Abstract
This study reports on the ability of poly(ethylene glycol) diacrylate (PEGDA) hydrogel scaffolds with pendant integrin-binding GRGDSP peptides (RGD-gels) to support the re-differentiation of cultured vascular smooth muscle cells (SMCs) toward a contractile phenotype. Human coronary SMCs were seeded on RGD-gels, hydrogels with other extracellular matrix derived peptides, fibronectin (FN) and laminin (LN). Differentiation was induced on RGD-gels with low serum medium containing soluble heparin, and the differentiation status was monitored by mRNA expression, protein expression, and intracellular protein organization of the contractile smooth muscle markers, smooth muscle α-actin, calponin and SM-22α. RGD-gels supported a rapid induction (2.7- to 25-fold up-regulation) of SMC marker gene mRNA, with expression levels that were equivalent to FN and LN controls. Marker protein levels mirrored the changes in mRNA expression, with levels on RGD-gels indistinguishable from FN and LN controls. Furthermore, these markers co-localized in stress fibers within SMCs on RGD-gels suggesting the recapitulation of a contractile apparatus within the cells. These results indicate that SMCs cultured on RGD-bearing hydrogels can re-differentiate toward a contractile phenotype suggesting this material is an excellent candidate for further development as a bioactive scaffold that regulates SMC phenotype.
1. INTRODUCTION
Tissue engineering approaches have the potential to drastically improve the function of synthetic vascular grafts by employing cells and tissues to provide the complex set of responses necessary to maintain long-term patency [1]. Over the past two decades, much progress has been made generating biologically functional tissue engineered blood vessels (TEBV) [2–4]. Vascular smooth muscle cells (SMCs) and the surrounding extracellular matrix (ECM) provide mechanical support and are responsible for mediating changes in vascular tone and therefore are a critical part of TEBVs. However, the mere presence of SMCs is not sufficient to recapitulate their function. In a variety of vascular pathologies, including restenosis after angioplasty and anastomotic intimal hyperplasia, normal SMCs, which exhibit a quiescent, contractile phenotype, de-differentiate into a phenotype characterized by proliferation and excessive synthesis of ECM [5]. These “synthetic” SMCs are implicated in the stenosis of the vascular reconstruction. De-differentiation to the synthetic phenotype also occurs rapidly in the expansion culture of primary SMCs [6], such as is used for ex vivo graft preparation. Scaffold materials designed to stimulate vascular regeneration must be able to regulate SMC phenotype to prevent these failure mechanisms.
Regulation of SMC phenotype is complex and related to soluble signaling factors, mechanical stimuli, and cell substrate [7]. A wide variety of signaling factors have been implicated that affect the balance between contractile and synthetic phenotype including platelet derived growth factor, basic fibroblast growth factor, insulin-like growth factors (IGFs), heparin, and transforming growth factor β1 among many others [8–14]. It also is well known that mechanical stress can affect the phenotype of SMCs [7, 15, 16]. Furthermore, primary SMCs seeded on fibronectin (FN) de-differentiate more quickly in culture than cells seeded on laminin (LN) [13, 17–20] and the RGD peptide alone is sufficient to mimic the effects of FN [21]. In general, these studies have focused on factors that mediate de-differentiation of SMCs in culture. Strategies to re-differentiate cultured SMCs toward a contractile phenotype have been investigated less extensively.
The use of synthetic polymeric scaffolds with peptide cell-adhesive ligands allows quantitative control of cell-scaffold interactions and minimizes the possibility of confounding results from ECM bound growth factors. In particular, photopolymerized poly(ethylene glycol) diacrylate (PEGDA) based hydrogels have been employed widely as tissue engineering scaffolds for a variety of tissues including bone, cartilage, and blood vessels [22–26]. PEGDA hydrogels resist the adsorption of exogenous proteins and non-specific cell attachment. However, the inclusion of synthetic peptides that are copolymerized with the network can provide sites for specific cell adhesion [23]. In particular, RGD-based peptides, derived from integrin-binding proteins such as fibronectin, have been used widely to mediate adhesion [22–24, 26].
PEGDA and other PEG-based hydrogels systems have been used as scaffolds to study SMC biology by several groups [24, 27–29]. This work has established the ability of these scaffolds to support basic cell functions, such as attachment, migration, and growth [28, 29]. However, prior work has not examined the effect these systems have on the phenotype of vascular SMCs in detail, focusing on indirect markers of phenotypic state, such as collagen production [24, 29] or by examining phenotypic markers using qualitative immunostaining techniques [27, 30]. In this report, our goal was to assess the ability of RGD-bearing PEGDA hydrogels (RGD-gels) to support an in vitro transition of cultured human vascular SMCs toward a contractile phenotype.
2. MATERIALS AND METHODS
2.1. Materials
All reagents were obtained from Sigma-Aldrich (St. Louis, MO) and used as received unless otherwise stated.
2.2. Preparation of PEGDA
PEG (MW 6000, dried in vacuo at 45 °C) was dissolved in anhydrous dichloromethane (0.2 g/ml), purged with argon, and placed on ice. Triethylamine (dried under molecular sieves, 1.1 moles per OH), then acryloyl chloride (1.1 moles per OH) were added drop-wise. The reaction was stirred overnight at 4 °C. The acrylated PEG product was filtered to remove triethylamine hydrochloride salt, precipitated in diethyl ether, collected by filtration, and dried in vacuo. Substitution was approximately 72%, as determined by 1H NMR (in CDCl3) using the magnitude of peaks at 5.0–6.5 ppm (vinyl protons) and 3.7 ppm (PEG backbone).
2.3. Preparation of peptide-PEG-acrylate derivatives
The following peptides were synthesized as candidates to mediate cell attachment to PEGDA hydrogels: GRGDSP (fibronectin-derived) [31], KQAGDV (fibrinogen-derived) [27, 32], GSWSGSPPRRARVT (fibronectin-derived, syndecan-binding peptide) [33], GKDGEA (collagen I-derived) [34], VAPG (elastin-derived) [35], and YIGSR (laminin-derived) [36]. Peptides were synthesized using a solid phase peptide synthesizer (Applied Biosystems, Model 433A, Foster City, CA) using standard Fmoc chemistry on an amide (Knorr) resin. Peptides were cleaved and deprotected using trifluoroacetic acid, precipitated in ether, dried, purified using reverse phase high performance liquid chromatography (HPLC), and stored lyophilized at −20 °C. Successful peptide synthesis was confirmed by matrix assisted laser desorption/ionization mass spectroscopy (MALDI-MS).
Peptides (added dropwise, 0–15 % molar excess) were reacted with acrylate-PEG-N-hydroxysuccinimide (ACRL-PEG-NHS, 15–55 mg/ml, approximate MW 3400, Layson Bio, Huntsville, AL) in aqueous sodium bicarbonate (pH = 8.4) under argon for at least 2 h. Salts and unreacted peptide were removed by dialysis against water for 1 day (1:400 volume ratio, 3 exchanges minimum). The purified product was lyophilized and stored at −20 °C. Conjugation was confirmed by comparison of MALDI-MS spectra of conjugates with the corresponding acrylate-PEG-COOH formed from hydrolyzed acrylate-PEG-NHS starting material.
2.4. Synthesis of hydrogel substrates
Glass coverslips were cleaned by sonication in chloroform followed by 15 min treatment per surface by radio frequency (RF) glow discharge from argon bubbled through water. Glass was then coated with γ-methacryloxypropyl trimethoxysilane in 95% ethanol/5% water (v/v) adjusted to pH 5 with glacial acetic acid (~0.7% v/v) for 2 h per side, rinsed with copious amounts of pure ethanol, and dried in vacuo in covered glass petri dishes at 110 °C to anneal and sterilize the silanized coverslips. A uniform water-air contact angle greater than 60° confirmed successful modification. Flexible poly(ethylene terephthalate) (PET) sheets (McMaster-Carr, Cleveland, OH) were cleaned by sonication in water then ethanol, treated by RF glow discharge, and sterilized with ethylene oxide.
Hydrogel films were formed on the silanized glass substrates. Hydrogel precursor solutions contained PEGDA (20% w/w), peptide-PEG-acrylate (5–10 mM, 2–4% w/w), 0.1% w/v Irgacure 2959 (1-[4-[2-Hydroxyethoxy]-phenyl]-2-hydroxy-2-methyl-1-propane-1-one, Ciba Specialty Chemicals, Tarrytown, NY) dissolved in phosphate buffered saline (PBS, pH 7.4). The resulting solution was sterilized by filtration (0.22 μm pore). In a sterile field, drops of precursor solution (1 drop per coverslip, 9 μl per cm2) were placed on PET sheets, covered with silanized glass coverslips, and polymerized for 10 min under ultraviolet irradiation (365 nm, 0.4–0.5 mW/cm2). The assembly was inverted, submerged in PBS, and the PET sheet peeled away leaving a thin hydrogel film covalently linked to the glass coverslip. The gel films were incubated in excess PBS for at least 2 h to leach unreacted material from the gels before use in cell experiments.
2.5. Cell culture
Human coronary artery SMCs (HCASMCs, Lonza, Walkersville, MD) were routinely cultured in SmGM-2 (Lonza) growth media which contains 5% fetal bovine serum (FBS) and proprietary amounts of basic fibroblast growth factor, epidermal growth factor, and insulin. Supplied antimicrobials were not added. All cell culture was performed at 37 °C, 5% CO2. All studies presented in this manuscript were performed at passage 7 with a single lot of cells (Lonza lot 6F4008) but similar studies (not shown) were performed with other cell lots and other passages (6–8) with similar results.
2.6. Differentiation of HCASMCs
Hydrogel films (D = 25 mm) formed with 5 mM peptide-PEG-acrylate were placed in 6-well culture places and secured with a ring of silicone tubing. Human fibronectin (FN, 1 μg/cm2) or murine laminin-1 (LN, 2 μg/cm2 from Engelbreth-Holm-Swarm sarcoma) was coated (1–3 h) on tissue culture polystyrene (TCPS) as controls. For immunofluorescence (IF) experiments FN and LN coatings were done on glass coverslips rather than TCPS. Prior to seeding, each surface was washed with PBS and preincubated with serum free attachment medium (SFAM: insulin-transferrin-selenium supplement [ITS-X, 1X, Invitrogen, Carlsbad, CA], taurine [5 mM], bovine serum albumin [BSA, 1 mg/ml] in Dulbecco’s Modified Eagle Medium [DMEM, Invitrogen]). HCASMCs were dissociated with trypsin, resuspended in SFAM, and seeded at a density of 2.2–4.0 × 104 cells/cm2 for mRNA and protein experiments or 1.5 × 104 cells/cm2 for IF experiments. After overnight seeding (14–17 h), SFAM was replaced with low serum (2% FBS) DMEM containing heparin (400 μg/ml) to induce differentiation (LSM+H) or SmGM-2 as a control. HCASMCs were cultured under these conditions for up to 6 d. Media was exchanged every 2 d. To assess ligand induced contractility, HCASMCs were exposed to 0–4 mM carbachol after 6 d of culture in LSM+H. HCASMC morphology was observed under phase contrast microscopy for 5 min after the addition of carbachol as described by others [13].
2.7. Real-time reverse transcription polymerase chain reaction (RT-PCR)
RNA was collected from experimental samples immediately after seeding (14–17 h) and after 2 d and 6 d of culture in LSM+H or SmGM using RNeasy spin columns (Qiagen, Valencia, CA) following the manufacturer’s instructions. Total RNA yield was determined spectroscopically and 130–320 ng RNA was reverse transcribed using the Superscript First Strand Synthesis System (Invitrogen). The resulting cDNA template was diluted 1:16.5 in ultra pure water (Millipore filtration system, resistivity ≥ 18.2 MΩ-cm) and was used for real-time PCR analysis using the iQ SYBR Green Supermix (Bio-rad, Hercules, CA) and an iCycler fluorescence detection system (Bio-rad). Gene specific primers (Table 1) were designed using Oligoperfect software (Invitrogen). All primers sets were designed to amplify all known transcript variants of each gene.
Table 1.
Primers used for real time RT-PCR
| Name | Gene | Product Size (BP) | Primers |
|---|---|---|---|
| Smooth Muscle α-Actin | ACTA2 | 144 | GGCCGAGATCTCACTGACTAAGTGGCCATCTCATTTTCAA |
| Smooth Muscle Basic Calponin | CNN1 | 226 | CCCAGAAGTATGACCACCAGTACTTGGTGATGGCCTTGAT |
| SM22α | TAGLN | 112 | CCAACAAGGGTCCTTCCTATCCACACTGCACTATGATCCA |
| Smooth Muscle Myosin Heavy Chain | MYH11 | 136 | CTGCAGCTTGGAAATATCGTGAGTGAGGATGGATCTGGTG |
| Glyceraldehyde-3-Phosphate Dehydrogenase | GAPDH | 237 | GACCTGACCTGCCGTCTAGTTGCTGTAGCCAAATTCGTT |
A standard curve of pooled cDNA and a no template control were run on each plate for each gene to relate threshold amplification cycle (Ct) to relative transcript abundance. Melt curve analysis was performed after each PCR to ensure single product amplification. Reaction wells with aberrant results were excluded from further analysis (generally fewer than 1 well per plate). Relative transcript abundance was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression assuming 100% PCR efficiency.
2.8. Western Blotting
Protein was collected from experimental samples after seeding (14–17 h) and after 2 d and 6 d of culture in LSM+H or SmGM-2 in radioimmunoprecipitation assay (RIPA) lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA) on ice. The soluble protein content was determined using the bicinchoninic acid (BCA) protein assay (Pierce Biotechnologies, Rockford, IL). Samples were suspended in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (final composition: 62.5 mM Tris [pH 6.8], 2% w/v SDS, 10% v/v glycerol, 5% v/v β-mercaptoethanol, 25 μg/ml bromophenol blue). Protein samples (5 μg per lane) were separated on a Tris-HCl Ready Gel (12% resolving, 4% stacking, Bio-rad) and transferred to a nitrocellulose membrane. Membranes were blocked in 4% (w/v) dried milk and probed for smooth muscle α-actin (200–400 ng/ml, clone 1A4, Santa Cruz), calponin (400 ng/ml, clone CALP, Santa Cruz), and SM-22α (800 ng/ml, polyclonal, H-75, Santa Cruz). The blot was visualized using horseradish peroxidase conjugated secondary antibodies (goat-anti-mouse: 80 ng/ml, goat-anti-rabbit: 100 ng/ml, Santa Cruz) and the SuperSignal West Pico Chemiluminescent detection system (Pierce) using CL-XPosure film (Pierce). Membranes were then stripped in stripping buffer (62.5 mM Tris, 2% SDS, 100 mM β-mercaptoethanol) for 20 min at 50 °C and reprobed for GAPDH (400 ng/ml, clone 0411, Santa Cruz) using the same procedure. Films were scanned using a Hewlitt-Packard ScanJet IIcx and densitometry was performed on the digitized images using ImageJ (NIH). Optical densities were normalized to lane 4 for each probe, then again to GAPDH for each lane before pooling data between experiments.
2.9. Immunofluorescent staining
After 6 d of culture, surfaces were rinsed twice with PBS, fixed in 4% paraformaldehyde in PBS for 10 min, permeabilized in 0.1% (v/v) Triton-X 100, and blocked in blocking buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, 2% BSA). Immunofluorescence (IF) experiments utilized the same primary antibodies as western blotting. For α-actin and calponin co-staining, surfaces were incubated with primary antibodies (500 ng/ml), washed, and visualized with Alexafluor 568 goat-anti-mouse IgG2a (Invitrogen) and Alexafluor 488 goat-anti-mouse IgG1 (Invitrogen), respectively. For visualization of SM-22α, substrates were also blocked with an avidin/biotin blocking kit (Invitrogen), then incubated with anti-SM-22α antibodies (2 μg/ml), washed, incubated with biotinlyated goat-anti-rabbit Fab fragment (1:400 dilution, Jackson Immunoresearch, West Grove, PA), washed, and visualized with Alexfluor-488 conjugated streptavidin (5 μg/ml, Invitrogen). Co-staining for α-actin followed the same procedure as above. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, 1 μM). Fluorescent images were acquired on a Nikon Diphot 100 inverted microscope using a 10X objective with fixed acquisition settings to allow qualitative assessment of protein levels and using a 40X objective with individually optimized acquisition settings to assess intracellular protein organization. Control samples were stained using the above protocols without primary antibodies.
2.10. Attachment to hydrogel substrates
Hydrogel films (D = 15 mm) formed with 10 mM peptide-PEG-acrylate were placed in 24 well culture places and retained with a small ring of silicone tubing to ensure the well bottom was covered only by hydrogel film. Peptide-PEG-acrylate incorporation, and therefore the final peptide concentration in the network, was assumed to be equivalent for all peptides examined. FN and LN were coated on TCPS as controls. Prior to seeding, each surface was washed with PBS and preincubated with SFAM. HCASMCs were non-enzymatically detached (Cellstripper, Mediatech, Manassas, VA), resuspended in SFAM, and seeded near confluence for 16 h. Each substrate was washed gently with Hank’s balanced salt solution supplemented with HEPES (25 mM), MgCl2 (1 mM), CaCl2 (1 mM) and BSA (1 mg/ml) and imaged by phase contrast microscopy. The wash buffer was removed and the samples frozen at −80 °C. Frozen cells were lysed at room temperature using CyQUANT lysis buffer (Invitrogen) containing PicoGreen (Invitrogen). DNA content of the lysate was measured using a fluorescent microplate reader. To account for variations in cell seeding density, results for each experiment were normalized to FN attachment before pooling data between experiments.
For GRGDSP-PEG-acrylate (10 mM) containing hydrogels only, inhibition of attachment by soluble peptide was determined. HCASMCs were incubated with free GRGDSP peptide (2 mM) for 30 min before seeding. Seeding duration was reduced to 1 h. Cell morphology and attachment were determined as described above.
2.11. Statistics
Statistical analysis was done using Microsoft Excel and Minitab 15. Data are represented as mean ± standard deviation of at least triplicate independent experiments. Single comparisons were made using an un-paired student’s t-test. Analysis of variance (ANOVA) followed by Tukey’s post hoc test was used for data sets with multiple comparisons. Statistical analysis of real time PCR data was performed in the logarithmic domain. A value of α < 0.05 was considered significant.
3. RESULTS
3.1. Analysis of functionalized PEG materials
Conjugation of peptides to ACRL-PEG-NHS resulted in a shift in the polymer chain distribution to higher molecular weight on MALDI-MS spectra compared with hydrolyzed ACRL-PEG-NHS approximately equal to the molecular weight of peptide. Hydrolyzed ACRL-PEG-NHS that was dialyzed, but not conjugated, did not result in a right shift of the distribution. Conjugation was also confirmed by a shift in the location of each peak relative to hydrolyzed ACRL-PEG-NHS, although it was not possible to resolve individual chain peaks in the MALDI-MS spectra for all of the conjugates studied.
3.2. Cell morphology during differentiation
Cell morphology was monitored over the course of culture in either LSM+H or SmGM-2 media. Within 2 d of media change, HCASMCs cultured in LSM+H became less-spindle shaped with a hypertrophied cytoplasm that spread to fill gaps between cells and self-oriented in aligned band patterns. HCASMCs cultured in SmGM also self oriented into aligned patterns, but remained smaller and more fusiform in shape. No differences in cell morphology were observed between substrates.
After 6 d of culture in LSM+H, HCASMCs on FN only were exposed to 0–4 mM carbachol. No changes in cell morphology were noted after 5 min of observation.
3.3. Expression of contractile marker mRNA
We observed decreased mRNA expression of α-actin (2.8-fold, p = 0.042), calponin (3.2-fold, p = 0.0012) and SM-22α (1.4-fold, p = 0.053) in HCASMCs seeded on LN compared with FN immediately after the seeding period in serum-free medium (Fig. 1). Expression of α-actin, calponin, and SM-22α was also decreased on LN compared with RGD-gels (1.9-, 2.1-, and 1.2-fold, respectively) but, with the exception of calponin (p = 0.01), these differences were not significant. No significant differences were observed in SM-MHC expression (p > 0.82) or in α-actin, calponin, and SM-22α expression between RGD-gels and FN (p > 0.12).
Figure 1.
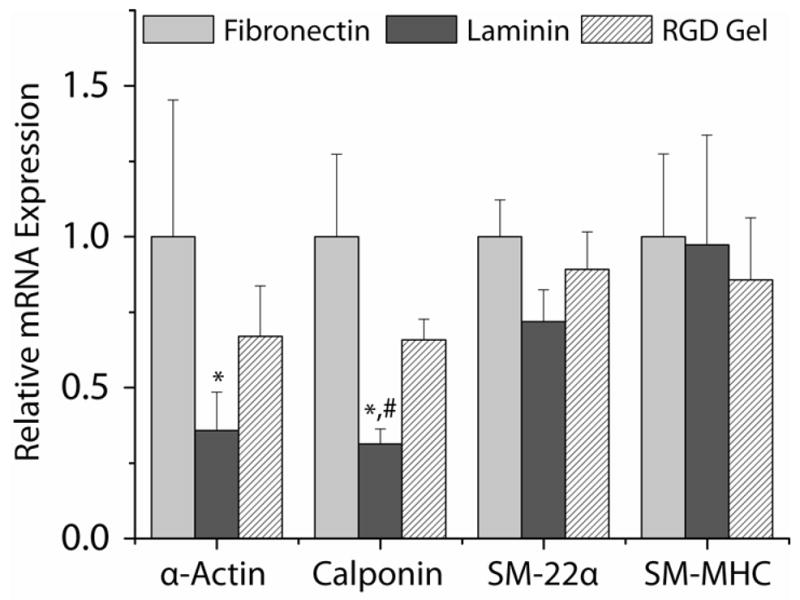
Expression of contractile marker gene mRNA after a 14–17 h seeding period in SFAM. The expression of each gene was normalized to the relative (to GAPDH) expression on fibronectin (FN). *: p < 0.05 with respect to FN, #: p < 0.05 with respect to RGD-gels.
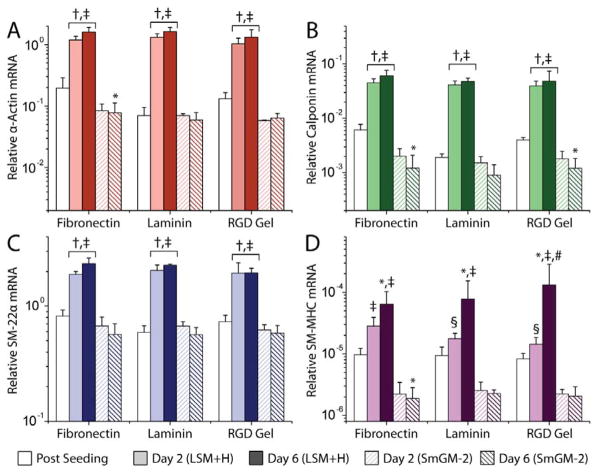
Over 6 d of culture, the pattern of α-actin, calponin, and SM-22α expression followed a similar pattern (Fig. 2ABC). On each substrate, the expression of these markers increased substantially after 2 d in culture in LSM+H compared with expression at the end of the seeding period (Fig. 2ABC, p < 0.0003). Marker expression continued to increase over the remaining 4 days of culture, but was not statistically significant (p > 0.89). The overall level of expression of each gene, relative to GAPDH, was indistinguishable between substrates after 2 and 6 d of culture in LSM+H (p > 0.95). Expression of markers in LSM+H also was significantly higher than cells cultured in parallel on the same substrates in SmGM-2 (Fig. 2ABC, p < 0.0003)
Figure 2.
Expression of α-actin (A), calponin (B), SM-22α (C), and SM-MHC (D) mRNA over 6 d of culture in LSM+H or SmGM. Expression levels shown are relative to GAPDH expression in the same sample. †: p < 0.0003 vs. post seeding level, *: p < 0.05 vs. post seeding level, ‡: p < 0.0003 vs. SmGM-2 (same day, same substrate), §: p < 0.023 vs. SmGM-2 (same day, same substrate), # p < 0.05 vs. Day 2 (same medium, same substrate).
Expression of SM-MHC also increased over 6 d of culture in LSM+H, but did so more slowly than α-actin, calponin, and SM-22α (Fig. 2D). After 2 d of culture in LSM+H, SM-MHC expression increased slightly (1.7–2.9 fold, p > 0.59), but did not become statistically greater than the post-seeding level until the end of the 6 d culture period (6.7–16 fold increase, p < 0.03). SM-MHC expression in LSM+H also was greater than SmGM-2 parallel cultures (Fig 4D, p < 0.03). SM-MHC transcript abundance was 4–6 orders of magnitude less than GAPDH.
Figure 4.
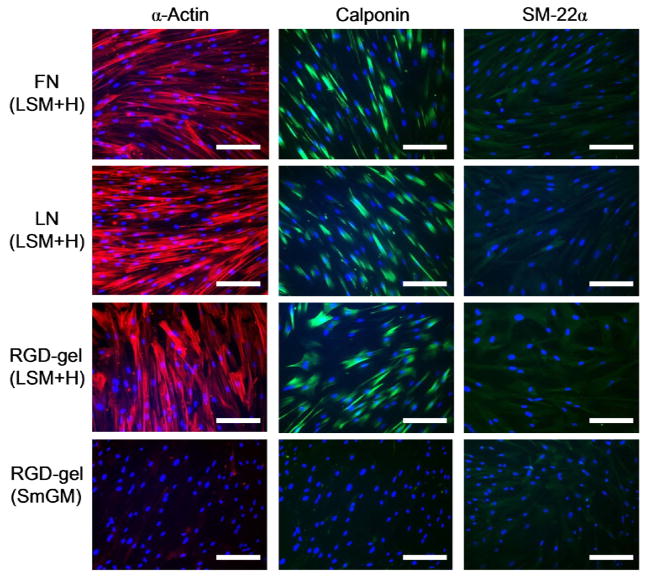
Expression and cellular distribution of α-actin (red, left column), calponin (green, center column), and SM-22α (green, right column) protein after 6 d of culture in LSM+H or SmGM-2 determined by immunofluorescent staining. Immunofluorescent stains of SMCs cultured with SmGM-2 on fibronectin and laminin (not shown) were similar to RGD-gels and showed substantially less staining than SMCs cultured in LSM+H. Staining and acquisition protocols were identical for all images for each protein. Nuclei were counterstained with DAPI (blue). Scale bar = 200 microns.
Expression of all marker proteins decreased slightly from the post-seeding level over 6 d of culture in SmGM-2 on all substrates (Fig. 2, p < 0.05 for several conditions). There were no differences in level of α-actin, calponin, SM-22α, or SM-MHC expression between substrates for SMCs cultured in SmGM (p > 0.95).
3.4. Expression of contractile marker proteins
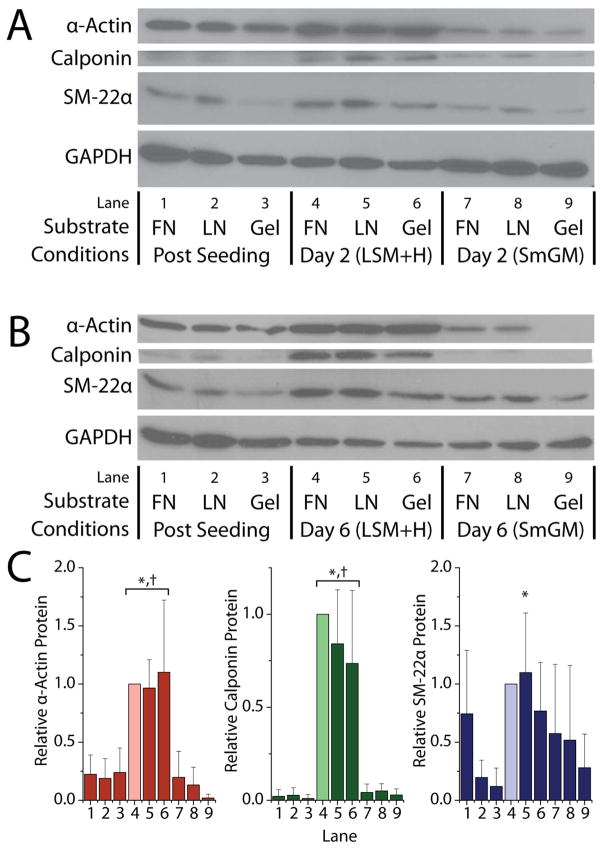
Changes in α-actin, calponin, and SM-22α protein, assessed by western blot (Fig. 3), generally were consistent with observed changes in mRNA expression (Fig. 2). After 2 d of culture in LSM+H, we observed a qualitative increase in band intensity for α-actin, calponin, and SM-22α (Fig. 3A). Semi-quantitative densitometry of these blots revealed a significant increase in α-actin only (p < 0.02) and extensive variability between samples. After 6 d of culture in LSM+H, we also observed a qualitative increase in band intensity for α-actin, calponin, and SM-22α (Fig. 3B) compared with the parallel cultures in SmGM-2 or HCASMCs after seeding. Changes in SM-22α band intensity were subtle, consistent with the modest 2–3 fold change in mRNA levels observed by RT-PCR (Fig 3AB). Semi-quantitative densitometry of these blots revealed significant increases in α-actin and calponin protein compared with post-seeding levels and with parallel cultures in SmGM-2 (p < 0.0012) and a slight increase in SM-22α levels (Fig. 3C). No qualitative or semi-quantitative differences were observed between substrates for any time-points or culture conditions for all three proteins (p > 0.25).
Figure 3.
Relative expression of α-actin, calponin, and SM-22α protein after 2 d (A) or 6 d (B) of culture in LSM+H or SmGM-2 on fibronectin (FN), laminin (LN) and RGD-gels (Gel). Densitometry (C) was performed on 6 d blots from independent experiments (N = 5) and normalized to lane 4 (lighter shading). *: p < 0.05 vs. post seeding levels, †: p < 0.001 vs. Day 6-SmGM-2 levels.
IF staining also revealed substantially more α-actin and calponin protein in cells cultured in LSM+H for 6 d compared with SmGM-2 (Fig. 4). No difference in the intensity of staining was observed between HCASMCs on FN, LN, or RGD-gels. Moderate variations in expression were observed within the cell population, but IF staining was still strong in nearly all of the cells, suggesting that the entire population was responding uniformly. In wide field images, no differences in SM-22α IF staining intensity between samples were observed (Fig. 4, right column).
3.5. Intracellular organization of contractile marker proteins
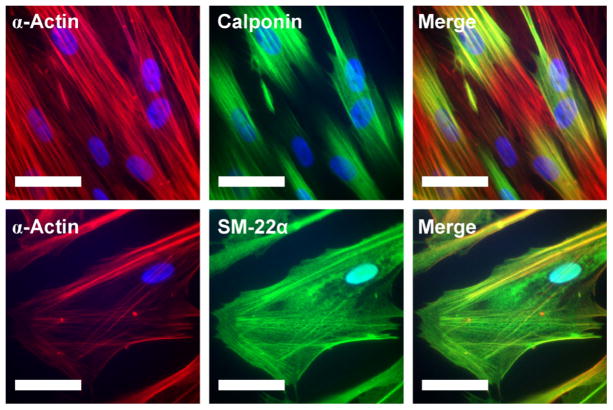
High magnification IF staining for α-actin, calponin, and SM-22α revealed that expressed proteins co-localized into filaments within the cytoplasm of HCASMCs cultured in LSM+H for 6 d (Fig 5). Filaments of α-actin were aligned with the long axis of the cells. Calponin co-localized most strongly with α-actin stress fibers in the central region of the cells and diminished in staining intensity towards the cell borders (Fig. 5A). SM-22α stained more weakly than calponin, but co-localized with α-actin stress fibers evenly throughout the entire cytoplasm (Fig 5B). SM-22α staining was not homogenous through the cell population, but was more prominent in a small subset of cells, with many cells showing minimal staining. No differences in co-localization were observed between FN, LN, and RGD-gel substrates. Controls stained without primary antibody resulted in weak, diffuse background staining with no filamentous α-actin, calponin, or SM-22α structures.
Figure 5.
Intracellular organization of α-actin (red), calponin (green, top row), and SM-22α (green, bottom row) protein after 6 d of culture in LSM+H on RGD-gels determined by immunofluorescent staining. Co-localization is indicated by yellow in the right column. Nuclei were counterstained with DAPI (blue). Scale bar = 50 microns.
3.6. Attachment to hydrogel substrates
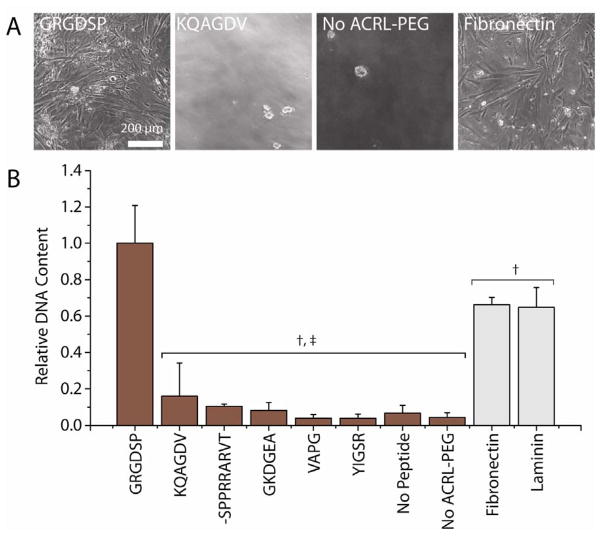
After a 16 h seeding period, SMCs attached and spread only on GRGDSP-PEG-ACRL containing hydrogels (RGD-gels). After washing, the adherent cells were well-spread on RGD-gels, FN, and LN substrates, but rounded cell morphology was observed for the sparse population of adherent cells on the other substrates (Fig. 6A). Compared with FN, LN, and RGD-gels, the DNA content of all of the other hydrogel substrates was reduced by at least 84% (p < 0.0001) (Fig. 6B). Among the non-RGD-gels, there were no significant differences in DNA content (p > 0.887). We also observed a small decrease (~35%) in measured DNA content on the FN and LN samples compared with the RGD-gels (p < 0.0001), which was not attributable directly to differences in the PicoGreen assay response to the hydrogel substrate compared with protein coated TCPS.
Figure 6.
Cell morphology (A) and attachment density (B) to additional peptide modified PEGDA hydrogels after 16 h in SFAM. Phase contrast micrographs (A) for peptide-gels not shown were similar to KQAGDV containing gels and gels containing no ACRL-PEG. Cell attachment and spreading on laminin was similar to fibronectin. Attachment was quantified by measuring DNA content (B) reported relative to GRGDSP-containing (10 mM) hydrogels. †: p < 0.0001 vs. GRGDSP containing gels, ‡: p < 0.0001 vs. FN or LN coated tissue culture polystyrene. Scale bar = 200 microns.
Soluble GRGDSP peptide (2 mM) reduced the post-seeding DNA content on GRGDSP-containing (10 mM) hydrogels by 60% (p < 0.03), which was similar to attachment to hydrogels with no peptide (p = 0.70). In the presence of GRGDSP peptide, the number of cells on the surface with a spread morphology was drastically reduced, with cell morphology generally more similar to that on unmodified hydrogels. Overall, cell attachment after 1 h incubation was reduced and attachment to GRGDSP hydrogels was less than FN controls.
4. DISCUSSION
The aim of this study was to investigate the ability of synthetic, peptide bearing hydrogel scaffolds to support the re-differentiation of cultured vascular SMCs toward a contractile cell phenotype. A photopolymerizable PEGDA hydrogel modified with pendant GRGDSP peptides was utilized as a scaffold material with well defined SMC-material interactions. This system allowed us to explore the role that a specific RGD ligand-cell interaction has on the ability of cultured HCASMCs to re-differentiate to a contractile phenotype in vitro. Once seeded on these substrates, we utilized low serum culture conditions and soluble heparin to drive SMC differentiation and monitored phenotype using a panel of markers at the level of mRNA, protein, and intracellular organization.
Here we showed that FN, LN, and RGD-gels support equivalent levels of expression of contractile markers, α-actin, calponin, and SM-22α, over a 6 d culture period at the transcriptional and translational level and that these markers are organized into filaments within the cell. By these measures, we achieved a significant shift in cell phenotype on our chemically well-defined RGD-gel system. These results are consistent with other studies of vascular SMC phenotype on PEG-based gels [27, 30] which demonstrate the qualitative presence of contractile SMC markers such as α-actin, calponin, and caldesmon detected by immunostaining. Our results quantify these changes at the mRNA and protein level, and indicate that with appropriate culture conditions, phenotype switching can occur rapidly, with significant changes in the transcriptional profile and protein levels after only 2 d of culture. Furthermore, we demonstrated that changes in marker levels were also associated with distinct organization of contractile proteins into fibrillar structures in the cell cytoplasm. This organization was critical to distinguish positive staining from diffuse intracellular background in IF studies, which was very similar to control slides without primary antibodies. We also noted weak fibrillar staining for α-actin, calponin, and SM-22α (Fig. 4) in SMCs cultured in SmGM-2, which without comparison to cells cultured in LSM+H may have caused them to appear differentiated. These issues suggest that IF studies alone are not sufficient to study the phenotype of isolated SMC cultures. While our study examined the contractile state of SMCs on selected substrates, we did not examine other issues that might impact the differentiation of SMCs such as variation in the ligand density of RGD or the mechanical properties of the underlying substrate. Since these issues have been shown to affect cell proliferation and collagen production [27, 29], the roles these factors play on SMC differentiation will be excellent candidates for additional study using the quantitative methods described here.
While dramatically bringing their marker profile closer to a contractile phenotype, the cells did not show evidence of contraction in response to carbachol. Furthermore, the overall level of expression of SM-MHC mRNA was significantly lower than the other markers and the SM-MHC protein was not detectable by western blotting or IF (not shown). This molecule is not only a marker of cell phenotype, but a critical component of the contractile machinery. Taken together, these observations suggest the contractile apparatus was not reformed completely. These results also illustrate the importance of completely characterizing cell phenotype quantitatively and that even with the achievement of quantitative increases in several marker proteins, more work is needed to restore a truly contractile phenotype to cultured SMCs.
Extensive literature suggests that FN plays an important role in the loss of contractile SMC phenotype during primary culture while LN may delay this transition [13, 17–20]. The effect of FN could also be mimicked by the RGD-ligand alone [21]. Our experiments were designed to assess the roles these ECM ligands have on the re-differentiation of cultured vascular SMCs, which has not been studied extensively. We observed decreased expression of contractile marker mRNA (with no differences in protein levels) on the LN substrate compared with FN and RGD-gels after seeding in serum free media. This result was the opposite of that anticipated if cultured cells behaved similarly to primary vascular SMCs. These results suggest that during expansion culture, the signaling machinery in native SMCs may be reprogrammed to respond differently to laminin. Such changes in signaling machinery have been observed in cultured SMCs with IGF-1, which initially helps to maintain the contractile phenotype of primary rat aortic SMCs, but after de-differentiation in culture stimulates proliferation and migration [37].
However, it is also possible that low initial contractile marker expression on LN may have resulted from poor initial adhesion on LN rather than altered response to SMC-LN interactions per se. Others have suggested that there may be a correlation between focal adhesion formation and SMC differentiation [27]. We observed differences in SMC morphology between LN and FN during the early stages of the adhesion process (after 0–4 h, not shown), but we found that after 4 h cells seeded on LN did not form attachments sufficient to withstand a change in medium. However, cell morphology was indistinguishable between the substrates after overnight seeding. Others have shown that cultured baboon SMCs plated on LN developed a well formed provisional FN matrix after 4 h of culture, effectively masking the LN substrate [38]. Although we did not measure FN production directly, our morphological observations were consistent with our human SMCs also organizing a FN matrix within the first few hours of culture which subsequently provided robust cell adhesion but delayed SMC marker expression. The production and organization of endogenous FN also may have explained why the initial differences in cell phenotype on LN did not persist during the remainder of the differentiation period, especially after the addition of LSM+H, which contains exogenous cell-adhesive serum proteins including FN.
To address this issue, we prepared hydrogels containing peptides from LN and other ECM proteins in an effort to directly assess the role of ligand identity on modulation of SMC differentiation. In contrast to LN coated TCPS, the hydrogel substrates were designed to resist the adsorption of exogenous protein. We screened a variety of peptides derived from ECM proteins including those known to modulate SMC phenotype: KQAGDV (fibrinogen) [27, 32], GKDGEA (type I collagen) [34], YIGSR (laminin) [36], and VAPG (elastin) [35]. We also assessed a heparin binding peptide, - SPPRRARVT [33], in an attempt to engage the syndecan family of receptors that is known to be important for SMC adhesion [39]. Except for GRGDSP, we did not observe attachment or spreading on any of the peptide-containing hydrogels, which precluded us from performing differentiation studies on these surfaces. This result was unanticipated since all of the above peptides have documented interactions with receptors known to be expressed on SMCs [39] or have been empirically shown to bind SMCs [27, 32]. Many of the published cell attachment studies used a solid substrate to present the peptides which does not resist protein adsorption to the same degree as PEG. In the PEGDA hydrogels we employed, no cell adhesion was observed unless the hydrogels were modified with a pendant peptide, making this a more rigorous system for assessing cell-peptide interactions in the context of cell adhesion.
However, in the case of KQAGDV and VAPG, SMC attachment has been reported on PEGDA hydrogels modified with these peptides [27, 40]. In these studies, specific attachment either was not confirmed by inhibition of attachment with soluble peptide [27] or soluble peptide did not inhibit attachment [40]. These studies also did not exclude the possibility of non-specific or serum dependent adhesion. These results also highlight the potential for SMC species differences, lot-to-lot differences in cell response, or changes in cell receptor library due to slightly different expansion culture techniques, although we found similar results for VAPG and YIGSR surfaces with two additional lots of human SMCs (not shown) and other groups have reported difficulty establishing SMC attachment to YIGSR-bearing hydrogels [28]. Our results, obtained by seeding in serum free media on protein adsorption resistant PEGDA hydrogels, suggests that there is variable cell response in SMC-peptide interactions and that, among the peptides tested, only the GRGDSP or its derivatives can provide reliable, peptide-specific cell attachment to these gels. We have also confirmed that SMC attachment to RGD-gels was inhibited by soluble peptide and did not occur on non-RGD bearing hydrogels. These results indicate that SMC attachment to these scaffolds, at least initially, is highly specific to the RGD ligand.
Due to the well-defined chemistry of the PEGDA system, the overall properties of the gel and the concentration of this ligand can be controlled quantitatively and independently [41]. Here, we have demonstrated that the RGD-gels can support differentiation of cultured SMCs toward a contractile phenotype. Consequently, this scaffold material will be an excellent candidate for further development as a bioactive scaffold for smooth muscle tissue engineering.
Furthermore, our results suggest that cultured SMCs may respond differently to the extracellular matrix than primary cells, which is an important design consideration for scaffold systems intended for ex vivo expanded cells. Since the population of SMCs that would likely populate a regeneration-inducing TEBV in vivo would also adopt a synthetic phenotype (in order to migrate and proliferate in the scaffold), scaffold-cell interactions might be best designed to accommodate a synthetic-to-contractile switch, rather than inhibit de-differentiation to the synthetic phenotype. Our results suggest that a fibronectin or fibronectin-mimicking scaffold, such as the RGD-gels employed here, may be superior for this purpose.
5. CONCLUSIONS
In this report, we demonstrated that FN, LN, and RGD-gels support equivalent levels of expression of the contractile markers, α-actin, calponin, and SM-22α over a 6 d culture period in low serum media at the transcriptional and translational level and that these markers are organized into filaments within the cell. These results indicate that SMCs cultured on RGD-bearing hydrogels can re-differentiate toward a contractile phenotype suggesting this material is an excellent candidate for further development as a bioactive scaffold that regulates SMC phenotype in TEBV designs.
Acknowledgments
The project described was supported by Grant Number 5R01EB002067 for the National Institute of Biomedical Imaging and Bioengineering and Grant Number 1R01HL087843 for the National Heart, Lung, and Blood Institute. JAB also was supported by NIH T32GM07250 and American Heart Association Predoctoral Fellowship 0715422B. We would like to acknowledge the technical assistance of Dr. Faina Kligman in the synthesis and purification of peptides, Dr. Meghan Pennini in developing RT-PCR assays, and Domenick Prosdocimo in developing western blot assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brewster LP, Bufallino D, Ucuzian A, Greisler HP. Growing a living blood vessel: insights for the second hundred years. Biomaterials. 2007;28(34):5028–5032. doi: 10.1016/j.biomaterials.2007.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231(4736):397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 3.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional arteries grown in vitro. Science. 1999;284(5413):489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 4.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12(3):361–365. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reidy MA. Regulation of Arterial Smooth Muscle Growth. In: Schwartz SM, Mecham RP, editors. The vascular smooth muscle cell: molecular and biological responses to the extracellular matrix. San Diego: Academic Press; 1995. p. xvi.p. 410. [Google Scholar]
- 6.Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev. 1979;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Stegemann JP, Hong H, Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol. 2005;98(6):2321–2327. doi: 10.1152/japplphysiol.01114.2004. [DOI] [PubMed] [Google Scholar]
- 8.Hedin U, Daum G, Clowes AW. Heparin inhibits thrombin-induced mitogen-activated protein kinase signaling in arterial smooth muscle cells. J Vasc Surg. 1998;27(3):512–520. doi: 10.1016/s0741-5214(98)70326-x. [DOI] [PubMed] [Google Scholar]
- 9.Pukac L, Huangpu J, Karnovsky MJ. Platelet-derived growth factor-BB, insulin-like growth factor-I, and phorbol ester activate different signaling pathways for stimulation of vascular smooth muscle cell migration. Exp Cell Res. 1998;242(2):548–560. doi: 10.1006/excr.1998.4138. [DOI] [PubMed] [Google Scholar]
- 10.Pukac LA, Carter JE, Ottlinger ME, Karnovsky MJ. Mechanisms of inhibition by heparin of PDGF stimulated MAP kinase activation in vascular smooth muscle cells. J Cell Physiol. 1997;172(1):69–78. doi: 10.1002/(SICI)1097-4652(199707)172:1<69::AID-JCP8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 11.Daum G, Hedin U, Wang Y, Wang T, Clowes AW. Diverse effects of heparin on mitogen-activated protein kinase-dependent signal transduction in vascular smooth muscle cells. Circ Res. 1997;81(1):17–23. doi: 10.1161/01.res.81.1.17. [DOI] [PubMed] [Google Scholar]
- 12.Rauch BH, Millette E, Kenagy RD, Daum G, Clowes AW. Thrombin- and factor Xa-induced DNA synthesis is mediated by transactivation of fibroblast growth factor receptor-1 in human vascular smooth muscle cells. Circ Res. 2004;94(3):340–345. doi: 10.1161/01.RES.0000111805.09592.D8. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi K, Saga H, Chimori Y, Kimura K, Yamanaka Y, Sobue K. Differentiated phenotype of smooth muscle cells depends on signaling pathways through insulin-like growth factors and phosphatidylinositol 3-kinase. J Biol Chem. 1998;273(44):28860–28867. doi: 10.1074/jbc.273.44.28860. [DOI] [PubMed] [Google Scholar]
- 14.Bjorkerud S. Effects of transforming growth factor-beta 1 on human arterial smooth muscle cells in vitro. Arterioscler Thromb. 1991;11(4):892–902. [PubMed] [Google Scholar]
- 15.Kim BS, Nikolovski J, Bonadio J, Mooney DJ. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol. 1999;17(10):979–983. doi: 10.1038/13671. [DOI] [PubMed] [Google Scholar]
- 16.Williams B. Mechanical influences on vascular smooth muscle cell function. J Hypertens. 1998;16(12 Pt 2):1921–1929. doi: 10.1097/00004872-199816121-00011. [DOI] [PubMed] [Google Scholar]
- 17.Hedin U, Bottger BA, Forsberg E, Johansson S, Thyberg J. Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol. 1988;107(1):307–319. doi: 10.1083/jcb.107.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayward IP, Bridle KR, Campbell GR, Underwood PA, Campbell JH. Effect of extracellular matrix proteins on vascular smooth muscle cell phenotype. Cell Biol Int. 1995;19(10):839–846. doi: 10.1006/cbir.1995.1019. [DOI] [PubMed] [Google Scholar]
- 19.Thyberg J, Hultgardh-Nilsson A. Fibronectin and the basement membrane components laminin and collagen type IV influence the phenotypic properties of subcultured rat aortic smooth muscle cells differently. Cell Tissue Res. 1994;276(2):263–271. doi: 10.1007/BF00306112. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, Yamamoto K, Noumura T. Type I collagen promotes modulation of cultured rabbit arterial smooth muscle cells from a contractile to a synthetic phenotype. Exp Cell Res. 1993;204(1):121–129. doi: 10.1006/excr.1993.1016. [DOI] [PubMed] [Google Scholar]
- 21.Hedin U, Bottger BA, Luthman J, Johansson S, Thyberg J. A substrate of the cell-attachment sequence of fibronectin (Arg-Gly-Asp-Ser) is sufficient to promote transition of arterial smooth muscle cells from a contractile to a synthetic phenotype. Dev Biol. 1989;133(2):489–501. doi: 10.1016/0012-1606(89)90052-3. [DOI] [PubMed] [Google Scholar]
- 22.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23(22):4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 23.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39(2):266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Mann BK, Gobin AS, Tsai AT, Schmedlen RH, West JL. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22(22):3045–3051. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 25.Nuttelman CR, Tripodi MC, Anseth KS. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J Biomed Mater Res A. 2004;68(4):773–782. doi: 10.1002/jbm.a.20112. [DOI] [PubMed] [Google Scholar]
- 26.Hwang NS, Varghese S, Zhang Z, Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006;12(9):2695–2706. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 27.Peyton SR, Raub CB, Keschrumrus VP, Putnam AJ. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27(28):4881–4893. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Fittkau MH, Zilla P, Bezuidenhout D, Lutolf MP, Human P, Hubbell JA, et al. The selective modulation of endothelial cell mobility on RGD peptide containing surfaces by YIGSR peptides. Biomaterials. 2005;26(2):167–174. doi: 10.1016/j.biomaterials.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Mann BK, West JL. Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J Biomed Mater Res. 2002;60(1):86–93. doi: 10.1002/jbm.10042. [DOI] [PubMed] [Google Scholar]
- 30.Hahn MS, McHale MK, Wang E, Schmedlen RH, West JL. Physiologic pulsatile flow bioreactor conditioning of poly(ethylene glycol)-based tissue engineered vascular grafts. Ann Biomed Eng. 2007;35(2):190–200. doi: 10.1007/s10439-006-9099-3. [DOI] [PubMed] [Google Scholar]
- 31.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 32.Mann BK, Tsai AT, Scott-Burden T, West JL. Modification of surfaces with cell adhesion peptides alters extracellular matrix deposition. Biomaterials. 1999;20(23–24):2281–2286. doi: 10.1016/s0142-9612(99)00158-1. [DOI] [PubMed] [Google Scholar]
- 33.Sagnella S, Anderson E, Sanabria N, Marchant RE, Kottke-Marchant K. Human endothelial cell interaction with biomimetic surfactant polymers containing Peptide ligands from the heparin binding domain of fibronectin. Tissue Eng. 2005;11(1–2):226–236. doi: 10.1089/ten.2005.11.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staatz WD, Fok KF, Zutter MM, Adams SP, Rodriguez BA, Santoro SA. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J Biol Chem. 1991;266(12):7363–7367. [PubMed] [Google Scholar]
- 35.Mecham RP, Hinek A, Entwistle R, Wrenn DS, Griffin GL, Senior RM. Elastin binds to a multifunctional 67-kilodalton peripheral membrane protein. Biochemistry. 1989;28(9):3716–3722. doi: 10.1021/bi00435a014. [DOI] [PubMed] [Google Scholar]
- 36.Graf J, Ogle RC, Robey FA, Sasaki M, Martin GR, Yamada Y, et al. A pentapeptide from the laminin B1 chain mediates cell adhesion and binds the 67,000 laminin receptor. Biochemistry. 1987;26(22):6896–6900. doi: 10.1021/bi00396a004. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi K, Shibata K, Morita T, Iwasaki K, Watanabe M, Sobue K. Insulin receptor substrate-1/SHP-2 interaction, a phenotype-dependent switching machinery of insulin-like growth factor-I signaling in vascular smooth muscle cells. J Biol Chem. 2004;279(39):40807–40818. doi: 10.1074/jbc.M405100200. [DOI] [PubMed] [Google Scholar]
- 38.Hedin UL, Daum G, Clowes AW. Disruption of integrin alpha 5 beta 1 signaling does not impair PDGF-BB-mediated stimulation of the extracellular signal-regulated kinase pathway in smooth muscle cells. J Cell Physiol. 1997;172(1):109–116. doi: 10.1002/(SICI)1097-4652(199707)172:1<109::AID-JCP12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Moiseeva EP. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res. 2001;52(3):372–386. doi: 10.1016/s0008-6363(01)00399-6. [DOI] [PubMed] [Google Scholar]
- 40.Gobin AS, West JL. Val-ala-pro-gly, an elastin-derived non-integrin ligand: smooth muscle cell adhesion and specificity. J Biomed Mater Res A. 2003;67(1):255–259. doi: 10.1002/jbm.a.10110. [DOI] [PubMed] [Google Scholar]
- 41.Beamish JA, Zhu J, Kottke-Marchant K, Marchant RE. The effects of monoacrylated poly(ethylene glycol) on the properties of poly(ethylene glycol) diacrylate hydrogels used for tissue engineering. J Biomed Mater Res. 2009 doi: 10.1002/jbm.a.32353. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]