Abstract
In skeletal muscle, STAT5a/b transcription factors are critical for normal postnatal growth, whole-animal glucose homeostasis, and local IGF-1 production. These observations have led us to hypothesize that STAT5a/b are critical for maintenance of normal muscle mass and function. To investigate this, mice with a skeletal muscle-specific deletion of the Stat5a/b genes (Stat5MKO) were used. Stat5MKO mice displayed reduced muscle mass, altered fiber-type distribution and reduced activity. On a molecular level, gene expression in skeletal muscle of Stat5MKO and control mice was analyzed by microarrays and real-time PCR, both in the presence and absence of growth hormone (GH) stimulation. Expression of several genes involved in muscle growth and fiber type were significantly changed. Specifically, in the quadriceps, a muscle almost exclusively composed of type II fibers, the absence of STAT5a/b led to increased expression of several genes associated with type I fibers and the de novo appearance of type I fibers. In addition, it is shown here that expression of the androgen receptor gene (Ar) is controlled by GH through STAT5a/b. The link between STAT5a/b and Ar gene is likely through direct transcriptional regulation, as chromatin immunoprecipitaion of the Ar promoter region in C2C12 myoblasts was accomplished by antibodies against STAT5a. These experiments demonstrate an important role for STAT5a/b in skeletal muscle physiology, and they provide a direct link to androgen signaling.—Klover, P., Chen, W., Zhu, B.-M., Hennighausen, L. Skeletal muscle growth and fiber composition in mice are regulated through the transcription factors STAT5a/b: linking growth hormone to the androgen receptor.
Keywords: chromatin immunoprecipitation, microarray
In mammals, skeletal muscle is composed of several types of fibers that are classified by the predominant type of myosin heavy-chain isoform they contain, including the major types I, IIa, IIb, and IIx. Type I fibers, also called “slow-twitch”, have increased oxidative capacity and ATP production compared to type II or “fast-twitch” fibers, which rely primarily on glycolysis for ATP production. Skeletal muscle shows remarkable plasticity of fiber composition, and differences therein are reflected in the capabilities of the muscle itself. For example, elite long-distance runners have a higher percentage of type I fibers, reflecting the increased oxidative capacity of their highly conditioned muscles (1). Type II fibers are increased with resistance training and are more quickly fatigued. Type II fibers are also likely important in maintaining whole-body insulin sensitivity and fatty acid oxidation (2).
Skeletal muscle growth and function are heavily influenced by hormones. While several hormones are known to influence the postnatal growth of skeletal muscle, growth hormone (GH) and IGF-1 appear to play a central role in this process. Patients with insensitivity to GH (Laron syndrome) or GH deficiency have diminished musculature and strength (3, 4). Complete deficiency of IGF-1 in mice results in a severe reduction in whole-body size at birth and in postnatal growth (5). IGF-1 also appears to be important in the maintenance of skeletal muscle mass with age. Unexpectedly, mice that do not respond to IGF-1 in the skeletal muscle demonstrate skeletal muscle hyperplasia as adults, possibly as a compensatory response, following hypoplasia in the first 3 wk of life (6). Both GH and IGF-1 levels decline with age, but it is unclear whether this is a cause of aging-related declines in muscle mass and function. Notably, muscle-specific overexpression of IGF-1 reverses hypotrophy associated with aging in mice (7), and chronic administration of low doses of IGF-1 have been shown to reverse aging-dependent oxidative damage and insulin resistance in rats (8).
Recent work by our group has demonstrated that the transcription factors STAT5a/b in skeletal muscle convey signals from hormones, such as GH, into gene expression that affects whole-animal growth, including body weight and length, and metabolism. STAT5a/b are essential for transcription of the IGF-1 gene in the liver and muscle (9, 10), making these transcription factors important molecules in the maintenance of skeletal muscle function associated with IGF-1. As evidence of this, IGF-1 mRNA levels are reduced ∼60% in skeletal muscle-specific Stat5a/b-knockout (Stat5MKO) mice. This decline, however, does not cause a severe reduction in circulating IGF-1 levels (9), suggesting that growth defects in these mice may be caused by reduced autocrine/paracrine IGF-1 signaling. Furthermore, mice with a conditional deletion of STAT5a/b in both the liver and muscle do not show growth defects beyond those observed in Stat5MKO mice, suggesting that local IGF-1 produced in skeletal muscle is more important than liver-derived IGF-1 for growth (9).
Maintenance of normal muscle mass and strength depends on the presence of the androgenic hormones testosterone and dihydroxytestosterone, which convey their signals through the androgen receptor (AR) (11). The Ar gene is expressed in myoblasts, myofibers, and satellite cells of both males and females. Complete deletion of the Ar gene in mice (ARKO mice) resulted in reduced muscle mass and strength in males but not females (12). Consistent with altered muscle-fiber composition, microarray analysis of ARKO muscle demonstrated the up-regulation of genes encoding slow-twitch muscle contractile proteins (12).
Using mouse genetics, researchers have now demonstrated that loss of GH signaling through its receptor (13), STAT5a/b (9), and IGF-1 (5), as well as loss of androgen signaling through the AR (12), results in reduced muscle mass and impaired function. However, it is not known whether there is a direct molecular link between androgen and GH signaling in muscle tissue. We have now investigated on a histological and molecular level muscle development in the absence of STAT5a/b. In particular, we used a histological approach and gene expression profiling to investigate to what extent GH-STAT5a/b signaling controls muscle fiber identity and activity and whether loss of STAT5a/b intersected with androgen signaling.
MATERIALS AND METHODS
Animals
Mice were housed and maintained according to National Institutes of Health (NIH) guidelines. Stat5MKO mice were generated previously as described (9). The genotype of these mice is Stat5fl/fl Myf5-cre, with cre recombinase gene expression under the control of the endogenous Myf5 promoter (14).
Cell lines and culture
The C2C12 mouse myoblast cell line was purchased from American Type Culture Collection (Manassas, VA, USA). C2C12 myoblasts were cultured in DMEM containing 10% heat-inactivated FBS (Invitrogen, Carlsbad, CA, USA) and Normocin antibiotic cocktail (Amaxa/Lonza, Gaithersburg, MD, USA).
Immunohistochemistry
Muscle tissue was extracted and immediately frozen in liquid nitrogen-cooled isopentane. Frozen sections were prepared by Histoserv (Germantown, MD, USA). Antibodies to myosin heavy chain-type I (slow) and pan type II (fast) were from Vector Laboratories (Burlingame, CA, USA). To calculate muscle fiber cross-sectional area, sections were stained with laminin α-2 clone 4H8–2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Fiber area was then measured using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA).
Microarray experiment and data analysis
Recombinant mouse GH was purchased from the National Hormone and Peptide Program, directed by A. F. Parlow (Harbor-University of California Los Angeles Medical Center, Torrance, CA, USA). Mice were injected in the morning with 50 μg of GH. After 3 h, animals were euthanized by carbon dioxide, and muscles were immediately frozen. RNA from muscle and liver was extracted using TRIzol reagent, followed by a cleanup step using the RNeasy RNA Plus extraction kit (Qiagen, Valencia, CA, USA). Two micrograms of RNA per sample was processed and analyzed by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Core Facility (NIH, Bethesda, MD, USA). A total of 12 samples was used for the 4 experimental conditions, (Stat5MKO vs. WT, each ± GH), with 3 replications of each experimental condition, respectively. The RNA quality was checked by an Agilent Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Affymetrix gene expression analysis arrays for Mouse430_2 were used (Affymetrix, Santa Clara, CA, USA). The microarray signals were analyzed using the Affymetrix RMA algorithm. Up- and down-regulated genes were selected based on values of P ≤ 0.05 and fold change ≥ 2 or ≤−2 as assessed by ANOVA results using Partek Pro software (Partek, St. Charles, MO, USA). Fold changes were calculated as mutant/control. If the value was <1, the negative reciprocal of this value was indicated. To determine the potential disruption of specific pathways based on changes in gene expression, the MetaCore (GeneGo, http://www.genego.com) commercial gene pathway analysis web tool was used. The total number of probe IDs in each specific pathway was extracted from the above tool, and the signal values of each probe set ID from the specific gene pathway lists were plotted by the commercial software GeneSpring7.2 (Agilent). Raw data from microarrays have been submitted to the Gene Expression Omnibus (GEO) repository (National Center for Biotechnology Information, NIH, Bethesda, MD, USA) under accession no. GSE14710.
Quantitative real-time PCR for verifying microarray results
Verification of genes from microarray analysis was performed by using TaqMan probes (Applied Biosystems; Foster City, CA) for real-time PCR. Real-time PCR was carried out using an ABI Prism 7900HT (Applied Biosystems). Individual PCRs were performed in triplicate on samples using mouse β-actin as a housekeeping gene and experimental probes to obtain average CT values for these genes. Average β-actin CT values were subtracted from experimental CT values to obtain ΔCT values. ΔΔCT values were then obtained by subtracting experimental sample ΔCT values from the control sample ΔCT. Relative gene expression was then calculated using 2−ΔΔCT.
Oxygen consumption and energy expenditure
Oxygen consumption was measured using the Oxymax system (Columbus Instruments, Columbus, OH, USA). Indirect calorimetry was used to measure the energy expenditure. Locomotor activity was measured on mice in metabolic cages by an infrared activity monitoring system (Columbus Instruments).
Chromatin immunoprecipitation assay
Constitutively active STAT5a retrovirus was produced by cotransfecting the retroviral plasmid pMSCV-IRES-GFP (MIG) empty vector or pMSCV-STAT5a-IRES-GFP (MIG STAT5a), and the EcoPack packaging construct (Clontec, Mountain View, CA, USA) in human embryonic kidney 293T cells using LipoD293 transfection reagent (SignaGen Laboratories, Gaithersburg, MD, USA). 5 × 106 C2C12 myoblasts were infected with either MIG retrovirus or MIG STAT5a-expressing retrovirus. The infection efficiency after 48 h was measured by flow cytometric analysis of green fluorescent protein (GFP)-positive cells. Infection efficiency > 50% was determined by FACS analysis of GFP expression. STAT5a expression was verified by Western blot of immunoprecipitates against STAT5a (Supplemental Fig. 2). Chromatin immunoprecipitation was carried out using the reagents and protocol from the chromatin immunoprecipitation kit (17-295; Millipore, Temecula, CA, USA). Cells were cross-linked in 1% formaldehyde for 10 min at 37 C. Cells were then harvested, sonicated, and immunoprecipitated in buffers containing protease inhibitors (Sigma). Sonication was carried out using the Misonix Sonicator 3000 (Misonix, Farmingdale, NY, USA) with 10 pulses of 10 s each at output level 5. Chromatin was diluted and precleared with protein A agarose and salmon sperm DNA. A sample of diluted chromatin before immunoprecipitation was taken as a control for input levels. To pull down chromatin associated with STAT5a proteins, chromatin was precipitated with either IgG (negative control), anti-histone 3 (positive control), or STAT5a antibody (R&D Systems, Minneapolis, MN, USA). Washed chromatin complexes were eluted from protein A agarose, reverse cross-linked, and phenol extracted before being subjected to real-time PCR.
Immunoprecipitated DNA was amplified by qPCR using Maxima SYBR Green qPCR Master Mix (Fermentas Life Sciences, St. Leon-Rot, Germany) on an ABI Prism 7900HT (Applied Biosystems). For qPCR, DNA was amplified using primers near suspected AR GAS sites. For the Socs2 GAS sequence, forward primer 5′-GGAGGGCGGAGTCGCAGGC-3′, reverse primer 5′-GACTTGGCAAGAGTTAACCGTC-3′. For the Ar GAS sequence, forward primer 5′-TTTCTAAAAGCTGCGGGAGA, reverse primer 5′-GCCTGCCTAGTCAGCTCCT. Primers for a region outside of a suspected STAT5a/b binding region were forward 5′-AGAAGGGGCGAGTTCTTAGC-3′, reverse 5′-CTGATCTCCTGCCTCTACCG-3′.
Statistical analysis
Statistics were performed using the Microsoft Excel Data Analysis function (Microsoft, Redmond, WA, USA). Two-tailed Student’s t test was used to calculate significance. Data are expressed as means ± se unless otherwise indicated.
RESULTS
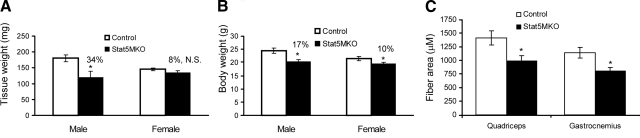
Previously, we observed a reduction in body weight of Stat5MKO male and female mice that was accounted for by a reduction in lean mass, but not fat mass, in these animals (9). These results were suggestive of a reduced skeletal muscle mass. To investigate the effect of STAT5a/b loss on skeletal muscle mass, the quadriceps muscle was removed from the hindlimbs and weighed. In 12-wk-old Stat5MKO males, a reduction in quadriceps mass of 34% was observed (Fig. 1A, left) compared to an overall reduction in body mass of 17% for the same group of animals (Fig. 1B, left). These results demonstrate a defect in skeletal muscle growth caused by lack of STAT5a/b. Female Stat5MKO mice did not display such a defect; however, and quadriceps mass was modestly reduced (change not statistically significant, P>0.05) (Fig. 1A, right). The lack of significant change in muscle mass in females was consistent with the relatively modest 10% reduction in body weight in these animals (Fig. 1B, right). To determine whether muscle fibers were reduced in size or number in Stat5MKO mice, muscle fiber cross-sectional areas of the gastrocnemius and quadriceps (rectus femoris) of male Stat5MKO mice were measured using image analysis software. Cross-sectional areas of quadriceps and gastrocnemius muscle fibers were significantly reduced in Stat5MKO mice compared to controls (Fig. 1C), consistent with reduced postnatal growth of the skeletal muscle rather than a developmental defect that altered fiber number.
Figure 1.
Muscle mass and fiber cross-sectional area in quadriceps of Stat5MKO and control mice. A) Quadriceps from 3-mo-old male Stat5MKO mice were reduced ∼35% in weight compared to controls (n=12), while quadriceps from females were reduced only 8% (n=12) B) Body weight of male Stat5MKO mice in panel A was reduced ∼15%, while body weight of females was reduced by 10%. C) Cross-sectional area of individual gastrocnemius and quadriceps muscle fibers (average of 100 fibers).
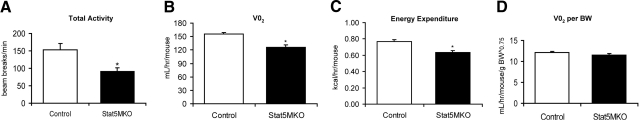
To determine whether Stat5MKO mice have altered voluntary physical activity, spontaneous locomotor activity was assessed by measuring infrared beam breaks over a 24-h period. Compared to controls, Stat5MKO mice exhibited a reduction in total activity by 30% (Fig. 2A). To determine whether respiration was affected by loss of STAT5a/b in muscle, oxygen consumption and energy expenditure were measured over a period of 24 h. Both total oxygen consumption (Fig. 2B) and energy expenditure (Fig. 2C) were reduced in Stat5MKO mice by ∼20%; however, after correcting for body weight, these parameters were unchanged (shown is total oxygen consumption per gram body weight, Fig. 2D). These experiments indicate that Stat5MKO mice have reduced activity, but energy expenditure and oxygen consumption per gram body weight were not significantly altered.
Figure 2.
Motor activity and energy expenditure in Stat5MKO mice. A) Activity of Stat5MKO and control mice, measured by breaks of a laser beam over 24 h (n=8). B) Oxygen consumption in Stat5MKO and control mice before adjusting for body weight. C) Energy expenditure in Stat5MKO and control mice before adjusting for body weight. D) Oxygen consumption did not differ between Stat5MKO mice adjusted for body weight. *P < 0.05; n = 8.
Gene expression analysis of Stat5MKO skeletal muscle
Our results demonstrate that the loss of STAT5a/b in skeletal muscle leads to several physiological changes in muscle mass and function. To identify STAT5a/b-dependent genes in the skeletal muscle that could account for these changes, microarray analysis was used to profile mRNAs in quadriceps muscle from Stat5fl/fl (control) and Stat5MKO male mice at 3 mo of age. To determine the role of GH-induced pathways acting through STAT5a/b in muscle, an additional microarray experiment was performed in which control and Stat5MKO males were treated with GH for 3 h. Raw data were analyzed by software analysis to identify pathways that may be affected by loss of STAT5a/b.
STAT signaling-related genes
The expression of several genes known to be regulated by STAT5a/b was altered in muscle tissue of Stat5MKO mice. These genes included Cish, Socs2, Socs3, and Stat3 (Table 1). Socs3 is a bona fide STAT3 target gene (15); therefore, increased Socs3 expression was likely a result of increased Stat3 expression. These changes are consistent with an altered balance of STAT signaling caused by the loss of STAT5a/b. The sharply reduced expression of Stat5a and Stat5b correlated with an almost complete absence of STAT5a/b protein in Stat5MKO muscle tissue (not shown). These results also indicate the existence of conserved JAK/STAT regulatory pathways present in skeletal muscle.
TABLE 1.
Microarray results: STATs and SOCS protein family member genes with expression changes in Stat5MKO quadriceps muscle
| Affymetrix ID | Symbol | Gene name | P value | Stat5MKO vs. control (basal) | Pvalue | Stat5MKO vs. control (GH) |
|---|---|---|---|---|---|---|
| 1448724_at | Cish | Cytokine inducible SH2-containing protein | 0.0152 | −7.09 | 6.16E-07 | −3.70 |
| 1450034_at | Stat1 | Signal transducer and activator of transcription 1 | 0.0308 | 1.51 | 0.0023 | 1.38 |
| 1460700_at | Stat3 | Signal transducer and activator of transcription 3 | 0.0055 | 2.30 | 3.48E-06 | 3.24 |
| 1450259_a_at | Stat5a | Signal transducer and activator of transcription 5A | 0.0029 | −2.47 | 4.31E-05 | −2.25 |
| 1422103_a_at | Stat5b | Signal transducer and activator of transcription 5B | 0.0003 | −6.88 | 2.35E-07 | −5.17 |
| 1449109_at | Socs2 | Suppressor of cytokine signaling 2 | 0.0015 | −4.78 | 1.81E-06 | −2.26 |
| 1416576_at | Socs3 | Suppressor of cytokine signaling 3 | 0.0127 | 2.04 | 1.26E-05 | 4.04 |
AR and polyamine biosynthetic gene regulation
Particularly relevant to the muscle growth reduction observed in male Stat5MKO mice, the nuclear hormone receptor gene Ar, a known regulator of skeletal muscle growth (2), was decreased >50% in muscle of untreated and GH-treated Stat5MKO mice (Table 2). Potential genes downstream of the AR were reduced as well. S-adenosylmethionine decarboxylase 1 (Amd1), ornithine decarboxylase (Odc1), and spermine oxidase (12) were all significantly reduced in either GH-treated or untreated Stat5MKO mice (Table 2). These genes play an important role in polyamine biosynthesis and have been shown to be reduced in skeletal muscle of AR-null mice (12). These results link the transcription factors STAT5a/b with AR expression and function.
TABLE 2.
Microarray results: genes involved in polyamine biosythesis with expression changes in Stat5MKO quadriceps muscle
| Affymetrix ID | Symbol | Gene name | P value | Stat5MKO vs. control (basal) | P value | Stat5MKO vs. control (GH) |
|---|---|---|---|---|---|---|
| 1437064_at | Ar | Androgen receptor | 0.0007 | −2.21 | 5.43E-07 | −2.15 |
| 1448484_at | Amd1 | S-adenosylmethionine decarboxylase 1 | 0.0010 | −2.11 | 7.24E-07 | −1.61 |
| 1438761_a_at | Odc1 | Ornithine decarboxylase, structural 1 | 0.0194 | −2.05 | 0.0007 | −1.34 |
| 1446509_at | Smox | Spermine oxidase | 0.0208 | −1.70 | 9.32E-05 | −1.65 |
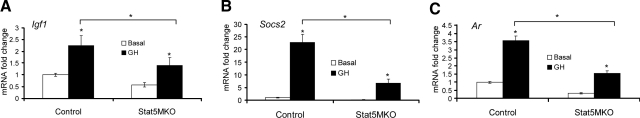
Microarray results suggested that Ar gene expression in skeletal muscle was dependent on STAT5a/b activity. To investigate the GH inducibiltiy of Ar expression in quadriceps muscle, 3-mo-old male mice were injected with GH for 3 h. GH-induced expression of the Ar gene and two known STAT5a/b target genes, Igf1 and Socs2, were determined by quantitative real-time PCR. As anticipated, both Igf1 and Socs2 were induced severalfold in muscle of control mice treated with GH. Levels of these genes after GH induction were reduced in Stat5MKO mice after treatment with GH (Fig. 3A, B, respectively), indicating a level of STAT5a/b dependence. In quadriceps from Stat5fl/fl controls, GH induced Ar expression by ∼3-fold. However, GH-induced expression of Ar was sharply reduced in Stat5MKO muscle (Fig. 3C), confirming that Ar expression is GH inducible and dependent on STAT5a/b activity.
Figure 3.
Confirmation of the GH and STAT5a/b dependence of Igf1, Socs2, and Ar genes. RNA was extracted from quadriceps of Stat5MKO and control animals injected i.p. in the morning with GH for 3 h. RNA was converted to cDNA and used for TaqMan real-time PCR. Graphs show relative gene expression of Stat5MKO compared to controls. A, B) GH-induced expression of expected STAT5a/b target genes Igf1 (A) and Socs2 (B). C) GH-induced expression of Ar. *P < 0.05, n = 5–8.
Skeletal muscle fiber-type-specific genes
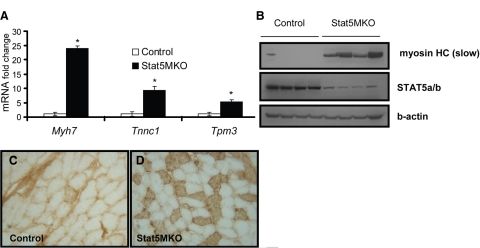
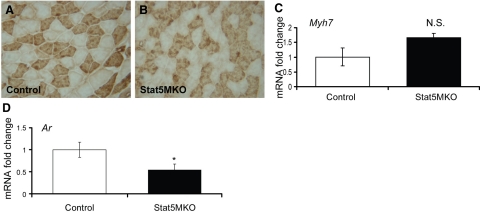
Functional analysis of genes in Stat5MKO muscle shows changes in many genes involved in muscle contraction (Table 3 and Supplemental Fig. 1). Notably, increased expression of several slow-twitch-specific genes, including Myh7, Tnnc1, Tmp3, Tnni1, and Myl2, was found (Table 3). Myh7 is the isoform of the myosin heavy chain found abundantly in slow-twitch fibers, but absent in fast-twitch fibers. Tnnc1 and Tnni1 are troponin isoforms associated with slow-twitch fibers. Validation of these changes by real-time PCR revealed between 5- and −20-fold increased mRNA levels of selected slow-twitch-specific genes in untreated Stat5MKO mice (Fig. 4A). To determine whether increased message levels correlated with increased protein expression, Western blot analysis of protein extracts from quadriceps muscles of untreated controls and Stat5MKO were probed with an antibody specific for the slow myosin heavy chain. A large increase in the myosin heavy chain slow isoform protein in Stat5MKO quadriceps muscle was observed, confirming that protein expression correlates with mRNA expression (Fig. 4B).
TABLE 3.
Microarray results: slow-twitch isoform of muscle contraction genes with increased expression in Stat5MKO quadriceps
| Affymetrix ID | Symbol | Gene name | P value | Stat5MKO vs. control (basal) |
|---|---|---|---|---|
| 1448553_at | Myh7 | Myosin, heavy polypeptide 7, cardiac muscle, beta, skeletal muscle, slow isoform | 0.0145 | 13.2 |
| 1448394_at | Myl2 | Myosin, light polypeptide 2, regulatory, cardiac, slow | 0.0210 | 2.98 |
| 1449996_a_at | Tpm3 | Tropomyosin 3, gamma, slow isoform | 1.23E-05 | 5.16 |
| 1418370_at | Tnnc1 | Troponin C, cardiac/slow skeletal | 0.0127 | 6.59 |
| 1450813_a_at | Tnni1 | Troponin I, skeletal, slow 1 | 0.0087 | 6.79 |
| 1419606_a_at | Tnnt1 | Troponin T1, skeletal, slow | 0.0270 | 5.56 |
Figure 4.
Type I fiber gene expression in quadriceps muscle fibers from Stat5MKO and control mice. A) Real-time PCR gene expression analysis of selected slow-isoform genes (n=3). B) Western blot analysis of 4 control and 4 Stat5MKO animals probed for slow-twitch myosin heavy chain (Myh7, top panel), STAT5a/b (middle panel) and b-actin (bottom panel) to show loading consistency. C, D) Control and Stat5MKO quadriceps muscles from 14-wk-old animals from each group were analyzed. Transverse sections were stained for myosin heavy chain slow isoform. C) Type I myosin staining in quadriceps muscle of control mice. D) Type I myosin staining in quadriceps muscle of Stat5MKO shows mostly fast-twitch; however, tissue contains areas of abundant type I fibers not seen in controls. View shows area of abundant type I fibers.
Because expression of several slow-twitch-related genes was highly increased in the quadriceps of Stat5MKO mice, skeletal muscle fiber-type analyses were made using immunohistochemistry. To identify slow-twitch fibers, quadriceps muscle sections were stained for type I myosin heavy-chain protein. In control mice, lack of type I myosin staining revealed that muscle fibers from quadriceps muscles were nearly 100% of the fast-twitch type (Fig. 4C). Consistently, however, in the quadriceps of Stat5MKO mice areas of abundant slow-twitch fibers were present (Fig. 4D), although fast-twitch fibers remain predominant. This corresponds to analysis of gene expression data, which identified several up-regulated genes that are associated with type I slow-twitch muscle fibers. In the soleus muscle, with its abundance of slow-twitch fibers, a shift toward increased slow fibers was not apparent (Fig. 5A, B; control vs. Stat5MKO). Consistent with this finding, gene expression of Myh7 was not significantly increased in soleus muscle (Fig. 5C). However, expression of Ar was reduced in the soleus of Stat5MKO mice (Fig. 5D), indicating that regulation of Ar gene expression by STAT5a/b occurs in both slow and fast muscle tissues. Together, these results indicate that loss of STAT5a/b in skeletal muscle caused a shift in muscle fiber composition in the fast-twitch quadriceps, but not the slow-twitch abundant soleus muscle.
Figure 5.
Ar expression is reduced in Stat5MKO soleus muscle, while slow-twitch fibers are unchanged. A, B) Transverse soleus muscle frozen sections stained for slow-twitch myosin heavy chain slow isoform. A) Control mice have abundant type I slow-twitch fibers. B) Stat5MKO soleus has similar slow-twitch fibers compared to control. C, D) Real-time PCR analysis of Myh7 and Ar expression in soleus muscle from untreated 3-mo-old male Stat5MKO and control mice. C) Myh7 expression is similar in control and Stat5MKO soleus, confirming that a fiber shift is not present in this muscle (n=6). D) Ar expression in soleus of Stat5MKO mice (n=6).
Ar promoter regulation by STAT5a/b
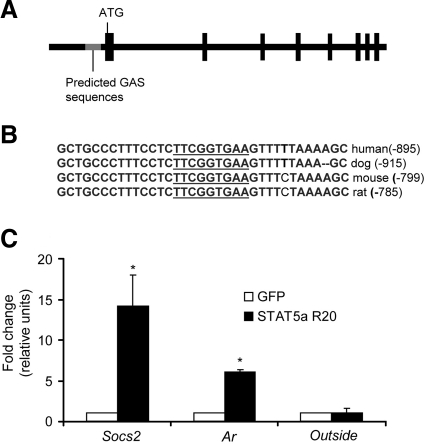
To investigate whether the Ar could be a direct STAT5a/b target gene, an analysis of the of the Ar promoter for STAT5a/b binding sites was performed. The consensus STAT5a/b binding motif (GAS site) is the palindromic TTCN3GAA sequence. Several GAS motifs were found upstream of the Ar transcription start site in the mouse Ar gene (see diagram of Ar gene, Fig. 6A). Strikingly, however, only one GAS site is conserved between species (Fig. 6B), which begins at −799 bp. The cross-species conservation of this region suggests it is critical for promoter activity of the Ar gene. To determine whether STAT5a/b can bind to this site, chromatin immunoprecipitation assays were performed in the mouse C2C12 myoblast cell line. To increase STAT5a levels in the nucleus, cells were infected with a retrovirus expressing STAT5a that has been truncated at the N terminus and demonstrates constitutive activation (16). In chromatin immunoprecipitates with STAT5a antibody, the region surrounding the Ar GAS site was increased 6-fold in immunoprecipitates from STAT5a infected cells compared to GFP-infected controls (Fig. 6C, left bars). As a positive control, STAT5a/b binding sites from the Socs2 gene was also increased in STAT5a-infected cells, but not GFP-infected controls (Fig. 6C, middle bars). Primers specific for a region outside of a suspected STAT5a/b-binding region in the IGF1 gene did not increase in STAT5a-expressing cells, confirming the sensitivity and specificity of the assay (Fig. 6C, right bars). In addition, STAT5a/b GAS sites were not pulled down in IgG immunoprecipitates (not shown). These results confirm that the AR gene is a direct target of STAT5a/b and links the GH/STAT5a/b pathway to the AR pathway by means of direct regulation of Ar gene expression.
Figure 6.
Existence and binding of STAT5a to a conserved GAS site in the Ar gene promoter. A) Schematic of the Ar gene. Vertical boxes indicate translated exon. Location of the conserved GAS sequence is indicated. Gray region containing GAS sequence represents an ∼340-bp region conserved between species. B) Conserved GAS sequence in the AR promoter from multiple mammalian species. C) Results from chromatin immunoprecipitation assays in C2C12 cells. Cells were infected with a constitutively active STAT5a (STAT5a R20)- or GFP-expressing retrovirus. Cross-linked protein/DNA complexes were sonicated and immunoprecipitated with anti-STATa or control antibodies. DNA was amplified from STAT5a-precipitated complexes using specific primers near known (Socs2) and suspected (Ar) GAS regions, respectively, and for a region outside of a known STAT5a/b binding site (outside). Data represent averages of 2 separate experiments with the range indicated.
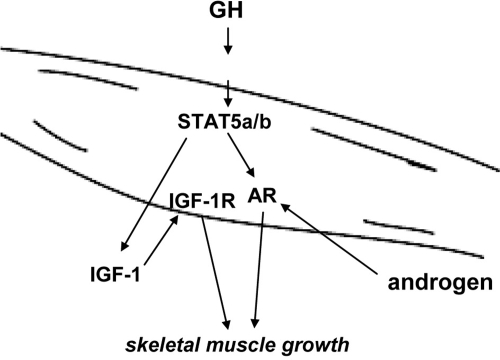
The regulation of Ar gene expression by GH/STAT5a/b in the skeletal muscle represents a novel means by which GH and androgen signaling interact and promote skeletal muscle growth. As illustrated in Fig. 7, we propose that GH acts to induce both IGF-1 and AR expression in skeletal muscle. GH induction of IGF-1 production plays an important role in both male and female postnatal growth of muscle and peripheral tissues, likely through an autocrine/paracrine mechanism (9). In addition, the androgens testosterone and dihydroxystestosterone promote muscle growth in males by acting on AR.
Figure 7.
Diagram of GH-STAT5a/b-AR regulation in muscle. We propose a model in which GH signals through STAT5a/b to induce AR expression in skeletal muscle tissues. GH can induce autocrine/paracrine IGF-1 production in skeletal muscle through STAT5a/b signaling that may act on skeletal muscle IGF-1 receptors (IGF-1R). In addition, GH/STAT5a/b activation induces expression of the AR. Androgens testosterone and dihydroxytestosterone in males then send an additional growth signal through the AR, controlling male-specific skeletal muscle growth.
DISCUSSION
The contributions of the transcription factors STAT5a/b to the physiology of skeletal muscle were addressed in this study. Changes in skeletal muscle growth, fiber composition, and gene expression profile observed in the absence of STAT5a/b revealed unappreciated critical roles for these transcription factors in muscle growth and fiber type specification. Previously, we discovered that despite only a modest reduction in circulating IGF-1, both male and female Stat5MKO mice have reduced body weight as the result of reduced lean mass. Here, it is demonstrated that skeletal muscle mass was preferentially reduced in males compared to their overall weight. Mechanistically, a paracrine role of IGF-1 is a likely explanation. This is supported by the severe effect on growth seen in the IGF-1−/− mice but not observed in the liver-specific IGF-1- knockout mouse (17). As the liver produces ∼75% of circulating IGF-1, a paracrine role was suspected. However, the picture is likely to be more complex, and factors other than IGF-1 may play a role in the GH-STAT5a/b pathway. Here, evidence is presented that the AR is affected by loss of STAT5a/b that may affect growth of skeletal muscle. A >30% loss of muscle mass in Stat5MKO males, but only ∼10% loss in females, further supports a role for reduced AR function. Buttressing this conclusion, Maclean et al. (12) found that in male, but not female, complete AR-null ARKO mice, muscle growth is impaired.
Increased expression of several slow-twitch-specific genes and the appearance of type I fibers were observed in the absence of STAT5a/b in quadriceps muscle, a tissue that is composed almost exclusively of type II fibers and lacks appreciable expression of type I marker genes. We do not understand the mechanism for this fiber-type shift currently. However, a remarkably similar phenotype has been reported by Baker and colleagues in the utrophin/dystrophin double-knockout mouse model of Duchenne musclular dystrophy (DMD) (18). The authors used microarray analysis to compare gene expression in muscle of these mice to the muscle of the distrophin-deficient mdx mouse that displays a less severe phenotype. Eight slow-twitch fiber-specific transcripts (Myl2, Myh7, Tnnc1, Tnnt1, Tnnt2, Tpm3, Tnni1, and Atp2a2) were up-regulated in the DMD mouse model compared to the mdx-deficient mouse. Each of these genes (Table 3; Atp2a2 1.6-fold increased) is changed in the same direction in the quadriceps of Stat5MKO mice. Also, Amd1 and Igh-6 expression were reduced in the DMD model and Stat5MKO mice. The consistency between these results suggests that STAT5a/b, urotrophin, and dystrophin are part of similar or overlapping pathways. It is also possible that reduction in AR has a role in the altered fiber composition as well. Maclean et al. (12) observed in the ARKO mice an up-regulation of slow-twitch isoforms of myosin light chain (Myl2) and troponin T (Tnnt1) mRNA in skeletal muscle 1.9- and 1.5-fold, respectively. This is consistent with, albeit less marked than, the changes observed in these genes in Stat5MKO muscle (Table 3).
It is apparent that STAT5a/b mediates a range of GH actions in skeletal muscle. We have shown previously the dependence of IGF-1 production in skeletal muscle on STAT5a/b. Beneficial aspects of exercise are mediated by STAT5a/b as well. Constitt et al. (19) have demonstrated that in healthy human subjects, aerobic exercise leads to phosphorylation of JAK2 and STAT5a/b. Consistent with this observation, the authors report that GH and, to a lesser extent, IGF-1 levels were transiently elevated after exercise, suggesting that GH activated a JAK2-Stat5 pathway leading to production of IGF-1 from an uncertain source. They also provided evidence that endogenously produced growth hormone is sufficient to activate STAT5a/b transcriptional activity in humans. That study, however, did not rule out other cytokines that could be involved in activation of the JAK2-STAT5 pathway, such as IL-6, which is known to be produced by skeletal muscle following exercise (20).
Expression of several transcription factors known to play a role in either muscle development or fiber-type determination were changed in muscle of untreated Stat5MKO mice (see microarray raw data, GEO GSE14710). FOXO1 was decreased >50% in GH-treated Stat5MKO mice. FOXO1 has been shown to control muscle fiber differentiation in mice (21) and to affect muscle fiber composition and function (21, 22). Down-regulation of the genes coding for the nuclear receptor PPARα and the transcriptional cofactor PGC-1α to <50% of GH-treated controls was found in Stat5MKO muscle. PPARα is known to be important for mitochondrial function (23), while PGC-1α has been reported to play a role in mitochondrial function and skeletal muscle fiber type (24, 25). These changes may reflect a change in mitochondrial function in Stat5MKO mice and may partially explain the reduced activity of these mice; however, this has not yet been investigated.
The role of STAT5a/b in the induction of Ar gene expression in skeletal muscle helps to explain the greatly reduced growth of skeletal muscle in male Stat5MKO mice and is in full agreement with the impaired muscle development observed in ARKO mice (12). Besides the increased slow-twitch fiber genes, a potentially reduced polyamine biosynthetic pathway is another common difference in skeletal muscle gene expression between the ARKO and Stat5MKO mice, and points to reduced AR activity in skeletal muscle of Stat5MKO mice. This is evidenced by the reduction in expression of two genes that are involved in polyamine biosynthesis, Amd1 and Smox, in ARKO (12) and Stat5MKO skeletal muscle (Table 2) .
The finding that the Ar gene is a direct target gene of STAT5a/b has implications for other cell types as well. In prostate cancer cells, a proposed transcriptional synergy between STAT5a/b and AR has been made (26). Specifically, STAT5a/b was demonstrated to interact with and enhance the prolactin-dependent transcriptional activity of the AR (26).
Our study presents evidence that STAT5a/b transcription factors play an important role in maintaining muscle size and fiber-type characteristics. Target genes of STAT5a/b transcription, including Igf1 and Ar, likely play critical roles in mediating GH actions throughout life. Although the full effect of GH-STAT5a/b signaling on the physiology of muscle tissue throughout life has not been fully elucidated, our study provides evidence that steroid and cytokine signaling are integrated. The combined roles of these two pathways in aging and disease-related declines in muscle mass warrants further investigation.
Supplementary Material
Acknowledgments
The authors thank George Poy (NIDDK Genomics Core Laboratory, Bethesda, MD, USA) for assistance with the microarrays. We also thank Dr. Oksana Gavrilova and William Jou (NIDDK Mouse Metabolism Core Laboratory) for conducting the locomotor activity and respiration experiments. Thanks to Dr. Richard Moriggl (Ludwig Boltzmann Institute for Cancer Research, Vienna, Austria) for providing the construct for the constitutively active STAT5a mutant. Finally, thanks to Alexandra Dr. McPherron and Dr. Kathleen Savage for technical expertise in muscle-fiber staining. This work was funded by the intramural program of NIH, NIDDK.
References
- Zierath J R, Hawley J A. Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS Biol. 2004;2:e348. doi: 10.1371/journal.pbio.0020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J, Hamilton J A, Ouchi N, LeBrasseur N K, Walsh K. Fast/glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7:159–172. doi: 10.1016/j.cmet.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Dov I, Gaides M, Scheinowitz M, Wagner R, Laron Z. Reduced exercise capacity in untreated adults with primary growth hormone resistance (Laron syndrome) Clin Endocrinol (Oxf) 2003;59:763–767. doi: 10.1046/j.1365-2265.2003.01920.x. [DOI] [PubMed] [Google Scholar]
- Gotherstrom G, Bengtsson B A, Sunnerhagen K S, Johannsson G, Svensson J. The effects of five-year growth hormone replacement therapy on muscle strength in elderly hypopituitary patients. Clin Endocrinol (Oxf) 2005;62:105–113. doi: 10.1111/j.1365-2265.2004.02181.x. [DOI] [PubMed] [Google Scholar]
- Liu J P, Baker J, Perkins A S, Robertson E J, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Fernandez A M, Dupont J, Farrar R P, Lee S, Stannard B, Le Roith D. Muscle-specific inactivation of the IGF-I receptor induces compensatory hyperplasia in skeletal muscle. J Clin Invest. 2002;109:347–355. doi: 10.1172/JCI13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton E R, Sweeney H L, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernandez M, Delgado G, Puche J E, Gonzalez-Baron S, Castilla Cortazar I. Low doses of insulin-like growth factor I improve insulin resistance, lipid metabolism, and oxidative damage in aging rats. Endocrinology. 2008;149:2433–2442. doi: 10.1210/en.2007-1190. [DOI] [PubMed] [Google Scholar]
- Klover P, Hennighausen L. Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: a role for autocrine/paracrine insulin-like growth factor I. Endocrinology. 2007;148:1489–1497. doi: 10.1210/en.2006-1431. [DOI] [PubMed] [Google Scholar]
- Davey H W, Xie T, McLachlan M J, Wilkins R J, Waxman D J, Grattan D R. STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology. 2001;142:3836–3841. doi: 10.1210/endo.142.9.8400. [DOI] [PubMed] [Google Scholar]
- Herbst K L, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:271–277. doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- MacLean H E, Chiu W S, Notini A J, Axell A M, Davey R A, McManus J F, Ma C, Plant D R, Lynch G S, Zajac J D. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J. 2008;22:2676–2689. doi: 10.1096/fj.08-105726. [DOI] [PubMed] [Google Scholar]
- Clark R P, Schuenke M, Keeton S M, Staron R S, Kopchick J J. Effects of growth hormone and insulin-like growth factor I on muscle in mouse models of human growth disorders. Horm Res. 2006;66:26–34. doi: 10.1159/000096620. [DOI] [PubMed] [Google Scholar]
- Tallquist M D, Weismann K E, Hellstrom M, Soriano P. Early myotome specification regulates PDGFA expression and axial skeleton development. Development. 2000;127:5059–5070. doi: 10.1242/dev.127.23.5059. [DOI] [PubMed] [Google Scholar]
- Auernhammer C J, Bousquet C, Melmed S. Autoregulation of pituitary corticotroph SOCS-3 expression: characterization of the murine SOCS-3 promoter. Proc Natl Acad Sci U S A. 1999;96:6964–6969. doi: 10.1073/pnas.96.12.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriggl R, Sexl V, Kenner L, Duntsch C, Stangl K, Gingras S, Hoffmeyer A, Bauer A, Piekorz R, Wang D, Bunting K D, Wagner E F, Sonneck K, Valent P, Ihle J N, Beug H. Stat5 tetramer formation is associated with leukemogenesis. Cancer Cell. 2005;7:87–99. doi: 10.1016/j.ccr.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu J L, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P E, Kearney J A, Gong B, Merriam A P, Kuhn D E, Porter J D, Rafael-Fortney J A. Analysis of gene expression differences between utrophin/dystrophin-deficient vs mdx skeletal muscles reveals a specific upregulation of slow muscle genes in limb muscles. Neurogenetics. 2006;7:81–91. doi: 10.1007/s10048-006-0031-7. [DOI] [PubMed] [Google Scholar]
- Consitt L A, Wideman L, Hickey M S, Morrison R F. Phosphorylation of the JAK2-STAT5 pathway in response to acute aerobic exercise. Med Sci Sports Exerc. 2008;40:1031–1038. doi: 10.1249/MSS.0b013e3181690760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth J P, Van Uum S H, Van Deuren M, Pesman G J, Van der Ven-Jongekrijg J, Van der Meer J W. Endurance run increases circulating IL-6 and IL-1ra but downregulates ex vivo TNF-α and IL-1β production. J Appl Physiol. 1995;79:1497–1503. doi: 10.1152/jappl.1995.79.5.1497. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Kitamura Y I, Funahashi Y, Shawber C J, Castrillon D H, Kollipara R, DePinho R A, Kitajewski J, Accili D. A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest. 2007;117:2477–2485. doi: 10.1172/JCI32054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, Nishino I, Ezaki O. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem. 2004;279:41114–41123. doi: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Chinetti G, Fruchart J C, Staels B. Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr P T, Zhang C Y, Wu Z, Boss O, Michael L F, Puigserver P, Isotani E, Olson E N, Lowell B B, Bassel-Duby R, Spiegelman B M. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur N K, Yan Z, Spiegelman B M. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007;282:30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- Tan S H, Dagvadorj A, Shen F, Gu L, Liao Z, Abdulghani J, Zhang Y, Gelmann E P, Zellweger T, Culig Z, Visakorpi T, Bubendorf L, Kirken R A, Karras J, Nevalainen M T. Transcription factor Stat5 synergizes with androgen receptor in prostate cancer cells. Cancer Res. 2008;68:236–248. doi: 10.1158/0008-5472.CAN-07-2972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.