Abstract
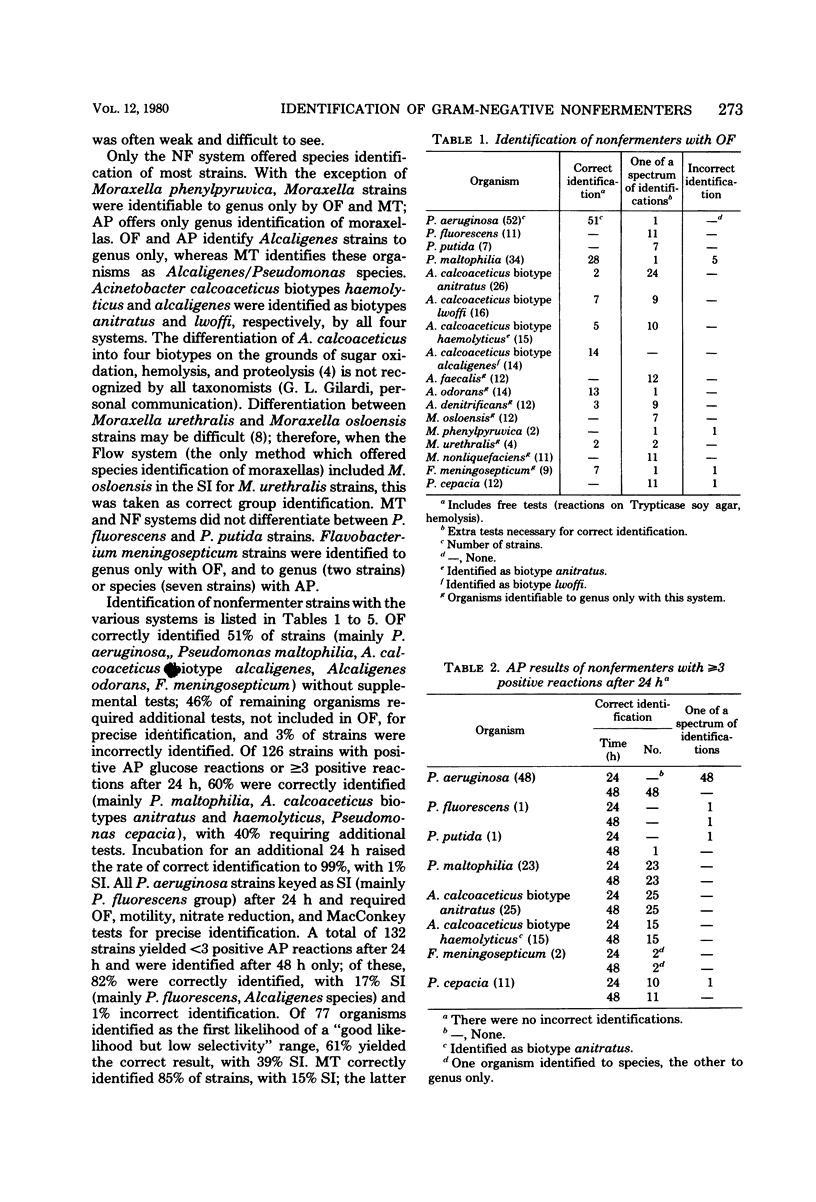
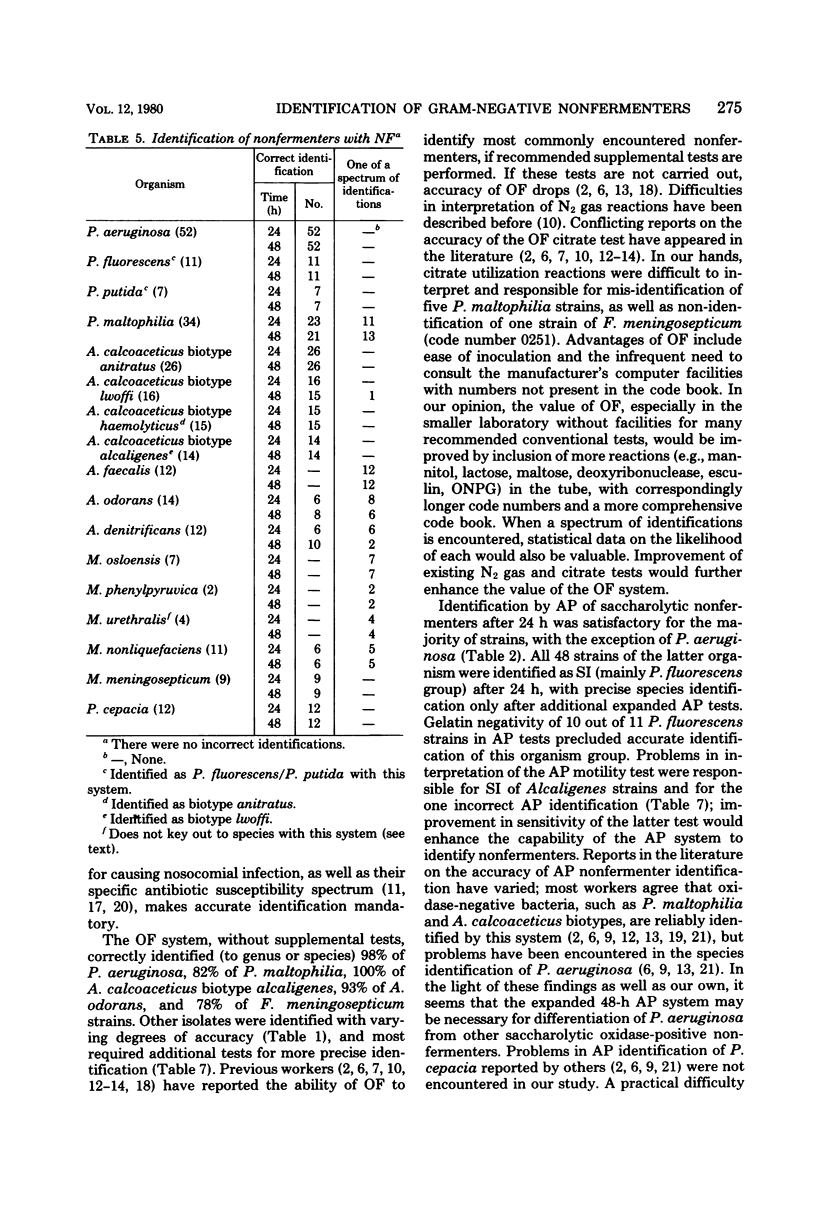
Four commercial kits, Oxi/Ferm (OF), API 20E (AP), Minitek (MT; BBL Microbiology Systems), and Flow N/F (NF), were evaluated, without additional tests, for identification of 258 gram-negative nonfermentative rods. OF and MT were read after 48 h of incubation, and AP and NF were read after both 24 and 48 h of incubation, respectively. Overall, OF correctly identified 51% of strains, with 46% as part (but not first) of a spectrum of identifications (SI), and 3% incorrect species identification. MT yielded 85% correct identification, with 15% SI. Of 126 glucose-positive strains, or those with greater than or equal to 3 positive AP reactions after 24 h, 60% were correctly identified, with 40% SI; incubation for an additional 24 h raised the rate of correct identification to 99%, with 1% SI. A total of 132 strains yield less than 3 positive AP reactions after 24 h and were identified after 48 h only; of these, 82% were correctly identified, with 17% SI and 1% incorrect species identification. NF correctly identified 79% of cultures after 24 h, with 21% SI; corresponding figures after an additional 24 h of incubation were 80% and 20%, respectively. All four commercial methods show promise; OF is easiest to inoculate, but requires extra tests for optimal identification. AP reliably identifies the majority of clinically important nonfermenters, with fairly good species identification of saccharolytic strains after 24 h. MT yields reliable identification of most nonfermenters and has the advantage of flexibility. NF is easy to inoculate, yields satisfactory identification rates, and may be read after 24 h of incubation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnishan J., Ayers L. W. Rapid identification of nonfermentative gram-negative rods by the Corning N/F system. J Clin Microbiol. 1979 Feb;9(2):239–243. doi: 10.1128/jcm.9.2.239-243.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowda H. Evaluation of two rapid methods for identification of commonly encountered nonfermenting or oxidase-positive, Gram-negative rods. J Clin Microbiol. 1977 Dec;6(6):605–609. doi: 10.1128/jcm.6.6.605-609.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Clare D., Moore M. H., Singer J. M. Rapid identification of Enterobacteriaceae from blood cultures with the Micro-ID system. J Clin Microbiol. 1979 Nov;10(5):693–697. doi: 10.1128/jcm.10.5.693-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofherr L., Votava H., Blazevic D. J. Comparison of three methods for identifying nonfermenting gram-negative rods. Can J Microbiol. 1978 Oct;24(10):1140–1144. doi: 10.1139/m78-186. [DOI] [PubMed] [Google Scholar]
- Isenberg H. D., Sampson-Scherer J. Clinical laboratory evaluation of a system approach to the recognition of nonfermentative or oxidase-producing gram-negative, rod-shaped bacteria. J Clin Microbiol. 1977 Mar;5(3):336–340. doi: 10.1128/jcm.5.3.336-340.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. J., Young V. M., Moody M. R. Evaluation of a multitest system for identification of saccharolytic pseudomonads. Am J Clin Pathol. 1978 Jan;69(1):41–47. doi: 10.1093/ajcp/69.1.41. [DOI] [PubMed] [Google Scholar]
- Nadler H., George H., Barr J. Accuracy and reproducibility of the Oxi/Ferm system in identifying a select group of unusual gram-negative bacilli. J Clin Microbiol. 1979 Feb;9(2):180–185. doi: 10.1128/jcm.9.2.180-185.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord C. E., Wretlind B., Dahlbäck A. Evaluation of two test-kits--API and Oxi Ferm tube--for identification of oxidative-fermentative Gram-negative rods. Med Microbiol Immunol. 1977 Jul 18;163(2):93–97. doi: 10.1007/BF02121824. [DOI] [PubMed] [Google Scholar]
- Oberhofer T. R. Comparison of the API 20E and Oxi/Ferm systems in identification of nonfermentative and oxidase-positive fermentative bacteria. J Clin Microbiol. 1979 Feb;9(2):220–226. doi: 10.1128/jcm.9.2.220-226.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhofer T. R., Rowen J. W., Cunningham G. F., Higbee J. W. Evaluation of the oxi/ferm tube system with selected Gram-negative bacteria. J Clin Microbiol. 1977 Dec;6(6):559–566. doi: 10.1128/jcm.6.6.559-566.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto L. A., Blachman U. Nonfermentative bacilli: evaluation of three systems for identification. J Clin Microbiol. 1979 Aug;10(2):147–154. doi: 10.1128/jcm.10.2.147-154.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto L. A., Pickett M. J. Rapid method for identification of gram-negative, nonfermentative bacilli. J Clin Microbiol. 1976 Jun;3(6):566–575. doi: 10.1128/jcm.3.6.566-575.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayegani M., Lee A. M., McLynn D. M. Evaluation of the Oxi/Ferm tube system for identification of nonfermentative Gram-negative bacilli. J Clin Microbiol. 1978 Jun;7(6):533–538. doi: 10.1128/jcm.7.6.533-538.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayegani M., Maupin P. S., McGlynn D. M. Evaluation of the API 20E system for identification of nonfermentative Gram-negative bacteria. J Clin Microbiol. 1978 Jun;7(6):539–545. doi: 10.1128/jcm.7.6.539-545.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwood N. M., Blazevic D. J., Hofherr L. Comparison of the API 20E and Corning N/F systems for identification of nonfermentative gram-negative rods. J Clin Microbiol. 1979 Aug;10(2):175–179. doi: 10.1128/jcm.10.2.175-179.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


