Abstract
Traditionally, animal models of schizophrenia were predominantly pharmacological constructs focused on phenomena linked to dopamine and glutamate neurotransmitter systems, and were created by direct perturbations of these systems. A number of developmental models were subsequently generated that allowed testing of hypotheses about the origin of the disease, mimicked a wider array of clinical and neurobiological features of schizophrenia, and opened new avenues for developing novel treatment strategies. The most thoroughly characterized (~100 primary research articles) is the neonatal ventral hippocampal lesion (NVHL) model, which is the subject of this review. We highlight its advantages and limitations, and how it may offer clues about the extent to which positive, negative, cognitive, and other aspects of schizophrenia, including addiction vulnerability, represent inter-related pathophysiological mechanisms.
1. Introduction
Schizophrenia is a complex psychiatric condition affecting ~1% of the world’s population. It is characterized by profound disturbances of mental functions and subtle brain abnormalities. Importantly, this disorder is thought to arise from a combination of genetic, developmental and environmental factors (Figure 1) [1]. Because of the diversity of symptoms, and heterogeneity of biological findings, schizophrenia may actually be a cluster of closely-related diseases, further complicating our understanding of its pathophysiology. Still, common pathophysiological pathways may exist while various modulatory factors may differentially alter disease processes within specific brain regions and/or circuits leading to differential expression patterns of positive, negative and cognitive symptoms [1–4]. This interpretation is reinforced by a number of studies that implicate a variety of brain structures in the pathophysiology of schizophrenia [1, 3–5], many of which are anatomically and functionally interconnected, and are crucial in determining appropriate decision making outcomes in response to external stimuli [3, 5].
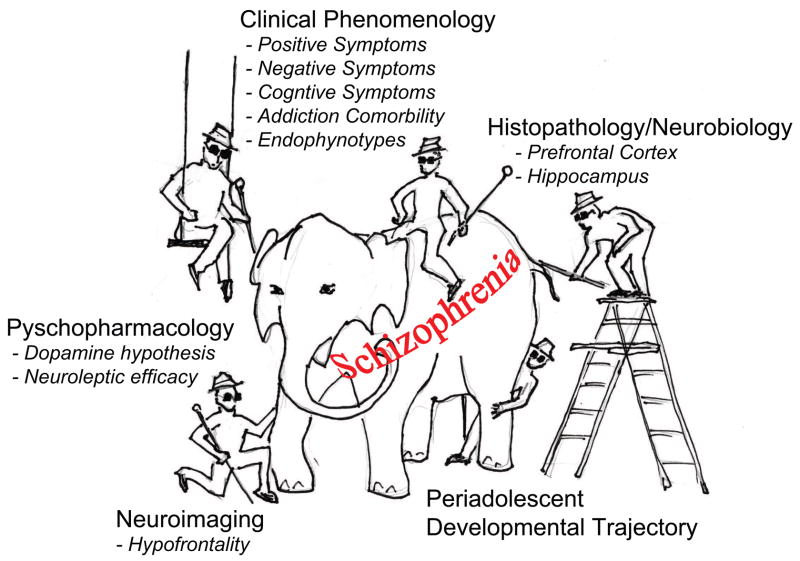
Figure 1.
Different methods used in schizophrenia research may lead to different conclusions dependent on one’s perspective. This notion is illustrated by a cartoon based on the tale, in which a group of blind men examining different parts of an elephant try to learn what it is like, and draw different conclusions. By generating multiple neurobiological and behavioral “parts” of schizophrenia, the NVHL comprehensively models the “elephant” as a developmental, neurocircuit-based pathophysiological pathway, through which converging genetic and environmental factors produce schizophrenia-spectrum clinical syndromes.
Over fifty animal models of schizophrenia have been described during the past 30 years (see Schizophrenia Research Forum - http://www.schizophreniaforum.org). Each of these models has their advantages and limitations, and they differ in the degree to which they model certain focal aspects of schizophrenia. Up until the early 1990’s, animal models of schizophrenia were predominantly pharmacological constructs focused on phenomena linked to dopamine and glutamate neurotransmitter systems, achieved by direct perturbations of these systems. A major heuristic limitation of these approaches was that they left out a number of key features attributed to schizophrenia such as its developmental pathogenesis, its cognitive deficits, and the impact on other neurotransmitter systems and neuroanatomy beyond those directly manipulated. Although the dopaminergic system has been strongly implicated in schizophrenia [1–4], and dopamine-based models have been important for screening drugs with dopamine blockade-based anti-psychotic efficacy [6–9], dopamine-linked behaviors are not uniquely prominent in this psychiatric condition and drugs with anti-dopaminergic efficacy do not always ameliorate schizophrenia symptoms. Thus, novel heuristic models that encompass a wider array of clinical and neurobiological features of schizophrenia (Figure 1) were needed to define major pathophysiological pathways or mechanisms that may be targeted by new treatments.
The most thoroughly characterized (~100 primary research articles) heuristic neurodevelopmental animal model of schizophrenia available today is the neonatal ventral hippocampal lesion (NVHL) model [3, 4, 10]. This model, conceived by Lipska, Weinberger and colleagues in the early 1990’s, was inspired by an attempt to capture prominent aspects of schizophrenia unaddressed by pharmacological models. Emerging brain imaging evidence at the time, pointed to a lateral ventricular enlargement and hippocampal changes in schizophrenia patients not consistent with acute, adult-age insults. Various studies suggested that environmental stressors such as obstetrical complications, infectious diseases affecting pregnant mothers in the second to third trimester of fetal life or malnutrition, carried increased risk of schizophrenia in the offspring. Putting these elements together parsimoniously, led to exploration of the impact of experimentally induced ventral hippocampal cell loss. Seven-day old rat pups (postnatal day -PD- 7) were used as this is a time point comparable to vulnerable phases of fetal hippocampal development during the second to third trimester of humans [11]. As a heuristic neurodevelopmental model, the NVHL triggers a number of behavioral, molecular and physiological changes reminiscent of a variety of aspects of schizophrenia that emerge at a particular time in development, i.e., around young adulthood/adolescence.
As reviewed next, a host of studies indicate that in NVHL rats, not only are multiple clinical features of the syndrome manifest, but the development and function of multiple brain systems are affected, including the frontal and medial temporal lobes, the ventral striatum, and the mesocorticolimbic dopamine system (Figure 2). Genetic background, environmental factors and pharmacological interventions can all modulate the behavioral and neurobiological aspects of the NVHL syndrome [1, 12], demonstrating the utility of this model for exploring the interaction of diverse causative factors in disease pathogenesis. We aim to provide a comprehensive overview of findings on the NVHL model, highlighting its advantages and limitations, and how it may offer clues about the extent to which positive, negative, cognitive, and other syndromal aspects of schizophrenia represent inter-related pathophysiological mechanisms.
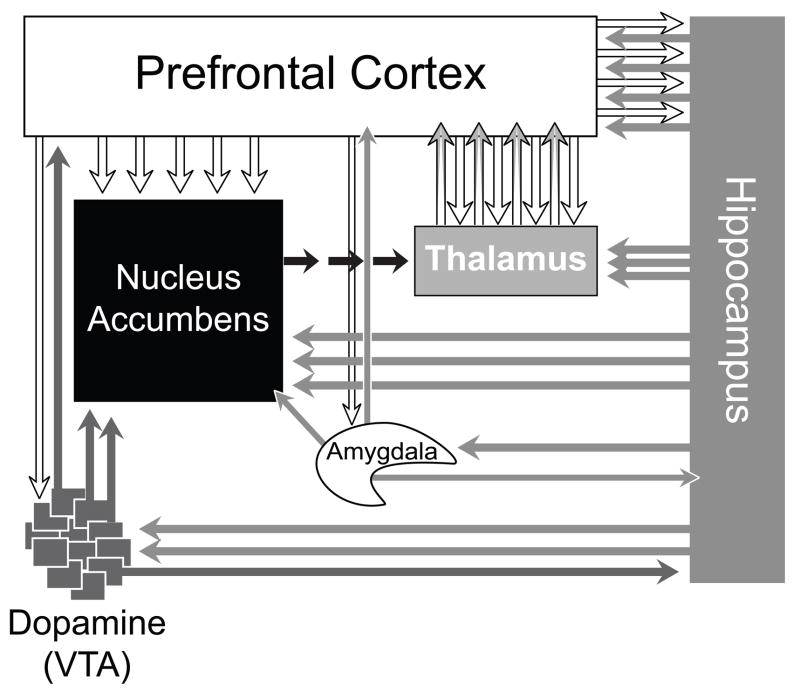
Figure 2.
A diagram illustrating major brain circuits thought to be involved in the pathophysiology of schizophrenia [1, 3–5]. They are anatomically and functionally interconnected, and are crucial in determining appropriate decision making outcomes in response to external stimuli [3, 5].
2. Behavioral Syndrome
It is widely accepted that a strong developmental component underlies the pathophysiology of schizophrenia [1–3]. Developmental models, while targeting different brain structures for producing diverse behavioral phenotypes, commonly aim to disrupt the normal developmental process of a given neurocircuitry. Studies focused on neonatal damage of the hippocampus (NVHL) in rats [3, 13–21] and in monkeys [22–25] involve compromise of hippocampal regions that directly project to the prefrontal cortex and ventral striatum (Figure 2). The ventral hippocampus in the rat corresponds to the anterior hippocampus in humans, a region that has been consistently implicated in human schizophrenia [26–36]. Developmental ventral hippocampal damage produces abnormalities in a number of dopamine-related behaviors that typically emerge late in adolescence and early adulthood, resembling the delayed onset of psychotic symptoms in schizophrenia (Figure 3) [4, 37]. Other NVHL-induced behavioral anomalies also bear close resemblance to those observed in schizophrenia, such as negative-like symptoms and cognitive deficits [3, 18, 38–43].
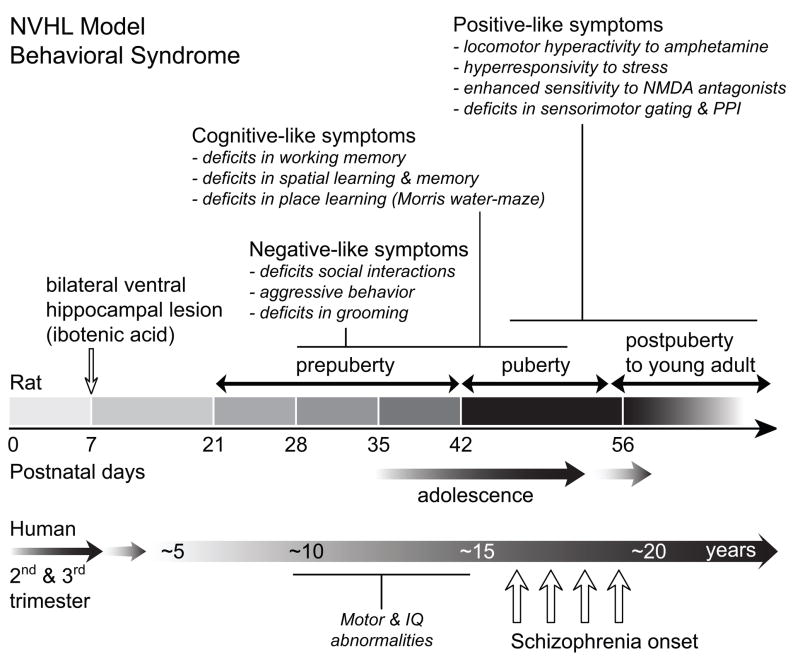
Figure 3.
A timeline of the emergence of behavioral changes following the neonatal ventral hippocampal lesion in the rat and a comparison with the emergence of symptoms in schizophrenia in humans. Developmental ventral hippocampal damage produces a number of dopamine-related behavioral abnormalities that emerge late in adolescence and early adulthood, resembling the delayed onset of psychotic symptoms in schizophrenia. Other NVHL-induced behavioral anomalies, such as negative-like symptoms and cognitive deficits [3, 18, 38–43], occur at both pre- (PD 35) and postpubertal (PD 65) ages, similar to subtle developmental anomalies in humans who later develop schizophrenia, and do not respond to antipsychotic drugs [48].
Rats with a bilateral NVHL exhibit a post-adolescent onset of hyperresponsivity to pharmacological or environmental stimuli known to provoke mesolimbic dopamine efflux (e.g. non-specific mild stress, novelty, amphetamine, apomorphine) [18]. Also, NVHL rats are less cataleptic to haloperidol and more sensitive to apomorphine compared to sham-operated rats [43]. These behavioral abnormalities can be reversed by treatment with both classical and atypical antipsychotic drugs [44]. Antipsychotic drugs given in adulthood (43) or during adolescence and up to adulthood (PD 35–56) prevent the exaggerated hyperactivity in response to amphetamine [45]. NVHL rats also show enhanced sensitivity to glutamate antagonists such as MK-801 and PCP, deficits in prepulse inhibition (PPI) and latent inhibition [12, 13, 17, 18, 38, 43, 44, 46], phenomena showing many parallels with positive symptoms of schizophrenia (Figure 3).
NVHL animals show deficits in social behaviors i.e., they spend less time engaging in social interactions and frequently demonstrate aggressive behavior [13]. It is thought that these deficits could be due to failures of social memory [47]. When tested as juveniles (PD <35), NVHL rats are less social than controls [48], but behave normally in motor tests involving exposure to stress and dopamine agonists. Interestingly, the appearances of social interaction deficits are not of developmental onset like the positive-like symptom traits. Social interaction deficits occur at both pre- (PD 35) and postpubertal (PD 65) ages and do not respond to antipsychotic drugs [48]. Deficits in grooming have also been reported in NVHL rats, perhaps in association with a decrease in social interactions [49] (Lipska, Tseng, Chambers, unpublished observations). However, neonatal lesion of the prefrontal cortex prevented the appearance of grooming deficits and novelty-induced hyperactivity in postpubertal NVHL rats but not deficits in social behaviors [49].
Spatial learning and memory tasks dependent on frontal-cortical/hippocampal cooperation (i.e., eight arm radial maze task) are profoundly altered in NVHL rats even before puberty and into adulthood [3]. Interestingly, NVHL rats demonstrate adequate sensorimotor functions and appropriate motivation to escape when tested in a cued Morris water-maze task [40]. However, NVHL rats show impairments in place learning and memory of the location of a submerged platform when compared to controls. These deficits become evident only after puberty. Similarly, NVHL rats exhibit deficits in both continuous delayed and discrete paired-trial variable-delay alternation tasks [50]. Importantly, the performance in these tasks remains unaltered in rats that received adult lesion of the ventral hippocampus [50]. These findings suggest that the neonatal lesion elicits long-lasting deficits in working memory tasks, in particular, the capacity to acquire and retain information in tests of spatial and avoidance learning.
In summary, these behavioral results show that an early developmental insult of the ventral hippocampus reproduces a broad spectrum of schizophrenia-related phenomena, some of which, but not all are responsive to treatment with classical and atypical antipsychotic drugs, despite the fact that the lesion does not target the dopaminergic system directly. Behavioral anomalies obtained in the NVHL model bear close resemblance to those observed in schizophrenia and are thought to mimic aspects of positive- and negative-like symptoms as well as cognitive deficits [3, 18, 38–43]. Thus, the NVHL model replicates and combines different phenomenological aspects of schizophrenia (Figure 1) into one experimental platform as a comprehensive developmental model (Figure 3).
3. Modifying Factors of the NVHL syndrome
3a. Sex and hormones
There are gender differences in quality of presentation of symptoms in schizophrenia, with males tending to have earlier onset in some cases [51]. In the NVHL model, the emergence of behavioral changes in adolescence appears, however, not to be associated with the surge of gonadal hormones during puberty as a similar temporal pattern of abnormalities is found in animals depleted of gonadal hormones prior to puberty [52] and no differences in cognitive/working memory effects of NVHL were observed between male and female rats [3]. However, differences were reported in the age of onset and intensity of abnormal motor responses, with male rats showing hyperactivity emerging earlier and being more pronounced than in females [53]. Thus, although neural circuits underlying NVHL-induced behavioral changes are robust and insensitive to hormonal modulation, there may be other developmental differences between the sexes that contribute to the different pattern of abnormal behaviors emerging as a consequence of the neonatal hippocampal lesion.
3b. Environmental context
It is thought that early life environment may alter adult behavioral phenotypes, and perhaps interact with developmental insults to produce a unique pattern of changes. One of the powerful modifiers of behavior is maternal care, shown to influence development of the hippocampus and cognitive functions in humans and in rodents [54]. Variations in maternal behavior (high levels of pup licking and grooming and arched-back nursing vs. low levels of these measures) can alter the robustness of the NVHL lesion phenotype [55, 56], possibly by interacting with the down stream developmental effects of NVHL on prefrontal cortical function.
Social-environmental conditions occurring later in pre-adult development may also impact adult behavior. For instance, peri-adolescent impoverishment of social contacts has long term behavioral effects in adulthood and has been proposed as a model of some aspects of schizophrenia in its own right [57]. Post-weaning (i.e., PD> 22) social isolation in normal rats (singly housed vs. housed in groups of three) produces part of the “positive” component syndrome (e.g., novelty-induced locomotion) resembling that observed in the NVHL model. Such early social isolation in NVHL rats further exaggerates neurochemical, morphological and behavioral consequences of the lesion consistent with complex biological-environmental stress diathesis theories describing the development of various psychiatric disorders [58, 59]. Non-social stressors also have detrimental effects on the NVHL phenotype. For instance, both tail pinch and restraint exaggerate changes in prefrontal cortical and nucleus accumbens chemistry [60–62], and acute foot-shock leads to enhanced and prolonged neural and neuroendocrine responses in NVHL animals [63]. Combining developmental/environmental contingencies with the NVHL model exaggerates the abnormal outcome and may be perhaps analogous to the effects of drugs of abuse, such as PCP, which also potentiate the NVHL syndrome [64]. These findings demonstrate the utility of the NVHL model for exploring multiple neurobiological/environmental hit hypotheses. In addition, the importance of developmental critical periods sensitive to environmental learning, and stress as contributors to the severity of pathophysiology in the NVHL model and in schizophrenia is highlighted.
3c. Developmental pathology: lesion timing, laterality and size
There is not doubt that developmental lesion models represent a rather crude technique to study the role of particular brain regions, transmitter systems or the connections between neurocircuits. They lack construct validity, as the schizophrenic brain does not manifest a “lesion” analogous to this model. However, the neurodevelopmental damage approach has generated animal models with great heuristic value, in particular for discovering neural and behavioral consequences of the initial injury distal to it both anatomically and in terms of developmental timeline. In this respect, the NVHL model appears to have face validity as it incorporates many aspects of behavioral, cellular and pharmacological phenomena as well as the temporal course of clinical onset of the disorder (Figure 3).
The effect of the NVHL is critically dependent on the timing of the lesion. When performed early, before or on PD 7, the behavioral changes are pronounced and appear with a developmental delay, whereas lesions performed later (e.g. PD 14, 21 and in adulthood) do not show a delayed profile of changes, abnormalities are less pronounced and qualitatively different [65–66]. Unilateral lesions, as compared with bilateral lesions, produce a smaller magnitude but same quality of effects in some domains (cognitive deficits) [3], but a qualitatively different pattern of effects in others (e.g. in response to natural and drug reinforces) [67].
It is not well understood how different phenotypic components of the NVHL syndrome could be differentially affected by lesion extent or laterality, but sparing of some elements of the hippocampal formation may be required for robust expression of the NVHL syndrome. The extent of ibotenic-acid induced NVHL damage that persists into adulthood ranges from local neuronal loss and cellular disarray to extensive ablation of the ventral hippocampus (Figure 4). While it has been reported that smaller NVHL damage results in weaker “positive” component phenotypes [12], larger lesions encompassing more of the dorsal hippocampus and portions of entorhinal cortex, can also weaken the phenotype [68].
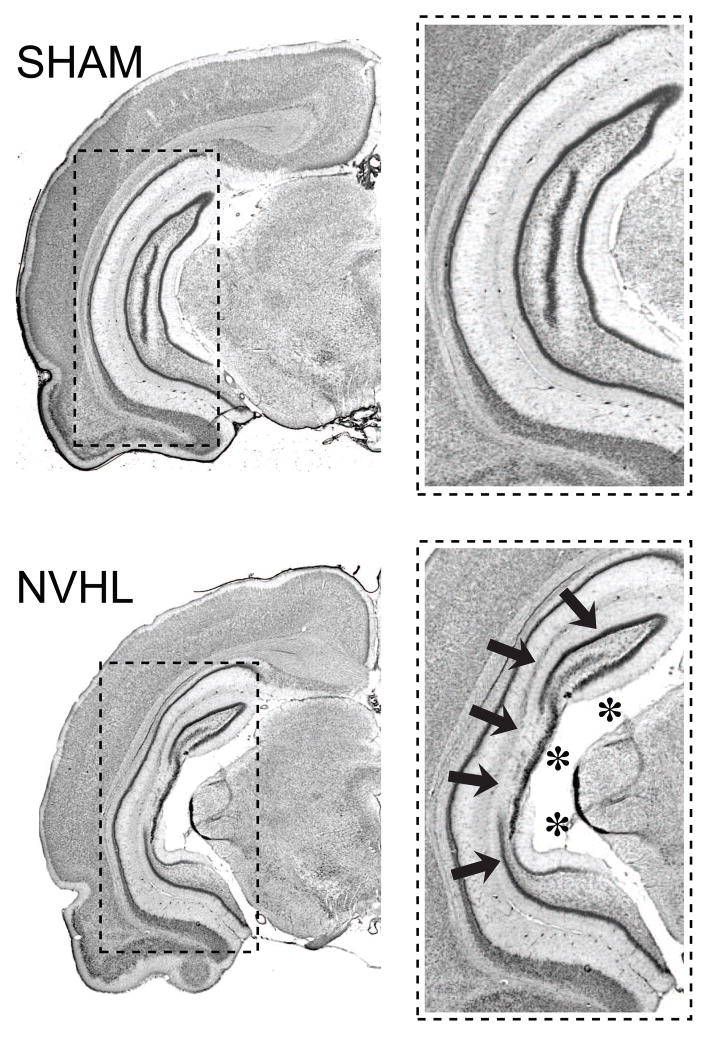
Figure 4.
Photomicrographs depicting the extent of a typical neonatal ventral hippocampal lesion. Coronal Nissl stained sections show the ventral hippocampus of a sham-operated rat (top, SHAM) and a characteristic neonatal ventral hippocampal lesion (bottom, NVHL), characterized by cell loss (arrows), and enlarged ventricles (asterisks).
Altogether, studies examining timing, laterality and lesion extent provide evidence that the NVHL syndrome is triggered by a spatially restricted defect during a specific phase of early cortico-limbic development. Such a triggering process may depend just as much, or more, on location and developmental timing, as on lesion modality or size. Indeed, developmentally transient inactivation of the ventral hippocampus, produced without gross anatomical changes, appears sufficient to generate an NVHL-like syndrome. Delivery of tetrodotoxin (TTX), a potent and specific blocker of voltage-gated sodium channels to the ventral hippocampus on PD7, results in behaviors and temporal patterns of symptom onset similar to those produced with ibotenic acid lesions [50]. As adults, neonatally TTX-infused rats exhibit exaggerated motor hyperactivity in response to novelty, mild stressors, and pharmacological stimulation (amphetamine and MK-801). Notably, analogous TTX infusions in adult animals do not alter these behaviors later in life. These results suggest that even transient loss of ventral hippocampal function during a critical phase of cortico-limbic maturation permanently changes the development of downstream neural circuits mediating certain dopamine- and NMDA-related behaviors. To the extent that neonatally-treated TTX rats do not experience a gross lesion of the ventral hippocampus, this approach improves the NVHL model in terms of anatomical construct validity. However, this improvement also comes at some cost in terms of experimental practicality. Precisely because the TTX manipulation delivers no gross lesion, confirming the location of the intervention is more difficult. Also, the magnitude of TTX-induced behavioral disruptions is smaller compared to NVHL animals such that NVHL rats exhibit about a 50% increase in spontaneous and amphetamine-induced locomotor activity above controls, whereas TTX increases these measures by about 20% [18, 42]. In light of the TTX model findings, the NVHL model appears to provide a more practical approach—that is both robust in effect, and easily verifiable by routine histological inspection—for achieving a developmental model that re-capitulates the complex aspects of schizophrenia.
3d. Genetics and Strain
Schizophrenia is a highly heritable disorder that probably involves multiple genes with small effects across large populations. Recently, numerous putative candidate genes have been recently identified [69]. Genetic mouse models offer great advantages in investigating roles of particular genes implicated in schizophrenia, and possibly gene-environment interactions. However, they also have serious limitations, e.g., genetic manipulations, which most often begin at conception, trigger various adaptive responses that may change or preclude emergence of behavioral phenotypes. The NVHL model enables testing responses to early insult in the hippocampus on different genetic backgrounds, rather than in the absence of a particular candidate risk gene. Indeed, by comparing rat strains receiving NVHL, it was shown that genetic factors can influence the behavioral anomalies induced by the neonatal disruption of the ventral hippocampus [12]. Lesions of the same size in Lewis, Fisher 344 and Sprague-Dawley rats result in different phenotypes and different age of onset of the “positive” components of the syndrome [12], indicating that genes convey susceptibility to NVHL phenotype and possibly latent traits observed in schizophrenia [1, 12]. Moreover, the results from the NVHL model suggest that variations in genes involved particularly in hippocampal development can be risk factors for schizophrenia. For example, variation in the DISC1 gene, produced via a balanced chromosomal translocation is implicated as a susceptibility factor for major mental illness. DISC1 is highly expressed in the hippocampus, especially during prenatal development. Based on animal studies, risk variants in DISC1 exert their pathological role by affecting hippocampal neurogenesis and neurite outgrowth [1, 70, 71]. In this context, the NVHL model may be viewed as a crude model of a genetic “lesion” in which early maturational events are severely disrupted.
4. Neurobiology underlying the NVHL syndrome
4a. Dysregulation of the prefrontal cortical network
Prefrontal cortical disruption in the NVHL model has been proposed to model cortical deficits observed in schizophrenia [72]. As previously discussed, many of the behavioral alterations in NVHL animals do not emerge until puberty and young adulthood [4, 37]. Treatment with antipsychotic drugs can reverse some of the behavioral impairments and physiological responses associated with the NVHL [41, 44, 73], suggesting that the functionality of the mesocorticolimbic dopamine system is altered in these animals. For example, recent in vivo experiments have demonstrated that prefrontal cortical neurons in NVHL rats exhibit an abnormal response to mesocortical inputs [72, 74]. These changes were observed in postpubertal rats with a NVHL. Lesions performed in adulthood failed to elicit the abnormal mesocortical responses, indicating that the altered prefrontal response is due to neurodevelopmental dysregulation of the mesocortico-dopamine-prefrontal system [4, 72, 74]. Removal of prefrontal cortical neurons restored some of the behavioral [37] and electrophysiological [75] alterations induced by the NVHL, suggesting that aberrant development of the prefrontal cortex triggered by an early disconnection of the hippocampus is critical for the NVHL syndrome.
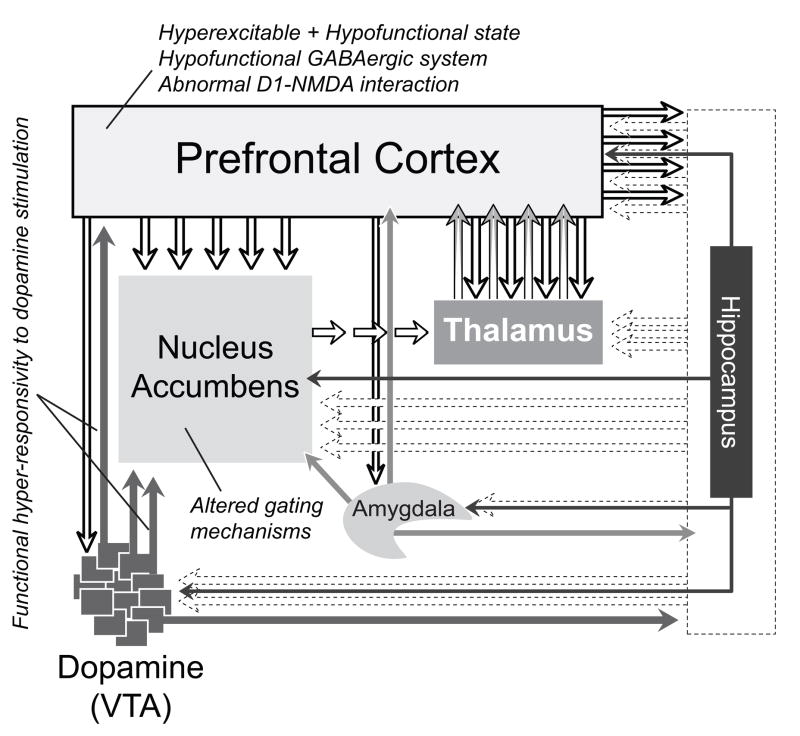
It is important to emphasize that anatomical findings from postmortem studies, and neuropsychological as well as neuroimaging studies of brain function in patients with schizophrenia have also implicated prefrontal cortical maldevelopment parallel to a developmental disconnection of the temporal and prefrontal cortices [76]. Similarly, anatomical studies in the NVHL model suggest that developmental loss of ventral hippocampal excitatory drive to the prefrontal cortex could trigger postsynaptic structural and molecular rearrangements. For instance, a decrease of pyramidal neuron dendritic length and spine density has been reported in the prefrontal cortex of NVHL animals [58, 77]. Importantly, social isolation and stress, two environmental factors known to exacerbate abnormal behavior induced by the NVHL, amplified these structural changes, providing further evidence that altered synaptic plasticity in the prefrontal cortex may lead to the development of the NVHL phenotype [58]. NVHL animals also exhibit reduced cortical levels of N-acetylaspartate (NAA) and BDNF, attenuated cortical expression of a membrane glutamate transporter EAAC1 and glutamate decarboxylase-67 (GAD67) (an enzyme for γ-aminobutyric acid -GABA- synthesis), altered cortical expression of activity-dependent transcription factors, such as c-fos and Δ-fosB, and altered firing pattern of prefrontal cortical pyramidal neurons in response to ventral tegmental area stimulation [42, 78–80]. Altogether, these findings indicate that prefrontal cortical malfunction may be due to aberrant cortical dopamine/glutamate/GABA interactions as a result of the NVHL (Figure 5). Interestingly, some of these alterations have been reported in stress- and psychostimulant-sensitization models [81–83], as well as in schizophrenia [1, 2, 76], suggesting that early developmental insult of the ventral hippocampus may facilitate sensitization of the mesocorticolimbic dopamine system, and thereby account for the adult onset of a variety of dopamine-related abnormalities. It should be noted again that unlike psychostimulant sensitization models, the NVHL model does not target the dopamine system directly. It seems likely that neuroadaptations postsynaptic to the mesocorticolimbic dopamine system predominantly underlie the subsequent behavioral and molecular phenomena associated with dopamine sensitization observed in the NVHL model.
Figure 5.
The development and function of multiple brain systems are affected in the NVHL model, including the frontal and medial temporal lobes, the ventral striatum, and the mesocorticolimbic dopamine system. Early developmental insult of the ventral hippocampus has the unique capacity to change function of a multitude of brain regions and may thus capture the complexity of the disease process of schizophrenia.
4b. Developmental disruption of prefrontal dopamine-glutamate interactions
It becomes clear from recent electrophysiological studies [72, 74, 84–86] that dopamine control of prefrontal mechanisms of inhibition and excitation is developmentally compromised in the NVHL model. Prefrontal cortical pyramidal neurons recorded from NVHL animals are more responsive to NMDA and D1 receptor activation. This selective postsynaptic-mediated excitability increase was observed only in the prefrontal cortex of post-pubertal (not pre-pubertal) NVHL animals, suggesting that an early disruption of the hippocampal input to the prefrontal cortex alters the normal postnatal development of prefrontal D1 and NMDA responses. Although the underlying cellular mechanisms accounting for these changes remain to be determined, it is known that D1 receptor activation increases cyclic adenosine monophosphate (cAMP) signaling and protein kinase A (PKA) activity [87, 88]. Postsynaptic elevation of cAMP-PKA-dependent signaling can potentiate NMDA-mediated excitation in the prefrontal cortex through a postsynaptic calcium-dependent mechanism [85, 86]. D1 receptor activation also induces NMDA receptor trafficking to the postsynaptic membrane [89], increasing surface expression of NMDA receptors. This interaction could selectively enhance prefrontal responses to NMDA, and not to AMPA, as observed in the NVHL rats [84]. Thus, it is tempting to speculate that an abnormal upregulation of these postsynaptic signaling cascades could be responsible for the augmented prefrontal response to D1 and NMDA in the NVHL model (Figure 5). These changes would, in turn, contribute to an altered balance of excitation and inhibition in the prefrontal cortex [90], triggering local mechanisms of neuronal adaptation that could lead to the hypofunctional prefrontal state observed in schizophrenia.
4c. Interneuronal dysfunction in the NVHL model: from genes to function
It is widely recognized that cortical inhibitory interneurons play an important role in determining the timing and spatial selectivity of pyramidal neuron firing [91, 92]. GABAergic interneurons in the prefrontal cortex also receive dopamine innervation from the ventral tegmental area [93–95], and express both D1 and D2 receptors [96–100]. Perhaps the most interesting observation is the powerful involvement of both D1 and D2 dopamine receptors in facilitating local GABAergic function in the adult prefrontal cortex [101] (Figure 5). Although we know little about how these interactions influence prefrontal function, evidence indicates that neonatal disconnection of the ventral hippocampus may alter the normal maturation of prefrontal interneuronal response to dopamine (Tseng et al., 2008 in revision). A deficit in prefrontal cortical interneuron has indeed been suggested by the selective downregulation of GAD-67 mRNA in the prefrontal cortex of NVHL animals [102, 103]. Consequently, the characteristic hyperreactive and hypofunctional prefrontal state observed in NVHL rats [72, 74] could reflect a postnatal disruption of dopamine regulation of prefrontal GABAergic function that normally matures during adolescence [101]. Although this hypothesis has yet to be examined, these changes could in part underlie abnormal cognitive performance in NVHL animals by setting inappropriate coordination between pyramidal neurons and GABAergic interneurons, which in turn, would alter the spatial selectivity of prefrontal neuronal responses to excitatory inputs (Figure 5). A similar cortical disruption, involving markers of GABAergic hypofunction, has been associated with schizophrenia [104]. Thus, acquisition of postpubertal dopamine control of inhibitory neurotransmission could be critical to fine-tuning prefrontal output activity responsible for mature cognitive processes, in particular, for engaging pyramidal neuron activity during working memory and goal-directed behaviors, two prefrontal-dependent functions that are refined during puberty and adolescence, and appear to be altered in the NVHL model as well as in schizophrenia.
4d. Dysregulation of subcortical circuits in the NVHL model
Subcortical function in the neonatally lesioned rats is altered in a fashion consistent with at least some reports on behavioral sensitization [105–108]. Striatal dopamine release is attenuated in response to stress and amphetamine, midbrain expression of dopamine transporter mRNA is reduced, and striatal expression of dynorphin (an opioid peptide co-localized with D1 receptors) and Δ-fosB (a transcription factor sensitive to persistent stimulation) are enhanced [37, 79]. It should be noted, however, that enhanced rather than attenuated striatal dopamine release has been observed under some conditions of behavioral sensitization to psychostimulants [109] as well as in a subgroup of schizophrenia patients observed in recent SPECT studies [110–112]. Similarly discrepant are the findings of synaptic morphology – increased synaptic densities and dendritic length and branching are reported in prefrontal cortex in sensitization models [113], whereas these parameters are decreased in schizophrenia [114] and in the NVHL model [77, 115]. Nevertheless, an array of behavioral and molecular changes associated with this model suggests that early developmental insult of the ventral hippocampus may facilitate some form of sensitization of the dopamine system likely related to alterations in the way postsynaptic systems respond to dopamine signaling [72, 74, 84]. Similar pathophysiological mechanisms have been hypothesized to underlie schizophrenia [116–118] in parallel to abnormal dopamine-related behaviors of the NVHL syndrome. Unlike psychostimulant sensitization models, however, the NVHL model does not target the dopamine system directly and similar sensitization-like phenomena are not seen following an analogous hippocampal lesion in adult animals. It is of considerable heuristic interest to determine how the developmental lesion initiates subsequent behavioral and molecular phenomena associated with dopamine sensitization.
As in the prefrontal cortex, the nucleus accumbens output neurons in NVHL rats respond to activation of dopamine projections with increased rather than decreased spike firing [73, 75]. Although the mechanisms underlying this increase remain to be determined, the effect appears after puberty. It seems plausible that enhanced mesocortical-evoked prefrontal firing in NVHL rats triggers abnormal spike firing in the nucleus accumbens as prefrontal cortical lesions in adult NVHL rats normalize the response [75] and improve abnormal behaviors in these animals [37]. Abnormalities of dopamine receptor expression resulting from the NVHL [16] could also mediate the postpubertal emergence of enhanced mesocorticolimbic response as the mesolimbic dopamine system matures after puberty [119, 120]. Given the role of the prefrontal cortex in the regulation of mesocorticolimbic dopamine activity, it is possible that increased prefrontal excitability resulting from an early hippocampal disconnection [72, 74, 84] initiates a vicious cycle of activation between the ventral tegmental area and the prefrontal cortex that, in turn, sustains activity in the nucleus accumbens and other targeted structures (Figure 5). Thus, cortical and subcortical alterations associated with the NVHL are likely linked [55], and normal mechanisms of filtering irrelevant information that mesocorticolimbic dopamine activation exerts [5] are impaired. In this regard, a prefrontal cortical lesion would block this altered recurrent corticostriatal activation, thereby restoring some functionality to the system as prefrontal lobotomy was used to alleviate positive symptoms in schizophrenia before the advent of antipsychotic drugs.
4e. Abnormal prefrontal cortical state in the NVHL model
Functional impairment of the prefrontal cortex, termed hypofrontality, is a landmark of schizophrenia and is characterized by a failure of the prefrontal cortex to support behavior [121, 122]. Although the neural substrates underlying this distinctive deficit are still under debate, hypofrontality commonly refers to a lack of prefrontal activation during working memory tasks [122]. However, recent studies also show absence of changes or even increased activation of the prefrontal cortex in schizophrenia [121, 123–126], suggesting that hypofrontality could be associated with different degrees of cortical hyperactivity as well as hypoactivity due to the non-linear relationship between prefrontal activation and task demand [122, 127–129]. Using a blood perfusion magnetic resonance imaging approach, Risterucci and co-workers [130] found that NVHL rats also exhibit an abnormal pattern of cortico-subcortical circuit activation reminiscent of classical neuroimaging findings described in schizophrenia. These findings may correlate with post-pubertal emergence of prefrontal cortical glutamatergic hyperactivity observed in NVHL rats (see sections 4b, 4c & 4d) [72, 74, 75, 84].
Depending on the strength of the stimulus, activation of the mesocortical dopamine system can elicit different forms of metabolic responses in the prefrontal cortex [74]. In the NVHL model, mesocortical activation elicits a hyper-metabolic response in the prefrontal cortex. However, increasing burst stimulation, which leads to increased prefrontal metabolic response in sham operated rats, results in a hypo-prefrontal metabolic state in the NVHL rats [74]. This inability to further increase the response may be a reflection of reduced PFC functional capacity in the lesioned animals. NVHL-induced neural abnormalities underlying such hyperexcitable prefrontal cortical state (see sections 4b & 4c) and reduced prefrontal functional capacity, could be a pathophysiological scenario useful in elucidating mechanisms of cognitive dysfunction in schizophrenia. In light of this conceptual framework (Figure 5), cortical hypofunctionality in schizophrenia could be compounded by a hyperglutamatergic and hyper-reactive prefrontal cortical state [72, 74, 75, 84], as two non-mutually exclusive pathophysiological conditions [122].
A functional attenuation of cortical GABAergic transmission could contribute to the hyperexcitable prefrontal cortex and higher metabolic activity observed in the NVHL [72, 74, 75, 84]. It is possible that an early inactivation of the hippocampal formation alters the postnatal maturation of inhibitory interneurons in the prefrontal cortex. Consequently, the exaggerated mesocortical-evoked prefrontal response [72, 74] observed in neonatally lesioned animals could reflect a developmental disruption of prefrontal GABAergic modulation by dopamine. These changes may result in reduction of a local inhibitory tone (i.e., D2-GABA interaction) and an abnormal potentiation of D1 and NMDA responses that normally mature during adolescence [84, 86, 101, 131]. Therefore, the underlying mechanisms mediating hypofrontality in schizophrenia may be associated with a reduction of cortical GABAergic function concurrent with a disruption of mesocortical dopamine modulation of prefrontal glutamatergic transmission (Figure 5).
5. Using NVHL model for understanding the neurobiology of schizophrenia
Research on the NVHL model has provided a number of new insights and avenues into understanding mechanisms of schizophrenia and related illnesses in ways that were not necessarily intended at the model’s conception. In this section, we will review the data on how the NVHL model influenced research on schizophrenia, and how it contributed to novel hypotheses about the pathophysiology of the disorder.
5a. Reinterpreting the dopamine hypothesis through the lens of the NVHL model
The dopamine hypothesis of schizophrenia is arguably the most influential and historically well-weathered neurochemical hypothesis of schizophrenia. Its foundation, based on conclusive evidence that antipsychotic efficacy depends on dopamine receptor blockade, and that simulation of dopamine transmission (e.g. with psychostimulants) can generate psychotic symptoms, remains firm. However, a literal interpretation of this hypothesis, suggesting that schizophrenic brains actually release more dopamine into mesolimbic circuits, has been elusive to define and/or largely unconfirmed over the last decades, even though pursuit of this evidence has been a golden grail in schizophrenia research. Quite possibly this research has been hampered by the frequency with which study subjects have current or past exposure to neuroleptics, which are themselves known to change parameters of dopamine system function [132]. Also, as suggested by several recent neuroimaging studies [110–112], the phenomena may require advanced approaches to detect what amounts to quite subtle changes in dopamine efflux, still untangled from the effects of acute administration of dopamine-active drugs.
Against this backdrop, investigations on the neurobiological mechanisms underlying the core positive-like symptom components of the model (e.g. behavioral hyper-responsiveness to stimuli that evoke dopamine release) are providing a compelling new interpretation of the dopamine hypothesis. As described in Section 4, the weight of the evidence indicates that neurobiological changes present in the model corresponding to the positive syndrome, are a manifestation of developmentally perturbed architecture and functionality of frontal cortical/ventral striatal neurons and networks that are postsynaptic to, and interpret dopamine signaling. Moreover, in all four studies published to date that have directly measured dopamine efflux into the nucleus accumbens of NVHL rats (using microdialysis or voltammetry), net dopamine efflux into the nucleus accumbens as a whole is either normal or actually decreased at baseline or in response to stimuli that produce the behavioral hyper-responsivity [15, 61, 133, 134]. These findings have major implications for where we should look for, and how we should understand the role of dopamine functionality in schizophrenia. Further, the notion that the dopamine hypothesis may be mechanistically manifest by abnormal responsivity of brain systems to dopamine efflux, rather than literally representing changes in dopamine efflux per se, connects well with emerging findings in research on both addictions and adolescent neurodevelopment, as described below.
5b. Dual diagnosis
Substance use disorder co-morbidity (i.e. dual diagnosis) in schizophrenia and other mental disorders is a public health problem of tremendous depth and scope. Schizophrenia patients suffer from addictions to nicotine, cocaine, alcohol, and cannabis at 2 to 4 fold rates greater than the general population [135]. This comorbidity is associated with poor outcomes across virtually all measures that have been examined, including greater rates or severity of treatment refractoriness and non-compliance, hospitalizations, psychiatric and medical illness and death, financial destitution, homelessness, and criminal incarceration [136, 137].
Application of the NVHL model in paradigms that examine motivational processes and addiction-related phenotypes has provided new insights into our understanding of mechanisms underlying dual diagnosis and addiction vulnerability. At least three key factors have contributed to the NVHL model serving as a fruitful investigative platform in this vein. First, it is a comprehensive and multi-faceted neurobiological and behavioral model of a mental illness. Similarly, vulnerability to addictions and the addiction process itself, involves events or traits involving many neural subsystems and behavioral components [138]. Second, and more specifically as reviewed above, key neurocircuits (e.g. frontal cortical-striatal) and transmitter systems (e.g. dopamine, glutamate, GABA) involved in motivational control and adaptation are altered in NVHL model, human schizophrenia, and the in the course of addictive disease [2]. Third, just as schizophrenia is a disorder of periadolescent vulnerability or onset, so are addictions [139]. Thus the periadolescent-developmental onset features of the NVHL model provide a potentially powerful platform for understanding the developmental basis of addictions and dual diagnosis.
Consistent with these considerations, NVHL rats tested in cocaine self-administration show signs of enhanced addiction vulnerability through several stages of the addiction process [67]. They acquire cocaine self-administration more avidly, respond in more impulsive/binging like patterns during maintenance of cocaine taking, and show enhanced or prolonged drug-seeking behavior after forced abstinence, or with cocaine-induced reinstatement of drug seeking. Enhanced installment of the addicted phenotype has also been demonstrated in self-administration of methamphetamine, a drug that is known to be particularly potent in generating psychosis, impulsive behavior and addictions in humans [140]. While trait-markers of behavioral impulsivity are of general interest as pre-morbid endophenotypes or vulnerability markers for addiction [141], NVHL rats show increased impulsive approach to natural (water) re-inforcers compared to controls prior to drug exposure that is exclusively exacerbated after experiencing a cocaine history [142]. Consistent with various descriptions of non-drug related, (e.g. natural) motivational dysregulation in schizophrenia [143], NVHL rats show various abnormalities in instrumental responding for food including increased acquisition, particularly when instrumental contingencies are relatively simple [41, 67, 144]. Consistent with enhancement of addiction comorbidity transcending addictive drug classes in schizophrenia, NVHL rats show increased behavioral activation to early doses of drugs of diverse psychoactive profiles including cocaine, nicotine, and alcohol [145–147]. Furthermore, a chronic repeating dosing regimen of all three of these drugs produces a similar, long-lasting behavioral sensitization profile in NVHL rats that is greater than in controls [145–147]. Given that behavioral sensitization to addictive drugs involves the same neuro-adaptive processes and neural substrates as those involved in addiction [148, 149], these findings suggest that NVHL rats, and by extension schizophrenia patients, are endowed with an involuntary, neurocircuit-based vulnerability to the behavior-sculpting actions of addictive drugs both prior to, and in the course of drug exposure. By establishing the unitary nature of a developmental neurocircuit context underlying schizophrenia and addiction vulnerability, this work renders past and future neurobiological findings in the NVHL model relevant to both schizophrenia and addictions research.
5c. Adolescent neurodevelopmental genesis of mental disorders
It is well known that synapses in the brain become stronger or weaker depending on how experience-driven changes impact synaptic connectivity in neurocircuits during postnatal development [150, 151]. Among different postnatal developmental stages, adolescent neurodevelopment is thought to be particularly sensitive to complex genetic-environmental-epigenetic phenomena, potentially leading to adult mental illness. While schizophrenia has long been recognized as a disorder of periadolescent exacerbation or onset, increasing attention is being paid to the realization that adolescent neurodevelopmental themes apply to a much larger array of mental disorders including addictions, affective disorders, impulse control disorders, anxiety disorders and personality disorders (Hulvershorn 2008 in press). Spanning these diagnostic contexts, a common theme emerges wherein, childhood psychiatric syndromes, or even healthy childhood presentations undergo a syndromal transformation through adolescence in which symptoms components are dropped, augmented, or qualitatively converted to produce new illness trajectories in adult life. These developmental transformations are collectively thought to involve an interaction between abnormal neural substrates underlying the genesis of mental illness and normative events of adolescent brain revision [152]. Studying both of these processes independently and in combination in animal models is expected to be a key avenue toward defining new treatment strategies for adult mental disorders that attempt to prevent or abort detrimental illness trajectories rather than offer chronic palliative treatment.
How specific genes-environment interactions impact and change the normal neurodevelopmental trajectory of a given neurocircuit remains elusive. One approach to make this problem more tractable is to identify and compare specific structural-functional trajectories of brain development over time as a phenotypic association between genes and behavior. Research on the NVHL model has been, and likely will continue to be both pioneering and exemplary in this vein. Initial publications of the NVHL model were among the first to describe delayed developmental effects of a brain lesion of any kind, as opposed to the slow, stable resolution of effects typical of adult lesions. The ability of the NVHL model to be examined and modified under a great diversity of tightly-controlled environmental conditions or pharmacological exposures, occurring throughout adolescent development offers a powerful approach to understanding neurocircuit-based interactions with the environment in the developmental genesis of mental disorders. The wealth of findings indicating the presence of neural alterations in the prefrontal cortex of NVHL rats, together with the predominance of the prefrontal cortex as a key region of interest in understanding normative neurodevelopmental revision, suggests that the NVHL model may have broad applicability to understanding the developmental transformational themes that transcend many psychiatric disorders beyond schizophrenia.
6. Summary and Conclusions
NVHL-based research has firmly established that early disruption of limbic inputs to frontal cortical-striatal circuits alters the normal development of mesocortical dopamine control of excitatory and inhibitory neurotransmission, which is critical for the acquisition of mature brain function emerging during late adolescence. In this respect, the clear capacity of early developmental insult of the ventral hippocampus to change gene expression, neural function and morphology in multiple brain regions (even though the genome itself is not changed) must be interpreted as a cautionary tale that cross-sectional genetic manipulations alone may have difficulty capturing the full picture of disease process and mechanism in mental disorders. Neuronal maturation in the prefrontal cortex occurs at late stages of postnatal development, typically during late adolescence, corresponding to a host of structural and functional refinements [153, 154] including the final maturation of dopamine innervation [85, 86, 101, 131]. Studies conducted in the NVHL model show that early developmental disruption of brain areas extrinsic to the prefrontal cortex (e.g. ventral hippocampus), however non-specific they may be, can produce what might be a quite general pathway of improper peri-adolescent assembly of prefrontal circuits. Because prefrontal networks and their responsiveness to dopamine are not normally fully developed until young adulthood, these early disruptions produce a delayed onset of impaired prefrontal functioning, most robustly emergent as a developmental derailment in peri-adolescence [74, 84, 86, 101, 131]. Together with the primary hippocampal failure in NVHL rats, this secondary, developmentally emergent form of frontal cortical-striatal dysfunction, is expressed as a complex syndrome encompassing positive, negative, and cognitive-like symptom components, and addiction vulnerability, resembling that observed in schizophrenia. By establishing a developmental and neurocircuit-based pathway of disease process, the NVHL model provides an important platform for continued investigations into the complex genetic, environmental, developmental, and neuronal based underpinnings of adult mental disorders as multilevel phenomena. Further investigations defining the details of this pathway in NVHL rats and conducted in parallel with research on schizophrenia patients, may lead to more effective and definitively preventative treatments for this devastating disorder.
Acknowledgments
We thank Dr. Daniel R. Weinberger for his creativity and vision, and many other contributions that led to generating the NVHL model. The authors are supported by Rosalind Franklin University/Chicago Medical School Start-up funds (K.Y.T), NIDA K08-DA019850 (R.A.C.) and the Intramural Program of the National Institutes of Health, NIMH (B.K.L.). We thank Dr. James McCutcheon and Dr. Michela Marinelli for helpful comments.
Footnotes
Hulvershorn, LA, Erickson, CA, Chambers, RA “Impact of Childhood Mental Health Problems” (in press) Young Adult Mental Health: Adolescence Transitions to Young Adulthood, Grant, J, Potenza, MN editors, Oxford University Press, London, UK.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 5. [DOI] [PubMed] [Google Scholar]
- 2.Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15:587–94. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- 4.Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–39. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 2003;17:429–35. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- 6.Cools AR, Brachten R, Heeren D, Willemen A, Ellenbroek B. Search after neurobiological profile of individual-specific features of Wistar rats. Brain Res Bull. 1990;24:49–69. doi: 10.1016/0361-9230(90)90288-b. [DOI] [PubMed] [Google Scholar]
- 7.Costall B, Naylor RJ. Behavioural interactions between 5-hydroxytryptophan, neuroleptic agents and 5-HT receptor antagonists in modifying rodent responding to aversive situations. Br J Pharmacol. 1995;116:2989–99. doi: 10.1111/j.1476-5381.1995.tb15954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markowitz R, Jacobson J, Bain G, Kornetsky C. Naloxone blockade of morphine analgesia: a dose-effect study of duration and magnitude. J Pharmacol Exp Ther. 1976;199:385–8. [PubMed] [Google Scholar]
- 9.McKinney WT, Moran EC. Animal models of schizophrenia. Am J Psychiatry. 1981;138:478–83. doi: 10.1176/ajp.138.4.478. [DOI] [PubMed] [Google Scholar]
- 10.Lipska BK, Luu S, Halim ND, Weinberger DR. Behavioral effects of neonatal and adult excitotoxic lesions of the mediodorsal thalamus in the adult rat. Behav Brain Res. 2003;141:105–11. doi: 10.1016/s0166-4328(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 11.Scheibel AB, Conrad AS. Hippocampal dysgenesis in mutant mouse and schizophrenic man: is there a relationship? Schizophr Bull. 1993;19:21–33. doi: 10.1093/schbul/19.1.21. [DOI] [PubMed] [Google Scholar]
- 12.Lipska BK, Weinberger DR. Genetic variation in vulnerability to the behavioral effects of neonatal hippocampal damage in rats. Proc Natl Acad Sci U S A. 1995;92:8906–10. doi: 10.1073/pnas.92.19.8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker A, Grecksch G, Bernstein HG, Hollt V, Bogerts B. Social behaviour in rats lesioned with ibotenic acid in the hippocampus: quantitative and qualitative analysis. Psychopharmacology (Berl) 1999;144:333–8. doi: 10.1007/s002130051015. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein HG, Grecksch G, Becker A, Hollt V, Bogerts B. Cellular changes in rat brain areas associated with neonatal hippocampal damage. Neuroreport. 1999;10:2307–11. doi: 10.1097/00001756-199908020-00016. [DOI] [PubMed] [Google Scholar]
- 15.Brake WG, Sullivan RM, Flores G, Srivastava LK, Gratton A. Neonatal ventral hippocampal lesions attenuate the nucleus accumbens dopamine response to stress: an electrochemical study in the adult rat. Brain Res. 1999;831:25–32. doi: 10.1016/s0006-8993(99)01477-8. [DOI] [PubMed] [Google Scholar]
- 16.Flores G, Wood GK, Liang JJ, Quirion R, Srivastava LK. Enhanced amphetamine sensitivity and increased expression of dopamine D2 receptors in postpubertal rats after neonatal excitotoxic lesions of the medial prefrontal cortex. J Neurosci. 1996;16:7366–75. doi: 10.1523/JNEUROSCI.16-22-07366.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grecksch G, Bernstein HG, Becker A, Hollt V, Bogerts B. Disruption of latent inhibition in rats with postnatal hippocampal lesions. Neuropsychopharmacology. 1999;20:525–32. doi: 10.1016/S0893-133X(98)00081-5. [DOI] [PubMed] [Google Scholar]
- 18.Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder H, Grecksch G, Becker A, Bogerts B, Hoellt V. Alterations of the dopaminergic and glutamatergic neurotransmission in adult rats with postnatal ibotenic acid hippocampal lesion. Psychopharmacology (Berl) 1999;145:61–6. doi: 10.1007/s002130051032. [DOI] [PubMed] [Google Scholar]
- 20.Wan FJ, Caine SB, Swerdlow NR. The ventral subiculum modulation of prepulse inhibition is not mediated via dopamine D2 or nucleus accumbens non-NMDA glutamate receptor activity. Eur J Pharmacol. 1996;314:9–18. doi: 10.1016/s0014-2999(96)00535-3. [DOI] [PubMed] [Google Scholar]
- 21.Wan RQ, Corbett R. Enhancement of postsynaptic sensitivity to dopaminergic agonists induced by neonatal hippocampal lesions. Neuropsychopharmacology. 1997;16:259–68. doi: 10.1016/S0893-133X(96)00217-5. [DOI] [PubMed] [Google Scholar]
- 22.Bachevalier J, Beauregard M, Alvarado MC. Long-term effects of neonatal damage to the hippocampal formation and amygdaloid complex on object discrimination and object recognition in rhesus monkeys (Macaca mulatta) Behav Neurosci. 1999;113:1127–51. doi: 10.1037//0735-7044.113.6.1127. [DOI] [PubMed] [Google Scholar]
- 23.Beauregard M, Bachevalier J. Neonatal insult to the hippocampal region and schizophrenia: a review and a putative animal model. Can J Psychiatry. 1996;41:446–56. doi: 10.1177/070674379604100710. [DOI] [PubMed] [Google Scholar]
- 24.Bertolino A, Saunders RC, Mattay VS, Bachevalier J, Frank JA, Weinberger DR. Altered development of prefrontal neurons in rhesus monkeys with neonatal mesial temporo-limbic lesions: a proton magnetic resonance spectroscopic imaging study. Cereb Cortex. 1997;7:740–8. doi: 10.1093/cercor/7.8.740. [DOI] [PubMed] [Google Scholar]
- 25.Saunders RC, Kolachana BS, Bachevalier J, Weinberger DR. Neonatal lesions of the medial temporal lobe disrupt prefrontal cortical regulation of striatal dopamine. Nature. 1998;393:169–71. doi: 10.1038/30245. [DOI] [PubMed] [Google Scholar]
- 26.Bogerts B. [The brains of the Vogt collection] Nervenarzt. 1990;61:315–6. [PubMed] [Google Scholar]
- 27.Bogerts B, Falkai P, Haupts M, Greve B, Ernst S, Tapernon-Franz U, et al. Post-mortem volume measurements of limbic system and basal ganglia structures in chronic schizophrenics. Initial results from a new brain collection. Schizophr Res. 1990;3:295–301. doi: 10.1016/0920-9964(90)90013-w. [DOI] [PubMed] [Google Scholar]
- 28.Eastwood SL, Burnet PW, Harrison PJ. Altered synaptophysin expression as a marker of synaptic pathology in schizophrenia. Neuroscience. 1995;66:309–19. doi: 10.1016/0306-4522(94)00586-t. [DOI] [PubMed] [Google Scholar]
- 29.Eastwood SL, Harrison PJ. Decreased synaptophysin in the medial temporal lobe in schizophrenia demonstrated using immunoautoradiography. Neuroscience. 1995;69:339–43. doi: 10.1016/0306-4522(95)00324-c. [DOI] [PubMed] [Google Scholar]
- 30.Eastwood SL, Harrison PJ. Hippocampal and cortical growth-associated protein-43 messenger RNA in schizophrenia. Neuroscience. 1998;86:437–48. doi: 10.1016/s0306-4522(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 31.Eastwood SL, Kerwin RW, Harrison PJ. Immunoautoradiographic evidence for a loss of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate-preferring non-N-methyl-D-aspartate glutamate receptors within the medial temporal lobe in schizophrenia. Biol Psychiatry. 1997;41:636–43. doi: 10.1016/S0006-3223(96)00220-X. [DOI] [PubMed] [Google Scholar]
- 32.Falkai P, Bogerts B. Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci. 1986;236:154–61. doi: 10.1007/BF00380943. [DOI] [PubMed] [Google Scholar]
- 33.Jeste DV, Lohr JB. Hippocampal pathologic findings in schizophrenia. A morphometric study. Arch Gen Psychiatry. 1989;46:1019–24. doi: 10.1001/archpsyc.1989.01810110061009. [DOI] [PubMed] [Google Scholar]
- 34.Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed) 1987;295:681–2. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med. 1990;322:789–94. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- 36.Weinberger DR. Cell biology of the hippocampal formation in schizophrenia. Biol Psychiatry. 1999;45:395–402. doi: 10.1016/s0006-3223(98)00331-x. [DOI] [PubMed] [Google Scholar]
- 37.Lipska BK, Weinberger DR. Prefrontal cortical and hippocampal modulation of dopamine-mediated effects. Adv Pharmacol. 1998;42:806–9. doi: 10.1016/s1054-3589(08)60869-8. [DOI] [PubMed] [Google Scholar]
- 38.Al-Amin HA, Weinberger DR, Lipska BK. Exaggerated MK-801-induced motor hyperactivity in rats with the neonatal lesion of the ventral hippocampus. Behav Pharmacol. 2000;11:269–78. doi: 10.1097/00008877-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Le Pen G, Gaudet L, Mortas P, Mary R, Moreau J. Deficits in reward sensitivity in a neurodevelopmental rat model of schizophrenia. Psychopharmacology. 2002;161:434–41. doi: 10.1007/s00213-002-1092-4. [DOI] [PubMed] [Google Scholar]
- 40.Le Pen G, Grottick AJ, Higgins GA, Martin JR, Jenck F, Moreau JL. Spatial and associative learning deficits induced by neonatal excitotoxic hippocampal damage in rats: further evaluation of an animal model of schizophrenia. Behav Pharmacol. 2000;11:257–68. doi: 10.1097/00008877-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Le Pen G, Moreau JL. Disruption of prepulse inhibition of startle reflex in a neurodevelopmental model of schizophrenia: reversal by clozapine, olanzapine and risperidone but not by haloperidol. Neuropsychopharmacology. 2002;27:1–11. doi: 10.1016/S0893-133X(01)00383-9. [DOI] [PubMed] [Google Scholar]
- 42.Lipska BK, Chrapusta SJ, Egan MF, Weinberger DR. Neonatal excitotoxic ventral hippocampal damage alters dopamine response to mild repeated stress and to chronic haloperidol. Synapse. 1995;20:125–30. doi: 10.1002/syn.890200205. [DOI] [PubMed] [Google Scholar]
- 43.Lipska BK, Weinberger DR. Delayed effects of neonatal hippocampal damage on haloperidol-induced catalepsy and apomorphine-induced stereotypic behaviors in the rat. Brain Res Dev Brain Res. 1993;75:213–22. doi: 10.1016/0165-3806(93)90026-7. [DOI] [PubMed] [Google Scholar]
- 44.Lipska BK, Weinberger DR. Subchronic treatment with haloperidol and clozapine in rats with neonatal excitotoxic hippocampal damage. Neuropsychopharmacology. 1994;10:199–205. doi: 10.1038/npp.1994.22. [DOI] [PubMed] [Google Scholar]
- 45.Richtand NM, Taylor B, Welge JA, Ahlbrand R, Ostrander MM, Burr J, et al. Risperidone pretreatment prevents elevated locomotor activity following neonatal hippocampal lesions. Neuropsychopharmacology. 2006;31:77–89. doi: 10.1038/sj.npp.1300791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Amin HA, Shannon Weickert C, Weinberger DR, Lipska BK. Delayed onset of enhanced MK-801-induced motor hyperactivity after neonatal lesions of the rat ventral hippocampus. Biol Psychiatry. 2001;49:528–39. doi: 10.1016/s0006-3223(00)00968-9. [DOI] [PubMed] [Google Scholar]
- 47.Becker A, Grecksch G. Social memory is impaired in neonatally ibotenic acid lesioned rats. Behav Brain Res. 2000;109:137–40. doi: 10.1016/s0166-4328(99)00163-1. [DOI] [PubMed] [Google Scholar]
- 48.Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology (Berl) 1997;132:303–10. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- 49.Flores G, Silva-Gomez AB, Ibanez O, Quirion R, Srivastava LK. Comparative behavioral changes in postpubertal rats after neonatal excitotoxic lesions of the ventral hippocampus and the prefrontal cortex. Synapse. 2005;56:147–53. doi: 10.1002/syn.20140. [DOI] [PubMed] [Google Scholar]
- 50.Lipska BK, Aultman JM, Verma A, Weinberger DR, Moghaddam B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology. 2002;27:47–54. doi: 10.1016/S0893-133X(02)00282-8. [DOI] [PubMed] [Google Scholar]
- 51.Messias EL, Chen CY, Eaton WW. Epidemiology of schizophrenia: review of findings and myths. Psychiatr Clin North Am. 2007;30:323–38. doi: 10.1016/j.psc.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lipska BK, Weinberger DR. Gonadectomy does not prevent novelty or drug-induced motor hyperresponsiveness in rats with neonatal hippocampal damage. Brain Res Dev Brain Res. 1994;78:253–8. doi: 10.1016/0165-3806(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 53.Black DW. Iowa record-linkage study: death rates in psychiatric patients. J Affect Disord. 1998;50:277–82. doi: 10.1016/s0165-0327(98)00019-6. [DOI] [PubMed] [Google Scholar]
- 54.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 55.Wood GK, Quirion R, Srivastava LK. Early environment contributes to developmental disruption of the MPFC after neonatal ventral hippocampal lesions in rats. Synapse. 2003;50:223–32. doi: 10.1002/syn.10265. [DOI] [PubMed] [Google Scholar]
- 56.Wood GK, R ME, Quirion R, Srivastava LK. Strain differences in the behavioral outcome of neonatal ventral hippocampal lesions are determined by the postnatal environment, not genetic factors. Eur J Neurosci. 2001;14:1030–4. doi: 10.1046/j.0953-816x.2001.01716.x. [DOI] [PubMed] [Google Scholar]
- 57.Silva-Gomez AB, Rojas D, Juarex I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Research. 2003;983:128–36. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- 58.Alquicer G, Morales-Medina JC, Quirion R, Flores G. Postweaning social isolation enhances morphological changes in the neonatal ventral hippocampal lesion rat model of psychosis. J Chem Neuroanat. 2008;35:179–87. doi: 10.1016/j.jchemneu.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Alquicer G, Silva-Gomez AB, Peralta F, Flores G. Neonatal ventral hippocampus lesion alters the dopamine content in the limbic regions in postpubertal rats. Int J Dev Neurosci. 2004;22:103–11. doi: 10.1016/j.ijdevneu.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Laplante F, Stevenson CW, Gratton A, Srivastava LK, Quirion R. Effects of neonatal ventral hippocampal lesion in rats on stress-induced acetylcholine release in the prefrontal cortex. J Neurochem. 2004;91:1473–82. doi: 10.1111/j.1471-4159.2004.02831.x. [DOI] [PubMed] [Google Scholar]
- 61.Lillrank SM, Lipska BK, Kolachana BS, Weinberger DR. Attenuated extracellular dopamine levels after stress and amphetamine in the nucleus accumbens of rats with neonatal ventral hippocampal damage. J Neural Transm. 1999;106:183–96. doi: 10.1007/s007020050150. [DOI] [PubMed] [Google Scholar]
- 62.Molteni R, Lipska BK, Weinberger DR, Racagni G, Riva MA. Developmental and stress-related changes of neurotrophic factor gene expression in an animal model of schizophrenia. Mol Psychiatry. 2001;6:285–92. doi: 10.1038/sj.mp.4000865. [DOI] [PubMed] [Google Scholar]
- 63.Chrapusta SJ, Egan MF, Wyatt RJ, Weinberger DR, Lipska BK. Neonatal ventral hippocampal damage modifies serum corticosterone and dopamine release responses to acute footshock in adult Sprague-Dawley rats. Synapse. 2003;47:270–7. doi: 10.1002/syn.10179. [DOI] [PubMed] [Google Scholar]
- 64.Hori T, Subramaniam S, Srivastava LK, Quirion R. Behavioral and neurochemical alterations following repeated phencyclidine administration in rats with neonatal ventral hippocampal lesions. Neuropharmacology. 2000;39:2478–91. doi: 10.1016/s0028-3908(00)00059-9. [DOI] [PubMed] [Google Scholar]
- 65.Wood GK, Lipska BK, Weinberger DR. Behavioral changes in rats with early ventral hippocampal damage vary with age at damage. Brain Research Developmental Brain Research. 1997;101:17–25. doi: 10.1016/s0165-3806(97)00050-3. [DOI] [PubMed] [Google Scholar]
- 66.Daenen EWPM, Van der Heyden JA, Kruse CG, Wolterink G, Van Ree JM. Adaptation and habituation to an open field and responses to various stressful events in animals with neonatal lesions in the amygdala or ventral hippocampus. Brain Res. 2001;918:153–65. doi: 10.1016/s0006-8993(01)02987-0. [DOI] [PubMed] [Google Scholar]
- 67.Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Swerdlow NR, Halim N, Hanlon FM, Platten A, Auerbach PP. Lesion size and amphetamine hyperlocomotion after neonatal ventral hippocampal lesions: More is less. Brain Res Bul. 2001;55:71–7. doi: 10.1016/s0361-9230(01)00492-0. [DOI] [PubMed] [Google Scholar]
- 69.Gogos JA, Gerber DJ. Schizophrenia susceptibility genes: emergence of positional candidates and future directions. Trends Pharmacol Sci. 2006;27:226–33. doi: 10.1016/j.tips.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 70.Kempermann G, Krebs J, Fabel K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry. 2008;21:290–5. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- 71.Lipska BK, Mitkus SN, Mathew SV, Fatula R, Hyde TM, Weinberger DR, et al. Functional genomics in postmortem human brain: abnormalities in a DISC1 molecular pathway in schizophrenia. Dialogues Clin Neurosci. 2006;8:353–7. doi: 10.31887/DCNS.2006.8.3/blipska. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Donnell P, Lewis BL, Weinberger DR, Lipska BK. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex. 2002;12:975–82. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- 73.Goto Y, O’Donnell P. Delayed mesolimbic system alteration in a developmental animal model of schizophrenia. J Neurosci. 2002;22:9070–7. doi: 10.1523/JNEUROSCI.22-20-09070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tseng KY, Amin F, Lewis BL, O’Donnell P. Altered prefrontal cortical metabolic response to mesocortical activation in adult animals with a neonatal ventral hippocampal lesion. Biol Psychiatry. 2006;60:585–90. doi: 10.1016/j.biopsych.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goto Y, O’Donnell P. Prefrontal lesion reverses abnormal mesoaccumbens response in an animal model of schizophrenia. Biol Psychiatry. 2004;55:172–6. doi: 10.1016/s0006-3223(03)00783-2. [DOI] [PubMed] [Google Scholar]
- 76.Weinberger DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophr Res. 1995;16:87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- 77.Flores G, Alquicer G, Silva-Gomez AB, Zaldivar G, Stewart J, Quirion R, et al. Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–70. doi: 10.1016/j.neuroscience.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 78.Bertolino A, Knable MB, Saunders RC, Callicott JH, Kolachana B, Mattay VS, et al. The relationship between dorsolateral prefrontal N-acetylaspartate measures and striatal dopamine activity in schizophrenia. Biol Psychiatry. 1999;45:660–7. doi: 10.1016/s0006-3223(98)00380-1. [DOI] [PubMed] [Google Scholar]
- 79.Lee K, Kornetsky C. Acute and chronic fluoxetine treatment decreases the sensitivity of rats to rewarding brain stimulation. Pharmacol Biochem Behav. 1998;60:539–44. doi: 10.1016/s0091-3057(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 80.O’Donnell P, Greene J, Pabello N, Lewis BL, Grace AA. Modulation of cell firing in the nucleus accumbens. Ann N Y Acad Sci. 1999;877:157–75. doi: 10.1111/j.1749-6632.1999.tb09267.x. [DOI] [PubMed] [Google Scholar]
- 81.Feldpausch DL, Needham LM, Stone MP, Althaus JS, Yamamoto BK, Svensson KA, et al. The role of dopamine D4 receptor in the induction of behavioral sensitization to amphetamine and accompanying biochemical and molecular adaptations. J Pharmacol Exp Ther. 1998;286:497–508. [PubMed] [Google Scholar]
- 82.Gambarana C, Masi F, Tagliamonte A, Scheggi S, Ghiglieri O, De Montis MG. A chronic stress that impairs reactivity in rats also decreases dopaminergic transmission in the nucleus accumbens: a microdialysis study. J Neurochem. 1999;72:2039–46. doi: 10.1046/j.1471-4159.1999.0722039.x. [DOI] [PubMed] [Google Scholar]
- 83.Vanderschuren LJ, Schmidt ED, De Vries TJ, Van Moorsel CA, Tilders FJ, Schoffelmeer AN. A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. J Neurosci. 1999;19:9579–86. doi: 10.1523/JNEUROSCI.19-21-09579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tseng KY, Lewis BL, Lipska BK, O’Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biol Psychiatry. 2007;62:730–8. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–9. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- 87.Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 88.Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–97. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 89.Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21:5546–58. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tseng KY, Mallet N, Toreson KL, Le Moine C, Gonon F, O’Donnell P. Excitatory response of prefrontal cortical fast-spiking interneurons to ventral tegmental area stimulation in vivo. Synapse. 2006;59:412–7. doi: 10.1002/syn.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 92.Szabadics J, Lorincz A, Tamas G. Beta and gamma frequency synchronization by dendritic gabaergic synapses and gap junctions in a network of cortical interneurons. J Neurosci. 2001;21:5824–31. doi: 10.1523/JNEUROSCI.21-15-05824.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benes FM, Vincent SL, Molloy R. Dopamine-immunoreactive axon varicosities form nonrandom contacts with GABA-immunoreactive neurons of rat medial prefrontal cortex. Synapse. 1993;15:285–95. doi: 10.1002/syn.890150405. [DOI] [PubMed] [Google Scholar]
- 94.Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sesack SR, Hawrylak VA, Melchitzky DS, Lewis DA. Dopamine innervation of a subclass of local circuit neurons in monkey prefrontal cortex: ultrastructural analysis of tyrosine hydroxylase and parvalbumin immunoreactive structures. Cereb Cortex. 1998;8:614–22. doi: 10.1093/cercor/8.7.614. [DOI] [PubMed] [Google Scholar]
- 96.Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–8. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- 97.Muly EC, 3rd, Szigeti K, Goldman-Rakic PS. D1 receptor in interneurons of macaque prefrontal cortex: distribution and subcellular localization. J Neurosci. 1998;18:10553–65. doi: 10.1523/JNEUROSCI.18-24-10553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci U S A. 1994;91:5720–4. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vincent SL, Khan Y, Benes FM. Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J Neurosci. 1993;13:2551–64. doi: 10.1523/JNEUROSCI.13-06-02551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vincent SL, Pabreza L, Benes FM. Postnatal maturation of GABA-immunoreactive neurons of rat medial prefrontal cortex. J Comp Neurol. 1995;355:81–92. doi: 10.1002/cne.903550110. [DOI] [PubMed] [Google Scholar]
- 101.Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–40. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lipska BK, Lerman DN, Khaing ZZ, Weickert CS, Weinberger DR. Gene expression in dopamine and GABA systems in an animal model of schizophrenia: effects of antipsychotic drugs. Eur J Neurosci. 2003;18:391–402. doi: 10.1046/j.1460-9568.2003.02738.x. [DOI] [PubMed] [Google Scholar]
- 103.Lipska BK, Lerman DN, Khaing ZZ, Weinberger DR. The neonatal ventral hippocampal lesion model of schizophrenia: effects on dopamine and GABA mRNA markers in the rat midbrain. Eur J Neurosci. 2003;18:3097–104. doi: 10.1111/j.1460-9568.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- 104.Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl) 2004;174:143–50. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- 105.Castner SA, al-Tikriti MS, Baldwin RM, Seibyl JP, Innis RB, Goldman-Rakic PS. Behavioral changes and [123I]IBZM equilibrium SPECT measurement of amphetamine-induced dopamine release in rhesus monkeys exposed to subchronic amphetamine. Neuropsychopharmacology. 2000;22:4–13. doi: 10.1016/S0893-133X(99)00080-9. [DOI] [PubMed] [Google Scholar]
- 106.Imperato A, Obinu MC, Carta G, Mascia MS, Casu MA, Gessa GL. Reduction of dopamine release and synthesis by repeated amphetamine treatment: role in behavioral sensitization. Eur J Pharmacol. 1996;317:231–7. doi: 10.1016/s0014-2999(96)00742-x. [DOI] [PubMed] [Google Scholar]
- 107.Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 108.Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- 109.Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–7. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- 110.Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155:761–7. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- 111.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 1997;94:2569–74. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93:9235–40. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–7. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 115.Lipska BK, Khaing ZZ, Weickert CS, Weinberger DR. BDNF mRNA expression in rat hippocampus and prefrontal cortex: effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur J Neurosci. 2001;14:135–44. doi: 10.1046/j.1460-9568.2001.01633.x. [DOI] [PubMed] [Google Scholar]
- 116.Duncan GE, Sheitman BB, Lieberman JA. An integrated view of pathophysiological models of schizophrenia. Brain Res Brain Res Rev. 1999;29:250–64. doi: 10.1016/s0165-0173(99)00002-8. [DOI] [PubMed] [Google Scholar]
- 117.Lieberman JA, Sheitman BB, Kinon BJ. Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology. 1997;17:205–29. doi: 10.1016/S0893-133X(97)00045-6. [DOI] [PubMed] [Google Scholar]
- 118.Meng ZH, Feldpaush DL, Merchant KM. Clozapine and haloperidol block the induction of behavioral sensitization to amphetamine and associated genomic responses in rats. Brain Res Mol Brain Res. 1998;61:39–50. doi: 10.1016/s0169-328x(98)00196-x. [DOI] [PubMed] [Google Scholar]
- 119.Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- 120.Voorn P, Kalsbeek A, Jorritsma-Byham B, Groenewegen HJ. The pre- and postnatal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the striatum of the rat. Neuroscience. 1988;25:857–87. doi: 10.1016/0306-4522(88)90041-3. [DOI] [PubMed] [Google Scholar]
- 121.Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–92. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 122.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60:285–98. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 123.Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, et al. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003;160:709–19. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- 124.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–15. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 125.Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 126.Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, et al. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry. 1999;45:1128–37. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 127.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 128.Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–6. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 129.Goldberg TE, Berman KF, Fleming K, Ostrem J, Van Horn JD, Esposito G, et al. Uncoupling cognitive workload and prefrontal cortical physiology: a PET rCBF study. Neuroimage. 1998;7:296–303. doi: 10.1006/nimg.1998.0338. [DOI] [PubMed] [Google Scholar]
- 130.Risterucci C, Jeanneau K, Schoppenthau S, Bielser T, Kunnecke B, von Kienlin M, et al. Functional magnetic resonance imaging reveals similar brain activity changes in two different animal models of schizophrenia. Psychopharmacology (Berl) 2005;180:724–34. doi: 10.1007/s00213-005-2204-8. [DOI] [PubMed] [Google Scholar]
- 131.Tseng KY, O’Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007;61:843–50. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sakai K, Gao XM, Hashimoto T, Tamminga CA. Traditional and new antipsychotic drugs differentially alter neurotransmission markers in basal ganglia-thalamocortical neural pathways. Synapse. 2001;39:152–60. doi: 10.1002/1098-2396(200102)39:2<152::AID-SYN6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 133.Corda MG, Piras G, Giorgi O. Neonatal ventral hippocampal lesions potentiate amphetamine-induced increments in dopamine efflux in the core, but not the shell, of the nucleus accumbens. Biol Psychiatry. 2006;60:1188–95. doi: 10.1016/j.biopsych.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 134.Wan FJ, Swerdlow NR. Sensorimotor gating in rats is regulated by different dopamine-glutamate interactions in the nucleus accumbens core and shell subregions. Brain Res. 1996;722:168–76. doi: 10.1016/0006-8993(96)00209-0. [DOI] [PubMed] [Google Scholar]
- 135.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drugs of abuse. Journal of the American Medical Association. 1990;264:2511–8. [PubMed] [Google Scholar]
- 136.Dixon L. Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophrenia Research. 1999;35:s93–s100. doi: 10.1016/s0920-9964(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 137.Drake RE, Wallach MA. Dual Diagnosis: 15 years of Progress. Psychiatric Services. 2000;51:1126–9. doi: 10.1176/appi.ps.51.9.1126. [DOI] [PubMed] [Google Scholar]
- 138.Volkow N, Li T-K. Drug addiction: The neurobiology of behavior gone awry. Nat Rev Neurosci. 2004;5:963–70. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- 139.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brady AM, McCallum SE, Glick SD, O’Donnell P. Enhanced methamphetamine self-administration in a neurodevelopmental rat model of schizophrenia. Pschopharmacology. 2008 doi: 10.1007/s00213-008-1195-7. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Redish AD, Jensen S, Johnson A. A unified framework for addiction: vulnerabilities in the decision process. Beh Brain Sci. 2008 doi: 10.1017/S0140525X0800472X. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chambers RA, Jones RM, Brown S, Taylor JR. Natural reward related learning in rats with neonatal ventral hippocampal lesions and prior cocaine exposure. Psychopharmacology. 2005;179:470–8. doi: 10.1007/s00213-004-2042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Luchins DJ. Repetitive behaviors in chronically institutionalized schizophrenic patients. Schizophrenia Research. 1992;8:119–23. doi: 10.1016/0920-9964(92)90027-3. [DOI] [PubMed] [Google Scholar]
- 144.Macedo CE, Sandner G, Angst MJ, Guiberteau T. Rewarded associative and instrumental conditioning after neonatal ventral hippocampal lesions in rats. Brain Res. 2008;1215:190–9. doi: 10.1016/j.brainres.2008.03.069. [DOI] [PubMed] [Google Scholar]
- 145.Berg SA, Chambers RA. Accentuated behavioral sensitization to nicotine in the neonatal ventral hippocampal lesion model of schizophrenia. Neuropharmacology. 2008;54:1201–7. doi: 10.1016/j.neuropharm.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chambers RA, Taylor JR. Animal modeling dual diagnosis schizophrenia: sensitization to cocaine in rats with neonatal ventral hippocampal lesions. Biol Psychiatry. 2004;56:308–16. doi: 10.1016/j.biopsych.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 147.Conroy SK, Rodd Z, Chambers RA. Ethanol sensitization in a neurodevelopmental lesion model of schizophrenia in rats. Pharmacology Biochemistry & Behavior. 2007;86:386–94. doi: 10.1016/j.pbb.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 149.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 150.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 151.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–7. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 152.Erickson CA, Chambers RA. Male Adolescence: Neurodevelopment and Behavioral Impulsivity. In: Grant J, Potenza MN, editors. Textbook of Men’s Mental Health. American Psychiatric Publishing; Washington, DC: 2006. [Google Scholar]
- 153.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]