Abstract
Background and Aims Quercus coccifera,
as a long-lived sprouter, responds plastically to environmental variation. In this study, the role of foliar plasticity as a mechanism of habitat selection and modification within the canopy and across contrasted habitats was characterized. An examination was made of the differential contribution of inner and outer canopy layers to the crown plasticity expressed in the field by adult individuals and its dependence on environmental and genetic factors.
Methods
Within-crown variation in eight foliar traits was examined in nine populations dominated by Q. coccifera. The difference between mean trait values at the inner and outer canopy layers was used as a proxy for crown plasticity to light. Correlations between geographic distances, environmental differences (climatic and edaphic) and phenotypic divergence (means and plasticities) were assessed by partial Mantel tests. A subset of field measurements was compared with data from a previous common garden experiment.
Key Results
Phenotypic adjustment of sun leaves contributed significantly to the field variation in crown plasticity. Plasticity in leaf angle, lobation, xanthophyll cycle pigments and β-carotene content was expressed in sun and shade leaves concurrently and in opposite directions. Phenotypic plasticity was more strongly correlated with environmental variation than mean trait values. Populations of taller plants with larger, thinner (higher specific leaf area) and less spiny leaves exhibited greater plasticity. In these populations, the midday light environment was more uniform at the inner than at the outer canopy layers. Field and common garden data ranked populations in the same order of plasticity.
Conclusions
The expression of leaf plasticity resulted in a phenotypic differentiation that suggests a mechanism of habitat selection through division of labour across canopy layers. Signs of plasticity-mediated habitat modification were found only in the most plastic populations. Intracanopy plasticity was sensitive to environmental variation but also exhibited a strong genetic component.
Key words: Habitat selection, habitat modification, leaf morphology, niche construction, phenotypic plasticity, population divergence, resprouter, within-crown division of labour, xanthophylls
INTRODUCTION
Long-lived organisms are expected to respond primarily by phenotypic plasticity to the environmental changes experienced throughout their lifespan (Sultan, 1987). This ability to perceive and respond to environmental cues by adopting alternative phenotypes (Bradshaw, 1965; Schlichting and Pigliucci, 1998) is relevant at different spatial and temporal scales. At the population level, phenotypic plasticity provides a mechanism by which species can tolerate wide-ranging environmental conditions without genetic change (Via, 1994). At a temporal scale, plastic response to environmental change facilitates survival and the potential for subsequent adaptive specialization (Ackerly, 2003). Genetic changes may either fix increased adaptive plasticity or attenuate environmental responsiveness when plasticity lowers fitness (Grether, 2005). At the individual level, with the expression of phenotypic plasticity comes the potential for discriminating between environmental qualities that are most suitable for growth. The plastic nature of plant modular construction allows the location of foraging organs in favourable resource patches, morphological and physiological integration among these, and even enables plant movements to reach appropriate habitats (e.g. by spread or fragmentation; Bazzaz, 1991). Plasticity enables habitat selection (sensu Donohue, 2003) to the extent that the response to cues changes the quality of the environment experienced subsequently, both in space (e.g. by tropisms) and in time (e.g. by bud-burst or germination timing). As a result of this ability, the phenotype constitutes both a result and a cause of the environment experienced by each leaf, branch or root segment (Sultan, 1987). This is particularly apparent when the expression of plasticity at the individual level transcends its role as a habitat-selecting mechanism and enables direct modification of the plant environment, i.e. niche construction (sensu Laland et al., 1999). It is known that expression of plasticity in architectural traits, such as leaf angle or size, results in habitat modifications with consequences for daily light interception and whole-plant carbon gain (Howell et al., 2002; Falster and Westoby, 2003).
Intracanopy plasticity in foliar traits seems to be elicited by both local environmental cues and regulatory signals originating from elsewhere in the plant (Miyazawa et al., 2006; Sack et al., 2006). With respect to the former, plants are expected to modulate their morphology and physiology in response to within-crown gradients in irradiance quantity and quality, temperature, vapour pressure deficit and wind speed (Sack et al., 2006). With respect to plant-wide signalling, plasticity as a local response is regulated by module integration, i.e. by the interaction effects due to the behaviour and communication of the structural and functional subunits of the plant (de Kroon et al., 2005). Integration of nutrient and photosynthate fluxes at the whole-plant level conditions the expression of plasticity in the foraging behaviour of the plant crown (Stoll and Schmid, 1998; Yang and Midmore, 2005). Within the canopy, this whole-plant integration may involve a feedback loop in which the expression of a given phenotype depends on carbon allocation from source leaves and, in turn, carbon assimilation by source leaves depends on the environmental modifications caused by the plasticity expressed elsewhere in the crown.
In long-lived woody sprouters, the phenotypic response to the immediate environment seems to afford a significant degree of control of resource availability through modification of environmental conditions, for instance by altering the light environment within the crown and at ground level (Bellingham and Sparrow, 2000). However, most research on the role of phenotypic plasticity in sprouters has been restricted to seedlings or saplings (but, for exceptions, see, e.g., Richardson et al., 2001; Valladares et al., 2005), which is a substantial drawback considering that these species exhibit lifespans 10–100 times longer than non-sprouters (Bond and Midgley, 2003). A sapling of a Mediterranean sprouter of the genus Quercus, for instance, represents a hundredth of its lifespan, and exhibits a crown that differs drastically from that of the adult in structure and degree of modularity. Previous reports on sprouting tree species suggest that the ultimate effect of phenotypic plasticity can only be observed in adults and in the field (Cavender-Bares et al., 2004; Bouvet et al., 2005). However, most studies have been conducted in common gardens and glasshouses, where the phenotypic range induced may never be expressed in natural environments (cf. paradaptive plasticity; Lortie and Aarssen, 1996) or may never reach the extreme phenotypes found in the field (Wayne and Bazzaz, 1993).
In the present study, field patterns of phenotypic variation were investigated in adult plants of Quercus coccifera, a characteristic evergreen sprouter of the Mediterranean Basin. Considering phenotypic plasticity as a major source of phenotypic variation in natural populations (cf. Schlichting and Pigliucci, 1998; Ackerly et al., 2000), it was predicted that these patterns would exhibit signs of habitat selection and modification. If so, intracanopy plasticity should not be limited to the emergence of a sheltered or shade phenotype at the inner canopy layers. As outlined above, the plasticity expressed by outer canopy leaves may be constraining to and be constrained by carbon gain and supply, and, in consequence, phenotypic responses to the within-crown environment were also expected to affect the expression of plasticity at the outer canopy layers. Thus, the first hypothesis is that crown plasticity in adults will involve phenotypic adjustments of both sun and shade leaves. This pattern should be consistent across populations in contrasting environments. In order to test this hypothesis, morphological and physiological variation at the leaf level expressed in the field within individual crowns and within and between populations was explored. As a secondary hypothesis, the aim was to assess whether differences among populations in field-expressed plasticity were associated with the variation in environmental factors and/or with genetic differences in population responsiveness to the environment. Since the strength of the latter genetic component of plasticity can be assessed by the correlation between field and common garden results (Givnish et al., 2004), phenotypic variation in the field was compared with that observed in the response to light in a previous common garden study (Balaguer et al., 2001).
MATERIALS AND METHODS
Study sites and experimental design
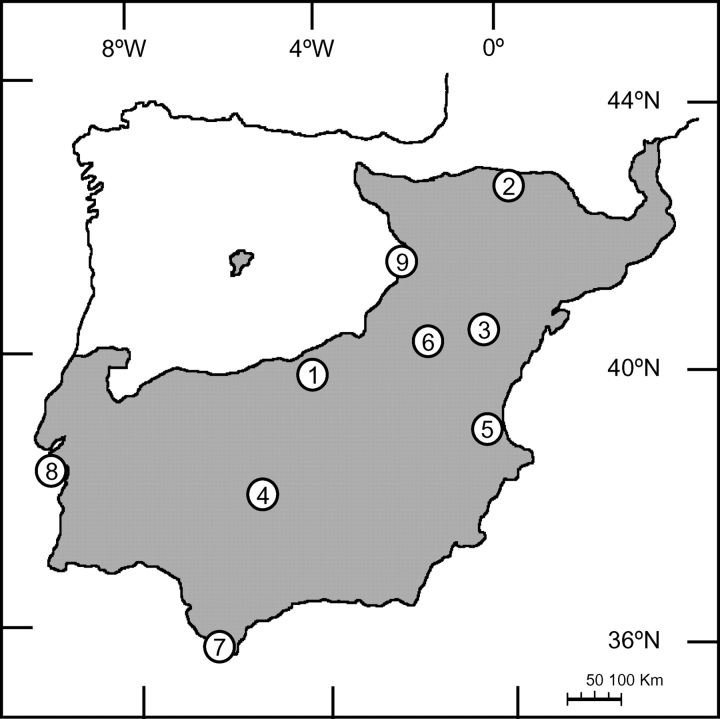
Nine populations encompassing all the habitats where vegetation is dominated by Quercus coccifera L. on the Iberian Peninsula were sampled (Table 1; Fig. 1; Cañellas and San Miguel, 2003). Three 50 × 50 m plots were marked out at seven localities. In the other two sites (ARR and SAL) the vegetation was too dense, and so plants were sampled along three transects across plots of the same size. A 27 × 27 matrix of geographic distances among sampling plots (GEO) was constructed. Five individuals per plot or transect were randomly chosen. In order to gather comparable data, all sampling was conducted in mid-summer, when plants are assumed to be in drought-induced aestivation (Cañellas and San Miguel, 2003). Only plants bearing fruit were included in the study in order to avoid juveniles. The individual crown was taken as the functional unit. Measurements and samples were taken on fully expanded, current year leaves in two exposures: south-facing, fully exposed (sun), and north-facing, in-crown (shade) leaves.
Table 1.
Populations of Quercus coccifera used in this study
| Population | Location | Latitude (°N) | Longitude (°W) | Altitude | Vegetation type |
|---|---|---|---|---|---|
| AIN | Ainsa, Huesca, Spain | 42°26′19″ | 0°5′6″ | 1549 m asl | Subalpine sunny-slope thicket |
| ARJ | Aranjuez, Madrid, Spain | 40°0′2″ | 3°36′27″ | 579 m asl | Calcicolous continental woodland |
| CÑV | Cañada de Verich, Teruel, Spain | 40°52′46″ | 0°6′79″ | 822 m asl | Stunted and widely spaced formation on a calcareous rock outcrop |
| CAR | Cardeña, Córdoba, Spain | 38°21′46″ | 4°19′20″ | 581 m asl | Silicicolous continental forest |
| SAL | El Saler, Valencia, Spain | 39°22′50″ | 0°19′40″ | 104 m asl | Impenetrable macchia on stabilized coastal dunes |
| GAR | Gargallo, Teruel, Spain | 40°51′87″ | 0°33′38″ | 1018 m asl | Calcicolous montane open woodland |
| FAC | Facinas, Cádiz, Spain | 36°9′46″ | 5°40′9″ | 118 m asl | Silicicolous coastal scrub |
| ARR | Serra da Arrábida, Setúbal, Portugal | 38°27′17″ | 9°0′62″ | 312 m asl | Impenetrable oceanic forest |
| TAR | Tarazona, Zaragoza, Spain | 41°50′35″N | 1°38′92″W | 694 m asl | Steppic xerophilous woodland |
Fig. 1.
Distribution of Kermes oak (Quercus coccifera) in Iberia, including geographic locations of the studied populations. 1, Aranjuez (ARJ); 2, Ainsa (AIN); 3, Cañada de Verich (CÑV); 4, Cardeña (CAR); 5, El Saler (SAL); 6, Gargallo (GAR); 7, Facinas (FAC); 8, Serra da Arrábida (ARR); 9, Tarazona (TAR).
Environmental data
Environmental data are presented in the Supplementary Information, available online. Climatic data included monthly mean 30-year maximum, minimum and mean temperatures, and rainfall. The mean annual number of ays of minimum temperatures below − 5 °C and below 0 ºC were also included, as winter cold seems to be limiting for Q. coccifera development (Martínez-Ferri et al., 2004). Soil data included pH and total N, P, K, Ca, Mg, Na, Zn and Mn. Climatic and edaphic data were z-standardized and combined into a single matrix of nine rows (one for each population) and 59 columns containing soil characteristics and meteorological data. This matrix was used to produce a dissimilarity matrix based on Euclidean distances between sampling plot means (ENV). For plots belonging to the same population, a constant distance (0·01) was assumed, while the distance assigned to any two plots belonging to different populations was the distance between populations. Photosynthetic photon flux density (PPFD) was measured at midday, between 1130 and 1230 h (solar time), on clear summer days with quantum sensors (SKP210; Skye Instruments Ltd, UK). Measurements were taken at the zenith and at the angle and orientation of 10 leaves per plant and exposure (sun/shade), in five plants per population. At midday during the Mediterranean summer, Q. coccifera plants reach the most negative stem water potential and the highest degree of dynamic photoinhibition (Martínez-Ferri et al., 2000). The role of crown architecture in buffering the variability of light intensity at this critical time was explored by comparing the coefficients of variation (CVs) of the PPFDs registered at the sun and shade leaves.
Morphological and pigment data
Specific leaf area (SLA), leaf area, angle, spininess (SPIN) index and lobation index (ILB) were calculated for ten leaves from each of the two exposures of 135 individuals (15 plants from nine populations), the height of which was measured. Leaf angle to the horizontal was measured in the field using a protractor on ten mature leaves per exposure. The ILB, formulated herein, was calculated as the difference between the measured perimeter and that of the ellipse that circumscribes the leaf blade, estimated by the YNOT formula (Maertens and Rousseau, 2000), and divided by the former. For the same leaves, a leaf SPIN index was formulated as the ratio between the number of marginal leaf spines and the theoretical perimeter, calculated for ILB. Three leaves of each exposure from the first three individuals of each plot were taken at solar midday and immediately stored in liquid nitrogen until their pigments were analysed (Martínez-Ferri et al., 2000). Chlorophylls and carotenoids were separated by HPLC (Waters Corp., Milford, MA, USA), following pigment extraction in cool acetone. The peaks were identified and quantified with pure commercial standards (VKI, Hørsholm, Denmark). Three light-responsive pigment pools were determined: total chlorophyll on a leaf area basis, and xanthophyll cycle pigments (VAZ) and β-carotene on a chlorophyll content basis. After z-standardization of the variables, two dissimilarity matrices, based on Euclidean distances between sampling plots, were calculated using eight traits (SLA, leaf area, SPIN, ILB, leaf angle, total chlorophyll, VAZ and β-carotene), a matrix for mean trait values (PHEN matrix) and another for the plasticity values (PhPl matrix).
Data analyses
Foliar data were averaged per plant, grouping these in plots and plots nested within populations, in a nested analysis of variance (ANOVA) of repeated measures, using exposure (sun–shade) as a within-subject factor. The individual mean trait value was assessed as the across-exposures average response. The difference between trait mean for the shade leaves and that for the sun leaves (the sign was retained) was used as a proxy for individual field-expressed plasticity to light environment. To validate this proxy and test for the genetic component of the observed phenotypic variation, field measurements were compared with those made of seedlings grown in a previous common garden experiment (Balaguer et al., 2001), based on the estimates of the four traits (SLA, leaf area, angle and ILB) from the three populations (ARR, GAR and CÑV) analysed in both studies. Merely for the purposes of this comparison, ILB was recalculated as the quotient between leaf perimeter and area (as defined in Balaguer et al., 2001). Correlations between all the dissimilarity matrices – geographic distances (GEO), environmental distances (ENV), mean trait values (PHEN) and trait phenotypic plasticities (PhPl) – were computed with Mantel tests (Mantel, 1967) using ZT software (Bonnet and van de Peer, 2002). Partial Mantel tests that corrected the effect of the geographical distance between populations were used in all matrix comparisons that did not include GEO. The significance of all other matrix correlations was assessed by 1 000 000 randomization tests.
RESULTS
Environmental, morphological and pigment data
As expected, a clear latitudinal cline was observed. Northern sampling sites (AIN, CÑV, GAR and TAR) appeared as significantly distinct for 27 of the 57 environmental parameters (P < 0·05; Supplementary Information, available online). Mean and minimum temperatures of winter, spring and autumn months, and maximum temperatures of winter and early spring months were colder, while summer rainfall was higher. The number of days with minimum temperatures below 0 °C for these sites was 51·28 days, and for the central and southernmost localities, 10·88 days.
Summer midday PPFD (Table 2) at zenith was 25·87 ± 3·67 % (s.e.) higher than that measured at the surface of the outer canopy leaves, and 91·50 ± 2·11 % (s.e.) higher than at the surface of the inner canopy leaves. This PPFD reduction was positively correlated with plant height at both the outer (r = 0·50, P < 0·001) and inner canopy layers (r = 0·48, P < 0·001). However, plant height did not explain the differences among populations in the PPFD reduction at the inner canopy layers. This reduction was less pronounced, i.e. crowns were more transparent at midday, at AIN, CÑV and TAR (l.s.d. test, P < 0·01). Across populations, shade leaves experienced midday PPFDs more heterogeneous (CV = 0·778) than those of sun leaves (CV = 0·187) but, when grouped, the southernmost populations (CAR, SAL, FAC and ARR) exhibited a light environment more uniform at the inner than at the outer canopy layers (shade CV = 0·111 vs. sun CV = 0·189).
Table 2.
Means (± s.e.) of leaf angle, area, β-carotene content on a total chlorophyll basis, total chlorophyll concentration on a leaf area basis (Chla+b), leaf lobation index (ILB; see text for details), midday photosynthetic photon flux density (PPFD) at leaf surface, specific leaf area (SLA), leaf spininess index (SPIN; see text for details) and pool of xanthophyll cycle pigments on a total chlorophyll basis for each population and exposure (VAZ), and mean population plant height
| AIN | ARJ | ARR | CAR | CÑV | FAC | GAR | SAL | TAR | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Angle (°)* | Shade | 32·94 (±2·50) | 29·46 (±2·57) | 26·05 (±2·23) | 21·69 (±1·42) | 31·37 (±1·91) | 20·97 (±1·30) | 27·59 (±1·80) | 21·84 (±1·50) | 33·90 (±2·06) |
| Sun | 42·05 (±2·01) | 57·09 (±2·49) | 68·53 (±1·61) | 61·53 (±1·52) | 54·11 (±2·68) | 63·38 (±1·05) | 57·01 (±2·67) | 47·98 (±2·70) | 42·79 (±2·69) | |
| Area (cm2)* | Shade | 2·14 (±0·15) | 5·25 (±042) | 8·42 (±0·63) | 8·16 (±0·72) | 2·04 (±0·18) | 6·50 (±0·41) | 3·12 (±0·17) | 5·94 (±0·39) | 2·74 (±0·16) |
| Sun | 1·14 (±0·07) | 1·36 (±0·13) | 2·43 (±0·12) | 2·78 (±0·30) | 0·77 (±0·06) | 1·47 (±0·10) | 1·05 (±0·08) | 1·66 (±0·12) | 1·01 (±0·07) | |
| β-carotene (mmol mol–1)† | Shade | 84·24 (±1·76) | 95·66 (±2·10) | 93·50 (±6·22) | 93·75 (±3·68) | 106·57 (±2·43) | 90·65 (±3·79) | 101·32 (±3·06) | 95·74 (±4·82) | 86·37 (±1·89) |
| Sun | 74·44 (±2·44) | 105·28 (±4·12) | 113·52 (±7·32) | 101·97 (±3·05) | 115·08 (±5·99) | 109·99 (±5·35) | 115·36 (±3·16) | 128·11 (±10·66) | 83·02 (±2·13) | |
| Chla+b (μmol m–2)† | Shade | 779·46 (±52·54) | 886·78 (±52·00) | 530·37 (±33·22) | 587·27 (±39·46) | 591·50 (±59·06) | 621·56 (±52·53) | 693·74 (±70·08) | 507·74 (±42·03) | 869·65 (±57·71) |
| Sun | 1000·67 (±49·40) | 855·82 (±45·42) | 568·92 (±42·18) | 636·68 (±27·76) | 683·64 (±53·58) | 653·07 (±50·37) | 602·77 (±89·70) | 690·28 (±80·70) | 902·34 (±29·63) | |
| ILB (cm cm−1)* | Shade | 0·25 (±0·01) | 0·31 (±0·01) | 0·26 (±0·01) | 0·24 (±0·01) | 0·23 (±0·01) | 0·25 (±0·02) | 0·28 (±0·02) | 0·28 (±0·01) | 0·26 (±0·01) |
| Sun | 0·21 (±0·01) | 0·22 (±0·01) | 0·15 (±0·01) | 0·18 (±0·01) | 0·18 (±0·01) | 0·21 (±0·01) | 0·23 (±0·02) | 0·21 (±0·01) | 0·19 (±0·01) | |
| PPFD (μmol m−2 s −1)‡ | Shade | 368·70 (±53·41) | 128·70 (±31·30) | 73·90 (±13·05) | 66·60 (±22·50) | 353·50 (±63·67) | 56·70 (±11·22) | 113·40 (±20·97) | 62·50 (±16·79) | 294·40 (±84·31) |
| Sun | 1631·90 (±55·35) | 1722·00 (±70·82) | 1020·80 (±123·14) | 1207·10 (±102·96) | 1617·50 (±67·28) | 1566·00 (±72·18) | 1342·50 (±124·04) | 1149·50 (±66·18) | 1752·88 (±25·80) | |
| SLA (m2 kg−1)* | Shade | 5·72 (±0·16) | 6·66 (±0·15) | 8·82 (±0·28) | 8·56 (±0·19) | 6·25 (±0·24) | 8·24 (±0·18) | 6·60 (±0·30) | 7·75 (±0·26) | 5·64 (±0·26) |
| Sun | 3·87 (±0·11) | 3·70 (±0·08) | 5·29 (±0·09) | 5·08 (±0·10) | 4·49 (±0·17) | 4·39 (±0·09) | 4·44 (±0·19) | 4·74 (±0·12) | 3·84 (±0·17) | |
| SPIN (spines cm−1)* | Shade | 2·16 (±0·11) | 1·61 (±0·09) | 1·50 (±0·06) | 1·36 (±0·04) | 2·42 (±0·12) | 1·68 (±0·05) | 2·06 (±0·12) | 1·72 (±0·05) | 1·74 (±0·09) |
| Sun | 2·09 (±0·12) | 1·83 (±0·11) | 1·51 (±0·11) | 1·46 (±0·07) | 2·85 (±0·16) | 2·46 (±0·12) | 2·45 (±0·16) | 2·17 (±0·08) | 1·73 (±0·14) | |
| VAZ (mmol mol−1)† | Shade | 49·73 (±2·06) | 63·81 (±2·72) | 63·15 (±1·78) | 91·12 (±5·22) | 72·51 (±3·65) | 78·44 (±3·35) | 66·42 (±2·47) | 70·06 (±8·02) | 68·30 (±3·52) |
| Sun | 64·28 (±3·53) | 92·14 (±2·88) | 99·95 (6·51) | 125·54 (±13·00) | 103·93 (±6·01) | 101·05 (±5·26) | 101·35 (±4·49) | 108·10 (±4·90) | 95·46 (±4·65) | |
| Mean plant height (m)§ | 1·16 (±0·06) | 2·25 (±0·22) | 2·09 (±0·17) | 2·53 (±0·30) | 0·74 (±0·10) | 1·31 (±0·09) | 1·32 (±0·17) | 1·84 (±0·13) | 1·14 (±0·16) |
* Fifteen means of 10 replicates.
† Nine means of three replicates.
‡ Five means of 10 replicates.
§ n = 15.
Mean population values for each trait for sun and shade leaves are shown in Table 2. Mean plant height ranged from < 1 m for the saxicolous plants at the rock outcrop of CÑV to > 2 m at ARJ, ARR and CAR. Smaller plants also exhibited smaller, spinier and thicker leaves (r > 0·32, P < 0·001). This was the predominant syndrome among northern populations (northern Iberia mean plant height of 1·1 m vs. 2 m for central and southern Iberia; leaf area = 1·75 vs. 4·4 cm2; SPIN = 2·2 vs. 1·7 lobes cm−1; SLA = 5·1 vs. 6·3 m2 kg−1). Among populations, all the tested traits exhibited significant differences, and the morphology of the outer canopy leaves was significantly different from that of the inner canopy leaves (Table 3). In the shade leaves, the decrease in photoprotective pigment contents (β-carotene and VAZ) was more marked than the increase in total chlorophyll content, indicating a decline in the frequency and/or intensity of saturation by high PPFD towards the inner crown. Variation in VAZ across the canopy was not sensitive to differences in environment or in crown architecture among populations (Table 3). In contrast, a significant interaction between population and exposure was found for leaf angle, area, ILB, SLA, SPIN and β-carotene content. Among these, mean plasticity in angle, area and SLA, assessed as field-expressed phenotypic range between exposures, displayed differentially lower values in the northern than in the central and southern populations (−18·0 vs. −35·7°; 1·5 vs. 4·9 cm2; 1·9 vs. 3·4 m2 kg−1, respectively). In general, there was a consistent pattern of increasing crown plasticity with increasing mean phenotypic trait values (Fig. 2). Among individuals, differences in plasticity values were due to the simultaneous variation of sun and shade phenotypes (Fig. 2). In seven out of eight characters, there was at least one population where crown plasticity correlated with the response of sun leaves. In leaf angle, for instance, this correlation was significant in all study populations (r > 0·51, P < 0·05, n = 15) and also in 27 % of the sampling plots (r > 0·88, P < 0·05, n = 5). Strikingly, crown plasticity in leaf angle, ILB, VAZ and β-carotene content was the result of adjustments in opposite directions in sun and shade leaves. These opposite phenotypic manifestations counterbalanced, to some extent, the correlation between crown plasticity and mean trait values. In VAZ, this correlation was significant in two out of nine populations; in leaf angle and β-carotene content, only in one population; and in ILB, in none of them.
Table 3.
Significance of phenotypic variation among populations (between-subject factor), sampling plots (nested in populations) and between exposures (within-subject factor) and of their interaction
| Trait | Population (8) | Plot (18) | Exposure (1) | Exposure by population (8) |
|---|---|---|---|---|
| Angle* | 5·99 (<0·0001) | 1·62 (0·0671) | 988·23 (<0·0001) | 23·59 (<0·0001) |
| Area* (log) | 56·40 (<0·0001) | 1·06 (0·3984) | 1719·44 (<0·0001) | 9·74 (<0·0001) |
| β-carotene† (log) | 21·87 (<0·0001) | 1·59 (0·0975) | 21·19 (<0·0001) | 4·99 (0·0001) |
| Chl a + b† (log) | 11·24 (<0·0001) | 1·51 (0·1229) | 7·24 (0·0150) | 2·29 (0·0689) |
| ILB* | 3·82 (0·0006) | 1·69 (0·0514) | 272·61 (<0·0001) | 3·49 (0·0013) |
| SLA* | 36·17 (<0·0001) | 1·60 (0·0727) | 1667·78 (<0·0001) | 16·91 (<0·0001) |
| SPIN* (log) | 18·91 (<0·0001) | 1·42 (0·1355) | 39·55 (<0·0001) | 7·73 (<0·0001) |
| VAZ† (log) | 15·54 (<0·0001) | 1·69 (0·0702) | 218·90 (<0·0001) | 1·25 (0·2901) |
See Table 2 for abbreviations used.
F-ratios and P-values are given. Degrees of freedom for each effect are indicated in parentheses. Bold face indicates significant effects after Bonferroni correction.
* Error d.f. = 108.
† Error d.f. = 54.
Fig. 2.

Relationship between sun (open circles), mean (dashed line) and shade (filled circles) phenotypes vs. crown plasticity values. Plasticity was calculated as the difference between trait mean for the shade leaves and that for the sun leaves of each individual. The value of r2 is given for each relationship together with the significance of the equivalent correlation coefficient r (*P < 0·05; **P < 0·01; ***P < 0·001). (A) Leaf angle; (B) leaf area; (C) lobation index; (D) spininess index; (E) specific leaf area; (F) β-carotene content on a total chlorophyll basis; (G) pool of xanthophyll cycle pigments on a total chlorophyll basis; (H) total chorophyll concentration on a leaf area basis.
Comparison of geographic, environmental and phenotypic patterns
According to a simple Mantel's association test, increased geographical distance resulted in a larger divergence in the environment (climate and soil; r = 0·58; P < 0·001), in the mean trait values (r = 0·32; P < 0·001) and in the plasticity values (r = 0·61; P < 0·001). A partial Mantel test was used to evaluate the correlations between phenotypic (PHEN and PhPl) and environmental (ENV) variations after controlling for the effect of geographical distance (GEO). As a result, although both phenotypic matrices were correlated (r = 0·23; P < 0·003), phenotypic plasticity was more strongly correlated with environmental variation (r = 0·30; P < 0·001) than mean trait values (r = 0·21; P < 0·01). Indeed, the relationship between mean trait values and environmental variation was not significant after Bonferroni correction. This result is consistent with the above observation that phenotypic tuning of some traits to environmental conditions involves the expression of plasticity in sun and shade leaves in opposite directions, leaving mean trait values largely invariable.
Congruency between field and greenhouse estimations of plasticity
Leaf angle, area, SLA and ILB were chosen to compare field measurements with the results of a previous common garden experiment (Balaguer et al., 2001) because their within-crown plasticity differed among populations in the field and these traits were assessed in both studies. When the standardized plasticity values were compared, field and nursery data consistently ranked the study populations (ARR, CNV and GAR) in the same order, in all cases, with the only exception of ILB between CNV and GAR (Fig. 3). Common garden estimates had a greater variation, most probably due to the smaller sample size (field data n = 15–27; common garden data n = 4–5).
Fig. 3.
Field (this study) vs. common garden (Balaguer et al., 2001) standardized plasticity values exhibited by three populations (CNV, GAR and ARR; Rock, Garrigue and Forest, respectively, in Balaguer et al., 2001) in response to light availability. Plasticity was computed as the difference between shade and sun values. The diagonal line indicates identical values for both studies. (A) Leaf area; (B) leaf angle; (C) specific leaf area; and (D) leaf perimeter/leaf area (ILB in Balaguer et al., 2001).
DISCUSSION
Within crown foliar plasticity
The present data showed a distinct contribution of the field variation expressed by sun-exposed leaves, at the outer canopy layers, to the plasticity of the crown in long-lived adult individuals. As a manifestation of its modular nature (cf. de Kroon et al., 2005), plasticity was expressed at the outer and inner canopy layers concurrently, and often in opposite directions. The expression of various phenotypes in sun-exposed leaves may be a response to shading by neighbouring plants or outcrops. However, this seems unlikely in this study since the contribution of sun leaves to individual crown plasticity was observed across populations regardless of their structure, from dense forests to scarcely vegetated rock outcrops. Sun phenotypes might also reflect the expression of plasticity in response to the variation in environmental factors other than light. The results suggest, however, that the expression of foliar plasticity in the field cannot be attributed to any single factor. Besides the observed environmental sensitivity of plasticity across populations, two findings are relevant at the population level: (a) the correlation between phenotypic variation in the leaf angle in the outer canopy and across the crown was often observed even at plot scale; and (b) crown plasticity in light-responsive traits, such as leaf angle, SLA and VAZ, resulting from simultaneous co-variation at the outer and inner layers of the canopy. While the former suggests high environmental patchiness probably associated with ontogenetic variation among resprouting adults, the latter reveals developmental flexibility elicited by such a degree of patchiness. The results are consistent with the hypothesis that plants modulate their morphology and physiology in order to be equally limited by all essential resources (Tilman, 1988).
Expression of plasticity in response to local environmental cues is modified by communication and behavioural integration of interconnected modules (de Kroon et al., 2005). In clonal plants, it is known that the interplay of plant integration and modular plasticity allows specialization in the performance of specific tasks and close co-operation by connected ramets that experience contrasting levels of resource availability (Stuefer et al., 1996; Alpert, 1999). Trees share with clonal plants a high degree of modularity (Watkinson and White, 1986). In woody species, sun leaves might be shading inner layers of the canopy, protecting them from photoinhibition (Howell et al., 2002). However, the findings do not fully support this hypothesis since foliar plasticity was minimal where plants are likely to experience maximal risk of photoinhibition due to lower temperatures and more transparent crowns (northern populations). The role of plasticity in self-shading appears to be restricted to more favourable environments. At midday, the light environment was more homogeneous at the inner than at the outer canopy layers in the more plastic populations from southern Iberia. Apart from sheltering inner layers of the canopy, the arrangement of sun leaves may also determine carbon gain by enhancing light interception at different sun-elevation angles (Falster and Westoby, 2003; Uemura et al., 2006). When leaves do not track sun movements, as occurs with Q. coccifera (Werner et al., 1999), leaf-angle plasticity would allow division of labour across the canopy. In this species, increasing plasticity implied steeper sun leaves and shallower angled shade leaves, which suggests that higher plasticity levels enable functional specialization for the photosynthetic exploitation of complementary time windows: morning/afternoon/winter (sun leaves) vs. midday/summer (shade leaves). Further experimental studies are needed to determine whether the internal specialization observed in Q. coccifera is a consequence of division of labour, as described for clonal plants (Stuefer et al., 1996).
Environmental sensitivity of field phenotypic variation
Leaf phenotypic plasticity in response to within-crown light availability, in the field and across a wide variety of environments, was found to be modulated by simultaneous variation in other environmental factors. This environmental sensitivity of field-expressed plasticity contrasted with the low responsiveness of mean trait values to environmental variation across populations. The simultaneous phenotypic adjustment in exposed and sheltered layers of the canopy to local environmental conditions not only explains the correlation between plasticity and environmental differences, but might also be the cause of the lower correlation observed between mean phenotypes and environment. The counter-directional tuning to sun and shade conditions within canopies may buffer the influence of the environment on the mean phenotypic response. This finding suggests a key role in adult plants of phenotypic plasticity in phenotype–environment matching. This is consistent with previous reports of active phenotypic plasticity in adult trees over a hundred years old (Richardson et al., 2001), and thereby does not support the lack of environmental responsiveness of adults previously suggested for woody species (Valladares et al., 2005).
The influence of plasticity on phenotypic variation of plants has seldom been studied in the field under natural conditions, especially in the case of long-lived organisms (Geber and Griffen, 2003). In the current study, foliar traits whose within-crown plasticity differed among populations were half as plastic in plants from northern sites (GAR, CNV, TAR and AIN). These plants were also shorter, with smaller, thicker and spinier leaves. This character syndrome is often associated with stressful and/or disturbance-prone environments (cf. Bond and Midgley, 2001; Ackerly et al., 2002). Winter temperatures, among other stress factors, have been reported to be particularly limiting for Q. coccifera (Martínez-Ferri et al., 2004). Under these circumstances, a restrained crown development could hypothetically explain the lesser foliar plasticity observed in the north, i.e. shade leaf phenotypes would be more similar to those of sun leaves in smaller, more transparent crowns, while within denser crowns, crown plasticity would result from the emergence of a shade phenotype. However, this limited plasticity seemed not to be solely a by-product of restricted growth. First, crown plasticity in the northern populations also resulted from the phenotypic adjustment at the outer canopy layers. Secondly, a greater degree of plasticity was associated across populations with larger and thinner leaves which have been considered indicative of low-stress, resource-rich environments (Cornelissen et al., 2003). Thus, our results support the hypothesis that plants of more productive habitats exhibit a higher plasticity as part of the foraging mechanisms (Grime et al., 1986).
Population responsiveness to environmental variation
The present results showed that field-expressed plasticity ranks a subset of populations of the study species in the same order as a previous common garden experiment (Balaguer et al., 2001). Environmental variation across populations hampers discrimination between immediate plastic response and evolved patterns of population responsiveness. However, this coincidence provides evidence for a genetic component in the phenotypic differences measured in mature trees in the field. This finding both confirms the relevant contribution of phenotypic plasticity to field phenotypic variation, previously inferred from nursery trials (Valladares et al., 2002), and indicates that field responses of Q. coccifera are determined by environmental variation plus heritable differences in the reaction norms. Across populations, leaf angle and SLA variation were significantly larger within individuals in the field than between seedlings in the common garden, which may be due to the contribution of alternative sources of variation (e.g. developmental instability) to the larger heterogeneity of natural habitats (Wayne and Bazzaz, 1993), or to an integrated response across plant modules that is not observed when comparisons are made between seedlings grown in separate pots.
In conclusion, the present results, although observational, confirm the involvement of foliar plasticity in the crown construction of adult plants of the long-lived sprouter Q. coccifera. Field observations lend support to the idea that local tuning of foliar traits to match environmental variation is optimized at the whole crown scale. As a foraging behaviour, the observed physiological and morphological responses depict a mechanism of habitat selection across canopy environmental gradients and across habitats of varying favourability. Only in the most plastic populations might habitat modification through self-shading be buffering light variation within the crown at the most favourable sites. However, the latter result is based on punctual measurements of the light environment, thus undermining the validity of the evidence. Within populations and sampling plots, the variation in the degree of individual plasticity (evinced by the consistent correlation between crown phenotypic range and within-crown responses) contrasts with the strength of the genetic component found at the population level. Experimental analyses are needed to assess whether this pattern is due to environmental or ontogenetic variation.
SUPPLEMENTARY INFORMATION
Supplementary Information for environmental data at the sites is available online at http://aob.oxfordjournals.org/. The data include monthly mean, maximum and minimum temperatures, monthly rainfall and soil characteristics.
ACKNOWLEDGEMENTS
We sincerely thank Raquel Pascual and Oscar Lozoya for their help in the field. The authors are also grateful to the two anonymous referees for their helpful suggestions and critical remarks. The research was financed by the Spanish Science and Technology Ministry (REN2000-2792-E and REN2000-2794-E).
LITERATURE CITED
- Ackerly DD. Community assembly, niche conservationism, and adaptive evolution in changing environments. International Journal of Plant Sciences. 2003;164:S165–S184. [Google Scholar]
- Ackerly DD, Dudley SA, Sultan SE, Schmitt J, Coleman JS, Linder CR, et al. The evolution of plant ecophysiological traits: recent advances and future directions. Bioscience. 2000;50:979–995. [Google Scholar]
- Ackerly DD, Knight CA, Weiss SB, Barton K, Starmer KP. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: contrasting patterns in species level and community level analyses. Oecologia. 2002;130:449–457. doi: 10.1007/s004420100805. [DOI] [PubMed] [Google Scholar]
- Alpert P. Effects of clonal integration on plant plasticity in Fragaria chiloensis. Plant Ecology. 1999;141:99–106. [Google Scholar]
- Balaguer L, Martínez-Ferri E, Valladares F, Pérez-Corona E, Baquedano FJ, Castillo FJ, Manrique E. Population divergence in the plasticity of the response of Quercus coccifera to the light environment. Functional Ecology. 2001;15:124–135. [Google Scholar]
- Bazzaz FA. Habitat selection in plants. American Naturalist. 1991;137:S116–S130. [Google Scholar]
- Bellingham PJ, Sparrow AD. Resprouting as a life history strategy in woody plant communities. Oikos. 2000;89:409–416. [Google Scholar]
- Bond WJ, Midgley JJ. Ecology of sprouting in woody plants: the persistence niche. Trends in Ecology and Evolution. 2001;16:45–51. doi: 10.1016/s0169-5347(00)02033-4. [DOI] [PubMed] [Google Scholar]
- Bond WJ, Midgley JJ. The evolutionary ecology of sprouting in woody plants. International Journal of Plant Sciences. 2003;164:S103–S114. [Google Scholar]
- Bonnet E, van de Peer Y. zt: a software tool for simple and partial Mantel tests. Journal of Statistical Software. 2002;7:1–12. [Google Scholar]
- Bouvet J-M, Vigneron P, Saya A. Phenotypic plasticity of growth trajectory and ontogenic allometry in response to density for Eucalyptus hybrid clones and families. Annals of Botany. 2005;96:811–821. doi: 10.1093/aob/mci231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics. 1965;13:115–155. [Google Scholar]
- Cañellas I, San Miguel A. La coscoja (Quercus coccifera L.): ecología, características y usos. Madrid: Instituto Nacional de Investigación y Tecnología Agraria; 2003. [Google Scholar]
- Cavender-Bares J, Kitajima K, Bazzaz FA. Multiple trait associations in relation to habitat differentiation among 17 floridian oak species. Ecological Monographs. 2004;74:635–662. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, et al. Handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany. 2003;51:335–380. [Google Scholar]
- Donohue K. Setting the stage: phenotypic plasticity as habitat selection. International Journal of Plant Sciences. 2003;164:S79–S92. [Google Scholar]
- Falster DS, Westoby M. Leaf size and angle vary widely across species: what consequences for light interception? New Phytologist. 2003;158:509–525. doi: 10.1046/j.1469-8137.2003.00765.x. [DOI] [PubMed] [Google Scholar]
- Geber MA, Griffen LR. Inheritance and natural selection of functional traits. International Journal of Plant Sciences. 2003;164:S21–S42. [Google Scholar]
- Givnish TJ, Montgomery RA, Goldstein G. Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: light regimes, static light responses, and whole-plant compensation points. American Journal of Botany. 2004;91:228–246. doi: 10.3732/ajb.91.2.228. [DOI] [PubMed] [Google Scholar]
- Grether GF. Environmental change, phenotypic plasticity, and genetic compensation. American Naturalist. 2005;166:115–123. doi: 10.1086/432023. [DOI] [PubMed] [Google Scholar]
- Grime JP, Crick JC, Rincon JE. The ecological significance of plasticity. Symposia of the Society for Experimental Biology. 1986;40:5–29. [PubMed] [Google Scholar]
- Howell CJ, Kelly D, Turnbull MH. Moa ghosts exorcised? New Zealand's divaricate shrubs avoid photoinhibition. Functional Ecology. 2002;16:232–240. [Google Scholar]
- de Kroon H, Huber H, Stuefer JF, van Groenendael JM. A modular concept of phenotypic plasticity in plants. New Phytologist. 2005;166:73–82. doi: 10.1111/j.1469-8137.2004.01310.x. [DOI] [PubMed] [Google Scholar]
- Laland KN, Odling-Smee FJ, Feldmand MW. Evolutionary consequences of niche construction and their implications for ecology. Proceedings of the National Academy of Sciences, USA. 1999;96:10242–10247. doi: 10.1073/pnas.96.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortie C, Aarssen LW. The specialization hypothesis for phenotypic plasticity in plants. International Journal of Plant Sciences. 1996;157:484–487. [Google Scholar]
- Maertens R, Rousseau R. Een nieuwe benaderde formule voor de omtrek van een ellips (A new approximate formula for the perimeter of an ellipse) Wiskunde & Onderwijs. 2000;26:249–258. [Google Scholar]
- Mantel N. The detection of disease clustering and a generalized regression approach. Oecologia. 1967;129:169–178. [PubMed] [Google Scholar]
- Martínez-Ferri E, Balaguer L, Valladares F, Chico JM, Manrique E. Energy dissipation in drought-avoiding and drought-tolerant tree species at midday during the Mediterranean summer. Tree Physiology. 2000;20:131–138. doi: 10.1093/treephys/20.2.131. [DOI] [PubMed] [Google Scholar]
- Martínez-Ferri E, Manrique E, Valladares F, Balaguer L. Winter photoinhibition in the field involves different processes in four co-occurring Mediterranean tree species. Tree Physiology. 2004;24:981–990. doi: 10.1093/treephys/24.9.981. [DOI] [PubMed] [Google Scholar]
- Miyazawa SI, Livingston NJ, Turpin DH. Stomatal development in new leaves is related to the stomatal conductance of mature leaves in poplar (Populus trichocarpa × P. deltoides) Journal of Experimental Botany. 2006;57:373–380. doi: 10.1093/jxb/eri278. [DOI] [PubMed] [Google Scholar]
- Richardson AD, Ashton PMS, Berlyn GP, McGroddy ME, Cameron IR. Within-crown foliar plasticity of western hemlock, Tsuga heterophylla, in relation to stand age. Annals of Botany. 2001;88:1007–1015. [Google Scholar]
- Sack L, Melcher PJ, Liu WH, Middleton E, Pardee T. How strong is intracanopy leaf plasticity in temperate deciduous trees? American Journal of Botany. 2006;93:829–839. doi: 10.3732/ajb.93.6.829. [DOI] [PubMed] [Google Scholar]
- Schlichting CD, Pigliucci M. Phenotypic evolution: a reaction norm perspective. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- Stoll P, Schmid B. Plant foraging and dynamic competition between branches of Pinus sylvestris in contrasting light environments. Journal of Ecology. 1998;86:934–945. [Google Scholar]
- Stuefer JF, de Kroon H, During HJ. Exploitation of environmental heterogeneity by spatial division of labor in a clonal plant. Functional Ecology. 1996;10:328–334. [Google Scholar]
- Sultan SE. Evolutionary implications of phenotypic plasticity in plants. Evolutionary Biology. 1987;21:127–178. [Google Scholar]
- Tilman D. Plant strategies and the dynamics and structure of plant communities. Princeton, NJ: Princeton University Press; 1988. [Google Scholar]
- Uemura A, Harayama H, Koike N, Ishida A. Coordination of crown structure, leaf plasticity and carbon gain within the crowns of three winter-deciduous mature trees. Tree Physiology. 2006;26:633–641. doi: 10.1093/treephys/26.5.633. [DOI] [PubMed] [Google Scholar]
- Valladares F, Arrieta S, Aranda I, Lorenzo D, Sánchez-Gómez D, Tena D, Suárez F, Pardos JA. Shade tolerance, photoinhibition sensitivity and phenotypic plasticity of Ilex aquifolium in continental Mediterranean sites. Tree Physiology. 2005;25:1041–1052. doi: 10.1093/treephys/25.8.1041. [DOI] [PubMed] [Google Scholar]
- Valladares F, Balaguer L, Martínez-Ferri E, Pérez-Corona E, Manrique E. Plasticity, instability and canalization: is the phenotypic variation in seedlings of sclerophyll oaks consistent with the environmental unpredictability of Mediterranean ecosystems? New Phytologist. 2002;156:457–467. doi: 10.1046/j.1469-8137.2002.00525.x. [DOI] [PubMed] [Google Scholar]
- Via S. The evolution of phenotypic plasticity: what do we really know? In: Real LA, editor. Ecological genetics. Princeton, NJ: Princeton University Press; 1994. pp. 35–85. [Google Scholar]
- Watkinson AR, White J. Some life-history consequences of modular construction in plants. Philosophical Transactions of the Royal Society B: Biological Sciences. 1986;313:31–51. [Google Scholar]
- Wayne PM, Bazzaz FA. Birch seedling responses to daily time courses of light in experimental forest gaps and shadehouses. Ecology. 1993;74:1500–1515. [Google Scholar]
- Werner C, Correia O, Beyschlag W. Two differential strategies of Mediterranean macchia plants to avoid photoinhibitory damage by excessive radiation levels during summer drought. Acta Oecologica. 1999;20:15–23. [Google Scholar]
- Yang Z, Midmore DJ. Modelling plant resource allocation and growth partitioning in response to environmental heterogeneity. Ecological Modelling. 2005;181:59–77. [Google Scholar]