Abstract
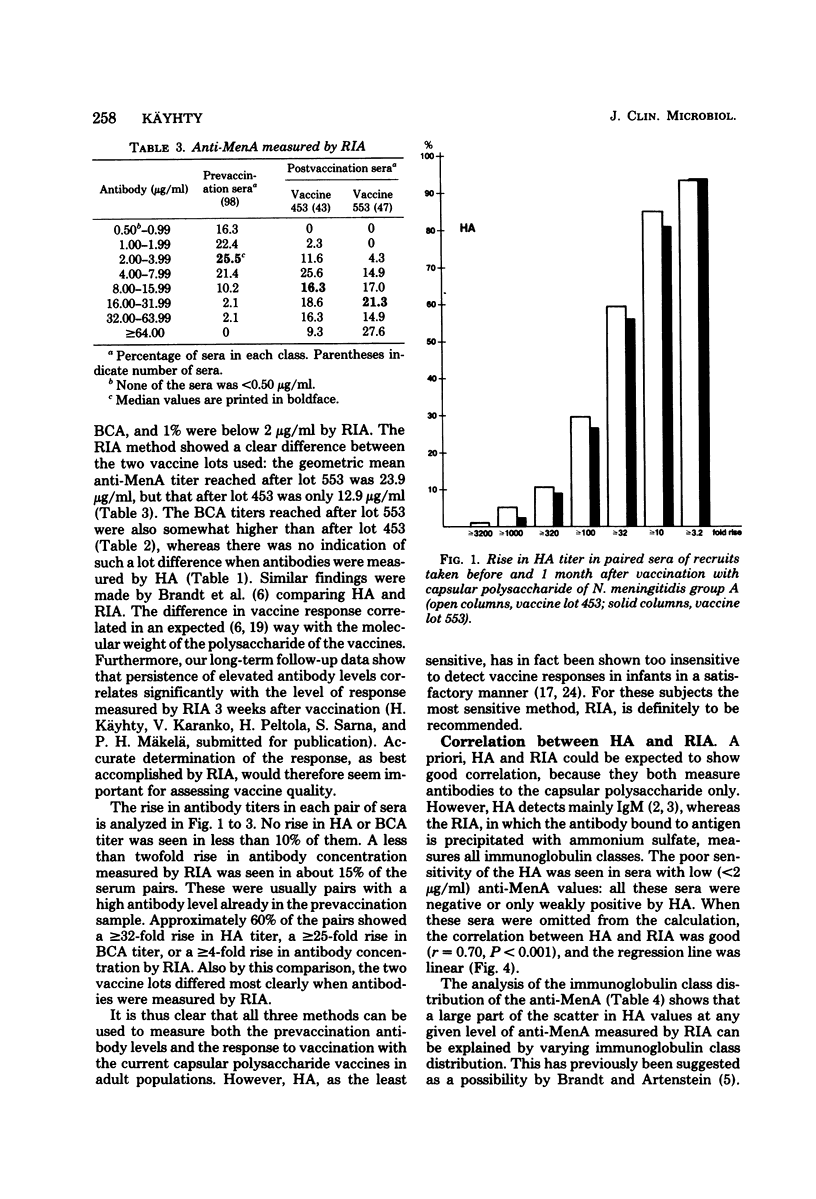
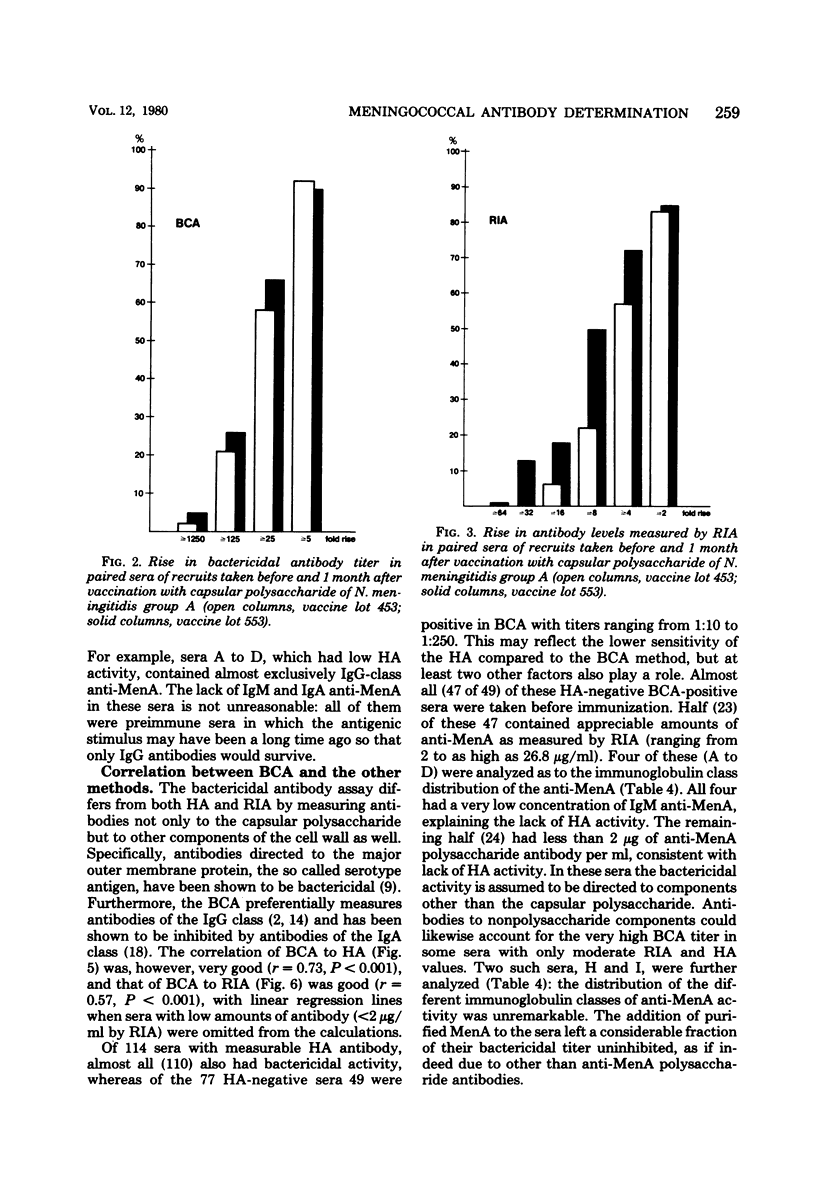
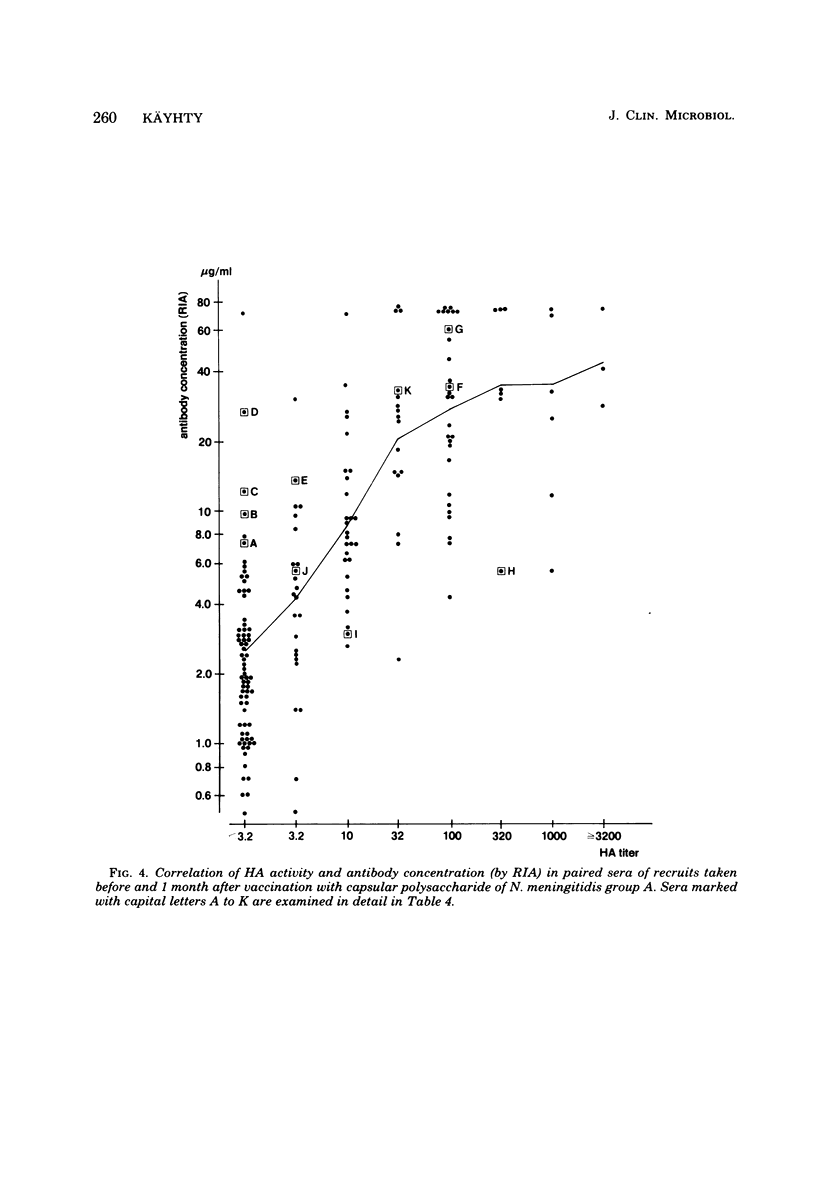
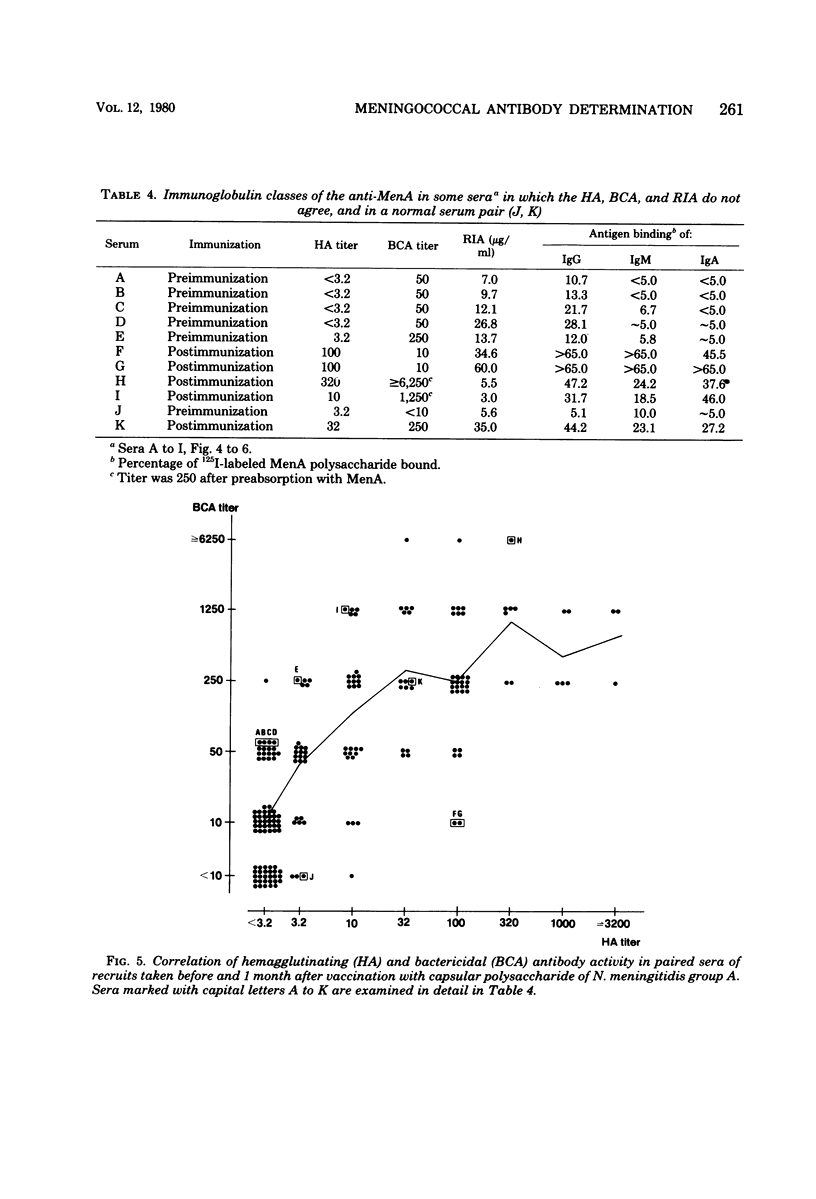
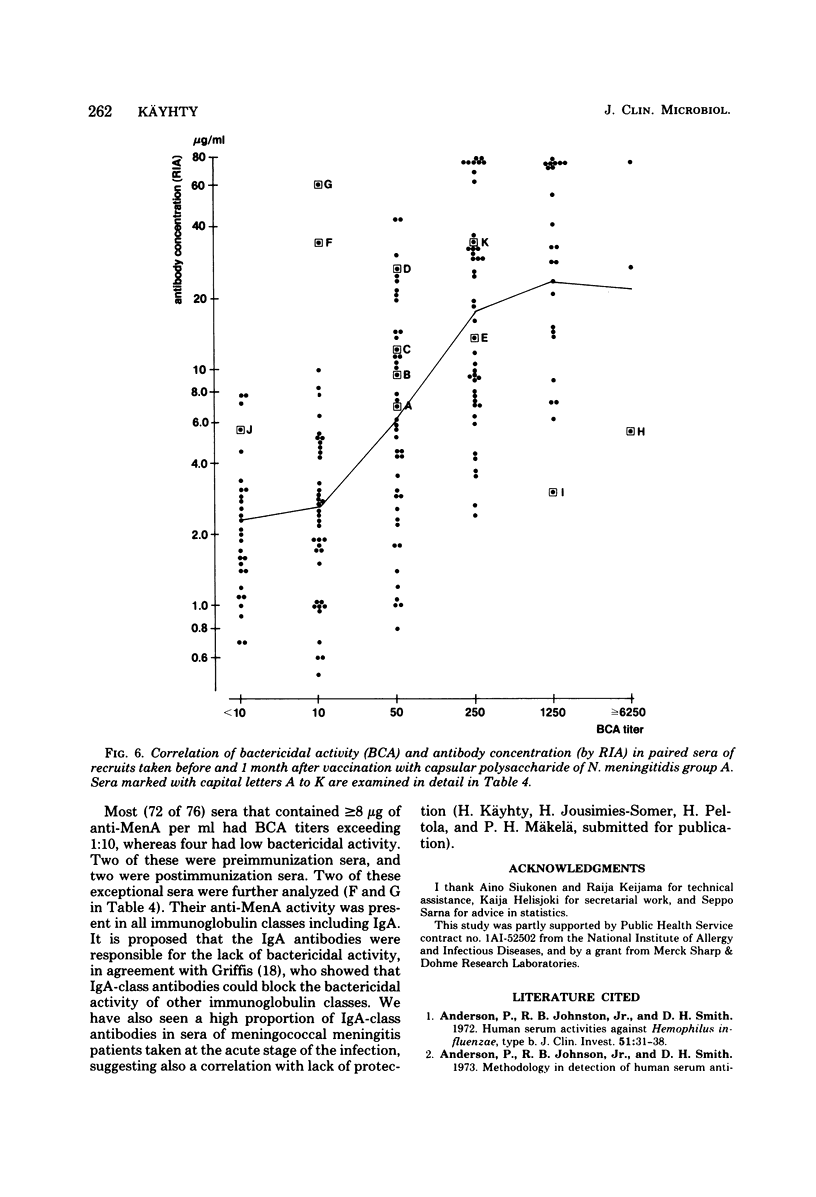
Passive hemagglutination (HA), a bactericidal activity test (BCA), and radioimmunoassay (RIA) were compared in measuring serum antibodies before and after group A meningococcal capsular polysaccharide vaccination of servicemen. The three methods were found satisfactory in demonstrating a response to vaccination in this age group. Of the postimmunization sera, 5% remained without HA and 1% remained without BCA activity; 1% of the postimmunization sera had less than 2 micrograms of antibody per ml as measured by RIA. Approximately 60% of the serum pairs showed a greater than or equal to 32-fold rise in HA titer, a greater than or equal 25-fold rise in BCA titer, or a greater than or equal to 4-fold rise in antibody concentration by RIA. A difference in response to two different vaccine lots was seen with RIA and BCA. Although the calculated correlation between the three methods was good, some individual sera gave discrepant results. These could be shown to be due mainly to one of the following factors: low HA titer was due to lack of the immunoglobulin M and A classes of antibodies, low BCA titer was due to the blocking effect of high immunoglobulin A content, and high BCA titer was due to antibodies directed to bacterial components other than the capsular polysaccharide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artenstein M. S., Brandt B. L., Tramont E. C., Branche W. C., Jr, Fleet H. D., Cohen R. L. Serologic studies of meningococcal infection and polysaccharide vaccination. J Infect Dis. 1971 Sep;124(3):277–288. doi: 10.1093/infdis/124.3.277. [DOI] [PubMed] [Google Scholar]
- Artenstein M. S., Gold R., Zimmerly J. G., Wyle F. A., Schneider H., Harkins C. Prevention of meningococcal disease by group C polysaccharide vaccine. N Engl J Med. 1970 Feb 19;282(8):417–420. doi: 10.1056/NEJM197002192820803. [DOI] [PubMed] [Google Scholar]
- Brandt B. L., Artenstein M. S. Duration of antibody responses after vaccination with group C Neisseria meningitidis polysaccharide. J Infect Dis. 1975 May;131 (Suppl):S69–S72. doi: 10.1093/infdis/131.supplement.s69. [DOI] [PubMed] [Google Scholar]
- Brandt B. L., Artenstein M. S., Smith C. D. Antibody responses to meningococcal polysaccharide vaccines. Infect Immun. 1973 Oct;8(4):590–596. doi: 10.1128/iai.8.4.590-596.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B. L., Wyle F. A., Artenstein M. S. A radioactive antigen-binding assay for Neisseria meningitidis polysaccharide antibody. J Immunol. 1972 Apr;108(4):913–920. [PubMed] [Google Scholar]
- Erwa H. H., Haseeb M. A., Idris A. A., Lapeyssonnie L., Sanborn W. R., Sippel J. E. A serogroup A meningococcal polysaccharide vaccine: studies in the Sudan to combat cerebrospinal meningitis caused by Neisseria meningitidis group A. Bull World Health Organ. 1973;49(3):301–305. [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Chapman S. S. Classification of Neisseria meningitidis group B into distinct serotypes. I. Serological typing by a microbactericidal method. Infect Immun. 1972 Jan;5(1):98–102. doi: 10.1128/iai.5.1.98-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R., Artenstein M. S. Meningococcal infections. 2. Field trial of group C meningococcal polysaccharide vaccine in 1969-70. Bull World Health Organ. 1971;45(3):279–282. [PMC free article] [PubMed] [Google Scholar]
- Gold R., Winklehake J. L., Mars R. S., Artenstein M. S. Identification of an epidemic strain of group C Neisseria meningitidis by bactericidal serotyping. J Infect Dis. 1971 Dec;124(6):593–597. doi: 10.1093/infdis/124.6.593. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969 Jun 1;129(6):1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y., Artenstein M. S. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med. 1969 Jun 1;129(6):1349–1365. doi: 10.1084/jem.129.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Etienne J., Sanborn W. R., Triau R., Cvjetanović B. The immunological responses observed in field studies in Africa with group A meningococcal vaccines. Prog Immunobiol Stand. 1971;5:485–491. [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Triau R., Sparks K. J. Quantitative determination of the human immune response to immunization with meningococcal vaccines. J Clin Invest. 1972 Jan;51(1):89–96. doi: 10.1172/JCI106801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J Immunol. 1975 Jun;114(6):1779–1784. [PubMed] [Google Scholar]
- KABAT E. A., BEZER A. E. The effect of variation in molecular weight on the antigenicity of dextran in man. Arch Biochem Biophys. 1958 Dec;78(2):306–318. doi: 10.1016/0003-9861(58)90354-0. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Wyle F. A., Formal S. B. Bactericidal antibody assay using 14 C-labeled Neisseria meningitidis. Proc Soc Exp Biol Med. 1972 Apr;139(4):1175–1180. doi: 10.3181/00379727-139-36324. [DOI] [PubMed] [Google Scholar]
- Lepow M. L., Goldschneider I., Gold R., Randolph M., Gotschlich E. C. Persistence of antibody following immunization of children with groups A and C meningococcal polysaccharide vaccines. Pediatrics. 1977 Nov;60(5):673–680. [PubMed] [Google Scholar]
- Monto A. S., Brandt B. L., Artenstein M. S. Response of children to Neisseria meningitidis polysaccharide vaccines. J Infect Dis. 1973 Apr;127(4):394–400. doi: 10.1093/infdis/127.4.394. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Käyhty H., Weckström P., Sivonen A., Renkonen O. V. Effect of group-A meningococcal vaccine in army recruits in Finland. Lancet. 1975 Nov 8;2(7941):883–886. doi: 10.1016/s0140-6736(75)92125-x. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Peltola H., Käyhty H., Jousimies H., Pettay O., Ruoslahti E., Sivonen A., Renkonen O. V. Polysaccharide vaccines of group A Neisseria meningtitidis and Haemophilus influenzae type b: a field trial in Finland. J Infect Dis. 1977 Aug;136 (Suppl):S43–S50. doi: 10.1093/infdis/136.supplement.s43. [DOI] [PubMed] [Google Scholar]
- Peltola H. Group A meningococcal polysaccharide vaccine and course of the group A meningococcal epidemic in Finland. Scand J Infect Dis. 1978;10(1):41–44. doi: 10.3109/inf.1978.10.issue-1.09. [DOI] [PubMed] [Google Scholar]
- Peltola H., Mäkelä H., Käyhty H., Jousimies H., Herva E., Hällström K., Sivonen A., Renkonen O. V., Pettay O., Karanko V. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med. 1977 Sep 29;297(13):686–691. doi: 10.1056/NEJM197709292971302. [DOI] [PubMed] [Google Scholar]
- Roberts R. B. The relationship between group A and group C meningococcal polysaccharides and serum opsonins in man. J Exp Med. 1970 Mar 1;131(3):499–513. doi: 10.1084/jem.131.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Artenstein M. S. Latex agglutination test for measurement of antibodies to meningococcal polysaccharides. Infect Immun. 1972 Mar;5(3):346–351. doi: 10.1128/iai.5.3.346-351.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahdan M. H., Rizk F., el-Akkad A. M., el-Ghoroury A. A., Hablas R., Girgis N. I., Amer A., Boctar W., Sippel J. E., Gotschlich E. C. A controlled field trial of a serogroup A meningococcal polysaccharide vaccine. Bull World Health Organ. 1973 Jun;48(6):667–673. [PMC free article] [PubMed] [Google Scholar]
- Wahdan M. H., Sallam S. A., Hassan M. N., Abdel Gawad A., Rakha A. S., Sippel J. E., Hablas R., Sanborn W. R., Kassem N. M., Riad S. M. A second controlled field trial of a serogroup A meningococcal polysaccharide vaccine in Alexandria. Bull World Health Organ. 1977;55(6):645–651. [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Barrera O., Sutton A., May J., Hochstein D. H., Robbins J. D., Robbins J. B., Parkman P. D., Seligmann E. B., Jr Standardization and control of meningococcal vaccines, group A and group C polysaccharides. J Biol Stand. 1977;5(3):197–215. doi: 10.1016/s0092-1157(77)80005-x. [DOI] [PubMed] [Google Scholar]


