedly less effective in reducing peripheral neuropathic pain in this model compared with effects using the formalin test (Table 1, over 5-fold difference in potency compared with phase 1 and 2). Using sub-antinociceptive doses of both peptides in combination, the A50 of the combination (125 pmol) was not statistically different from the theoretical additive A50 (203 pmol), indicating that the combination was additive (Fig 2C, Fig. 5).
Fig. 2.
Effect of intrathecally injected peptides over time on tactile hypersensitivity in rats with a spinal nerve ligation. Following measurement of hind paw withdrawal thresholds (g), rats were i.t. injected with either peptide or vehicle and tested once every 30 min. Intrathecal injection of either conG (A) or MVIIA (B) dose-dependently increased withdrawal thresholds. By contrast, vehicle did not alter withdrawal thresholds. C: Intrathecal injection of the highest dose of the combination of conG and MVIIA increased withdrawal thresholds. The total dose (pmol) of the combination is shown. N = 7/group. Data are expressed as mean ± S.E.M.
Fig. 5.
Isobologram of the combination of conantokin-G and conotoxin MVIIA in rats with a spinal nerve ligation. The A50 of MVIIA is demarcated on the horizontal axis and the A50 of conG is marked on the vertical axis. The S.E.M. is the thick line overlying the axis. The line connecting the A50 values is the theoretical additive line. The experimentally determined A50 of the combination falls within the S.E.M. of the line of additivity. Thus, the combination is merely additive.
3.4 Spinal cord injury
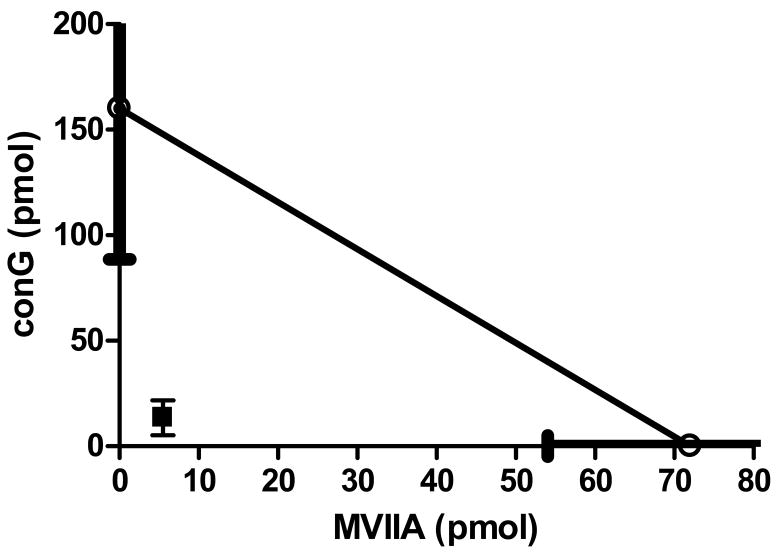
Increasing doses of both MVIIA and conG resulted in significantly increased withdrawal thresholds (Table 1, Fig. 3 A,B). The peak antinociceptive effect of conG was reached at 60–90 min post-injection, and sustained for at least 120 min. A significant antinociceptive effect was observed for MVIIA as early as 30 min post-injection, which was maintained for the two hr observation period. In contrast with both the formalin test and nerve ligation models, MVIIA was markedly more potent in reducing nociception in the spinal cord injury model (by 3- to 5-fold). Antinociceptive potency of conG alone was similar in both the peripheral and central neuropathic pain models (Table 1). In contrast to the additive antinociceptive effects of the peptide combination in the other models, the A50 of the combination (19 pmol) in the spinal cord injury pain model was significantly less than the theoretical additive A50 (118 pmol; P < 0.05). Therefore, the combination was synergistic (Fig. 3C, Fig. 6).
Fig. 3.
Effect of intrathecally injected peptides over time on tactile hypersensitivity in rats with a spinal compression injury. Following measurement of withdrawal thresholds (g) of the hind paws, rats were i.t. injected with either peptide or vehicle and tested once every 30 min. Intrathecal injection of either conG (A) and MVIIA (B) dose-dependently increased withdrawal thresholds. By contrast, vehicle injection did not alter withdrawal thresholds. C: Intrathecal injection of the combination of conG and MVIIA dose-dependently increased withdrawal thresholds. The total dose (pmol) of the combination is shown. N = 6/group. Data are expressed as mean ± S.E.M.
Fig. 6.
Isobologram of the combination of conantokin-G and conotoxin MVIIA in rats with a spinal compression injury. The A50 of MVIIA is demarcated on the horizontal axis and the A50 of conG is marked on the vertical axis. The S.E.M. is the thick line overlying the axis. The line connecting the A50 values is the theoretical additive line. The experimentally determined A50 of the combination falls below the S.E.M. of the line of additivity. Thus, the combination is synergistic.
3.5 Rotarod test
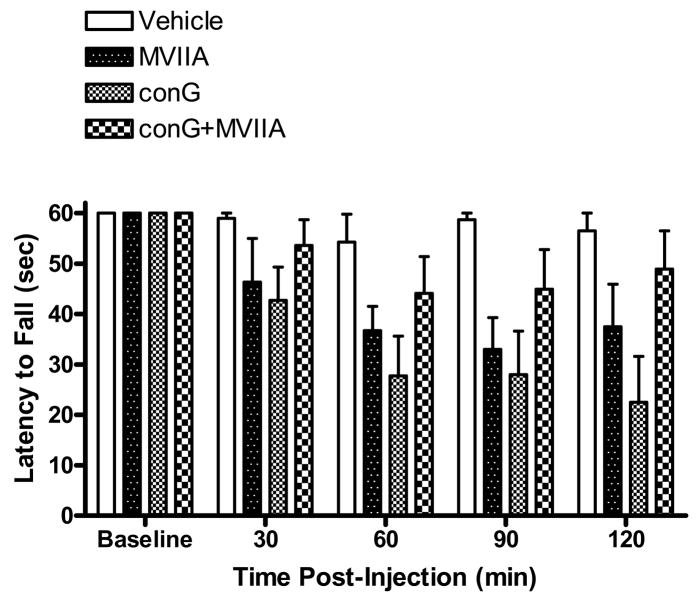
Rats that did not fall off the rotarod prior to the 60 sec. cut-off were assigned a latency of 60 sec. At baseline, prior to i.t. injection, the rats were able to stay on the accelerating rotarod for the full 60 sec (Fig. 7). Intrathecal injection of vehicle did not significantly disrupt coordinated locomotion. By contrast, beginning at 30 min post-injection, antinociceptive doses of either MVIIA (300 pmol) or conG (300 pmol) significantly decreased the latency to fall from the rotarod (P < 0.05 vs. baseline). At 60, 90, and 120 min post-injection, the latencies of MVIIA and conG-treated rats were significantly reduced compared to that of vehicle (P < 0.05). With the highest tested dose of the peptide combination, slight decreases in the latency to fall were observed but these were not significantly different from either baseline or vehicle latencies (P > 0.05).
Fig. 7.
Effect of intrathecally injected peptides over time on coordinated locomotion in the rotarod test. Following baseline measurement of the latency to fall (sec) from the rotarod, rats were i.t. injected with either peptide or vehicle and tested once every 30 min. Injection of either conG (300 pmol) or MVIIA (300 pmol) significantly decreased the latency to fall. By contrast, vehicle did not affect locomotion. Also, injection of the combination conG+MVIIA (87.5 pmol) did not significantly impair locomotion. N = 6/group. Data are expressed as mean ± S.E.M.
The highest tested dose of MVIIA (1000 pmol; Fig. 1) in this study was not used in the rotarod test due to the long duration (6–12 hrs) of movement-evoked tremor at this dose.
4. Discussion
Intrathecal injection of peptides with distinct molecular targets, MVIIA, a N-type voltage-gated Ca2+ channel blocker, and conG, a NMDA receptor antagonist, led to robust antinociceptive effects in rat models of persistent pain. In the formalin test and in rats with peripheral neuropathic pain, combination of the two peptides resulted in additive antinociceptive effects, but in rats with neuropathic SCI pain, a significant synergistic interaction was obtained. The potencies of the peptides differed across models which suggest varying degrees of involvement of the N-type Ca2+ channel and the NR2B-containing NMDA receptor across the models. The differential involvement of these molecular targets could also explain the additive antinociceptive effect of the peptides in some pain states compared to the super-additive (synergistic) effect in SCI pain. The current data also suggest that combining these peptides may be a novel approach to clinical pain control.
Persistent pain following tissue injury, including SCI, is a significant clinical problem and few pharmacological treatments are effective (Finnerup et al., 2001; Namaka et al., 2004). There are drugs that are effective for peripheral neuropathic pain, although the same drugs may not be as effective for neuropathic SCI pain, suggesting that drugs may have varying efficacies depending on the pain state (Finnerup and Jensen, 2004). Thus, despite similarities in symptoms, the various chronic pain states have distinct underlying mechanisms. Greater clarification of the pain mechanisms may lead to drugs with improved efficacy and safety and possibly activity across a spectrum of pain states.
Lesioning of the spinal cord leads to extensive neural and gliotic responses including the formation and release of excitatory amino acids, neuropeptide transmitters, inflammatory substances and cations (Yezierski, 2005). The initial release of excitatory substances triggers further exocytosis, spreading to adjacent intact tissue, mediated by voltage-gated and ligand-gated ion channels (Millan, 1999). The elevated influx of cations not only leads to exocytosis but also stimulation of Ca2+ dependent protein kinases, which have a number of modulatory effects on gene expression and receptor function (Millan, 1999). These abnormal and persistent changes in spinal cord homeostasis, in turn, underlie cutaneous hypersensitivity and spontaneous pain (Yezierski, 2005). Thus, blocking injury-associated ion entry into neurons may significantly reduce neuropathic SCI pain.
N-methyl-D-aspartate receptor antagonists markedly attenuate spinal cord tissue pathology and accompanying cutaneous hypersensitivity caused by spinal vascular ischemia (Hao et al., 1991). Systemic NMDA receptor antagonists have been shown to be antinociceptive in other models of chronic pain and in some types of clinical neuropathic but not acute pain (Eide et al., 1994; Nelson et al., 1997). However, the maximum tested doses were accompanied by adverse side-effects, such as dizziness and fatigue, discouraging widespread clinical use. N-methyl-D-aspartate receptors are localized in the dorsal horn of the spinal cord, so i.t. antagonist delivery attenuated pain with greatly diminished side-effects compared to systemic drug administration (Bennett et al., 2000; Sato et al., 1993; Tolle et al., 1993). Alternatively, antagonists selective for the NR2B subunit of the NMDA receptor may be useful for the pain treatment. Selective antagonists attenuate pain-related symptoms in rat neuropathic pain models, with few observable side-effects following systemic administration and are also neuroprotective (Boyce et al., 1999; Chazot, 2004; Chizh et al., 2001). The effects of small molecule selective antagonists have not been evaluated in neuropathic SCI pain.
A previous study utilizing various mouse pain models demonstrated robust antinociception following i.t. conG injection (Malmberg et al., 2003). The current study also demonstrated antinociceptive effects in rats, confirming the notion that antagonism of spinal NR2B-containing NMDA receptors with conG leads to antinociception across chronic pain models with diverse etiologies. In the dorsal horn, the NR2B subunits are found on primary afferent central terminals, which suggests that the antinociceptive effect of conG is due to reducing neurotransmitter release (Furuyama et al., 1993; Marvizon et al., 2002; Monaghan and Cotman, 1985; Tolle et al., 1993). The exact role, however, of spinal NR2B-containing NMDA receptors in nociception is controversial. Despite unambiguous localization of the subunit protein central terminals, i.t. injection of NR2B-selective small molecule antagonists do not attenuate nociception (Coderre, 1993; Nakazato et al., 2005). Also, in the case of SCI, NMDA receptor function is decreased, as measured by 3[H]MK-801 binding (Krenz and Weaver, 1998). These observations imply that conG may have mechanisms of action beyond NMDA receptor antagonism (Barton et al., 2004).
Similar to antagonism of pre-synaptic central terminal NMDA receptors, block of pre-synaptic N-type Ca2+ channels inhibit transmitter release and decreases spinal cord tissue pathology due to ischemia (Burns et al., 1999; Smith et al., 2002). Also, N-type Ca2+ channel blockers applied to post-synaptic dorsal horn neurons in rat models of chronic pain reduce spontaneous activity and abnormal responses to peripheral stimuli (Heinke et al., 2004; Matthews and Dickenson, 2001; Takemura et al., 1989; Vanegas and Schaible, 2000; Yusaf et al., 2001). Blocking other Ca2+ channel subtypes, such as P/Q- and L-type, in the spinal cord has little effect on chronic pain behavior (Chaplan et al., 1994b). Although i.t. ziconotide has been FDA approved for use in severe chronic pain, serious side-effects have been reported, including psychosis, dizziness and hypotension, which appear to be drug-mediated (Olivera and Teichert, 2007; Penn and Paice, 2000). N-type Ca2+ channel block may not be efficacious against some types of neuropathic pain (Yamamoto and Sakashita, 1998). To circumvent these issues, a low dose of MVIIA may be combined with other analgesics, the resulting mixture having significantly greater efficacy with markedly reduced side-effects compared to an equivalent dose of MVIIA (Wallace et al., 2008; Wang et al., 2000).
In the formalin test, the pain-related behaviors of the first phase is mediated through an acute activation of primary afferent nociceptors and the second phase is mediate by a sustained dorsal horn neuron activity (Yaksh et al., 1999). Block of dorsal horn N-type Ca2+ channels and NMDA receptors prior to formalin injection results in a slight antinociceptive effect in the first phase but a significant attenuation in the second phase, indicating that these channels and receptors are involved in the initiation of injury-induced persistent nociception (Coderre et al., 1993; Malmberg and Yaksh, 1994). The current data suggests that a combination of a NR2B-selective antagonist with a N-type Ca2+ channel blocker may prevent post-injury persistent nociception in an additive manner. Thus, such pretreatments would be useful prior to surgical procedures to diminish post-operative pain (Atanassoff et al., 2000; Choe et al., 1997; Ong et al., 2005).
Although numerous clinical analgesic drugs have been tested in rat models of neuropathic SCI pain, MVIIA has not been extensively evaluated (Hama and Sagen, 2007; Xu et al., 1992). In the current study MVIIA dose dependently ameliorated below-level hypersensitivity. Likewise, a recent case report demonstrated efficacy on below-level, but not at-level, neuropathic SCI pain (Saulino, 2007). Thus, block of spinal dorsal horn N-type Ca2+ channels may be effective for some types of SCI pain. Large-scale clinical trials are needed to confirm the efficacy of N-type Ca2+ channel blockers in neuropathic SCI pain.
Interestingly, MVIIA was significantly more potent than conG on SCI below-level hypersensitivity. The possible underlying source of increased potency of MVIIA includes increased channel affinity to MVIIA, but the exact functional disposition of N-type Ca2+ channels following SCI is not known. Characterizing changes in other ion channel function and distribution following SCI may also lead to the development of novel analgesic drugs.
Conversely, it appears that conG is more potent in the formalin test than in long-term neuropathic pain models. In fact, it appears that i.t. NMDA receptor antagonists are more potent in the formalin test than in spinal nerve injury pain (Chaplan et al., 1997). The dissimilar potencies suggest significant mechanistic differences between the models. Perhaps the inactivity of the NMDA receptors, prior to formalin injury, allows for antinociception to be obtained at low doses. As mentioned earlier, drugs that are applied prior to injury, as they are in the formalin test, may have utility as pre-operative analgesics (Atanassoff et al., 2000; Choe et al., 1997; Ong et al., 2005).
Similar to a previous study in mice, the highest tested doses of conG and MVIIA (300 pmol) in the current study resulted in impaired coordinated locomotion (Malmberg et al., 2003), thus, it is possible that motor impairment masqueraded as antinociception. However, motor impairment cannot entirely explain the effects of the peptides since the antinociceptive effects observed in the current study were dose-dependent and not limited to the highest dose. Motor impairment without antinociception can be observed with other drugs. For example, diazepam (3 mg/kg), which disrupts rotarod performance, is not antinociceptive (data not shown; (Carter, 1991; Franklin and Abbott, 1993)).
It is possible that in addition to enhanced antinociception of the combination, the side-effects are comparably worsened. Co-administration of conG and MVIIA resulted in an additive antinociception and in the case of neuropathic SCI pain, synergism. However, in the rotarod test, the combination did not significantly disrupt performance, in contrast to the markedly degraded performance with antinociceptive doses of the peptides alone. The lack of either additivity or synergism with the combination suggests distinct roles of NR2B-containing NMDA receptors and N-type Ca2+ channels in locomotion and sensation. It is unknown if other adverse side effects of ziconotide, which are difficult to discern in rats (e.g. dizziness), are affected by the addition of conG -- further studies are needed.
The current data suggest that acute i.t. injection of cone snail peptides, especially in combination, leads to robust antinociception. It has yet to be determined if efficacy is maintained with extended i.t. infusion of conG (Olivera and Teichert, 2007). Although these are peptides, which limits the route of administration, there are a number of potential ways that they could be continuously delivered for pain relief, including novel gene therapy (Glorioso et al., 2003) or cellular transplantation approaches (Eaton, 2006). Such methods may be of utility especially for chronic neuropathic SCI pain since few pharmacotherapies have long term efficacy.
Acknowledgments
We thank Dr. Shyam Gajavelli for supportive discussions and Dr. Maria Collado for technical assistance. Supported in part by the Miami Project to Cure Paralysis, NIH grant NS51667 and by the Craig H. Nielsen Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atanassoff PG, Hartmannsgruber MW, Thrasher J, Wermeling D, Longton W, Gaeta R, Singh T, Mayo M, McGuire D, Luther RR. Ziconotide, a new N-type calcium channel blocker, administered intrathecally for acute postoperative pain. Reg Anesth Pain Med. 2000;25:274–278. doi: 10.1016/s1098-7339(00)90010-5. [DOI] [PubMed] [Google Scholar]
- Barton ME, White HS, Wilcox KS. The effect of CGX-1007 and CI-1041, novel NMDA receptor antagonists, on NMDA receptor-mediated EPSCs. Epilepsy Res. 2004;59:13–24. doi: 10.1016/j.eplepsyres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Bennett G, Serafini M, Burchiel K, Buchser E, Classen A, Deer T, Du Pen S, Ferrante FM, Hassenbusch SJ, Lou L, Maeyaert J, Penn R, Portenoy RK, Rauck R, Willis KD, Yaksh T. Evidence-based review of the literature on intrathecal delivery of pain medication. J Pain Symptom Manage. 2000;20:S12–36. doi: 10.1016/s0885-3924(00)00204-9. [DOI] [PubMed] [Google Scholar]
- Boyce S, Wyatt A, Webb JK, O’Donnell R, Mason G, Rigby M, Sirinathsinghji D, Hill RG, Rupniak NM. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology. 1999;38:611–623. doi: 10.1016/s0028-3908(98)00218-4. [DOI] [PubMed] [Google Scholar]
- Burns LH, Jin Z, Bowersox SS. The neuroprotective effects of intrathecal administration of the selective N-type calcium channel blocker ziconotide in a rat model of spinal ischemia. J Vasc Surg. 1999;30:334–343. doi: 10.1016/s0741-5214(99)70145-x. [DOI] [PubMed] [Google Scholar]
- Carter RB. Differentiating analgesic and non-analgesic drug activities on rat hot plate: effect of behavioral endpoint. Pain. 1991;47:211–220. doi: 10.1016/0304-3959(91)90207-E. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994a;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280:829–838. [PubMed] [Google Scholar]
- Chaplan SR, Pogrel JW, Yaksh TL. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J Pharmacol Exp Ther. 1994b;269:1117–1123. [PubMed] [Google Scholar]
- Chazot PL. The NMDA receptor NR2B subunit: a valid therapeutic target for multiple CNS pathologies. Curr Med Chem. 2004;11:389–396. doi: 10.2174/0929867043456061. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Headley PM, Tzschentke TM. NMDA receptor antagonists as analgesics: focus on the NR2B subtype. Trends Pharmacol Sci. 2001;22:636–642. doi: 10.1016/s0165-6147(00)01863-0. [DOI] [PubMed] [Google Scholar]
- Choe H, Choi YS, Kim YH, Ko SH, Choi HG, Han YJ, Song HS. Epidural morphine plus ketamine for upper abdominal surgery: improved analgesia from preincisional versus postincisional administration. Anesth Analg. 1997;84:560–563. doi: 10.1097/00000539-199703000-00017. [DOI] [PubMed] [Google Scholar]
- Coderre TJ. Potent analgesia induced in rats by combined action at PCP and polyamine recognition sites of the NMDA receptor complex. Eur J Neurosci. 1993;5:390–393. doi: 10.1111/j.1460-9568.1993.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- Eaton MJ. Cell and molecular approaches to the attenuation of pain after spinal cord injury. J Neurotrauma. 2006;23:549–559. doi: 10.1089/neu.2006.23.549. [DOI] [PubMed] [Google Scholar]
- Eide PK, Jorum E, Stubhaug A, Bremnes J, Breivik H. Relief of post-herpetic neuralgia with the N-methyl-D-aspartic acid receptor antagonist ketamine: a double-blind, cross-over comparison with morphine and placebo. Pain. 1994;58:347–354. doi: 10.1016/0304-3959(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Jensen TS. Spinal cord injury pain--mechanisms and treatment. Eur J Neurol. 2004;11:73–82. doi: 10.1046/j.1351-5101.2003.00725.x. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Johannesen IL, Sindrup SH, Bach FW, Jensen TS. Pain and dysesthesia in patients with spinal cord injury: A postal survey. Spinal Cord. 2001;39:256–262. doi: 10.1038/sj.sc.3101161. [DOI] [PubMed] [Google Scholar]
- Franklin KB, Abbott FV. Pentobarbital, diazepam, and ethanol abolish the interphase diminution of pain in the formalin test: evidence for pain modulation by GABAA receptors. Pharmacol Biochem Behav. 1993;46:661–666. doi: 10.1016/0091-3057(93)90558-b. [DOI] [PubMed] [Google Scholar]
- Furuyama T, Kiyama H, Sato K, Park HT, Maeno H, Takagi H, Tohyama M. Region-specific expression of subunits of ionotropic glutamate receptors (AMPA-type, KA-type and NMDA receptors) in the rat spinal cord with special reference to nociception. Brain Res Mol Brain Res. 1993;18:141–151. doi: 10.1016/0169-328x(93)90183-p. [DOI] [PubMed] [Google Scholar]
- Glorioso JC, Mata M, Fink DJ. Exploiting the neurotherapeutic potential of peptides: targeted delivery using HSV vectors. Expert Opin Biol Ther. 2003;3:1233–1239. doi: 10.1517/14712598.3.8.1233. [DOI] [PubMed] [Google Scholar]
- Hama A, Sagen J. Behavioral characterization and effect of clinical drugs in a rat model of pain following spinal cord compression. Brain Res. 2007;1185:117–128. doi: 10.1016/j.brainres.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Hama A, Woon Lee J, Sagen J. Differential efficacy of intrathecal NMDA receptor antagonists on inflammatory mechanical and thermal hyperalgesia in rats. Eur J Pharmacol. 2003;459:49–58. doi: 10.1016/s0014-2999(02)02828-5. [DOI] [PubMed] [Google Scholar]
- Hao JX, Xu XJ, Aldskogius H, Seiger A, Wiesenfeld-Hallin Z. The excitatory amino acid receptor antagonist MK-801 prevents the hypersensitivity induced by spinal cord ischemia in the rat. Exp Neurol. 1991;113:182–191. doi: 10.1016/0014-4886(91)90174-b. [DOI] [PubMed] [Google Scholar]
- Heinke B, Balzer E, Sandkuhler J. Pre- and postsynaptic contributions of voltage-dependent Ca2+ channels to nociceptive transmission in rat spinal lamina I neurons. Eur J Neurosci. 2004;19:103–111. doi: 10.1046/j.1460-9568.2003.03083.x. [DOI] [PubMed] [Google Scholar]
- Horvath G, Brodacz B, Holzer-Petsche U. Blood pressure changes after intrathecal co-administration of calcium channel blockers with morphine or clonidine at the spinal level. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:270–275. doi: 10.1007/s00210-002-0591-5. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Effect of spinal cord transection on N-methyl-D-aspartate receptors in the cord. J Neurotrauma. 1998;15:1027–1036. doi: 10.1089/neu.1998.15.1027. [DOI] [PubMed] [Google Scholar]
- Kristensen JD, Svensson B, Gordh T., Jr The NMDA-receptor antagonist CPP abolishes neurogenic ‘wind-up pain’ after intrathecal administration in humans. Pain. 1992;51:249–253. doi: 10.1016/0304-3959(92)90266-E. [DOI] [PubMed] [Google Scholar]
- Kvarnstrom A, Karlsten R, Quiding H, Gordh T. The analgesic effect of intravenous ketamine and lidocaine on pain after spinal cord injury. Acta Anaesthesiol Scand. 2004;48:498–506. doi: 10.1111/j.1399-6576.2003.00330.x. [DOI] [PubMed] [Google Scholar]
- Layer RT, Wagstaff JD, White HS. Conantokins: peptide antagonists of NMDA receptors. Curr Med Chem. 2004;11:3073–3084. doi: 10.2174/0929867043363901. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Gilbert H, McCabe RT, Basbaum AI. Powerful antinociceptive effects of the cone snail venom-derived subtype-selective NMDA receptor antagonists conantokins G and T. Pain. 2003;101:109–116. doi: 10.1016/s0304-3959(02)00303-2. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Voltage-sensitive calcium channels in spinal nociceptive processing: blockade of N- and P-type channels inhibits formalin-induced nociception. J Neurosci. 1994;14:4882–4890. doi: 10.1523/JNEUROSCI.14-08-04882.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, McRoberts JA, Ennes HS, Song B, Wang X, Jinton L, Corneliussen B, Mayer EA. Two N-methyl-D-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J Comp Neurol. 2002;446:325–341. doi: 10.1002/cne.10202. [DOI] [PubMed] [Google Scholar]
- Matthews EA, Dickenson AH. Effects of spinally delivered N- and P-type voltage-dependent calcium channel antagonists on dorsal horn neuronal responses in a rat model of neuropathy. Pain. 2001;92:235–246. doi: 10.1016/s0304-3959(01)00255-x. [DOI] [PubMed] [Google Scholar]
- Max MB, Byas-Smith MG, Gracely RH, Bennett GJ. Intravenous infusion of the NMDA antagonist, ketamine, in chronic posttraumatic pain with allodynia: a double-blind comparison to alfentanil and placebo. Clin Neuropharmacol. 1995;18:360–368. doi: 10.1097/00002826-199508000-00008. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Cotman CW. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985;5:2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato E, Kato A, Watanabe S. Brain but not spinal NR2B receptor is responsible for the anti-allodynic effect of an NR2B subunit-selective antagonist CP-101,606 in a rat chronic constriction injury model. Pharmacology. 2005;73:8–14. doi: 10.1159/000081069. [DOI] [PubMed] [Google Scholar]
- Namaka M, Gramlich CR, Ruhlen D, Melanson M, Sutton I, Major J. A treatment algorithm for neuropathic pain. Clin Ther. 2004;26:951–979. doi: 10.1016/s0149-2918(04)90171-3. [DOI] [PubMed] [Google Scholar]
- Nelson KA, Park KM, Robinovitz E, Tsigos C, Max MB. High-dose oral dextromethorphan versus placebo in painful diabetic neuropathy and postherpetic neuralgia. Neurology. 1997;48:1212–1218. doi: 10.1212/wnl.48.5.1212. [DOI] [PubMed] [Google Scholar]
- Olivera BM, Teichert RW. Diversity of the neurotoxic Conus peptides: a model for concerted pharmacological discovery. Mol Interv. 2007;7:251–260. doi: 10.1124/mi.7.5.7. [DOI] [PubMed] [Google Scholar]
- Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg. 2005;100:757–773. doi: 10.1213/01.ANE.0000144428.98767.0E. table of contents. [DOI] [PubMed] [Google Scholar]
- Penn RD, Paice JA. Adverse effects associated with the intrathecal administration of ziconotide. Pain. 2000;85:291–296. doi: 10.1016/s0304-3959(99)00254-7. [DOI] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Park HT, Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. Neuroreport. 1993;4:1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- Saulino M. Successful reduction of neuropathic pain associated with spinal cord injury via of a combination of intrathecal hydromorphone and ziconotide: a case report. Spinal Cord. 2007;45:749–752. doi: 10.1038/sj.sc.3102027. [DOI] [PubMed] [Google Scholar]
- Scott DA, Wright CE, Angus JA. Actions of intrathecal omega-conotoxins CVID, GVIA, MVIIA, and morphine in acute and neuropathic pain in the rat. Eur J Pharmacol. 2002;451:279–286. doi: 10.1016/s0014-2999(02)02247-1. [DOI] [PubMed] [Google Scholar]
- Smith MT, Cabot PJ, Ross FB, Robertson AD, Lewis RJ. The novel N-type calcium channel blocker, AM336, produces potent dose-dependent antinociception after intrathecal dosing in rats and inhibits substance P release in rat spinal cord slices. Pain. 2002;96:119–127. doi: 10.1016/s0304-3959(01)00436-5. [DOI] [PubMed] [Google Scholar]
- Takemura M, Kiyama H, Fukui H, Tohyama M, Wada H. Distribution of the omega-conotoxin receptor in rat brain. An autoradiographic mapping. Neuroscience. 1989;32:405–416. doi: 10.1016/0306-4522(89)90089-4. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298:865–872. [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of Pharmacological Calculations with Computer Programs. Springer-Verlag; New York, NY: 1981. [Google Scholar]
- Tallarida RJ, Porreca F, Cowan A. Statistical analysis of drug-drug and site-site interactions with isobolograms. Life Sci. 1989;45:947–961. doi: 10.1016/0024-3205(89)90148-3. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Raffa RB. Testing for synergism over a range of fixed ratio drug combinations: replacing the isobologram. Life Sci. 1996;58:PL 23–28. doi: 10.1016/0024-3205(95)02271-6. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Shinjo K, Mizutani M, Shimada K, Ishikawa T, Menniti FS, Nagahisa A. Antinociceptive activity of CP-101,606, an NMDA receptor NR2B subunit antagonist. Br J Pharmacol. 1997;122:809–812. doi: 10.1038/sj.bjp.0701445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolle TR, Berthele A, Zieglgansberger W, Seeburg PH, Wisden W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. J Neurosci. 1993;13:5009–5028. doi: 10.1523/JNEUROSCI.13-12-05009.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas H, Schaible H. Effects of antagonists to high-threshold calcium channels upon spinal mechanisms of pain, hyperalgesia and allodynia. Pain. 2000;85:9–18. doi: 10.1016/s0304-3959(99)00241-9. [DOI] [PubMed] [Google Scholar]
- Wallace MS, Kosek PS, Staats P, Fisher R, Schultz DM, Leong M. Phase II, open-label, multicenter study of combined intrathecal morphine and ziconotide: addition of ziconotide in patients receiving intrathecal morphine for severe chronic pain. Pain Med. 2008;9:271–281. doi: 10.1111/j.1526-4637.2007.00355.x. [DOI] [PubMed] [Google Scholar]
- Wang YX, Gao D, Pettus M, Phillips C, Bowersox SS. Interactions of intrathecally administered ziconotide, a selective blocker of neuronal N-type voltage-sensitive calcium channels, with morphine on nociception in rats. Pain. 2000;84:271–281. doi: 10.1016/s0304-3959(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Wermeling D, Drass M, Ellis D, Mayo M, McGuire D, O’Connell D, Hale V, Chao S. Pharmacokinetics and pharmacodynamics of intrathecal ziconotide in chronic pain patients. J Clin Pharmacol. 2003;43:624–636. [PubMed] [Google Scholar]
- Wheeler-Aceto H, Cowan A. Standardization of the rat paw formalin test for the evaluation of analgesics. Psychopharmacology (Berl) 1991;104:35–44. doi: 10.1007/BF02244551. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Hao JX, Aldskogius H, Seiger A, Wiesenfeld-Hallin Z. Chronic pain-related syndrome in rats after ischemic spinal cord lesion: a possible animal model for pain in patients with spinal cord injury. Pain. 1992;48:279–290. doi: 10.1016/0304-3959(92)90070-R. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Hua XY, Kalcheva I, Nozaki-Taguchi N, Marsala M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc Natl Acad Sci U S A. 1999;96:7680–7686. doi: 10.1073/pnas.96.14.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sakashita Y. Differential effects of intrathecally administered N- and P-type voltage-sensitive calcium channel blockers upon two models of experimental mononeuropathy in the rat. Brain Res. 1998;794:329–332. doi: 10.1016/s0006-8993(98)00306-0. [DOI] [PubMed] [Google Scholar]
- Yezierski RP. Spinal cord injury: a model of central neuropathic pain. Neurosignals. 2005;14:182–193. doi: 10.1159/000087657. [DOI] [PubMed] [Google Scholar]
- Yusaf SP, Goodman J, Pinnock RD, Dixon AK, Lee K. Expression of voltage-gated calcium channel subunits in rat dorsal root ganglion neurons. Neurosci Lett. 2001;311:137–141. doi: 10.1016/s0304-3940(01)02038-9. [DOI] [PubMed] [Google Scholar]